Abstract
Marine pollution caused by heavy metals has emerged as a significant environmental concern, garnering increased attention in recent years. The accumulation of heavy metals in the tissues of marine organisms poses substantial threats to both marine ecosystems and human populations that rely on seafood as a primary food source. Fish and crustaceans are effective biomonitors for assessing heavy metal contamination in aquatic environments. In this study, we determined the concentrations of several heavy metals, including cadmium (Cd), lead (Pb), nickel (Ni), mercury (Hg), and tin (Sn), in four fish species (Mugil cephalus, Mugil capito, L. aurata, and Morone labrax) and five crustacean species (S. rivulatus, Cerastoderma glaucum, Paratapes undulatus, R. decussatus, Callinectes sapidus, and Metapenaeus Stebbingi) from Temsah Lake during both winter and summer seasons. To evaluate the potential ecological and health risks associated with consuming these fish and crustacean species, we calculated the metal pollution index (MPI), weekly intake (EWI), target hazard quotient (THQ), and carcinogenic risk (CR) values. The results revealed a noticeable increase in metal levels during the summer compared to winter in the studied samples. Moreover, the concentration of heavy metals in the muscles of the species generally exceeded those in the liver and gills. The MPI values indicated that Morone labrax exhibited the highest values during winter, while L. aurata showed the highest values during summer. Mugil cephalus demonstrated the lowest MPI values in both seasons. The EWI values for the studied metals were found to be lower than the corresponding tolerable weekly intake (TWI) values. Additionally, under average exposure conditions, the THQ and HI data were generally below one for most study species in the area. The calculated CR values for investigated metals in the studied species indicated acceptable carcinogenic risk levels. Therefore, this suggests that consuming studied species within Temsah lake does not present any potential health hazards for consumers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Marine pollution with heavy metals is a significant environmental issue that has garnered increasing attention in recent years. Heavy metals, such as cadmium, nickel, lead, mercury, and tin can accumulate in the tissues of marine organisms over time, posing a serious threat to the health of both marine ecosystems and human populations that rely on seafood as a primary source of nutrition1,2.
The sources of marine pollution with heavy metals are numerous and diverse, ranging from industrial activities, such as mining, smelting, and metal processing, to agricultural runoff, sewage disposal, and atmospheric deposition. These metals can enter the marine environment through various pathways, including direct discharge into waterways, leaching from landfills and waste disposal sites, and atmospheric deposition from industrial emissions and other sources3,4,5,6,7.
Once in the marine environment, heavy metals can be transported over long distances and can persist in sediments and biota for many years8,9. The accumulation of heavy metals in marine organisms can lead to a range of negative impacts, including reduced growth and reproduction, altered behavior and physiology, and increased susceptibility to disease and predation10.
Moreover, heavy metals can bioaccumulate and biomagnify in the food chain, meaning that organisms at higher trophic levels can accumulate greater concentrations of these metals than those at lower trophic levels7. This can result in significant health risks to humans who consume contaminated seafood, including neurological damage, developmental disorders, and cancer11,12.
Fish and crustaceans are important sources of protein and nutrients for human populations worldwide and are a critical component of many traditional diets13,14. However, consuming contaminated seafood can pose significant health risks to humans, particularly in terms of exposure to heavy metals such as mercury, lead, and cadmium15,16. The health effects of heavy metal exposure can range from neurological damage and developmental disorders to cancer and cardiovascular disease17,18,19.
The World Health Organization (WHO) has established guidelines for safe levels of heavy metals in seafood products, but many studies have found that a significant proportion of seafood consumed worldwide exceeds these limits WHO20. Connelly et al.21 found that more than half of the seafood samples tested in the United States exceeded the safe limit for mercury.
Efforts to address the issue of heavy metal contamination in seafood have focused on a range of strategies, including improved regulations and management practices for industrial and agricultural activities, increased monitoring and surveillance of seafood products, and the development of innovative technologies for removing heavy metals from contaminated sediments and waterways22,23,24. In addition, consumer education and awareness campaigns have been developed to help people make informed decisions about the types and amounts of seafood they consume25.
Bivalves are widely used in ecotoxicological studies as bioindicator organisms because of their broad distribution, sessile nature, filter-feeding behavior, and tolerance to many pollutants26,27. Rahman et al.28 found that fish from the Ganges River in India were contaminated with high levels of lead and cadmium, while Apeti et al.29 and Medina-Morales et al.30 reported that Fish and shellfish from the Gulf of Mexico had elevated levels of mercury. The groundbreaking research conducted by Parrino et al.31 significantly advances our comprehension of the influence of environmental pollution and feeding habits on the blood parameters of two teleost fish species.
Numerous marine organisms have been recognized as dependable bioindicators for surveilling heavy metal pollution in coastal and estuarine waters. The levels of heavy metals in the tissues of these organisms are influenced by both their sampling location and dietary preferences32. The use of bioindicator species in biomonitoring programs provides valuable information on the levels of heavy metal contamination in coastal and estuarine waters, which is essential for effective environmental management and the protection of public health33. These programs can inform policy decisions aimed at reducing the environmental impacts of heavy metal pollution and promoting sustainable fisheries management practices.
Despite the wealth of information available on metal contamination in fish from other aquatic environments, there are still significant gaps in knowledge regarding the concentrations of metals in various fish organs (gills, livers, and muscles) and crustacean species from Temsah Lake, Suez Canal. To address these gaps in knowledge, further research is needed to provide valuable insights into the levels of metal contamination in a wider range of fish and crustacean species from Temsah Lake and to investigate the distribution of metals in different organs of these aquatic species. In addition, comprehensive risk assessments should be conducted to determine the potential health risks associated with consuming contaminated seafood from Temsah Lake.
By identifying the metal contamination levels in seafood, researchers can inform policy decisions and management strategies to mitigate the risks associated with metal pollution in aquatic ecosystems.
The specific objective of this study was to investigate the concentrations of metals in various organs of fish and crustacean tissue species derived from Temsah Lake while assessing the potential health risks posed to consumers. The novelty of this study lies in the fact that it is the first analysis conducted in this particular region, marking a significant contribution to the existing knowledge base. Temsah Lake holds substantial importance as a fish source for communities in Egypt, underscoring the necessity of understanding the levels of metal contamination present in these aquatic species to ensure the safety and well-being of consumers. By accomplishing these objectives, this research endeavors to provide invaluable insights into the extent of metal contamination found in fish from Temsah Lake, along with an assessment of potential health risks to consumers. Such information holds great significance in guiding management and regulatory strategies aimed at mitigating the risks associated with heavy metal pollution in aquatic ecosystems. Additionally, these findings can facilitate the promotion of safe and sustainable consumption practices for seafood products, enhancing the overall welfare of both individuals and the environment.
