Abstract
Normal tissue complication probability (NTCP) models for radiation pneumonitis (RP) in lung cancer patients with stereotactic body radiation therapy (SBRT), which based on dosimetric data from treatment planning, are limited to patients who have already received radiation therapy (RT). This study aims to identify a novel predictive factor for lung dose distribution and RP probability before devising actionable SBRT plans for lung cancer patients. A comprehensive correlation analysis was performed on the clinical and dose parameters of lung cancer patients who underwent SBRT. Linear regression models were utilized to analyze the dosimetric data of lungs. The performance of the regression models was evaluated using mean squared error (MSE) and the coefficient of determination (R2). Correlational analysis revealed that most clinical data exhibited weak correlations with dosimetric data. However, nearly all dosimetric variables showed "strong" or "very strong" correlations with each other, particularly concerning the mean dose of the ipsilateral lung (MI) and the other dosimetric parameters. Further study verified that the lung tumor ratio (LTR) was a significant predictor for MI, which could predict the incidence of RP. As a result, LTR can predict the probability of RP without the need to design an elaborate treatment plan. This study, as the first to offer a comprehensive correlation analysis of dose parameters, explored the specific relationships among them. Significantly, it identified LTR as a novel predictor for both dose parameters and the incidence of RP, without the need to design an elaborate treatment plan.
Similar content being viewed by others
Introduction
Lung cancer is one of the most frequently diagnosed malignancies and is the leading cause of cancer-associated mortality in both sexes1,2,3. Radiotherapy is a crucial treatment option for lung cancer4,5,6. The aim of radiotherapy is to prescribe the desired dose to the target volume while protecting the surrounding critical organs in the best way7,8. Multiple studies have demonstrated the superiority of stereotactic body radiation therapy (SBRT) in achieving enhanced local tumor control and sparing critical neighboring normal structures from radiation-induced injury compared to conventional fractionated radiation therapy (CFRT) techniques9,10,11. Currently, SBRT is a precise, targeted radiation treatment technique that can serve as a standard or preferred treatment option for patients with either inoperable or operable lung cancer with small tumors12,13. Nonetheless, this therapeutic approach can still induce certain acute or late toxicities14. Among these adverse effects, radiation pneumonitis (RP) is the most common toxicity, warranting particular attention15. The incidence of RP has been reported to range from 9 to 49%16,17. Although most cases are asymptomatic (grade 1) or mild (grades 2 and 3), up to 12% of patients can be severe and potentially life-threatening (grades 4 and 5)10,18,19. Having a deep understanding of the incidence of RP in SBRT for lung cancers is crucial.
Many studies have investigated various prognostic factors for RP, and specific dose-volume thresholds for RP in SBRT for lung cancers have been identified20,21. However, symptomatic RP (RP2) typically occurs within 3–6 months, and sometimes even several years, after the completion of SBRT18. Gaining knowledge of whether a patient will suffer from RP2 after SBRT requires a long follow-up period or multiple physical reexaminations, which is detrimental to post-treatment care for patients. The study aims to identify a novel factor that can predict lung dose distributions and the incidence of RP before formulating actionable SBRT treatment plans for lung cancer patients, without the need to design an elaborate treatment plan.
Materials and methods
Study population and SBRT methods
The present study retrospectively examined the medical records of patients with lung cancer who underwent SBRT from September 2021 to December 2023 at Renmin Hospital of Wuhan University. This study was performed in accordance with the Declaration of Helsinki and approved by the Clinical Research Ethics Committee of Renmin hospital of Wuhan University (approval number: WDRY2024-K033). The inclusion criteria for the cohort were: (1) lung cancer patients treated with a dose greater than 5 Gy per fraction with no prior or subsequent radiation therapy (RT); (2) RT plans with retrievable dosimetric data; (3) a conformal index (CI), defined as the volume receiving the prescription dose divided by the planned target volume (PTV), should not exceed 1.2; (4) a verification passing rate (\(\gamma\) rate) for the RT planning of more than 95% with dose and positional deviations less than 3% and 3 mm, respectively.
