Abstract
Cerium oxide nanoparticles are known for their antibacterial effects resulting from Ce3+ to Ce4+ conversion. Application of such cerium oxide nanoparticles in dentistry has been previously considered but limited due to deterioration of mechanical properties. Hence, this study aimed to examine mesoporous silica (MCM-41) coated with cerium oxide nanoparticles and evaluate the antibacterial effects and mechanical properties when applied to dental composite resin. Cerium oxide nanoparticles were coated on the MCM-41 surface using the sol–gel method by adding cerium oxide nanoparticle precursor to the MCM-41 dispersion. The samples were tested for antibacterial activity against Streptococcus mutans via CFU and MTT assays. The mechanical properties were assessed by flexural strength and depth of cure according to ISO 4049. Data were analyzed using a t-test, one-way ANOVA, and Tukey’s post-hoc test (p = 0.05). The experimental group showed significantly increased antibacterial properties compared to the control groups (p < 0.005). The flexural strength exhibited a decreasing trend as the amount of cerium oxide nanoparticle-coated MCM-41 increased. However, the flexural strength and depth of cure values of the silane group met the ISO 4049 standard. Antibacterial properties increased with increasing amounts of cerium oxide nanoparticles. Although the mechanical properties decreased, silane treatment overcame this drawback. Hence, the cerium oxide nanoparticles coated on MCM-41 may be used for dental resin composite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
For the past generation, amalgam has been widely used in dentistry for traditional tooth preparations. However, its usage has declined gradually due to disadvantages such as color disharmony and mercury poisoning1. Moreover, development in resin composite has increased their popularity2,3. Resin composites are a combination of inorganic fillers and monomers, and unlike amalgam, they offer advantages, such as an esthetic tooth-like appearance, ease of application, and excellent biocompatibility. However, one of the main drawbacks of resin composite is that the resin composite contracts as the monomer molecules bond closer together during polymerization. This phenomenon, known as polymerization shrinkage4,5, creates gaps between the tooth and the restoration. Microleakage and poor oral hygiene habits may lead to the infiltration of dental tissues by caries-producing bacteria, such as Streptococcus mutans, resulting in secondary caries6,7. Many studies have focused on enhancing the antibacterial properties of resin composites to minimize the risk of secondary caries8.
The inorganic fillers in resin composite mainly consist of silica and zirconia particles, which are added to enhance strength. Additionally, various inorganic substances, such as zinc (Zn) and silver (Ag), are incorporated as nanoparticles to confer antibacterial properties9,10. Recently, cerium (Ce), an inorganic material known for its antibacterial properties, has gained renewed attention, leading to numerous studies11. Cerium is one of the most common rare earth elements with various applications due to its unique transition from Ce3+ to Ce4+ valence states. In particular, cerium oxide nanoparticles (CeO2) are known for their superoxide dismutase (SOD)-mimicking properties11. The term “nanoezyme” refers to a nano-inorganic compound mimicking enzymes12. The H2O2 produced in this process can specifically target bacteria and exhibit antibacterial activity13. However, when nanoparticles, such as zinc, silver, and cerium, are added to resin composites in their raw form, they tend to aggregate due to the high specific surface area of the nanoparticles14. This aggregation interferes with bonding to monomer, thus decreasing mechanical strength14,15. Therefore, many studies have focused on uniformly dispersing particles by coating antibacterial inorganic materials with other inorganic materials or applying antibacterial inorganic materials onto substrates, such as silica16,17. This study used mesoporous silica, MCM-41, to coat the cerium oxide nanoparticles within the mesoporous structure and incorporated this combination as an inorganic component in the resin composite. MCM-41 is mesoporous silica characterized by pores typically ranging from 2 to 8 nm in size18. It is also known for its drug delivery capabilities, ensuring uniform drug release over a specific period19,20,21. MCM-41 enhances mechanical strength through increased bonding with monomers because of its large surface area22,23. This interaction counteracts the reduction in mechanical strength resulting from nanoparticles added to the resin composite via the large surface area of MCM-4123,24.
Hence, this study aimed to assess the antibacterial capacity by varying the amount of cerium oxide nanoparticles coated on MCM-41 while also evaluating the corresponding mechanical properties. The null hypothesis of this study is that there will be no significant difference in antibacterial properties between the group using mesoporous silica (MCM-41) coated with cerium oxide nanoparticles as a filler for resin composite and the group using mesoporous silica (MCM-41) as a filler for resin composite. There will be no significant difference in mechanical strength between the groups using and not using mesoporous silica (MCM-41) coated with cerium oxide nanoparticles as a filler for resin composite.