Materials and methods
Study area
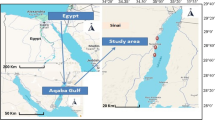
Temsah Lake is a vital aquatic ecosystem located in Egypt, with a geographical location of 30.78°N latitude and 31.23°E longitude. The lake covers an area of approximately 12,000 hectares and has a maximum depth of 3 m. It is situated in the Suez Canal region and is surrounded by agricultural land, making it vulnerable to pollution from agricultural runoff and other sources4.
The lake is an important habitat for diverse aquatic species, including fish, shrimp, and crabs. It is also a significant breeding and feeding ground for migratory birds, such as the white pelican and the great egret34.
El-Temsah Lake is a major source of fish, crustaceans, bivalves, and shellfish that are consumed by local populations. However, the northern and western boundaries of the lake have been rapidly developed for tourism purposes, aquatic sports, and recreational activities35. In addition to its ecological significance, Temsah Lake is an important economic resource for local communities. It supports a thriving fishing industry, with various fish and shellfish species caught and sold in local markets. The lake is also used for recreational activities, such as boating and birdwatching, and is a popular tourist destination36.
Despite its ecological and economic importance, Temsah Lake faces various environmental challenges, including pollution from agricultural runoff, untreated sewage, and industrial activities. These pollutants can significantly impact the lake's water quality, the health of its aquatic species, and the health of people who consume contaminated seafood. As a result, these conditions have threatened the health of the lake, interfered with its recreational purpose, and reduced the richness and diversity of indigenous fish, phytoplankton, zooplankton, plants, and animal populations36.
To address these challenges, various management and conservation strategies have been implemented, including developing monitoring and surveillance programs, establishing protected areas, and promoting sustainable fishing practices. Ongoing research and monitoring efforts are critical to maintaining Temsah Lake’s ecological and economic value and ensuring its long-term sustainability.
Sampling
Several fish species, including Mugil cephalus, Mugil capito, L. aurata, and Morone labrax were collected from Temsah Lake, Suez Canal (Fig. 1). In addition, five specimens of crustaceans, namely S. rivulatus, Cerastoderma glaucum, Paratapes undulatus, R. decussatus, Callinectes sapidus, and Metapenaeus Stebbingi. The specimens utilized in this study were sourced from licensed fishermen who possess the necessary authorization to operate within the pristine waters of the lake. These fishermen provided us with non-live samples for our research purposes. The collected samples' gills, liver, and muscle tissues were isolated and washed with distilled water before being weighed in separate containers. The samples were meticulously packed in polyethylene bags and transported to the laboratory under refrigeration at a temperature of −4 °C to maintain their integrity and preserve their quality. Upon arrival, they were promptly frozen at −20 °C to ensure optimal conditions for subsequent chemical analysis, which aimed to determine the concentrations of heavy metals present in the samples. The analysis was conducted according to the37 protocol. Additionally, the soft tissues of the mussels were separated from the shells and stored in a freezer until chemical analysis was conducted to determine the concentrations of heavy metals.
Map illustrating the sampling site of Temsah Lake, Suez Canal (The map was sourced from Google Maps, https://earth.google.com/web).
Determination of metal concentrations
The tissue samples were meticulously weighed and subsequently subjected to digestion in Teflon PTFE tubes using HNO3 acid in a microwave oven. After reaching room temperature, the samples were transferred to 25 ml volumetric flasks and diluted to a final volume of 25 ml using 2% HNO3. To achieve appropriate levels, the diluted samples were adjusted with de-ionized water within the range of standards prepared from stock standard solutions of the metals (Merck). Before analysis, a 0.45 µm nitrocellulose membrane filter was employed to filter all samples. All digested solutions were analyzed in triplicate using an Atomic Absorption Spectrophotometer (Shimadzu Model AA-6800 with graphite furnace), employing a hydride unit for mercury and tin determination. The instrument was operated according to the manufacturer's specifications and settings. In the laboratory, sample blanks were prepared similarly to the field samples, and all determinations were conducted in triplicate. The concentrations of heavy metals in fish samples were reported as mg/kg dry weight. This analytical procedure is a reliable and widely accepted method for quantifying heavy metal concentrations in various biological samples. The utilization of triplicate measurements and sample blanks ensures the accuracy and precision of the results. To guarantee accuracy and precision in the analysis, analytical-grade reagents were employed, and the glassware was soaked in 10% nitric acid, followed by rinsing with distilled water to prevent potential metal contamination.
Instrumentation, quality control, and quality assurance
Quantification of Pb, Cd, and Ni was performed using an atomic absorption spectrophotometer (Shimadzu AA-6800) equipped with a graphite furnace (GFA-EX7) and an autosampler (ASC-6100). High-density graphite tubes were employed for elements that are not easily carbonized, such as Pb and Cd, while pyrolytic graphite-coated graphite tubes were used for elements that are easily carbonized, such as Ni. Hydride vapor generation-atomic absorption spectrophotometry (HVG-AAS) (Shimadzu Model: HVG-1) and cold vapor-atomic absorption spectrophotometry (CVAAS) (Shimadzu Model: MVU-1A) techniques were employed for the quantification of Sn and Hg, respectively. Hollow cathode lamps (HCL) were operated according to the manufacturer’s recommendation for Cd, Pb, Ni, Hg, and Sn, serving as radiation sources for AAS. The argon and acetylene gases used had a purity of 99.99%. The analytical conditions of AAS employed for the measurement of heavy metals in the aqueous solution of fish samples are summarized in (Table 1).
To prevent contamination, all glassware and equipment were meticulously cleaned with 2% HNO3 and rinsed with deionized water. The calibration line method was employed to ensure accuracy, maintaining optimal analytical conditions. Standard solutions for the elements were purchased from Merck, Germany, and were traceable to SRM from NIST. Working standard solutions were prepared by diluting the stock solution (1000 mg/L) using deionized water. For recovery checks, dilutions of 0.3, 12, 6, 20, and 2 µg/L were used for Cd, Pb, Ni, Sn, and Hg, respectively. Blank samples were spiked with the analyte to verify the calibration of the instrument, which also provided an indication of accuracy38. After every 5 samples, a calibration blank and an independent calibration verification standard were analyzed to confirm the calibration status of the AAS. Matrix interference (blank) was less than 1% for all elements. Calibration curves with an R2 value of at least 0.999 were considered for concentration calculations. The recoveries of the different analytes were as follows: Pb: 101%, Cd: 106%, Ni: 96%, Hg: 90%, and Sn: 99%.
The recovery efficiency of the metals investigated in this research aligns with findings from other studies employing diverse analytical techniques such as (Memon et al.39) and (Memon et al.40).
Statistical analysis
A tri-factorial analysis of variance (ANOVA) was employed to elucidate significant variations in metal levels across different species and organs throughout distinct seasons. Additionally, a unifactorial analysis of variance (ANOVA) was utilized to evaluate metal quantities among diverse crustacean species and seasons in crustaceans. Statistical significance was determined at a threshold of p < 0.05. Prior to conducting the study, the data underwent thorough examinations to ensure both homogeneity of variances and normality. In cases where the data did not exhibit normal distribution or homogeneity, the Kruskal–Wallis one-way analysis of variance (ANOVA) on ranks and the Mann–Whitney U test were employed.