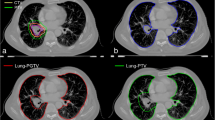
Out of 110 lung SBRT patients, only 57 cases met the inclusion criteria. Among them, 21 had tumors in the right upper lung (RUL), 26 in the left upper lung (LUL), two in the left lower lung (LLL), and eight in the right lower lung (RLL). The volume of the ipsilateral lung (IL) was determined by excluding the PTV from the lung volume, while the contralateral lung (CL) referred to the lung on the opposite side of the IL. The total lung (TL) volume was calculated as the sum of IL and CL volumes, measured in cubic centimeters (cc).
All patients in this cohort underwent supine 4D CT simulation with appropriate immobilization for stereotactic treatment. The gross tumor volume (GTV) was delineated across all respiratory phases of 4D CT. An internal target volume (ITV) was generated to encompass the GTV throughout all respiratory phases. The PTV was then derived by adding additional anisotropic margins of 5 mm in the axial plane and 7–10 mm in the longitudinal direction to the ITV. In this study, only the ITV and the PTV were considered. All treatment plans were generated using two treatment planning systems (TPS): ten cases were from Monaco (Elekta, v5.40.04), while 47 plans were acquired from Pinnacle (Philips, v9.10). Image-guided radiation therapy (IGRT) was performed to verify the isocenter location during each treatment. Clinical data for all patients in this cohort are described in Table 1.
Prescriptions varied among oncologist, with fractions ranging from 5 to 12 and single doses ranging from 5 to 10 Gy. A total dose of 40–72 Gy was delivered to the isocenter of the PTV using a 6 MV photon beam. To correct for differences in the number of fractions, the planning CT scans, dose distribution, and structure sets of all treatment plans were exported from the TPS to MIM software (Version 7.3.4, MIM Software Inc., Cleveland, OH) to transform accumulated dose distributions into 2 Gy biologically equivalent doses (EQD2). After inputting the number of fractions and matching the structures with appropriate α/β values, the EQD2 distribution can be obtained. This distribution was expressed in Gray (Gy) and derived from the linear-quadratic model (LQ model)22. Typically, an α/β ratio of 10 Gy is applied to the target, and an α/β ratio of 3 Gy is used for normal lung tissue11. In this study, the corrected dose-volume histograms (DVHs) of the lungs and the planned target volume (PTV) were calculated to accommodate the effects of dose per fraction. This correction allows for a more accurate assessment of the dose distribution within the target and surrounding tissues, which is critical for evaluating potential radiation-induced toxicities such as RP.
The normal tissue complication probability (NTCP) models
The probability of observing a specified complication after irradiation to dose \(D\) of subvolume \(V\) (expressed as a fraction of the whole organ) can be modelled using a cumulative normal distribution in the standard Lyman model23:
with
The parameter TD50 is the tolerance dose corresponding to a 50% probability of complication after irradiation of the reference volume. The parameter m is inversely related to the slope of the dose–response curve for the complication in question, while n represents the volume parameter. Based on a recursive algorithm proposed by Lyman, the model proposed by Kutcher and Burman (LKB)24 suggested that the exposure dose D can be represented by the effective uniform dose Deff to the entire volume. A special case of the LKB model is when n equals 1, where Deff can be replaced by the mean lung dose (MLD). In this study, the mean dose of total lungs (MT) corresponds to the MLD in the LKB model:
Therefore
In this equation, Di is the dose to subvolume vi, and the sum extends over all dose bins in the DVH.
Data analysis
In this study, Pearson's correlation coefficient (Pearson's r)25,26 was utilized to evaluate the relationship between clinical and dosimetric parameters. The strength of the correlation increases with the absolute values of r (|r|). Table 2 provides guidelines for interpreting the strength of the correlation coefficient in this cohort, employing terms such as "perfect", "very strong", "strong", "moderate", "weak", and "poor". A correlation was deemed significant if the associated p-value was < 0.05. Linear regression models were employed to explore specific relationships among various dosimetric data. The performance of these regression models was evaluated using mean squared error (MSE) and the coefficient of determination, R-squared (R2).