Materials and method
Materials
A glycerolate dimethacrylate (Bis-GMA), tri(ethylene glycol) dimethacrylate (TEGDMA), camphorquinone (CQ), ethyl-4-dimethylaminobenzoate (4-EDMAB), cetyltrimethylammonium bromide (CTAB), tetraethyl orthosilicate (TEOS), ammonium hydroxide (NH4 OH) solution, cerium (III) nitrate hexahydrate (Ce(NO3)3·6H2 O), (3-Aminopropyl)triethoxysilane (APTES) and sodium hydroxide (NaOH) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All chemicals were used without further purification.
Preparation of mesoporous silica (MCM-41) coated with cerium oxide nanoparticles (MSN-C)
Mesoporous silica (MCM-41) particles were synthesized using a modification of the procedure reported by Boccardi et al.25. Briefly, this method is a combination of the standard pathway and the modified Stöber method in which 0.2 g of n-hexadecyltrimethylammonium bromide (CTAB) was dissolved in 18 mL of ethanol, 18.5 mL of ammonia, and 11 mL of deionized water mixture. Then, 1 mL of tetraethyl orthosilicate (TEOS) was added under continuous stirring. After stirring, the precipitate was centrifuged and washed with deionized water and ethanol several times. The MCM-41 particles were calcined at 60 °C (2 °C/min) for 12 h and 550 °C (2 °C/min) for 6 h. Cerium oxide solution was prepared using a peptization method reported by Oh et al.26. 1 g of cerium oxide nanoparticles were dispersed in 20 mL of deionized water, and the pH was adjusted to 0.1 by nitric acid. The cerium oxide solution was heated for 2 h at 40 °C. After heating, the cerium oxide solution was cooled down to room temperature. Finally, MCM-41 coated with cerium oxide nanoparticles was synthesized following the method suggested by Oh et al.26, and 0.1 g of MCM-41 particles were dispersed in 30 mL of deionized water. 1.0–4.0 mL of cerium oxide solution was added to the MCM-41 solution. After stirring for 30 min, the mixing solution was adjusted to pH 6.8 by ammonium hydroxide solution and stirred at 60 °C for 4 h. The resulting particles were centrifuged, washed with deionized water several times, dried at 50 °C for 24 h, washed again with deionized water, and dried at 90 °C. The details of MCM-41 coated with cerium oxide nanoparticle groups are listed in Table 1.
Functionalization of synthesized fillers with silane
The synthesized MSN-C and MCM-41 particles were grafted surface using APTES reported by Chen and Brauer27. In short, APTES and n-propylamine were added to 5 mL of cyclohexane in a 2% volume solution. Then, 100 mg of the synthesized particles were added to the mixed solution and stirred for 2 h. The surface-modified particles were washed thrice and dried under 0.1 mbar for 4 h. For the confirmation of the silane treatment, Fourier Transform Infrared Spectroscopy (FT-IR, INVENIO, Bruker, Billerica, MA, USA) analyses were carried out.
Characterization of MSN-C
The MSN-C particle structure was identified through transmission electron microscopy (TEM) (JEM-F200, JEOL, Tokyo, Japan). Energy dispersive X-ray spectroscopy (EDS) (JEM-F200, JEOL, Tokyo, Japan) mapping was used for the elemental distribution analysis of silicon (Si) and cerium (Ce) elements. Before the TEM-EDS analysis, MSN-C particles (mass of 1% or less) were dispersed in deionized water and then dropped onto carbon-coated copper grids. BET equipment (Autosorb IQ, Quantachrome, USA) was used to measure the porosity of the particles. Pore size and pore volume were calculated using the Barrett–Joyner–Halenda (BJH) method28. Ce4+ and Ce3+ values were calculated using X-ray photoelectron spectroscopy (XPS) (K-alpha, Thermo U. K., USA). The XPS analysis was performed using Al Kα radiation (1486.6 eV) at 12 kV and 3 mA over a rectangular area of 400 × 400 μm. To confirm the released Ce ions from the dental resin composite prepared as following methods, the specimens were immersed in distilled water for 24 h and 48 h at 37 °C. Then the obtained extracts were subjected to the inductively coupled plasma optical emission spectrometer (ICP-OES, ICP Optima 8300, Perkin Elmer, Waltham, MA, USA).
Preparation of the resin composite
The resin matrix was prepared by mixing 49.5 wt% of Bis-GMA, 49.5 wt% of TEGDMA, and photoinitiators of CQ and 4-EDMAB (0.2 and 0.8 wt%, respectively). The resin composite was then prepared by mixing 85 wt% (88.33 vol%) of the resin matrix as prepared in accordance to ratio stated earlier and 15 wt% (11.67 vol%) of resin filler, based on the previous result16. The detailed filler content of the experimental group is listed in Table 2.
The filler was added to the resin and uniformly dispersed using the speed mixer (Hauschild SpeedMixer DAC 150, FlackTech Inc., Hauschild, Germany) at 3500 rpm for 1 min. The specimen was prepared by placing a resin composite in a metal mold and curing it with a light irradiator (Elipar™ DeepCure-L Curing Light, 3 M ESPE, Seefeld, Germany).