Following the completion of the analysis of variance (ANOVA), the Tukey honestly significant difference (HSD) multiple range test was employed to ascertain the specific location of the variation. The statistical calculations were conducted using SPSS 26.0 for Windows.
Ecological risk assessment
The metal pollution index (MPI) serves as a tool to assess and compare the overall metal contamination levels in the studied species across different seasons. MPI is determined using the following formula:
In this equation, Mn represents the concentration of a specific metal expressed in mg/kg of wet weight, as outlined by Usero et al.41. The MPI calculation incorporates the individual metal concentrations of interest and calculates the geometric mean, providing an indicator of the overall metal pollution level. The Ministry of Environment in Egypt adheres to the minimum limits for heavy metal concentrations established by the Food and agriculture organization (FAO) and the world health organization (WHO) in 1989.
Health risk assessment
Estimated weekly intake (EWI)
The estimated weekly intake (EWI) of heavy metals depends on various factors, such as the concentration of the metal, food consumption, and body weight. To assess the potential risk of consuming fish with high heavy metal content, a number of assumptions were made in this study. Firstly, it was assumed that the amount of ingested dose was equivalent to the absorbed pollutant dose, according to USEPA42 guidelines. Secondly, the effect of cooking on the pollutants was disregarded, as per the findings of Chien et al.43. Thus, the EWI of heavy metals was calculated using the following formula.
where C represents the concentration of heavy metals in fish (measured in mg/kg wet weight), Ccons denotes the average weekly consumption of fish in the local area (55.34 g/day bw), and Bw represents the body weight (70 kg).
Determination of target hazard quotient (THQ)
The determination of THQ, which stands for Target Hazard Quotient, involves calculating the ratio of the exposure dose to the reference dose (RfD) in order to assess the risk of noncarcinogenic effects. If the THQ value is less than 1, the exposure level is lower than the RfD. This implies that daily exposure at this level is unlikely to result in adverse effects throughout an individual's lifetime and vice versa. The dose calculations for THQ were carried out using standard assumptions from the integrated US EPA risk analysis44. The model for estimating THQ was based on the following equation, as proposed by Chien et al.43:
EF represents the exposure frequency, which is 52 days per year for individuals who consume fish once a week. ED stands for exposure duration and is 70 years for adults and 7 years for children. CM signifies the metal concentration, which indicates the amount of metal in a given sample or environment. FIR denotes the fish ingestion rate of 55.34 g per person per day, according to FAO45. Cf is the conversion factor (0.208) used to convert the fresh weight (Fw) of fish to its dry weight (Dw), considering a moisture content of 79% in the fish. WAB represents the target body weight of 70 kg for adults and 32 kg for children, as specified by USEPA46. RfD represents the oral reference dose in micrograms per gram per day. The following values are assigned to specific metals according to47,48: Cd = 0.001, Pb = 0.004, Ni = 0.02, Hg = 0.0001, Sn = 0.6. ATn indicates the average time for non-carcinogens, calculated by multiplying the exposure frequency (EF) by the exposure duration (ED). It estimates the average time an individual is exposed to non-carcinogenic substances. AT represents the average time for carcinogens and is determined by multiplying the exposure frequency (52 days per year) by the exposure duration for adults (70 years).
Cancer risk (CR)
The cancer risk (CR) calculation for metals involves utilizing the cancer slope factor, specifically provided by USEPA42 for this particular metal. The lifetime cancer risk is determined using the following Equation42:
In this equation, CSF represents the oral carcinogenic slope factor obtained from USEPA’s Integrated Risk Information System (IRIS) online database49.
Hazard index (HI)
The hazard index (HI) measures the potential risk of multiple hazardous substances. It is calculated by summing up the hazard quotients (HQs) of each individual substance, as per the guidelines provided by the USEPA50.
Results and discussions
The levels of heavy metals found in the tissues of fish and crustaceans
Figure 2 provides a comprehensive overview of the data, showing that the liver consistently exhibited higher concentrations of heavy metals compared to the gills and muscles. Interestingly, the figure also demonstrates that crustacean muscles generally contained higher levels of heavy metals compared to fish organs. Furthermore, the study revealed significant variations in heavy metal concentrations across different fish and crustacean species in both seasons.
The findings consistently demonstrated a specific order of heavy metal concentrations in the organs of fish and crustaceans: liver > gills > muscle (except for Pb). The distribution of heavy metals followed the order of Pb > Ni > Cd > Sn > Hg. These metals possess the potential to accumulate in the tissues of fish and crustaceans, which occupy higher trophic levels in the aquatic food chain, thus posing potential risks to human health. Throughout all seasons, the liver and gill tissues consistently displayed the highest concentrations of heavy metals. These metabolically active tissues tend to accumulate significant levels of heavy metals. Notably, the liver of fish and the tissues of crustacean species contained notably higher levels of Ni, Sn, Cd, and Hg compared to other tissues. Conversely, gill tissue exhibited higher concentrations of Pb compared to the liver and muscle tissues. This suggests that the liver serves as the primary target for heavy metals, accumulating higher levels of Ni, Sn, Cd, and Hg, possibly due to the presence of metallothionein protein. The liver plays a crucial role in detoxification and storage, making it a vital organ for the accumulation and removal of heavy metals from the system. The gills of fish, responsible for respiratory and excretory functions, accumulate high concentrations of Pb, reflecting the levels of metals present in the surrounding water and making fish susceptible to their toxicity. Despite being the primary edible part, fish muscles do not significantly accumulate heavy metals, resulting in lower levels of heavy metals in muscle tissues compared to gill and liver tissues. This indicates the lower affinity of fish muscles for environmental metals.
Comparing the levels of metals in fish tissues with previous studies, the present investigation revealed lower levels compared to those reported51,52,53 for fish from the Mediterranean Sea. Moreover, the concentrations of heavy metals observed in this study varied compared to levels reported in other studies of crustaceans and fish. Interestingly, seasonal differences were noted in this study for all metals in all tissues of the studied species. During the summer, there was a noticeable increase in Cd, Pb, Ni, Hg, and Sn levels in the studied samples compared to winter (Fig. 3). This rise in metal content in the tissues of fish and crustaceans could potentially be attributed to their heightened physiological activity during the summer months, as well as an increased input of metals into the bay (Supplementary Materials). As suggested by Zubcov et al.54, the accelerated growth rate of fish during summer could also lead to a higher accumulation of metals in their tissues. Interestingly, similar findings have been reported by Kargin et al.55 and Çoğun et al.51 regarding the elevated levels of metals during summer in Mediterranean fish and crustaceans.
The present investigation unveiled significant differences (p < 0.05) in metal concentrations among various fish and crustacean species, highlighting the role of both species and organs in determining heavy metal levels. A statistical analysis using analysis of variance (ANOVA) demonstrated that seasonal variations had a significant influence on the variability of Cd, Pb, Ni, and Sn (p < 0.05).