Ethics approval
The study was approved by the Clinical Research Ethics Committee of Renmin hospital of Wuhan University (approval number: WDRY2024-K033). Due to the retrospective nature of the study, the Clinical Research Ethics Committee of Renmin hospital of Wuhan University waived informed consent.
Results
The dosimetric data
In this study, dose-volume (Vd) was defined as the percentage of volume receiving equal to or greater than the specified radiation dose (in Gy). Dosimetric factors considered in this cohort included mean dose (MD), as well as V5, V10, V20, V25, and V30 of IL, CL, and TL. The dose distribution among patients exhibited significant individual differences. In terms of IL, MD ranged from 2.63 to 17.91 Gy, with a median value of 7.32 Gy. The highest and lowest V5 values were 49.29% and 9.27%, respectively. V10 ranged from 6.10 to 35.87%, V20 from 1.47 to 26.39%, and V25 from 2.41 to 23.32%. Even V30 showed considerable variation, ranging from 1.92 to 20.28%, with a median value of 8.86%. Radiation exposure to CL appeared more favorable, with low percentages of V5 and V10 recorded and a minimal MD. The received dose of TL balanced the effects of IL and CL, with the low radiation exposure to CL significantly reducing the dose level of TL. The characteristics of MD and Vd are illustrated in Fig. 1.
Correlational analysis of clinical and dosimetric data
To explore the relationships among various characteristics, a correlation analysis was conducted on all clinical and dosimetric data considered as continuous variables. Pearson's r and the corresponding p-values for this cohort are depicted in Fig. 2. The analysis revealed that clinical parameters, such as age, tumor size (ITV and PTV), the prescription dose (D), the number of fractions (F), the dose per fraction (d), and CI, exhibited only "poor" or "weak" correlations with dosimetric data, suggesting they could be disregarded in this study as well.
The most important aspect is that almost all dosimetric data (MD and Vd) exhibited "strong" or "very strong" correlations with each other, particularly between the mean dose of ipsilateral lung (MI) and other dosimetric variables. Specifically, MI showed a "very strong" correlation with Vd of IL, and it was strongly correlated with the mean dose of the total lung (MT) and the mean dose of the contralateral lung (MC) as well. Pearson's \(r\) ranged from 0.70 to 0.95 for these relationships, with all p-values approximating 0. Furthermore, MT exhibited "very strong" correlations with Vd of TL, while MC strongly correlated with Vd of CL. These findings suggested that dose distribution variables could be interconnected through certain linear regression relationships in SBRT of lung cancers. These results are consistent with findings in existing literature. Matsue et al.17 observed that lung metrics (MT and V5-40) were highly correlated, with Pearson's \(r\) ranging from 0.61 to 0.99. Similarly, Liu et al.10 suggested strong linear correlations between MT, V20 of TL, and MI. However, their study only examined correlations between a few dosimetric parameters without explicitly describing the relationships among the dosimetric data. This study has offered a more comprehensive correlation analysis of dose parameters, and the specific relationships among these parameters will be further explored in the subsequent section.
Linear regression for dosimetric data
Given the strong and significant correlations observed among all dosimetric variables in the preceding section and the literature, it is more appropriate to condense the various dosimetric variables into a single representative dose parameter for the analysis of NTCP of RP. As previously mentioned, MI, which exhibited "strong" or "very strong" correlations with other dosimetric variables, can be considered the most suitable choice for the representative dose parameter.
This section employed a machine learning method, linear regression, to clarify the relationships among different dose distribution variables. All dose distribution data in this cohort were divided into a training set and a test set, with the test set accounting for 12% of each dataset. A "random_state" parameter was utilized to ensure result consistency for every linear regression model. The fitting results are depicted in Fig. 3 and Table 3. The parameters w and b represent the slope and intercept of the linear regression model, respectively.