Antibacterial activity
Streptococcus mutans (ATCC 25175, S. mutans) was used for antibacterial activity. Streptococcus mutans was subcultured on brain heart infusion (BHI) broth medium at 37 °C. The antibacterial properties of the synthesized particles were evaluated using the minimum inhibitory concentration (MIC) method. Streptococcus mutans suspension was adjusted to 0.5 McFarland (1.5 × 108 colony forming units (CFU)/mL) and then added to synthesized particles. The mixed suspension was incubated in a 37 °C incubator for 24 h. The MIC was determined using a microplate spectrophotometer (Epoch; BioTek, Winooski, VT, USA) at 550 nm29. The antibacterial activity was evaluated (according to the previous method)30 on particles surface-modified with silane and particles without modification. First, the resin composite was poured into Φ10 × 2 mm metal molds and then cured for 60 s. All specimens (n = 10) were sterilized with EO gas before testing. The specimens were immersed in S. mutans suspension (1 mL, 6 × 107/mL, OD600 nm = 0.6) at 37 °C for 24 h. After incubation, the eluted suspension was tested by colony forming units (CFU) and MTT (3-(4,5-dimetylthiazol-2-yl)-2,5-dyphenyltetrazolium bromide). CFU was evaluated by diluting the S. mutans suspension, followed by spreading (100 µL) on an agar plate. The colonies were then counted by naked eyes, and CFU was calculated by converting the values in accordance to initial dilution factors. MTT (0.5 mg/mL) analysis used a colorimetric indicator. 10 µL of MTT solution was mixed with eluted S. mutans suspension (100 µL) and then stored in a 37 °C incubator for 3 h. Next, 100 µL of dimethyl sulfoxide (DMSO) was added into the mixed suspension and stored at room temperature for 30 min. The absorbance was measured at 570 nm using a microplate spectrophotometer. The bacterial adhesion test was conducted using the same method described above. 1 mL of S. mutans suspension (OD600 nm = 0.6) was placed on the disc-shaped specimen (as above) and cultured for 24 h.
Flexural strength
The flexural strength was measured for groups with filler particle surface-modified both with and without silane. The specimens (n = 10) were prepared by a 2 × 2 × 25 mm stainless steel mold. Using a universal testing machine (Instron 5942, Norwood, MA, USA), 1 kN load was applied at a crosshead speed of 1 mm/min. Flexural strength (σ) was calculated using the following equations:
where F represents the maximum load applied to the specimen (N), l is the distance between the supports (20 mm), b and h are the width and height (mm) of the specimen. After flexural test, fractured surface of the resin was observed using scanning electron microscopy (SEM, IT-500HR, JEOL, Tokyo, Japan).
Depth of cure
Depth of cure was tested 10 times according to ISO 4049:2019 using the particles mentioned above (surface-modified and non surface-modified with silane). The resin composite was poured using a Φ4 × 6 mm stainless steel mold, covered with a transparent film, and cured for 20 s using a light irradiator. The uncured resin was removed using a spatula, and the length of the cured resin was measured using a micrometer with 0.1 mm accuracy. The measured length was divided by 2.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics 23 software (IBM, Armonk, NY, USA). All data were tested for homogeneity using Levene's test, and an independent sample t-test was conducted between the group surface-modified with silane and the group without surface modification. The groups treated with silane and those without treatment were analyzed using one-way ANOVA, followed by Tukey’s post hoc analysis, with p < 0.05 considered statistically significant.
Results
Characterization of MSN-C
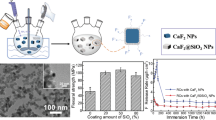
MSN-C particles were identified using TEM-EDS (Fig. 1). TEM-EDS images showed that cerium oxide was evenly coated on MCM-41, and MSN-C had spherical morphology. The EDS confirmed the composition of the elements Si and Ce (Table 3). The results showed that the amount of Ce increased constantly for each particle group. MSN-C4 had the most Ce at 3.83 wt%, and MSN-C1 had the least Ce at 1.89 wt%. The MSN-C particles showed a mesoporous structure, as demonstrated by BET results (Fig. 2a). In the BET results, all MSN-C particles had hysteresis loops at a relative pressure (P/P0) of 0.4–1.0 and type IV isotherm, which proved to be mesoporous structures30. All MSN-C particles showed a 3.8 nm pore size, but pore volume and surface area showed different results. The MSN particles without Ce had the largest surface area, but pore volume showed the lowest results. The MSN-C4 particles with the most Ce had the smallest surface area, but pore volume showed the highest results (Table 4)31. The ICP-OES analyses showed presence of cerium ions in extractions of all groups except for MS, confirming that cerium ions were released from all groups except for MS (Fig. 2b). Furthermore, the amount of released cerium ions was proportional to the coated amount on MS, with the MSC4 group exhibiting the highest ion release. The amount of released Ce ion for 48 h was 0 mg/L, 0.35 mg/L, 0.55 mg/L, 0.67 mg/L, and 0.69 mg/L for MS, MSC1, MSC2, MSC3, and MSC4, respectively.
