Abstract
Pre-hospital end-tidal carbon dioxide (EtCO2) monitoring and arterial to end-tidal carbon dioxide gradient (Pa-EtCO2) have been associated with mortality in patients with traumatic brain injury. Our study aimed to analyze the association between alveolar EtCO2 or Pa-EtCO2 and mortality in patients admitted in intensive care unit (ICU) with neurological injuries. In our retrospective analysis from using large de-identified ICU databases (MIMIC-III and -IV and eICU databases), we included 2872 ICU patients with neurological injuries, identified according to the International Classification of Diseases (ICD-9 and -10), who underwent EtCO2 monitoring. We performed logistic regression and extended Cox regression to assess the association between mortality and candidate covariates, including EtCO2 and Pa-EtCO2. In-hospital mortality was 26% (n = 747). In univariate analysis, both the Pa-EtCO2 gradient and EtCO2 levels during the first 24 h were significantly associated with mortality (for a 1 mmHg increase: OR = 1.03 [CI95 1.016–1.035] and OR = 0.94 [CI95 0.923–0.953]; p < 0.001). The association remained significant in multivariate analysis. The time-varying evolution of EtCO2 was independently associated with mortality (for a 1 mmHg increase: HR = 0.976 [CI95 0.966–0.985]; p < 0.001). The time-varying Pa-EtCO2 gradient was associated with mortality only in univariate analysis. In neurocritical patients, lower EtCO2 levels at admission and throughout the ICU stay were independently associated with mortality and should be avoided.
Similar content being viewed by others
Introduction
Acute neurological disorders are a leading cause of disability and are a primary reason for ICU admission. Mortality can be as high as 40% at one year for patients with non-traumatic acute brain injuries, such as post-cardiac arrest encephalopathy, malignant stroke, or meningitis1. In contrast, patients admitted to the ICU with traumatic brain injury (TBI) have a 6-month mortality rate of 21%, with 43% experiencing at least severe disability at 6 months2.
Factors independently associated with outcomes in neurocritical patients include age, duration of mechanical ventilation, intracranial hemorrhage, and inoperable neoplasm1. Additionally, the International Mission for Prognosis and Analysis of Clinical Trials in TBI (IMPACT-TBI: www.tbi-impact.org) and the CENTER-TBI studies identified several prognostic factors for patients with moderate to severe TBI. These factors include the motor component of the Glasgow Coma Scale (GCS), occurrence of hypoxia, hypotension, hyperglycemia, anemia, pupil size abnormalities, subarachnoid hemorrhage, and diffuse lesion on CT scan admission2.
Two recent studies have demonstrated an association between the quantification of pre-hospital arterial to end-tidal carbon dioxide gradient (Pa-EtCO2) or end-tidal dioxide carbon (EtCO2) and mortality in patients with TBI. Doppman et al. found that Pa-EtCO2 at admission in a cohort of 181 TBI patients may be independently associated with mortality3. However, their logistic regression model did not account for covariates previously shown to influence Pa-EtCO2 levels, such as hemodynamic and respiratory failures4,5. Bossers et al.6 observed in 2589 TBI patients that pre-hospital EtCO2 values were associated with 30-day mortality, with an increased risk observed when EtCO2 values were below 35 mmHg.
Recently, a brain-lung interaction has been hypothesized, suggesting that isolated brain injury may trigger inflammatory pathways leading to lung injury7. Consequently, EtCO2 and Pa-EtCO2 may serve as sensitive markers of the severity of neurological injury.
The objective of our study was to evaluate the association between EtCO2 and/or Pa-EtCO2 and mortality, adjusting for hemodynamic and respiratory dysfunction, in a population of neurologically injured patients admitted to the intensive care unit.
Methods
Data source
We conducted a retrospective analysis of three extensive medical databases available on the Physionet repository of freely-available medical research data8, managed by the Massachusetts Institute of Technology Laboratory for Computational Physiology (https://physionet.org). This repository was supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) under NIH grant number R01EB030362. We utilized data from three primary sources: (1) the Medical Information Mart for Intensive Care III and IV databases (MIMICs)9,10, which includes critical care data from over 76,000 patient stays at the Beth Israel Deaconess Medical Center spanning from 2008 to 2019 and; (2) the eICU collaborative research database11, a large multi-center critical care database comprising data from over 200,000 patient stays across various institutions in the United States.