The liver exhibited significantly higher mean levels of Cd, Pb, Ni, and Sn, while the gills and muscles displayed considerably lower mean levels of these metals (p < 0.05). However, there was no statistically significant difference in the average levels of Pb between the liver and gills (p > 0.05). Furthermore, the hepatic tissues of the examined fish species exhibited elevated concentrations of heavy metals compared to the muscular and branchial tissues. This finding aligns with previous research suggesting that the liver can serve as a reliable biomarker for assessing water pollution caused by heavy metals. The concentrations of metals in the liver directly reflect those present in the surrounding environment56.
The study investigated the interactions among seasons, fish organs, and fish species. It was found that the two-way interactions between season and fish organs were not statistically significant for most metals. However, a significant interaction was observed for Sn levels (p < 0.05). Conversely, significant interactions were observed between fish species and seasons for Cd and Ni (p < 0.05), while no significant interactions were found for Pb and Sn (p > 0.05). Additionally, there were no significant associations between fish species and fish organ interactions and the levels of Cd, Pb, Ni, and Sn (p > 0.05). No statistically significant three-way interactions were observed between season, fish organs, and fish species for any of the metals (p > 0.05).
In terms of average metal concentrations, L. aurata exhibited significantly higher amounts of Cd, Pb, Ni, and Sn compared to other species (p < 0.05). Conversely, Mugil capito displayed significantly lower average concentrations of Cd, Pb, and Ni compared to other species (p < 0.05). Furthermore, Mugil cephalus had significantly lower average levels of Sn (p < 0.05). It is worth noting that there were no significant differences in the average concentrations of Cd and Sn between Mugil cephalus and Mugil capito (p > 0.05). Similarly, no notable disparities were found in the average levels of Ni between Mugil cephalus and Morone labrax, as well as between L. aurata and Mugil capito (p > 0.05). The observed variations in metal concentrations among species can be attributed to various biological factors, including age, sex, maturity, dietary preferences, and species-specific metal accumulation abilities57,58.
Hg concentrations in fish and crustacean species were found to deviate from a normal distribution (p < 0.05), as indicated by the Kolmogorov–Smirnov (K-S) test. No statistically significant differences (p > 0.05) were identified among the fish and crustacean species. However, a significant distinction (p < 0.01) was observed between the two seasons using the non-parametric Kruskal–Wallis test (K-W). The study revealed that Hg levels during winter were significantly higher compared to those observed during summer for both fish and crustacean species (Mann–Whitney U post hoc test; p = 0.002 and 0.009, respectively). The measured Hg concentrations during the summer season exhibit a noteworthy standard deviation. This phenomenon can be attributed to the influence of biological characteristics of organisms, encompassing factors such as habitat preferences, feeding habits, and metabolic processes, which impact the accumulation and distribution of metals within their tissues. Variances in species composition and their unique responses to metal exposure contribute to divergent metal concentrations observed among the samples. These biological factors play a pivotal role in shaping the patterns of Hg accumulation and highlight the complexity of metal dynamics within aquatic ecosystems59.
Metal pollution index (MPI)
The metal pollution index (MPI) visually represents the cumulative metal buildup within a fish sample, offering a comprehensive and informative assessment of metal contamination, as described by Ali and Khan60. This study employed MPI to investigate the bioaccumulation trends of six metals in the organisms under investigation and assess pollution levels.
Figure 4 displays the investigated metals (Cd, Pb, Ni, Hg, and Sn) measured in different organs (liver, gills, and muscles) of the studied species within Temsah lake during both winter and summer.
The highest MPI values in fish species were observed in Morone labrax during winter and L. aurata during summer, while Mugil cephalus exhibited the lowest values in both seasons. These differences can be attributed to these species' distinct ecological behaviors and habitat preferences and the influence of seasonal variations in biological activity and productivity on metal availability and accumulation in the environment. It's important to note that these patterns may vary depending on specific locations, environmental conditions, and the sources and types of metal pollutants present within the ecosystem. Factors such as feeding habits, metabolic rates, and the specific metal-contaminated environments all contribute to the observed differences in MPI values between Morone labrax and L. aurata during winter and summer, respectively.
In comparison, crustacean species, particularly Paratapes undulate, displayed the highest MPI values during both winter and summer (Fig. 5). The elevated MPI values in clams can be attributed to their physiology and body composition. Clams possess a relatively large surface area relative to their body mass, facilitating enhanced uptake and accumulation of metals from their surrounding environment. Additionally, their ability to retain metals in their tissues for extended periods contributes to higher concentrations and, subsequently, higher MPI values.
On the other hand, low MPI values were observed in the muscle tissues, which were comparable to those found in the gills and liver. This can be attributed to the low affinity of muscle tissue for metal accumulation, primarily due to its high-fat content with low metal-combination affinity coupled with lower metabolic activity61.
All fish and crustacean species examined in this study exhibited low metal pollution index values, indicating that these fish are safe for human consumption. These results align with findings from previous studies by62,63,64,65 which reported similar or higher MPI values.
Human health risk assessment
To assess potential health risks associated with the consumption of fish and crustacean species, the estimation values of weekly intake (EWI), target hazard quotient (THQ), hazard index (HI), and carcinogenic risk (CR) were calculated. Studies calculate these values based on a consumption frequency of seven times a week to evaluate health risks related to fish consumption. However, it can be assumed that due to the high price of fish, it is not commonly consumed at such a high frequency in Egypt. Therefore, health risk indices were calculated based on the assumption of consuming fish and crustacean species once a week.
The values of EWI, THQ, HI, and CR were calculated separately for both child and adult consumers. The calculations considered the average body weight and lifetime as provided by the Environmental Protection Agency (USEPA) in 200066, which indicated a mean body weight of 70 kg and a lifetime of 70 years for adults. For children, the EPA data 200846 indicated an average body weight of 32 kg and a lifetime of 7 years.
By considering these factors and performing the necessary calculations, the health risks associated with the consumption of fish and crustacean species can be estimated for different consumption frequencies, providing valuable insights for consumer safety.
As depicted in (Table 2), the estimated weekly intake (EWI) exhibited higher values during the summer compared to the winter. Furthermore, it was observed that children had higher EWI values than adults. Notably, the EWI values for studied metals were found to be lower than the corresponding tolerable weekly intake (TWI) values. Therefore, this suggests that the weekly intake of these specific metals through the consumption of fish species within the investigated area does not present any potential health hazards for consumers.
The order of EWI, from highest to lowest, was Pb > Ni > Sn > Cd > Hg. Among the studied fish samples, L. aurata exhibited the highest EWI values, whereas Paratapes undulatus showed the highest EWI values among the studied crustacean samples. These findings indicate potential health risks associated with the weekly intake of Paratapes undulatus.