The XPS results confirmed the bonding between Ce and Si (Fig. 3a). According to the reference paper, the peak at binding energy 529 nm showed Ce–O–Si bonding, while synthesized MSN-C particles also showed a peak at 529 nm32. Ce had a unique mechanism achieved through Ce (IV) to Ce (III) conversion because Ce is a strong oxidant. The XPS results showed six peaks from 880 to 920 eV (Fig. 3b). 885.3 and 903.9 eV indicated Ce 3d5/2 and Ce 3d3/2 states of Ce3+. 898.1, 900.8, 907.4, and 916.6 eV indicated 3d3/2 states of Ce4+ and 882.2, 888.8 eV indicated Ce 3d5/2 state12. We determined the area of the peak and calculated the Ce (IV)/Ce (III) ratio to investigate the redox state of Ce. MSN-C1 was 22.58%, MSN-C2 was 19.51%, MSN-C3 was 18.22%, and MSN-C4 was 17.49%. Finally, FT-IR results of silane-treated groups, MSN-41 and MSN-C4, demonstrated the –CH2 peaks from 2932 to 2883 cm−1, which originated from the silanol groups of APTES33 (Fig. 3c).
X-ray photoelectron spectroscopy of MSN-C particles. (a) O 1s spectra of MSN-C. The peaks show Ce–O–Si bonding. (b) Ce 3d spectra of MSN-C. The peaks showed that the electronic state of Ce changed from Ce (IV) to Ce (III). (c) FT-IR results of both with and without silane treatment of MCM-41 and MSN-C4 particles.
Antibacterial activity
Streptococcus mutans was used for the antibacterial activity test. The antibacterial activity of the synthesized particle MSN-C was measured using MIC. The antibacterial activity of MSN-C particles was proved using the MIC test, confirming that antibacterial properties began to appear in the group containing more than 2 mL of cerium oxide solution (Table 5).
There was a tendency for the antimicrobial capacity to increase in all groups. The CFU values for resin composite specimens showed significantly increased antibacterial properties as more cerium oxide nanoparticles coated on MCM-41 (Fig. 4a) (p < 0.05). There was a significant difference with and without silane treatment except in the MSC4 group (p < 0.05). MSN-C4 showed the highest antibacterial properties. There was no significant difference between MSC1 and MS (p < 0.05).
(a) Colony-forming unit (CFU) counts and (b) MTT results of S. mutans on the resin composite. Asterisks(*) denote significance for comparison between the surface-modified and non surface-modified groups within each group (p < 0.05). Bars with upper case alphabetical letters indicate significant differences among the non surface-modified groups and lower case letters indicate significant differences among the surface-modified groups (p < 0.05).
The MTT results of resin composites had the same tendency as CFU results (Fig. 4b). The MSC4 group showed the highest antibacterial properties compared to MS, MSC1, MSC2, and MSC3 groups. The antibacterial activity tests showed that the MS group prepared with MCM-41 had no antibacterial properties while the antibacterial properties increased with increasing amounts of cerium oxide nanoparticles.
Flexural strength
Figure 5a demonstrates the results of the flexural strength test for the resin composite performed for both groups surface-modified with silane and non-modified particles. The flexural strength values were higher for surface-modified groups than for non-modified groups (p < 0.05). As the cerium oxide content increased, the mechanical strength tended to decrease in both groups with and without surface modification. The surface-modified MS showed the highest flexural strength (107 MPa). MSC4 with an unmodified surface showed the lowest flexural strength (34 MPa). The flexural strength values in surface-modified groups, except MSC4, were within the required values stated in ISO 4049 (80 MPa). The flexural strength values in surface-modified groups, except MSC4, were within the required values stated in ISO 4049 (80 MPa). Figure 5b shows that the strain–stress curve represents the representative values of the experimental groups, and all groups showed similar trends.
(a) Flexural strength of resin composite. Asterisks(*) denote significance for comparison between the surface-modified and non surface-modified groups within each group (p < 0.05). Bars with upper case alphabetical letters indicate significant differences among the non surface-modified groups and lower case letters indicate significant differences among the surface-modified groups (p < 0.05). (b) Strain–stress curve of dental resin composite.
The SEM images in Fig. 6 demonstrated the fractured surfaces of groups incorporated with silane surface-modified particles. It was evident from the SEM images that the particles were evenly distributed across all groups in the dental resin composites.
Depth of cure
The depth of cure of the resin composite significantly decreased when non surface-modified MSC was added to the resin composite (Fig. 7) (p < 0.05). Surface-modified MS showed the highest value at 2.96 mm. Non surface-modified MSC4 showed the lowest value at 1.00 mm. There was a significant difference between the surface-modified group and the non surface-modified group, and all surface-modified groups showed higher values than the non surface-modified groups (p < 0.05).