MIMICs and eICU data comply with Health Insurance Portability and Accountability Act of 1996, and access for all investigators was approved by PhysioNet8. Database availability was individually authorized by PhysioNet for the data manager (ALG). Due to the de-identified nature of the data, informed consent was waived.
Patients’ selection
Patients aged 18 years and older with a diagnosis of acute neurological conditions based on ICD-9 or 10 (International Classification of Diseases 9th and 10th Edition, Clinical Modification [ICD-9-CM] and [ICD-10-CM]) were included (see Additional file 1). We selected admissions where the diagnosis was categorized as “Primary” or “Major” in the eICU database, or as the first to third priority in MIMICs. In MIMICs, the first listed diagnosis was considered “Primary”. Only subjects with at least one recorded EtCO2 measurement during their ICU stay were included in the final statistical analysis.
The ICU admission details, including sex, date of birth, transfer and discharge information were accessible. Primary and secondary diagnosis codes, diagnosis-related groups, procedure codes (in Current Procedural Terminology format). In the eICU database, in-hospital mortality was recorded. In the MIMIC databases, in-hospital mortality and one-year survival status were available.
The time-varying variables of interest encompassed documented clinical observations, laboratory and microbiology test results, prescriptions, fluid balance, and physiological scores. A detailed list of variables considered for statistical analysis is provided in Table 1.
Data management and data missingness
The data management and statistical analysis were conducted using R software: A language and environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org). The RICU package12 was used for data retrieval, subject selection and variable extraction process.
After extracting the complete dataset, a post-processing calculation step was performed, which included: (1) calculating the Pa-EtCO2 as the difference between arterial partial pressure of carbon dioxide (PaCO2) and EtCO2; (2) computing the ratio of arterial partial pressure of oxygen (PaO2) to inspired fraction of oxygen (FiO2) (PaO2/FiO2), and; (3) determining the body mass index (BMI) as weight divided by squared height. These calculations were conducted when data where available, with a minimum 1-h time-lag between two consecutive datapoints. Finally, continuous variables and calculations were median day averaged.
Unadjusted statistics were initially conducted on complete cases. Adjusted statistics were then performed following imputation step to handle missing data. Missing data were assumed to be missing at random, and multiple imputations were carried out using the R package MICE13. A random forest algorithm was applied for both continuous and categorical variables. This process resulted in 4 datasets containing imputed non-missing data, in addition to the the original dataset. All variable calculations or manipulations were completed prior to the multiple imputation step.
After imputation, the results were averaged, and multivariate models were selected based on the methodology described by Schomaker and Heumann14, implemented using the MAMI R package15,16.
Statistical analysis
Descriptive statistics were computed on the original non-imputed dataset, using data collected within the first 24 h following ICU admission. Continuous variables were presented as mean (± SD), ordinal categorical variables as median (interquartile range), and nominal categorical variables as number (percentages). Between-group comparisons were conducted using Student’s t-test, Wilcoxon rank sum test or Chi-square test, as applicable.
We carefully selected the candidate variables according to known factors described in literature. To assess their association with mortality, unadjusted and covariate-adjusted logistic regression models were applied to the datasets available during the first 24 h, after multiple imputation procedure. Model selection based on Akaike criterion minimization was performed using the ‘mami’ function of the eponym package16. The risk of in-hospital mortality is expressed as odd-ratio (OR) with a 95% confidence interval (CI95).
To assess the association between time-varying candidate variables and mortality, both unadjusted and covariate-adjusted extended Cox regression models were applied to the imputed datasets. The analysis accounted for competitive events such as endotracheal tube withdrawal or death by right-censoring data from the day of mechanical ventilation weaning. Model selection followed the approach described above. The risk of in-hospital mortality is reported as hazard ratio (HR) with a 95% confidence interval (CI95).
The CI95 for both the logistic and extended Cox regression models were estimated using a bootstrapping method involving 25 resamples14.