Assessing human health risks associated with consuming contaminated fish often involves the calculation of established or recommended indices, such as the target hazard quotient (THQ) and hazard index (HI). To further evaluate the safety of fish consumption, the THQ was utilized as a meaningful assessment index for potential health hazards to consumers69. It has been recognized that the THQ can estimate the risk of non-carcinogenic effects on human health resulting from pollutant ingestion70. The THQ represents the ratio between the exposure to a pollutant and its reference dose58.
In this study, the THQs for the studied metals were estimated based on the consumption of the investigated species, following the assumptions provided by the United States Environmental Protection Agency (USEPA) in 201771. As depicted in (Table 3), the THQ data for both adults and children, under average exposure conditions, were all found to be less than 1. These results indicate that there is no significant potential health risk to the general population, suggesting that fish consumption may confer benefits and consumers can be considered safe57,70.
Based on the aforementioned analysis, it can be concluded that the consumption of the selected aquatic species is unlikely to pose any apparent health risks to the general population. However, special attention should be paid to individuals with lower body weight72.
It is important to note that exposure to multiple pollutants can lead to antagonistic, synergistic, additive, and/or interactive effects70. Thus, the health risk associated with the combined presence of multiple metals (Cd, Pb, Ni, Hg, and Sn) in fish was assessed by summing the individual THQ values obtained for each metal.
Assessing the hazard index (HI), which considers multiple metal elements, is crucial. If the HI value surpasses 1, it indicates a potential health risk for consumers73. In this study, the HI values have not exceeded the recommended limit, indicating that adult consumers would not experience non-carcinogenic health effects from the consumption of the investigated fish and crustaceans (Fig. 6). Consequently, there is no observed risk of developing chronic general effects attributed to the consumption of the examined fish and crustacean muscles for each metal, except for Paratapes undulatus and R. decussatus in the case of children, where the values were slightly higher. Furthermore, the muscle values in the HI index for other species, which reflect the combined effects of all metals, also remain below the acceptable threshold. Even if this fish is consumed seven times a week, the risk of non-carcinogenic health effects related to the metal elements would not occur. The values obtained in this study are consistent with previous research conducted on various fish species in different geographical locations62,63,74.
The carcinogenic risk (CR) associated with Pb and Cd was calculated and presented in (Table 4). Generally, CR values above 10−6 are considered unacceptable, values below 10−6 are deemed insignificant, and values between 10−4 and 10−6 are considered acceptable carcinogenic risks according to EPA guidelines 201075. The calculated CR values for Pb in the studied species were lower than the specified limits. In the study area, all CR exposures were found to be negligible. However, the CR value for Cd in crustaceans during winter exceeded the negligible limit for children. If crustacean species were consumed every day of the week, the CR value would approach the danger limits. Nevertheless, it is well-known that crustacean species are not consumed on a daily basis. Therefore, the values obtained in this study remain below the hazard limits. Consequently, the carcinogenic risk for consumers due to heavy metal exposure through the consumption of the two fish species from Temsah Lake was determined to be low. Our findings are in line with the results obtained by Demirak et al.74 in his comparative study of Lake Köyceğiz in Turkey and Lake Võrtsjärv in Estonia.
Table 5 presents a comprehensive comparative analysis between the findings of MPI, HI, EWI, and CR from the current study and previous research conducted in various global regions. Notably, our results indicate lower metal concentrations compared to studies conducted in the Persian Gulf (Iran), the Black Sea (Turkey), the Arabian Gulf (Saudi Arabia), Manzalah Lake (Egypt), and the Kirtankhola River (Bangladesh).
Conclusion
In conclusion, this study analyzed the concentrations of cadmium (Cd), lead (Pb), nickel (Ni), mercury (Hg), and tin (Sn) in four fish species (Mugil cephalus, Mugil capito, L. aurata, and Morone labrax) and five crustacean species (S. rivulatus, Cerastoderma glaucum, Paratapes undulatus, R. decussatus, Callinectes sapidus, and Metapenaeus Stebbingi) collected from Temsah Lake during both winter and summer seasons. Our findings revealed a noticeable increase in metal levels during summer compared to winter in the studied samples. Furthermore, the concentration of heavy metals in the muscles of the species generally exceeded those in the liver and gills. The MPI values indicated that Morone labrax exhibited the highest values during winter, while L. aurata showed the highest values during summer. Mugil cephalus consistently demonstrated the lowest MPI values in both seasons. Significantly, the EWI values for the studied metals fell below the corresponding Tolerable Weekly Intake (TWI) values, suggesting that the consumption of these species is within safe limits. Additionally, the THQ and HI data, under average exposure conditions, were generally below 1 for most species in the study area, indicating low health risks associated with heavy metal consumption. The calculated CR values for Pb in the studied species indicated acceptable carcinogenic risk levels. Based on these findings, it can be concluded with confidence that consuming the studied fish and crustacean species from Temsah Lake does not pose any significant health hazards to consumers. However, it is recommended to continue ongoing monitoring and research efforts to ensure the sustained safety and sustainability of seafood consumption in the region.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Younis, A. M. Accumulation and rate of degradation of organotin compounds in coastal sediments along the red sea Egypt. Egypt. J. Aquat. Biol. Fish. 24, 413–436. https://doi.org/10.21608/ejabf.2020.108918 (2020).
Haseeb-ur-Rehman, M., Munshi, A. B., Atique, U. & Kalsoom, S. Metal pollution and potential human health risk assessment in major seafood items (fish, crustaceans, and cephalopods). Mar. Pollut. Bull. 188, 114581. https://doi.org/10.1016/j.marpolbul.2023.114581 (2023).
Younis, A. M., Aly-Eldeen, A. M. & Elkady, M. E. Effect of different molecular weights of chitosan on the removal efficiencies of heavy metals from contaminated water. Egypt. J. Aquat. Biol. Fish. 23, 149–158. https://doi.org/10.21608/ejabf.2019.52591 (2019).
Younis, A. M., Soliman, N. F., Elkady, E. M. & Mohamedein, L. I. Distribution and ecological risk evaluation of bioavailable phosphorus in sediments of El Temsah lake Suez Canal. Oceanologia 64, 287–298. https://doi.org/10.1016/j.oceano.2021.12.001 (2022).
Soliman, N. F., Elkady, E. M. & Younis, A. M. Chemical fractions and ecological risk of metals in sediments of the bitter lakes Egypt. Egypt. J. Aquat. Biol. Fish. 24, 167–196. https://doi.org/10.21608/ejabf.2020.110915 (2020).
Nour, H. E., Helal, S. A. & Abdel Wahab, M. Contamination and health risk assessment of heavy metals in beach sediments of red sea and gulf of aqaba, Egypt. Mar. Pollut. Bull. 177, 113517. https://doi.org/10.1016/j.marpolbul.2022.113517 (2022).
Jeong, H., Byeon, E., Kim, D.-H., Maszczyk, P. & Lee, J.-S. Heavy metals and metalloid in aquatic invertebrates: A review of single/mixed forms, combination with other pollutants, and environmental factors. Mar. Pollut. Bull. 191, 114959. https://doi.org/10.1016/j.marpolbul.2023.114959 (2023).