Depth of cure of resin composite. Asterisks(*) denote significance for comparison between the surface-modified and non surface-modified groups within each group (p < 0.05). Bars with upper case alphabetical letters indicate significant differences among the non surface-modified groups and lower case letters indicate significant difference among the surface-modified groups (p < 0.05).
Discussion
Dental resin composite is typically composed of a monomer and inorganic fillers. However, secondary caries can be caused by gaps resulting from insufficient restoration resin or poor oral hygiene habits. Bacteria, such as S. mutans, can penetrate these gaps, leading to the development of caries. Previous research has linked secondary caries to restoration and sealant34. Nowadays, numerous studies seek to improve antibacterial properties by adding antibacterial inorganic fillers, such as zinc (Zn), fluoride (F), or silver (Ag). Cerium (Ce) is one of the rare earth elements with a unique mechanism that confers antibacterial properties. This mechanism involves the conversion of Ce3+ to Ce4+35. Adding inorganic fillers to resin composite increases their antibacterial properties but decreases their mechanical properties.
Silica nanoparticle-based resin composite was widely used in dentistry due to its ability to enhance mechanical properties. In addition, mesoporous particles have been diversly investigated in dental field due to its prolong antibacterial effet and strengthening dental resin16,36. Among silica nanoparticles, MCM-41, a type of mesoporous silica has gained attention37. MCM-41 has a drug-delivery system by containing an antibacterial agent38,39 and could enhance mechanical properties by improving the interfacial connection with resin monomers23. Therefore, we synthesized mesoporous silica (MCM-41) coated with cerium oxide nanoparticles to improve antibacterial properties without compromising mechanical properties.
MSN-C particles were prepared using a modified Stöber method and sol–gel method. As shown in Fig. 3a, cerium oxide nanoparticles were chemically bonded with MCM-41. Figure 1 showed that the synthesized MSN-C particles were spherical, and the more Ce was coated, the Table 3 confirmed that the cerium element weight percent in MSN-C1 particles was 1.39 wt%, and increased to 3.83 wt% in MSN-C4 particles. Figure 3c presents the FT-IR spectra which showed peaks at 2932 cm−1 and 2864 cm−1, corresponding to the CH2. These observations confirm the presence of the propyl chain of the APTES used in silane23. BET results (Table. 4) showed that surface area and pore volume decreased with increasing amounts of Ce revealing a trend similar to the previous study, the cerium oxide nanoparticle did not create severe pore blockage and opened the surfaces of synthesized particles31. According to XPS results the Ce(IV)/Ce(III) ratio decreased with increasing amounts of cerium, probably due to the size of cerium oxide nanoparticles. The increased size of cerium oxide led to increased surface(Fig. 3b). Diffusion of oxygen atoms, which hindered oxygen vacancies in cerium oxide, resulting in decreased Ce3+ concentrations40. MSN-C4 accumulated more cerium oxide than MSN-C1, and the size of the cerium oxide particle was relatively large, resulting in less Ce (IV)/Ce (III) conversion.
Antibacterial property is the keystone of this study. This study investigated the antibacterial properties using CFU and MTT. According to a previous study, the MIC value for cerium oxide nanoparticles was 25 µg/mL41. However, as shown in Table 5, when the MIC test was conducted using synthesized MSN-C particles, the antibacterial properties of the particles became apparent from 50 mg/mL of MSN-C2. Therefore, the experimental groups were set as from MSN-C1 to MSN-C4 according to the MIC results. When evaluating antibacterial properties by mixing synthesized MSN-C particles with resin monomers to prepare a resin composite, the non surface-modified MSC2 group began to exhibit antibacterial properties. In Fig. 2b, cerium ion release studies revealed continuous Ce ion release over 24 and 48 h except for MS. Notably, MSC4 demonstrated the highest antibacterial activity, which is correlated with the degree of cerium ion release. The amount of released Ce ions over 24 h was 0 mg/L, 0.17 mg/L, 0.32 mg/L, 0.37 mg/L, and 0.46 mg/L for MS, MSC1, MSC2, MSC3, and MSC4, respectively. The release of Ce ion was continued for 48 h, resulting in additional releases of 0 mg/L, 0.18 mg/L, 0.23 mg/L, 0.3 mg/L, and 0.23 mg/L of more Ce ions for MS, MSC1, MSC2, MSC3, and MSC4, respectively. These results suggest that the dental resin incorporated with cerium could have a lasting antibacterial effect. The released Ce ion which was confirm through ICP-OES acted as an antibacterial agent by the transition from Ce3+ to Ce4+ valence states. As a result, the groups containing MSC demonstrated antibacterial effect against S. mutans.