For IL and TL, V5, V10, V20, V25 and V30 could be linearly represented by their corresponding MD. Apart from the MSE for V5 of IL versus MI, which exceeded 9, other regression results were quite satisfactory, with MSE less than 3 and R2 greater than 0.80. Due to smaller tumor sizes and stricter dose limitations in SBRT compared to CFRT for lung cancers, the delivered dose to the CL was relatively low. This was directly reflected in the percentage of volume receiving a dose greater than 10 Gy of CL, which was approximately zero. Thus, only regression algorithms for V5 and V10 versus MC were performed, with MSE and R2 values of 2.89, 0.94 and 0.59, 0.80 for V5 and V10, respectively.
Another exciting discovery was that both MT and MC could be linearly expressed by MI, with MSE and R2 values of 0.04 and 0.97 for MT, and 0.02 and 0.88 for MC, respectively. In summary, in SBRT of lung cancers, the Vd for IL, MT, and MC could be linearly represented by MI with a good fit. Furthermore, MT and MC could linearly express the Vd of TL and CL. Thus, according to MI, all dose distributions of IL, CL and TL in SBRT for lung cancers can be linearly predicted.
The predictor of MI
In this study, MI can be regarded as a representative dose for all dose distributions. However, factors such as gender, age, tumor location, and TPS in the cohort did not appear to be significantly associated with MI, as detailed in Fig. 4.
Some references28 have pointed out that the volume of the lung or tumor size (usually referred to as PTV) is generally regarded as significant clinical factor associated with RP in SBRT of lung cancers. This is because a larger tumor size increases the coverage area of the dose isoline delivered to the lung, and a smaller lung volume adds to the value of Vd. However, in this study, their individual effects on dose distributions of lungs were not noticeable, even though PTV was strongly and significantly correlated with MI (p = 0.00). As seen in Fig. 5, overall, MI increased with the addition of PTV and decreased with increasing volume of IL. However, there were no specific formulas to describe the relationships between them.
Baker et al.18 previously proposed that the ratio of PTV to normal lung volume was highly significant for developing RP2 in SBRT for lung cancers. However, their study did not describe the relationship between the ratio and RP2 or dosimetric data. Based on this, the lung tumor ratio (LTR), defined as the volume of IL divided by PTV, was introduced in this section to combine the information of PTV and the volume of IL. Its value ranged from 9.18 to 151.97, with a median of 36.26 and a mean of 44.88 in the cohort. Figures 6 illustrate that compared to the PTV or the volume of IL, the LTR was a more reliable predictive factor for MI. The fitting curve could be expressed as a power function, with MSE and R2 being 13.51 and 0.62, respectively. Namely, MI decreased as a power exponential of the LTR. Thus, in this study, the LTR can be used to predict MI, and then MI can be used to calculate the Vd of IL, MC, and MT. Furthermore, the Vd of CL and TL can be obtained by MC and MT, respectively. Overall, the LTR was a suitable predictor for MI and could be used to predict all dose distributions in SBRT for lung cancers.
The normal tissue complication probability (NTCP) model
Generally, to optimize RT treatment planning, estimating the NTCP is necessary. This probability relies on individual patient specifics, such as radiation sensitivity, cellular repair ability, organ reserve capacity, smoking history, physical conditions, pre-RT, and the actual physical dose delivered (including the fractionation scheme). Several studies29,30,31 have proposed that predictive models incorporating these factors might be more appropriate than models relying solely on dosimetric parameters to predict RP. However, in practice, acquiring proximal knowledge of the spatial dose distribution supplied by the RT treatment planning is relatively straightforward. Additionally, it has been found that the accuracy of predictive models based solely on dosimetric data substantially improves when patients with extremely inferior pre-RT pulmonary function or re-RT are excluded32.