Ethics approval and consent to participate
MIMIC-IV and eICU data are Health Insurance Portability and Accountability Act of 1996 compliant, and all the investigators with data access were approved by PhysioNet8. In occurrence, the databases availability was personally credentialed by PhysioNet for the data manager (ALG). Because of the de-identified nature of the data, informed consent was waived.
Prior abstract presentation
This work has been presented at the national congress of French anesthetists and intensivist in September 2022, in Paris (France).
Results
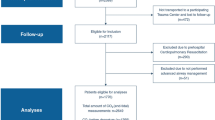
We screened 49,325 admissions based on our ICD-9 and -10 criteria (Fig. 1), with 21,239 (43%) from the eICU database and 18,407 (37%) from the MIMIC-IV database. The remaining 9679 (20%) subjects initially from MIMIC-III database were excluded in subsequent selection steps. Among patients with EtCO2 recordings, 2872 subjects were included: 1868 (65%) from MIMIC-IV and 1004 (35%) from eICU database. Only one subject from MIMIC-III was included in the final analysis. By the end of the follow-up, 747 (26%) subjects had died, with 50 (2%) dying on the first day and 587 (20%) while on ventilator support. The median time-to-death was 4 [2–9] days.
Flow chart. (MIMIC: Medical Information Mart for Intensive Care, ICU: Intensive care unit). Complete cases refer to the number of patients for whom both EtCO2 or Pa-EtCO2 and the outcome variable were available simultaneously. Specifically, this includes patients with EtCO2 or Pa-EtCO2 data from the first day and one-year mortality data. Additionally, it encompasses those with EtCO2 or Pa-EtCO2 data from the day of death or ventilator weaning.
The primary diagnosis was neurological for 1869 (65%) admissions while for 1003 (35%) admissions neurological conditions were classified under major diagnoses. Most patients were admitted due to intracranial brain injuries (96%), with TBI being the leading primary diagnosis for ICU admission (20%). Figure 2 illustrates the relationship between associated diagnoses. We conducted a sub-group analysis specifically focusing on TBI patients (See Additional file 2).
Table 1 presents the admission characteristics of the study population. The mean age was 59 ± 18 years. The median motor Glasgow Coma Score (mGCS) during the first 24 h was 5 [1–6]. Among 247 patients, the mean intracranial pressure measured within the first 24 h of admission was 10 ± 7 mmHg. The average Pa-EtCO2 was 7 ± 10 mmHg.
There were no missing data for the in-hospital mortality outcome. Regarding EtCO2, as per protocol, all subjects had at least one recording during their ICU stay. However, for our primary objective, we noted 22% missing rate for EtCO2 among the 2599 intubated patients on the first day of ICU admission.
Figure 3 illustrates the unadjusted time-evolution of EtCO2 and Pa-EtCO2 in relation to vital status at ICU-discharge. We observed higher EtCO2 values and lower Pa-EtCO2 values in survivors compared to non survivors (p < 0.001 for both). Significant time-dependent changes in Pa-EtCO2 were also noted.
Time-evolution of end-tidal carbon dioxide (EtCO2) values and arterial to end-tidal carbon dioxide (Pa-etCO2) gradients. The figure illustrates the results of a mean fractional polynomial regression performed on the complete-cases dataset. Analyzing the interaction between time and EtCO2 (left) and time and Pa-etCO2 (right). ANCOVA was conducted to compare the EtCO2 and Pa-etCO2 between survivors and non-survivors (p < 0.001 for both) and to evaluate the effect of time on the two dead-space surrogates. Notably, only Pa-etCO2 demonstrated significantly increase over time.
Table 2 and Additional file 3 present the results of logistic regression analysis, assessing the association between candidate covariates measured within the first 24 h and in-hospital mortality. In the univariate analysis, both the Pa-EtCO2 gradient and EtCO2 levels measured within the first 24th hours were associated with in-hospital mortality (for a 1 mmHg increase: OR = 1.03 [CI95 1.016–1.035] and OR = 0.94 [CI95 0.923–0.953], respectively; p < 0.001 for both). In the multivariate analysis, both EtCO2 and Pa-EtCO2 remained independently associated with in-hospital mortality (for a 1 mmHg increase: OR = 0.997 [CI95 0.994–1]; p = 0.045 and OR = 1.003 [1–1.006]; p = 0.022, respectively). Figure 4 depicts the predicted mortality in relation to EtCO2 and Pa-EtCO2 measured during the first 24 h.