However, the antibacterial properties of resin composite containing silane-treated particles began emerging from MSC3. Non surface-modified MSC4 exhibited the best antibacterial properties in CFU and MTT assays, showing approximately twice the antibacterial activity in CFU compared to MSC1. Additionally, a significant difference was observed in the antibacterial properties between surface-modified groups and non surface-modified groups except MSC4. This disparity could attributed to the absolute molecular weight of cerium in the resin. For the surface modification using silane on MSN-C particles, silane was applied to the surface modification group that the molecular weight of applied silane was added up to the surface modification group. In conclusion, the amount of cerium varies between the surface-modified group and the non surface-modified group when same weight of particles were used. Therefore, the surface-modified group which had less cerium demonstrated low antibacterial properties than the non surface-modified group. Additionally, the silane layer on the surface of mesoporous silica may hinder the release of cerium ions, which are responsible for the antibacterial action. This can result in a lower concentration of active cerium ions available for antibacterial activities and therefore result in higher number of CFU counts42.
Mechanical properties are also important in resin composite. When nanoparticles are added to resin composite, they tend to aggregate with each other due to the large specific surface area of the nanoparticles, resulting in a decrease in the mechanical properties of resins14,15. However, the MCM-41 specific surface could overcome this drawback. In Fig. 6, the particles were uniformly distributed in the resin matrix due to the low surface area of the nanoparticles. In this study, cerium oxide nanoparticles were coated on MCM-41 to prevent aggregation and enhance mechanical strength through its mesoporous structure. According to the flexural strength test results, higher cerium oxide content in all groups, regardless of silane treatment, led to reduced strength. The mesoporous silica acted as mechanical polymer network to resin monomer23. Besides, the methyoxysilane groups of APTES reacts with the methacrylate functional group of resin matrix which resulted in higher mechanical strength and depth of cure for surface-modified groups than the non surface-modified groups43. Specifically, the MSC4 group, without silane treatment, showed significantly lower results. However, silane treatment increased the strength compared to the non-silane-treated group. This trend was also observed in the results for depth of cure, where increasing cerium oxide content made polymerization more challenging due to the optical properties of cerium oxide, resulting in lower flexural strength and polymerization depth44. Cerium oxide is known for its strong light absorption properties in the near-ultraviolet region. The photopolymer used in resin composite typically operates within a light wavelength range of 430–480 nm close to the ultraviolet region. However, cerium oxide exhibits its highest absorption rates between 250 and 356 nm, with some references indicating absorption extending up to 500 nm44. When cerium oxide is added to the resin composite, it could absorb light, reducing the formation of free radicals in the monomer. This light absorption is believed to hinder the polymerization process, and therefore may have resulted in reduction of flexular strength. Nevertheless, silane treatment partially overcame these issues as the results showed that the silane-treated groups achieved flexural strength values of 80 MPa or more, which meets the requirements set by the International Standard ISO 4049, with the exception of the MSC4 group, which had a value of 77 MPa. It is important to note that the flexural strength values obtained in this study may not be as high as those of commercially available resins. This can be attributed to the lower filler content in our experimental materials, which were developed based on a previous study16 to specifically focus on the effect of cerium. The lower filler content was a deliberate choice to isolate the impact of cerium on the resin's properties. However, despite this limitation, most of the experimental groups exceeded the minimum flexural strength value set by ISO 4049. This finding suggests that the incorporation of silane-treated cerium filler particles can enhance the flexural strength of dental resins, even at lower filler contents.
The dental resin composite incorporated with the MSN-C showed antibacterial effect which can be supported by lasting Ce ion released fro the specimens. In addition, the resin composite with silane treated MSN-C demonstrated enhanced flexural strength. These results go along with the other studies on various mesoporous particles45,46. Besides the results related to the mesoporous silica nanoparticles, this study also compare the properties of MSN-C with and without the silane treatment. From the analysis on the dental resin composite incorporated with the MSN-C, the silane coated MSN-C could be used as dental resin composite filler.
Conclusions
Mesoporous silica (MCM-41) coated with cerium oxide nanoparticles was synthesized using the modified Stöber method. The cerium oxide nanoparticle was successfully coated on mesoporous silica (MCM-41). Cerium oxide nanoparticles exhibited antibacterial properties that increased with higher amounts. Conversely, there was a tendency for mechanical properties to decrease as the amount of cerium oxide nanoparticles increased. However, silane treatment of the silane treatment of the MSN-C particles could overcome this drawback. In conclusion, cerium oxide nanoparticles coated on mesoporous silica (MCM-41) may be used for dental resin composite filler.
Data availability
The data will be shared at a reasonable request to the corresponding author.
References
DeRouen, T. A. et al. Neurobehavioral effects of dental amalgam in children: A randomized clinical trial. Jama 295, 1784–1792 (2006).