It is important to note that although all dosimetric parameters have demonstrated a good correlation with the incidence of RP in references10,29,33, it has become a consensus that MT (i.e., MLD in references) is the best predictor of RP. In this cohort, for each patient, the DVH of their lungs (including MT) can be simplified to a single parameter (MI), which is a dependent variable of LTR as described in the preceding section. Therefore, utilizing the LTR to predict the probability of RP will significantly streamline the construction process of its NTCP model. Referring to Table 3, MT can be expressed as a function of MI:
Furthermore, as depicted in Fig. 5, MI can be predicted by the LTR:
Hence, based on the MT model and the combined formulas (4), (5) and (6), the integration variable of NTCP for RP can be expressed as a function of the LTR:
It should be noted that the inconsistency in model parameters posed a challenge, as they varied among different study cohorts. As indicated by Tsougos et al.32, the impact of parameter uncertainties on dose–response curves can be significant. Borst et al.34 analyzed data from 128 lung cancer patients treated with doses per fraction ranging from 6 to 12 Gy, with fitted parameters of TD50 = 19.6 Gy and m = 0.43. Swlvaraj et al.35, examined a multi-institutional dataset of 1015 lung cancer patients treated with SBRT, suggesting the best-fit parameters as TD50 = 47.3 Gy and m = 0.43. However, regardless of the specific values of the model parameters, the ability to predict the NTCP of RP using the LTR according to Eq. (7) remains unaffected for lung cancer patients before undergoing actionable SBRT planning.
Additionally, there exists another simplified RP-MT model known as the QUANTEC-model36, derived from a pooled analysis of 10 different retrospective datasets. Combining this model with formulas (5) and (6), it can also be expressed as a function of the LTR:
In theory, regardless of its form, all predictive NTCP models for RP based on dosimetric data in SBRT for lung cancers can be utilized by employing the LTR factor, as the LTR can be used to predict dose distributions of lungs.
Discussion
RP is one of the most extensively researched toxicities in SBRT for lung cancers37,38. It is desirable to estimate the patient-specific risk of developing severe RP before the start of treatment, utilizing factors available from the planned dose distribution and the patient's medical history. Numerous factors, including parameters related to the patient, tumor, and treatment dosimetric factors, appear to be associated with the risk of developing RP39,40. Onishi et al.41 observed that fatal RP frequently occurred after SBRT in lung cancer patients with pretreatment pulmonary interstitial change (PIC). Baker et al.18 pointed out that factors such as female gender, pack-years of smoking, larger GTV and PTV, and the ratio of PTV to normal lung volume, were highly significant in the context of SBRT for lung cancers. Zhao et al.28 conducted a meta-analysis of 88 studies on thoracic cancers treated with SBRT, demonstrating that older age and larger tumor size were significant adverse risk factors for RP. Kong et al.42 updated Zhao's study and reviewed 97 studies, indicating that various dosimetric parameters were reported to be associated with RP. Although there was no apparent threshold “tolerance dose-volume” level in their review, most studies noted safe treatment, with a rate of RP2 of less than 10% to 15% after lung SBRT, with the MT being 8 Gy in 3 to 5 fractions and V20 less than 10% to 15%. Liu et al.10 also proposed that a history of respiratory comorbidity, previous thoracic radiation, tumor location, MI, MT, and V20 of the TL were all significantly associated with the risk of RP2.
Physicists and oncologists can adjust only dosimetric parameters prior to treatment among the characteristics associated with RP mentioned above. Numerous studies have specified the relationship between dose threshold levels and the risk probability of RP. Barriger et al.43 demonstrated that RP2 was noted in 4.3% of patients with MD \(\le\) 4 Gy compared with 17.6% of patients with MD > 4 Gy. Additionally, the crude incidence of RP2 for V20 > 4%, 5%, 7% and 10% of TL was 16%, 18%, 16.6%, and 15.7%, respectively. Matsuo et al.17 proposed that V25 of the TL and PTV volume were significant factors related to RP2. Ryckman et al.44 suggested that future trials should consider MD and V20 of TL as constraints associated with the development of RP2. Liu et al. 10 indicated that the risk for RP2 exceeding 50% was a function of the dose and the volume of IL. To limit the rate of RP2 to less than 10%, they pointed out that the threshold for MT, MI, and V20 of TL were 5.9 Gy, 19.7 Gy, and 10% for all patients, while 13.6 Gy, 21.2 Gy, and 13.2% for patients without prior radiation, respectively. Kita et al.2 proposed that V8, V10, V20, and MD of TL correlated with RP. Their study especially demonstrated that V10 exceeding 16.7% was the most reliable indicator of RP2 among the dose parameters analyzed. The literature underscored that MD, V10, V20, and V25 were acknowledged as crucial dosimetric parameters associated with RP2. Therefore, this study incorporated these metrics and, consistent with clinical practice, added V5 and V30 as additional evaluation criteria.