Hanafy, S., Younis, A. M., El-Sayed, A. Y. & Ghandour, M. A. Spatial, seasonal distribution and ecological risk assessment of Zn, Cr, and Ni in red sea surface sediments, Egypt. Egypt. J. Aquat. Biol. Fish. 25, 513–538. https://doi.org/10.21608/ejabf.2021.191624 (2021).
Elnaggar, D. H., Mohamedein, L. I. & Younis, A. M. Risk assessment of heavy metals in mangrove trees (Avicennia marina) and associated sea water of Ras Mohammed protectorate, red sea Egypt. Egypt. J. Aquat. Biol. Fish. 26, 117–135. https://doi.org/10.21608/ejabf.2022.258908 (2022).
Chahouri, A. et al. Assessment of heavy metal contamination and ecological risk in Morocco’s marine and estuarine ecosystems through a combined analysis of surface sediment and bioindicator species: Donax trunculus and Scrobicularia plana. Mar. Pollut. Bull. 192, 115076. https://doi.org/10.1016/j.marpolbul.2023.115076 (2023).
El-Naggar, M., Hanafy, S., Younis, A. M., Ghandour, M. A. & El-Sayed, A.-A.Y. Seasonal and temporal influence on polycyclic aromatic hydrocarbons in the red sea coastal water Egypt. Sustainability 13, 11906. https://doi.org/10.3390/su132111906 (2021).
Younis, A. M. et al. Polycyclic aromatic hydrocarbons (PAHs) in Egyptian red sea sediments: Seasonal distribution, source Identification, and toxicological risk assessment. Arab. J. Chem. 16, 104999. https://doi.org/10.1016/j.arabjc.2023.104999 (2023).
Vinothkannan, A., Charles, P. E. & Rajaram, R. Consumption of metal-contaminated shellfish from the Cuddalore coast in Southeastern India poses a hazard to public health. Mar. Pollut. Bull. 181, 113827. https://doi.org/10.1016/j.marpolbul.2022.113827 (2022).
Prabakaran, K., Sompongchaiyakul, P., Bureekul, S., Wang, X. & Charoenpong, C. Heavy metal bioaccumulation and risk assessment in fishery resources from the Gulf of Thailand. Mar. Pollut. Bull. 198, 115864. https://doi.org/10.1016/j.marpolbul.2023.115864 (2024).
Amin, H. F., Ahmed, O. M., Rasmey, A.-H.M., Younis, A. & Bekhitd, A. E. D. A. Effect of technological processing on the safety of Indian mackerel (Rastrelliger kangurata) from Suez Egypt. Egypt. J. Aquat. Biol. Fish. 22, 283–294. https://doi.org/10.21608/ejabf.2018.22009 (2018).
Stankovic, S., Jovic, M., Stankovic, A. R. & Katsikas, L. Environmental chemistry for a sustainable world: Volume 1: Nanotechnology and health risk. In Heavy Metals in Seafood Mussels Risks for Human Health (eds Lichtfouse, E. et al.) (Springer, 2012).
Nordberg, G. F. & Costa, M. Handbook on the Toxicology of Metals: Volume I: General Considerations (Academic press, 2021).
Hossain, M. B. et al. Heavy metals in four marine fish and shrimp species from a subtropical coastal area: Accumulation and consumer health risk assessment. Biology 11, 1780. https://doi.org/10.3390/biology11121780 (2022).
Taghavi, M. et al. Health risk assessment of heavy metal toxicity in the aquatic environment of the persian Gulf. Mar. Pollut. Bull. 202, 116360. https://doi.org/10.1016/j.marpolbul.2024.116360 (2024).
WHO. WHO Guidelines for the Safe Use of Wasterwater Excreta and Greywater: Volume 4: Excreta and Greywater Use in Agriculture (World Health Organization, 2006).
Connelly, N. A., Lauber, T. B., McCann, P. J., Niederdeppe, J. & Knuth, B. A. Estimated exposure to mercury from fish consumption among women anglers of childbearing age in the great lakes region. Environ. Res. 171, 11–17. https://doi.org/10.1016/j.envres.2019.01.005 (2019).
FAO. The State of World Fisheries and Aquaculture 2018 - Meeting the sustainable development goals. http://www.fao.org/3/i9540en/I9540EN.pdf (Food and Agriculture Organization of the United Nations, 2018).
Traven, L. et al. Assessment of health risks associated with heavy metal concentration in seafood from North-Western Croatia. Sci. Rep. 13, 16414. https://doi.org/10.1038/s41598-023-43365-7 (2023).
Jolaosho, T. L. et al. Occurrence, distribution, source apportionment, ecological and health risk assessment of heavy metals in water, sediment, fish and prawn from Ojo river in Lagos Nigeria. Environ. Monit. Assess. 196, 109. https://doi.org/10.1007/s10661-023-12148-y (2024).
USFDA. Seafood safety. Retrieved from https://www.fda.gov/food/buy-store-serve-safe-food/seafood-safety. (US Food and Drug Administration, Washington, DC, USA, 2021).
Bruno, F. et al. Heavy metals bioaccumulation in Mytilus galloprovincialis and Tapes decussatus from Faro lake (Messina) Italy. Biol. Trace Elem. Res. https://doi.org/10.1007/s12011-024-04128-1 (2024).
Parrino, V. et al. Trace elements (Al, Cd, Cr, Cu, Fe, Mn, Ni, Pb and Zn) in Mytilus galloprovincialis and Tapes decussatus from Faro and Ganzirri lakes (Sicily, Italy): Flow cytometry applied for hemocytes analysis. J. Trace Elem. Med Biol. 68, 126870. https://doi.org/10.1016/j.jtemb.2021.126870 (2021).
Rahman, M. M. et al. Health risks from trace elements in muscles of some commonly available fish in Australia and India. Environ. Sci. Pollut. Res. 27, 21000–21012. https://doi.org/10.1007/s11356-020-08600-y (2020).
Apeti, D. A., Lauenstein, G. G. & Evans, D. W. Recent status of total mercury and methyl mercury in the coastal waters of the northern Gulf of Mexico using oysters and sediments from NOAA’s mussel watch program. Mar. Pollut. Bull. 64, 2399–2408. https://doi.org/10.1016/j.marpolbul.2012.08.006 (2012).
Medina-Morales, S. A. et al. Mercury (Hg) and selenium (Se) content in the shark Mustelus henlei (Triakidae) in the Northern Mexican Pacific. Environ. Sci. Pollut. Res. 27, 16774–16783. https://doi.org/10.1007/s11356-020-08198-1 (2020).
Parrino, V. et al. Comparative study of haematology of two teleost fish (Mugil cephalus and Carassius auratus) from different environments and feeding habits. Eur. Zool. J. 85, 193–199. https://doi.org/10.1080/24750263.2018.1460694 (2018).