Jun, S.-K., Kim, D.-A., Goo, H.-J. & Lee, H.-H. Investigation of the correlation between the different mechanical properties of resin composites. Dent. Mater. J. 32, 48–57 (2013).
Kundie, F., Azhari, C. H., Muchtar, A. & Ahmad, Z. A. Effects of filler size on the mechanical properties of polymer-filled dental composites: A review of recent developments. J. Phys. Sci. 29, 141–165 (2018).
Araújo, G. S. A., Sfalcin, R. A., Araújo, T. G. F., Alonso, R. C. B. & Puppin-Rontani, R. M. Evaluation of polymerization characteristics and penetration into enamel caries lesions of experimental infiltrants. J. Dent. 41, 1014–1019 (2013).
Kaisarly, D. & Gezawi, M. E. Polymerization shrinkage assessment of dental resin composites: A literature review. Odontology 104, 257–270 (2016).
Khvostenko, D. et al. Cyclic mechanical loading promotes bacterial penetration along composite restoration marginal gaps. Dent. Mater. 31, 702–710 (2015).
Maske, T. T., Kuper, N. K., Cenci, M. S. & Huysmans, M.-C.D. Minimal gap size and dentin wall lesion development next to resin composite in a microcosm biofilm model. Car. Res. 51, 475–481 (2017).
Zalega, M. & Bociong, K. Antibacterial agents used in modifications of dental resin composites: A systematic review. Appl. Sci. 14, 3710 (2024).
Weng, Y. et al. A novel antibacterial resin composite for improved dental restoratives. J. Mater. Sci.: Mater. Med. 23, 1553–1561 (2012).
Abebe, B., Zereffa, E. A., Tadesse, A. & Murthy, H. A. A review on enhancing the antibacterial activity of ZnO: Mechanisms and microscopic investigation. Nanoscale Res. Lett. 15, 1–19 (2020).
Heckert, E. G., Karakoti, A. S., Seal, S. & Self, W. T. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 29, 2705–2709 (2008).
Jin, J. et al. Cerium oxide nanozymes confer a cytoprotective and bio-friendly surface micro-environment to methacrylate based oro-facial prostheses. Biomaterials 296, 122063 (2023).
Luo, Q. et al. Stabilizing ultrasmall ceria-cluster nanozyme for antibacterial and antibiofouling applications. Small 18, 2107401 (2022).
Jancar, J. et al. Current issues in research on structure–property relationships in polymer nanocomposites. Polymer 51, 3321–3343 (2010).
Reynaud, E., Jouen, T., Gauthier, C., Vigier, G. & Varlet, J. Nanofillers in polymeric matrix: A study on silica reinforced PA6. Polymer 42, 8759–8768 (2001).
Bai, X. et al. Preparation of Zn doped mesoporous silica nanoparticles (Zn-MSNs) for the improvement of mechanical and antibacterial properties of dental resin composites. Dent. Mater. 36, 794–807 (2020).
Moszner, N. & Klapdohr, S. Nanotechnology for dental composites. Int. J. Nanotechnol. 1, 130–156 (2004).
Sayari, A., Yang, Y., Kruk, M. & Jaroniec, M. Expanding the pore size of MCM-41 silicas: Use of amines as expanders in direct synthesis and postsynthesis procedures. J. Phys. Chem. B 103, 3651–3658 (1999).
Oh, J. Y., Yang, G., Choi, E. & Ryu, J.-H. Mesoporous silica nanoparticle-supported nanocarriers with enhanced drug loading, encapsulation stability, and targeting efficiency. Biomater. Sci. 10, 1448–1455 (2022).
Szegedi, A., Popova, M., Goshev, I. & Mihály, J. Effect of amine functionalization of spherical MCM-41 and SBA-15 on controlled drug release. J. Solid State Chem. 184, 1201–1207 (2011).
Vallet-Regi, M., Rámila, A., Del Real, R. & Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 13, 308–311 (2001).
Wang, N., Shao, Y., Shi, Z., Zhang, J. & Li, H. Influence of MCM-41 particle on mechanical and morphological behavior of polypropylene. Mater. Sci. Eng.: A 497, 363–368 (2008).
Samuel, S. P. et al. Mechanical properties of experimental dental composites containing a combination of mesoporous and nonporous spherical silica as fillers. Dent. Mater. 25, 296–301 (2009).
Yang, Q., Lin, Y.-H., Li, M., Shen, Y. & Nan, C.-W. Characterization of mesoporous silica nanoparticle composites at low filler content. J. Compos. Mater. 50, 715–722 (2016).
Boccardi, E. et al. Biodegradabiliy of spherical mesoporous silica particles (MCM-41) in simulated body fluid (SBF). Amer. Mineral. 103, 350–354 (2018).
Oh, M.-H., Lee, J.-S., Gupta, S., Chang, F.-C. & Singh, R. K. Preparation of monodispersed silica particles coated with ceria and control of coating thickness using sol-type precursor. Colloids Surf. A: Physicochem. Eng. Aspect. 355, 1–6 (2010).