The comprehensive dosimetric analyses and studies of dosimetric risk factors in the literature vary widely in the use of dose fractionation schemes. Many studies do not account for the dose differences caused by various fractionation schemes. This study recommends that all treatment plans used in dosimetric analyses should be adjusted for differences in the number of fractions by transforming accumulated dose distributions to EQD2. Additionally, there are inconsistencies in the MD and Vd calculations regarding whether the lung volumes should exclude tumor targets (GTV/PTV). The study suggests that the prescription dose be delivered to the PTV, thus the volume of normal lung tissue should exclude the PTV. Furthermore, while most literatures associated MD and Vd with TL when evaluating the effect of lung exposure dose on RP. However, radiographic films from the study of Rancati et al.45 demonstrated that all RPs were located in IL. Yorke et al.46 proposed in a study that no index calculated for the CL was significantly correlated with RP. This study also suggests that the dose distribution of IL is more appropriate for assessing RP in lung cancers after SBRT than TL. This recommendation stems from the observation that the dose exposure level of TL might be neutralized by the lower dose level received by the CL. Thus, focusing on the dose distribution of IL could provide a clearer understanding of the relationships.
Furthermore, this study has established the relationship between LTR and lung dosimetry data for lung cancers treated with SBRT. By integrating this relationship with the well-known LKB model, particularly under the condition where n = 1, the potential of LTR in predicting RP was inferred. However, the parameters of the LKB model heavily rely on the number of patients and specific inclusion criteria. Due to the limited sample size of our cohort, the statistical significance of the clinical NTCP distribution is insufficient to directly establish the relationship between LTR and NTCP. Additionally, the diverse patient inclusion criteria imply that applying fitting parameters directly from the literature to predict NTCP using LTR would result in significant deviations. Therefore, at present, this study can only offer theoretical insights into predicting RP probability using LTR. The future work will aim to collect a sufficient number of cases to refine this aspect.
In conclusion, the discovery of LTR has the potential to predict the incidence of RP without the need to design an elaborate treatment plan. It was also deemed beneficial for physicists and oncologists to balance lung protection and tumor treatment in devising the best radiotherapy scheme. However, the study had several limitations. Firstly, it was a retrospective study conducted at a single institution. Therefore, large-scale multicenter studies are necessary to validate the results obtained. Secondly, the NTCP model was derived from a public report, leading to a reliance on patient data from the literature. Consequently, it is essential to collect data on RP at a later stage to construct a more coherent NTCP model.
Conclusion
This study, as the first to offer a comprehensive correlation analysis of dose parameters, explored the specific relationships among them. Significantly, it identified LTR as a novel predictor for both dose parameters and the incidence of RP, without the need to design an elaborate treatment plan. Defining this predictive factor may provide a foundation for oncologists and physicists to tailor personalized RT planning, potentially refining lung dose constraints more effectively mitigate RP. However, further prospective confirmation of these results is necessary.
Data availability
All relevant data and materials have been included in the article. Further inquiries can be directed to the corresponding authors.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71(3), 209–249 (2021).
Kita, N. et al. Clinical and dosimetric factors for symptomatic radiation pneumonitis after stereotactic body radiotherapy for early-stage non-small cell lung cancer. Clin. Transl. Radiat. Oncol. 41, 1–6 (2023).
Zheng, R. S. et al. Cancer incidence and mortality in China, 2016. J. Natl. Cancer Center 2(1), 1–9 (2022).
Zhang, Z. et al. Radiomics and dosiomics signature from whole lung predicts radiation pneumonitis: A model development study with prospective external validation and decision-curve analysis. Int. J. Radiat. Oncol. Biol. Phys. 15(3), 746–758 (2023).
Lahiri, A. et al. Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol. Cancer 22(1), 37 (2023).
Ye, F. S. et al. Predicting radiation pneumonitis in lung cancer: A EUD-based machine learning approach for volumetric modulated arc therapy patients. Front. Oncol. 14, 11 (2024).