Baki, M. A. et al. Concentration of heavy metals in seafood (fishes, shrimp, lobster and crabs) and human health assessment in Saint Martin Island Bangladesh. Ecotoxicol. Environ. Saf. 159, 153–163. https://doi.org/10.1016/j.ecoenv.2018.04.035 (2018).
Abbas, M. M. M. Heavy metal levels and cancer risk assessments of the commercial denis, Sparus aurata collected from Bardawil lake and private fish farm waters as a cultured source Egypt. Biol. Trace Elem. Res. 202, 2864–2877. https://doi.org/10.1007/s12011-023-03880-0 (2024).
Soliman, N. F., Younis, A. M. & Elkady, E. M. An insight into fractionation, toxicity, mobility and source apportionment of metals in sediments from El Temsah lake Suez Canal. Chemosphere 222, 165–174. https://doi.org/10.1016/j.chemosphere.2019.01.009 (2019).
Kaiser, M. F., Amin, A. S. & Aboulela, H. A. Advances in geoscience and remote sensing. In Environmental Hazards in the El-Temsah Lake, Suez Canal District, Egypt (ed. Jedlovec, G.) (IntechOpen, 2009).
Madkour, F., Aamer, M. & El-Sherbiny, M. Assessment of eutrophication in lake Timsah, Suez Canal Egypt. Egypt. J. Aquat. Res. 32, 259–272 (2006).
UNEP/FAO/IAEA/IOC. Sampling of selected marine organisms and sample preparation for trace metals. Ref. Methods Mar. Pollut. Stud. 7, 19 (1984).
Dalziel, J. & Baker, C. Analytical methods for measuring metals by atomic absorption spectrophotometry. FAO Fish. Tech. 212, 14–21 (1983).
Memon, A. G. et al. Utilization of unmodified gold nanoparticles for label-free detection of mercury (II): Insight into rational design of mercury-specific oligonucleotides. J. Hazard. Mater. 321, 417–423. https://doi.org/10.1016/j.jhazmat.2016.09.025 (2017).
Memon, A. G. et al. Label-free colorimetric nanosensor with improved sensitivity for Pb2 + in water by using a truncated 8–17 DNAzyme. Front. Environ. Sci. Eng. 13, 12. https://doi.org/10.1007/s11783-019-1094-7 (2019).
Usero, J., González-Regalado, E. & Gracia, I. Trace metals in the bivalve molluscs Ruditapes decussatus and Ruditapes philippinarum from the Atlantic coast of Southern Spain. Environ. Int. 23, 291–298. https://doi.org/10.1016/S0160-4120(97)00030-5 (1997).
USEPA. Risk Assessment Guidance for Superfund. Volume I: Human Health Evaluation Manual (Part A) (Office of Emergency and Remedial Response, 1989).
Chien, L. C. et al. Daily intake of TBT, Cu, Zn, Cd and As for fishermen in Taiwan. Sci. Total Environ. 285, 177–185. https://doi.org/10.1016/S0048-9697(01)00916-0 (2002).
USEPA. Supplementary Guidance for Conducting Health Risk Assessment of Chemical Mixtures (US Environmental Protection Agency, 2000).
FAO. The state of world fisheries and aquaculture. Towards blue transformation. https://doi.org/10.4060/cc0461en (Food and Agriculture Organization of the United Nations Rome, Italy, 2022).
USEPA. Child-specific exposure factors handbook. Report No. EPA/600/R- 06/096F. http://cfpub.epa.gov/ncea/cfm/recordisplay.cfm?deid=199243 (US Environmental Protection Agency, Washington, DC, USA, 2008).
USEPA. Regional Screening Levels (RSLs)—Generic Tables. (US Environmental Protection Agency, Washington, DC, USA, 2023).
Yu, B., Wang, X., Dong, K. F., Xiao, G. & Ma, D. Heavy metal concentrations in aquatic organisms (fishes, shrimp and crabs) and health risk assessment in China. Mar. Pollut. Bull. 159, 111505. https://doi.org/10.1016/j.marpolbul.2020.111505 (2020).
USEPA. Integrated Risk Information System. https://www.epa.gov/iris/. (US Environmental Protection Agency, Washington, DC, USA, 2016).
USEPA. Exposure Factors Handbook (US EPA Office of Research and Development, 2011).
Çoğun, H., Yüzereroğlu, T., Kargin, F. & Firat, Ö. Seasonal variation and tissue distribution of heavy metals in shrimp and fish species from the yumurtalik coast of iskenderun gulf, mediterranean. Bull. Environ. Contam. Toxicol. 75, 707–715. https://doi.org/10.1007/s00128-005-0809-6 (2005).
Kucuksezgin, F., Altay, O., Uluturhan, E. & Kontas, A. Trace metal and organochlorine residue levels in red mullet (Mullus barbatus) from the Eastern Aegean, Turkey. Water Res. 35, 2327–2332. https://doi.org/10.1016/S0043-1354(00)00504-2 (2001).
Yilmaz, A. B. Levels of heavy metals (Fe, Cu, Ni, Cr, Pb, and Zn) in tissue of Mugil cephalus and Trachurus mediterraneus from Iskenderun Bay Turkey. Environ. Res. 92, 277–281. https://doi.org/10.1016/S0013-9351(02)00082-8 (2003).
Zubcov, E., Zubcov, N., Ene, A. & Biletchi, L. Assessment of copper and zinc levels in fish from freshwater ecosystems of Moldova. Environ. Sci. Pollut. Res. 19, 2238–2247. https://doi.org/10.1007/s11356-011-0728-5 (2012).
Kargin, F., Donmez, A. & Cogun, H. Y. Distribution of heavy metals in different tissues of the shrimp Penaeus semiculatus and Metapenaeus monocerus from the Iskenderun Gulf, Turkey: Seasonal variations. Bull. Environ. Contam. Toxicol. 66, 102–109. https://doi.org/10.1007/s001280000211 (2001).
Dural, M., Göksu, M. Z. L. & Özak, A. A. Investigation of heavy metal levels in economically important fish species captured from the Tuzla lagoon. Food Chem. 102, 415–421. https://doi.org/10.1016/j.foodchem.2006.03.001 (2007).
Łuczyńska, J., Paszczyk, B. & Łuczyński, M. J. Fish as a bioindicator of heavy metals pollution in aquatic ecosystem of Pluszne Lake, Poland, and risk assessment for consumer’s health. Ecotoxicol. Environ. Saf. 153, 60–67. https://doi.org/10.1016/j.ecoenv.2018.01.057 (2018).
Ramos-Miras, J. J. et al. Potentially toxic elements in commonly consumed fish species from the western Mediterranean Sea (Almería Bay): Bioaccumulation in liver and muscle tissues in relation to biometric parameters. Sci. Total Environ. 671, 280–287. https://doi.org/10.1016/j.scitotenv.2019.03.359 (2019).
Storelli, M. M., Barone, G., Storelli, A. & Marcotrigiano, G. Trace metals in tissues of Mugilids (Mugil auratus, Mugil capito, and Mugil labrosus) from the Mediterranean Sea. Bull. Environ. Contam. Toxicol. 77, 43–50. https://doi.org/10.1007/s00128-006-1030-y (2006).