Chen, T. & Brauer, G. Solvent effects on bonding organo-silane to silica surfaces. J. Dent. Res. 61, 1439–1443 (1982).
Barrett, E. P., Joyner, L. G. & Halenda, P. P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Amer. Chem. Soc. 73, 373–380 (1951).
Pop, O. L. et al. Cerium oxide nanoparticles and their efficient antibacterial application in vitro against gram-positive and gram-negative pathogens. Nanomaterials 10, 1614 (2020).
Jin, J. et al. Molecular weight tuning optimizes poly(2-methoxyethyl acrylate) dispersion to enhance the aging resistance and anti-fouling behavior of denture base resin. Biomater. Sci. 10, 2224–2236. https://doi.org/10.1039/D2BM00053A (2022).
Khalil, K. M. S. Cerium modified MCM-41 nanocomposite materials via a nonhydrothermal direct method at room temperature. J. Colloid Interface Sci. 315, 562–568 (2007).
Sahir, S. et al. Investigation of the effect of different cleaning forces on Ce–O–Si bonding during oxide post-CMP cleaning. Appl. Surf. Sci. 545, 149035 (2021).
Majoul, N., Aouida, S. & Bessaïs, B. Progress of porous silicon APTES-functionalization by FTIR investigations. Appl. Surf. Sci. 331, 388–391 (2015).
Askar, H. et al. Secondary caries: What is it, and how it can be controlled, detected, and managed?. Clin. Oral Investig. 24, 1869–1876 (2020).
Zhang, M. et al. Antibacterial mechanism and activity of cerium oxide nanoparticles. Sci. China Mater 62, 1727–1739 (2019).
Shi, M. et al. Europium-doped mesoporous silica nanosphere as an immune-modulating osteogenesis/angiogenesis agent. Biomaterials 144, 176–187 (2017).
Attik, N. et al. Mesoporous silica fillers and resin composition effect on dental composites cytocompatibility. Dent. Mater. 33, 166–174 (2017).
Chen, H., Wang, R., Zhang, J., Hua, H. & Zhu, M. Synthesis of core-shell structured ZnO@ m-SiO2 with excellent reinforcing effect and antimicrobial activity for dental resin composites. Dent. Mater. 34, 1846–1855 (2018).
Yan, H. et al. Chlorhexidine-encapsulated mesoporous silica-modified dentin adhesive. J. Dent. 78, 83–90 (2018).
Qiu, L., Liu, F., Zhao, L., Ma, Y. & Yao, J. Comparative XPS study of surface reduction for nanocrystalline and microcrystalline ceria powder. Appl. Surf. Sci. 252, 4931–4935 (2006).
Alirezaei, S., Mahmoudi, H., Ghodratizadeh, S., Mohammadi Khah, M. & Titidej, A. Investigating cytotoxicity and the effect of Zn doped CeO2 nanoparticles on tooth-damaging Streptococcus mutans bacteria. Nanomed. Res. J. 8, 16–23 (2023).
Wang, Y. et al. Antibiotic-loaded, silver core-embedded mesoporous silica nanovehicles as a synergistic antibacterial agent for the treatment of drug-resistant infections. Biomaterials 101, 207–216 (2016).
Matinlinna, J. P., Lassila, L. V., Özcan, M., Yli-Urpo, A. & Vallittu, P. K. An introduction to silanes and their clinical applications in dentistry. Int. J. Prosthodont. 17 (2004).
Devaraju, M. K., Yin, S. & Sato, T. Morphology control of cerium oxide particles synthesized via a supercritical solvothermal method. ACS Appl. Mater. Interfaces 1, 2694–2698 (2009).
Jo, J.-K. et al. Rechargeable microbial anti-adhesive polymethyl methacrylate incorporating silver sulfadiazine-loaded mesoporous silica nanocarriers. Dent. Mater. 33, e361–e372 (2017).
Li, Z., Barnes, J. C., Bosoy, A., Stoddart, J. F. & Zink, J. I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 41, 2590–2605 (2012).
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1C1C1010304).
Author information
Authors and Affiliations
Contributions
S.Y.B., A.R.H., K.M.K., and J.S.K., conceived and designed the experiments. S.Y.B. conducted all the experiments. S.Y.B. and J.S.K. interpreted and analyzed the data. S.Y.B conceived the study and wrote the manuscript. A.R.H., K.M.K., and J.S.K. provided manuscript writing assistance and critically revised the manuscript, adding important intellectual content. All authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Byun, SY., Han, A.R., Kim, KM. et al. Antibacterial properties of mesoporous silica coated with cerium oxide nanoparticles in dental resin composite. Sci Rep 14, 18014 (2024). https://doi.org/10.1038/s41598-024-69102-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69102-2
- Springer Nature Limited