Gul, O. V., Sengul, A. & Demir, H. Effects of radiation at different dose rates on hematologic parameters in rats. J. Radiat. Res. Appl. Sci. 17(2), 5 (2024).
Gul, O. V. Experimental evaluation of out-of-field dose for different high-energy electron beams and applicators used in external beam radiotherapy. Radiat. Phys. Chem. 215, 9 (2024).
Amini, A. et al. Stereotactic body radiation therapy (SBRT) for lung cancer patients previously treated with conventional radiotherapy: A review. Radiat. Oncol. 9, 1–8 (2014).
Liu, Y. M. et al. Risk factors for symptomatic radiation pneumonitis after stereotactic body radiation therapy (SBRT) in patients with non-small cell lung cancer. Radiother. Oncol. 156, 231–238 (2021).
Schroder, C. et al. Re-irradiation in the thorax—An analysis of efficacy and safety based on accumulated EQD2 doses. Radiol. Oncol. 152, 56–62 (2020).
Chang, J. Y. et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Oncol. 16(6), 630–637 (2015).
Sogono, P. et al. Safety, efficacy, and patterns of failure after single-fraction stereotactic body radiation therapy (SBRT) for oligometastases. Int. J. Radiat. Oncol. Biol. Phys. 109(3), 756–763 (2021).
Maquilan, G. & Timmerman, R. Stereotactic body radiation therapy for early-stage lung cancer. Cancer J. 22(4), 274–279 (2016).
Liu, H. et al. Predicting radiation pneumonitis after stereotactic ablative radiation therapy in patients previously treated with conventional thoracic radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 84(4), 1017–1023 (2012).
Ueki, N. et al. Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. J. Thorac. Oncol. 10(1), 116–125 (2015).
Matsuo, Y. et al. Dose-volume metrics associated with radiation pneumonitis after stereotactic body radiation therapy for lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 83(4), E545–E549 (2012).
Baker, R. et al. Clinical and dosimetric predictors of radiation pneumonitis in a large series of patients treated with stereotactic body radiation therapy to the lung. Int. J. Radiat. Oncol. Biol. Phys. 85(1), 190–195 (2013).
Yamashita, H. et al. Exceptionally high incidence of symptomatic grade 2–5 radiation pneumonitis after stereotactic radiation therapy for lung tumors. Radiat. Oncol. 2, 11 (2007).
Cella, L. et al. Radiation-induced dyspnea in lung cancer patients treated with stereotactic body radiation therapy. Cancers 13(15), 9 (2021).
Yamaguchi, S. et al. Stereotactic body radiotherapy for lung tumors in patients with subclinical interstitial lung disease: The potential risk of extensive radiation pneumonitis. Lung Cancer 82(2), 260–265 (2013).
Wennberg, B. M. et al. NTCP modelling of lung toxicity after SBRT comparing the universal survival curve and the linear quadratic model for fractionation correction. Acta Oncol. 50(4), 518–527 (2011).
Lyman, J. T. Complication probability as assessed from dose-volume histograms. Radiat. Res. 104, S13–S19 (1985).
Kutcher, G. J. & Burman, C. Calculation of complication probability factors for non-uniform normal tissue irradiation—The effective volume method. Int. J. Radiat. Oncol. Biol. Phys. 16(6), 1623–1630 (1989).
Harrington, P. D., Urbas, A. & Tandler, P. J. Two-dimensional correlation analysis. Chemom. Intell. Lab. Syst. 50(2), 149–174 (2000).
Armstrong, R. A. Should Pearson’s correlation coefficient be avoided?. Ophthalm. Physiol. Opt. 39(5), 316–327 (2019).
Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 18(3), 91–93 (2018).
Zhao, J. et al. Simple factors associated with radiation-induced lung toxicity after stereotactic body radiation therapy of the thorax: A pooled analysis of 88 studies. Int. J. Radiat. Oncol. Biol. Phys. 95(5), 1357–1366 (2016).
Seppenwoolde, Y. et al. Comparing different NTCP models that predict the incidence of radiation pneumonitis. Int. J. Radiat. Oncol. Biol. Phys. 55(3), 724–735 (2003).