Ali, H. & Khan, E. Assessment of potentially toxic heavy metals and health risk in water, sediments, and different fish species of River Kabul Pakistan. Hum. Ecol. Risk Assess. 24, 2101–2118. https://doi.org/10.1080/10807039.2018.1438175 (2018).
Barath Kumar, S., Padhi, R. K. & Satpathy, K. K. Trace metal distribution in crab organs and human health risk assessment on consumption of crabs collected from coastal water of South East coast of India. Mar. Pollut. Bull. 141, 273–282. https://doi.org/10.1016/j.marpolbul.2019.02.022 (2019).
Shaaban, N. A., El-Rayis, O. A. & Aboeleneen, M. S. Possible human health risk of some heavy metals from consumption of tilapia fish from lake Mariut Egypt. Environ. Sci. Pollut. Res. 28, 19742–19754. https://doi.org/10.1007/s11356-020-12121-z (2021).
Rakib, M. R. J. et al. Levels and health risk assessment of heavy metals in dried fish consumed in Bangladesh. Sci. Rep. 11, 14642. https://doi.org/10.1038/s41598-021-93989-w (2021).
El-Batrawy, O. A., El-Gammal, M. I., Mohamadein, L. I., Darwish, D. H. & El-Moselhy, K. M. Impact assessment of some heavy metals on tilapia fish, Oreochromis niloticus, in Burullus Lake Egypt. J. Basic Appl. Zool. 79, 13. https://doi.org/10.1186/s41936-018-0028-4 (2018).
Kumar, R., Srivastava, P. K. & Srivastava, S. P. Leaching of heavy metals (Cr, Fe, and Ni) from stainless steel utensils in food simulants and food materials. Bull. Environ. Contam. Toxicol. 53, 259–266. https://doi.org/10.1007/BF00192042 (1994).
USEPA. Guidance for Assessing Chemical Contaminant Data for Use in Fish Advisories, Risk Assessment and Fish Consumption Limit (USEPA Office of Water and Office of Science and Technology, 2000).
JECFA. Evaluation of Certain Food Additives and Contaminants. Seventy-Third Report of the Joint FAO /WHO Expert Committee on Food Additives (World Health Organization, 2011).
WHO. Guidelines for Drinking-Water Quality: First Addendum to the Fourth Edition (World Health Organization, 2017).
Soltani, N., Moore, F., Keshavarzi, B., Sorooshian, A. & Javid, R. Potentially toxic elements (PTEs) and polycyclic aromatic hydrocarbons (PAHs) in fish and prawn in the Persian Gulf Iran. Ecotoxicol. Environ. Saf. 173, 251–265. https://doi.org/10.1016/j.ecoenv.2019.02.005 (2019).
Miri, M. et al. Health risk assessment of heavy metal intake due to fish consumption in the Sistan region Iran. Environ. Monit. Assess. 189, 583. https://doi.org/10.1007/s10661-017-6286-7 (2017).
USEPA. Integrated Risk Information System (IRIS)-Chemical Assessment Summary (US Environmental Protection Agency, 2017).
Liu, H. et al. Occurrence, potential health risk of heavy metals in aquatic organisms from Laizhou Bay China. Mar. Pollut. Bull. 140, 388–394. https://doi.org/10.1016/j.marpolbul.2019.01.067 (2019).
Ahmed, A. S. S., Rahman, M., Sultana, S., Babu, S. M. O. F. & Sarker, M. S. I. Bioaccumulation and heavy metal concentration in tissues of some commercial fishes from the Meghna river Estuary in Bangladesh and human health implications. Mar. Pollut. Bull. 145, 436–447. https://doi.org/10.1016/j.marpolbul.2019.06.035 (2019).
Demirak, A. et al. Bioaccumulation and health risk assessment of heavy metals in European eels taken from lakes Köyceğiz (Turkey) and Võrtsjärv (Estonia). Environ. Sci. Pollut. Res. 29, 1620–1633. https://doi.org/10.1007/s11356-021-16822-x (2022).
USEPA. Risk-Based Concentration Table (US Environmental Protection Agency, 2010).
Kalipci, E., Cüce, H., Ustaoğlu, F., Dereli, M. A. & Türkmen, M. Toxicological health risk analysis of hazardous trace elements accumulation in the edible fish species of the Black Sea in Türkiye using multivariate statistical and spatial assessment. Environ. Toxicol. Pharmacol. 97, 104028. https://doi.org/10.1016/j.etap.2022.104028 (2023).
Aljohani, N. S. et al. Bioaccumulation of heavy metals in fish collected from the Eastern coast of Saudi Arabia and human health implications. Reg. Stud. Mar. Sci. 62, 102986. https://doi.org/10.1016/j.rsma.2023.102986 (2023).
Abdel-Kader, H. H. & Mourad, M. H. Estimation of Cadmium in muscles of five freshwater fish species from Manzalah lake, and possible human risk assessment of fish consumption (Egypt). Biol. Trace Elem. Res. 201, 937–945. https://doi.org/10.1007/s12011-022-03188-5 (2023).
Monier, M. N., Soliman, A. M. & Al-Halani, A. A. The seasonal assessment of heavy metals pollution in water, sediments, and fish of grey mullet, red seabream, and sardine from the Mediterranean coast, Damietta North Egypt. Reg. Stud. Mar. Sci. 57, 102744. https://doi.org/10.1016/j.rsma.2022.102744 (2023).
Ali, M. M. et al. Seasonal behavior and accumulation of some toxic metals in commercial fishes from Kirtankhola tidal river of Bangladesh—A health risk taxation. Chemosphere 301, 134660. https://doi.org/10.1016/j.chemosphere.2022.134660 (2022).
Zaghloul, G. Y., Ezz El-Din, H. M., Mohamedein, L. I. & El-Moselhy, K. M. Bio-accumulation and health risk assessment of heavy metals in different edible fish species from Hurghada City, Red Sea Egypt. Environ. Toxicol. Pharmacol. 95, 103969. https://doi.org/10.1016/j.etap.2022.103969 (2022).
Ikem, A., Ayodeji, O. J. & Wetzel, J. Human health risk assessment of selected metal(loid)s via crayfish (Faxonius virilis; Procambarus acutus acutus) consumption in Missouri. Heliyon 7, e07194. https://doi.org/10.1016/j.heliyon.2021.e07194 (2021).
Author information
Authors and Affiliations
Contributions
Authors A and D wrote the main manuscript text. Author B collected the samples and prepared the figures. Author C conducted the analysis. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Younis, A.M., Hanafy, S., Elkady, E.M. et al. Assessment of health risks associated with heavy metal contamination in selected fish and crustacean species from Temsah Lake, Suez Canal. Sci Rep 14, 18706 (2024). https://doi.org/10.1038/s41598-024-69561-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69561-7
- Springer Nature Limited