Predicted mortality in relation to end-tidal carbon dioxide (EtCO2) values and arterial minus end-tidal carbon dioxide (Pa-etCO2) gradients measured within the first 24 h of ICU admission. A logistic regression with natural cubic spline transformation was applied to the complete-cases dataset (refer to the density plot for data distribution) to model the predicted mortality.
The results of the extended Cox regression analysis are presented in Table 2 and in Additional file 4. When accounting for time-varying covariates, both the evolution of Pa-EtCO2 and the EtCO2 were predictive of mortality. A 1 mmHg increase in Pa-EtCO2 gradient was associated with a 4% increase in the instantaneous mortality hazard (HR = 1.04 [CI95 1.021–1.052]; p = 0.001). Conversely, a 1 mmHg increase in EtCO2 was associated with a 6% decrease in the hazard of mortality (HR = 0.94 [CI95 0.932–0.956]; p < 0.001). In the multivariate analysis, only the protective association between EtCO2 and mortality remained significant (for 1 mmHg increase, HR = 0.976 [CI95 0.966–0.985]; p < 0.001) (Fig. 4).
Discussion
In this retrospective analysis of the MIMIC-IV and of the eICU databases, involving 2,872 ICU admissions for acute neurological injuries, we found that EtCO2 levels at ICU-admission and their evolution during ICU stay were independently associated with mortality. While admission Pa-EtCO2 levels was independently associated with mortality, their evolution reaches statistical significance only in the univariate analysis.
Interestingly, while pre-hospital EtCO2 monitoring is recommended for managing patients with TBI17 and more broadly for neurocritical care patients18, only a few studies have explored its association with mortality. A recent study demonstrated that pre-hospital EtCO2 values were independently associated with 30-days mortality6. They found that EtCO2 levels below 35 mmHg were linked to increased mortality, whereas hypercapnia was not. Our findings align with this study, highlighting the importance of in-hospital EtCO2 monitoring.
Similarly, we found that the magnitude of Pa-EtCO2 was associated with mortality. This finding aligns with observations by Doppman et al.3. Physiological dead space, represented by Pa-EtCO2, has previously been linked to mortality in patients with septic shock4 or with acute respiratory distress syndrome (ARDS)19. We hypothesized that the association between Pa-EtCO2 and mortality observed by Doppman et al.3 could be influenced by cofounding factors such as respiratory or hemodynamic dysfunction. After adjusting for hemodynamic and respiratory indicators, the association with both Pa-EtCO2 magnitude, EtCO2 levels and mortality remained statistically significant, although the strength of the association was reduced. This observation can be explained in two ways: (1) EtCO2 reflects PaCO2 levels, and lower EtCO2 is associated with hypocapnia. Our findings, along with those of Bossers et al.6 suggest a strong association between hypocapnia and mortality. Hypocapnia below 37 mmHg induces arteriolar vasoconstriction in cerebral territories, which may help reduce refractory intracranial hypertension. However, studies have shown that a decrease in PaCO2 from 37 to 30 mmHg reduces cerebral blood flow, increases cerebral oxygen extraction ratio while maintaining cerebral oxygen consumption, indicating potential ischemic consequences of mild hypocapnia20,21. In TBI patients, hyperventilation has been associated with prolonged ICU stays and poor outcomes at 90 days21. International guidelines and experts on TBI management21,22 recommend using transient mild hypocapnia (30–32 mmHg) only for intracranial hypertension that is refractory to first- and second-line therapies, ideally monitored with cerebral blood flow, tissue brain oxygenation, or jugular venous bulb oxygen saturation measurements. Outside these conditions, physicians should carefully adjust ventilatory parameters to avoid hypocapnia; (2) Pa-EtCO2 is known to be a surrogate for physiological dead space, which can be defined as the ratio between Pa-etCO2 and PaCO2. Therefore, when PaCO2variations are minimal, changes in physiological dead space translate into EtCO2 variations. Although the link between neurological injury and physiological dead space is not immediately obvious, a brain-lung interaction as described by Ziaka et al.7 could explain this relationship. The “blast injury” theory suggests that elevated hydrostatic pressure caused by the catecholaminergic storm associated with brain injury23 can lead to neurogenic pulmonary edema24. Additionally, the pulmonary venule adrenergic hypersensitivity theory24 proposes that brain-mediated immuno-inflammatory pathways contribute to acute lung injury by increasing the permeability of the alveolo-capillary membrane. Consequently, Pa-EtCO2 and EtCO2, through their associations with physiological dead space, can be viewed as sensitive markers of neurological injury even in patients without respiratory or hemodynamic failure. For these reasons, our findings are physiological plausible.