Brink, C., Berg, M. & Nielsen, M. Sensitivity of NTCP parameter values against a change of dose calculation algorithm. Med. Phys. 34(9), 3579–3586 (2007).
Martel, M. K. Advanced radiation treatment planning and delivery approaches for treatment of lung cancer. Hematol.-Oncol. Clin. N. Am. 18(1), 231–243 (2004).
Tsougos, I. et al. NTCP modelling and pulmonary function tests evaluation for the prediction of radiation induced pneumonitis in non-small-cell lung cancer radiotherapy. Phys. Med. Biol. 52(4), 1055–1073 (2007).
Marks, L. B. et al. Physical and biological predictors of changes in whole-lung function following thoracic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 39(3), 563–570 (1997).
Borst, G. R. et al. Radiation pneumonitis in patients treated for malignant pulmonary lesions with hypofractionated radiation therapy. Radiother. Oncol. 91(3), 307–313 (2009).
Selvaraj, J. et al. Modeling radiation pneumonitis of pulmonary stereotactic body radiotherapy: The impact of a local dose-effect relationship for lung perfusion loss. Radiother. Oncol. 132, 142–147 (2019).
Marks, L. B. et al. Radiation dose-volume effects in the lung. Int. J. Radiat. Oncol. Biol. Phys. 76(3), S70–S76 (2010).
Fernandes, M. G. et al. Estimating how contouring differences affect normal tissue complication probability modelling. Phys. Imaging Radiat. Oncol. 29, 7 (2024).
Niezink, A. G. H. et al. External validation of NTCP-models for radiation pneumonitis in lung cancer patients treated with chemoradiotherapy. Radiother. Oncol. 186, 8 (2023).
Heiden, B. T. et al. Assessment of duration of smoking cessation prior to surgical treatment of non-small cell lung cancer. Ann. Surg. 277(4), e933–e940 (2023).
Dennstädt, F., Medová, M., Putora, P. M. & Glatzer, M. Parameters of the Lyman model for calculation of normal-tissue complication probability: A systematic literature review. Int. J. Radiat. Onco. Biol. Phys. 115(3), 696–706 (2023).
Onishi, H. et al. Stereotactic body radiation therapy for patients with pulmonary interstitial change: High incidence of fatal radiation pneumonitis in a retrospective multi-institutional study. Cancers 10(8), 11 (2018).
Kong, F. M. et al. Organs at risk considerations for thoracic stereotactic body radiation therapy: What is safe for lung parenchyma?. Int. J. Radiat. Oncol. Biol. Phys. 110(1), 172–187 (2021).
Barriger, R. B. et al. A dose-volume analysis of radiation pneumonitis in non-small cell lung cancer patients treated with stereotactic body radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 82(1), 457–462 (2012).
Ryckman, J. M. et al. Correlation of dosimetric factors with the development of symptomatic radiation pneumonitis in stereotactic body radiotherapy. Radiat. Oncol. 15(1), 15 (2020).
Rancati, T. et al. Factors predicting radiation pneumonitis in lung cancer patients: A retrospective study. Radiother. Oncol. 67(3), 275–283 (2003).
Yorke, E. D. et al. Dose-volume factors contributing to the incidence of radiation pneumonitis in non-small-cell lung cancer patients treated with three-dimensional conformal radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 54(2), 329–339 (2002).
Funding
This work was supported by the Research Foundation on Cutting-edge Cancer Supportive Care (Grant/Award Number: cphcf-2022-146).
Author information
Authors and Affiliations
Contributions
X Y, ZY D, and HB S conducted the literature search and study design. X Y, HB S, XP L and HY G collected the data, processed statistical information, and contributed to data interpretation. X Y and ZY D drafted the manuscript, which was subsequently revised by XP L and HY G.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, X., Dai, Z., Song, H. et al. A novel predictor for dosimetry data of lung and the radiation pneumonitis incidence prior to SBRT in lung cancer patients. Sci Rep 14, 18628 (2024). https://doi.org/10.1038/s41598-024-69293-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69293-8
- Springer Nature Limited