Yet, our study has several limitations including the retrospective design and methodological concerns related to the internal validity of our analysis. Specifically, the inclusion criteria were based on ICD-9 and -10 codes associated with acute neurological insults identified as primary or major diagnoses for ICU admission. However, the diagnosis classification in these American databases is derived from insurance classification. Consequently, we may have inadvertently included patients whose true primary diagnosis responsible for ICU admission does not align with our target population, introducing a potential selection bias that we could not mitigate.
Another potential selection bias arises from the EtCO2 monitoring requirements, as only patients on invasive mechanical ventilation were included, given that EtCO2 measurements are not available for off-ventilator patients. Therefore, our findings are applicable only to the most severe cases.
In addition, the usefulness of CO2 monitoring in neurologically injured patients was assessed regardless of the specific diagnosis for ICU admission. Adopting a pragmatic approach, we did not limit our analysis to a particular pathology, thus including patients with both intracranial and peripheral neurological injuries of traumatic or non-traumatic origin. However, a more specific approach could introduce further selection bias due to the aforementioned challenges in accurately identifying the true primary diagnosis for ICU admission within our three databases.
Conclusions
In patients admitted to the ICU for acute neurological injury, monitoring of the EtCO2 and the Pa-EtCO2 gradient can serve as a non-invasive and responsive indicator of mortality prognosis. Utilizing these measurements during the ICU-patient stay may assist physicians in better identifying critical situations, whether they are associated with hemodynamic or respiratory failure or not. Our findings align with recent publications, underscoring the importance of carbon dioxide monitoring in neurological critical care settings. Further prospective studies are needed to confirm our observations in specific populations of acute brain injuries.
Data availability
The datasets generated and analyzed during the current study are available in the Physionet repository, https://physionet.org.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- BMI:
-
Body mass index
- CI95 :
-
95% Confidence interval
- EtCO2:
-
End-tidal carbon dioxide
- FiO2 :
-
Inspired fraction of oxygen
- GCS:
-
Glasgow coma scale
- HR:
-
Hazard ratio
- ICD:
-
International classification of diseases
- ICU:
-
Intensive care unit
- mGCS:
-
Motor component of the Glasgow coma scale
- MIMIC:
-
Medical Information Mart for Intensive Care
- OR:
-
Odd ratio
- PaCO2 :
-
Arterial partial pressure of carbon dioxide
- Pa-EtCO2:
-
Arterial to end-tidal carbon dioxide gradient
- PaO2 :
-
Arterial partial pressure of oxygen
- TBI:
-
Trauma brain injury
References
Kiphuth, I. C. et al. Predictors for good functional outcome after neurocritical care. Crit. Care 14(4), R136. https://doi.org/10.1186/cc9192 (2010).
Steyerberg, E. W. et al. Predicting outcome after traumatic brain injury: Development and international validation of prognostic scores based on admission characteristics. PLoS Med. 5(8), e165. https://doi.org/10.1371/journal.pmed.0050165 (2008).
Doppmann, P. et al. End-tidal to arterial carbon dioxide gradient is associated with increased mortality in patients with traumatic brain injury: A retrospective observational study. Sci. Rep. 11(1), 10391 (2021).
Vallée, F. Cutaneous ear lobe PCO2 at 37°C to evaluate microperfusion in patients with septic shock. CHEST J. 138(5), 1062. https://doi.org/10.1378/chest.09-2690 (2010).
Bonifazi, M. et al. End-tidal to arterial PCO2 ratio: A bedside meter of the overall gas exchanger performance. ICMx. 9(1), 21. https://doi.org/10.1186/s40635-021-00377-9 (2021).
Bossers, S. M. et al. Association between prehospital end-tidal carbon dioxide levels and mortality in patients with suspected severe traumatic brain injury. Intensive Care Med. 49(5), 491–504. https://doi.org/10.1007/s00134-023-07012-z (2023).
Ziaka, M. & Exadaktylos, A. Brain–lung interactions and mechanical ventilation in patients with isolated brain injury. Crit. Care 25(1), 358. https://doi.org/10.1186/s13054-021-03778-0 (2021).
Goldberger, A. L. et al. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation. 101, 23. https://doi.org/10.1161/01.CIR.101.23.e215 (2000).
Johnson, A. E. W. et al. MIMIC-III, a freely accessible critical care database. Sci. Data 3(1), 160035 (2016).
Johnson, A. et al. MIMIC-IV. PhysioNet. (2020, accessed 21 Jan 2022). https://physionet.org/content/mimiciv/0.4/.
Pollard, T. J. et al. The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Sci. Data. 5(1), 180178 (2018).
Bennett, N. & Plecko, D. ricu: R’s interface to intensive care data. R package version 052. https://cran.r-project.org/package=ricu (2012).
van Buuren, S. & Groothuis-Oudshoorn, K. mice : Multivariate imputation by chained equations in R. J. Stat. Soft. 45, 3 (2011).
Schomaker, M. & Heumann, C. Model selection and model averaging after multiple imputation. Comput. Stat. Data Anal. 71, 758–770 (2014).
Schomaker, M. Model Averaging and Model Selection after Multiple Imputation using the {R}-package MAMI. http://mami.r-forge.r-project.org (2017).
Schomaker, M. {MAMI}: Model Averaging (and Model Selection) after multiple Imputation. R package version 0.9.13. http://mami.r-forge.r-project.org (2018).
Geeraerts, T. et al. Prise en charge des traumatisés crâniens graves à la phase précoce (24 premières heures). Anesthés. Réanimation 2(6), 431–453 (2016).
Le Roux, P. et al. Consensus summary statement of the International Multidisciplinary Consensus Conference on Multimodality Monitoring in Neurocritical Care: A statement for healthcare professionals from the Neurocritical Care Society and the European Society of Intensive Care Medicine. Intensive Care Med. 40(9), 1189–1209. https://doi.org/10.1007/s00134-014-3369-6 (2014).
Nuckton, T. J. & Pittet, J. F. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. New Engl. J. Med. 2002, 6 (2002).
Coles, J. P. et al. Hyperventilation following head injury: Effect on ischemic burden and cerebral oxidative metabolism*. Crit. Care Med. 35(2), 568–578 (2007).
Gouvea Bogossian, E., Peluso, L., Creteur, J. & Taccone, F. S. Hyperventilation in adult TBI patients: How to approach it?. Front. Neurol. 11, 580859. https://doi.org/10.3389/fneur.2020.580859/full (2021).
Chesnut, R. et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: The Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 46(5), 919–929. https://doi.org/10.1007/s00134-019-05900-x (2020).
Woiciechowsky, C. & Volk, H. D. Increased intracranial pressure induces a rapid systemic interleukin-10 release through activation of the sympathetic nervous system. Acta Neurochir. Suppl. 95, 373–376 (2005).
Davison, D. L., Terek, M. & Chawla, L. S. Neurogenic pulmonary edema. Crit. Care 16(2), 212. https://doi.org/10.1186/cc11226 (2012).
Singer, M. et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 315(8), 801. https://doi.org/10.1001/jama.2016.0287 (2016).
Acknowledgements
We thank the DOMASIA team (Pr Marc Cuggia, Dr Guillaume Bouzille and Dr Boris Delange) for their useful advises in data management and statistical analyses.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception and design. Analyzes were performed by ALG, and YL. The first draft of the manuscript was written by ALG, and all the authors commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Le Gall, A., Eustache, G., Coquet, A. et al. End-tidal carbon dioxide and arterial to end-tidal carbon dioxide gradient are associated with mortality in patients with neurological injuries. Sci Rep 14, 19172 (2024). https://doi.org/10.1038/s41598-024-69143-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69143-7
- Springer Nature Limited