Abstract
Cardiovascular disease (CVD) is a significant health concern, particularly among patients with diabetes. The weight-adjusted waist circumference index (WWI), a novel metric that accounts for central obesity, has shown potential in predicting obesity-related health risks. This study aimed to evaluate the association of WWI with CVD and mortality in patients with diabetes. Utilizing data from the National Health and Nutrition Examination Survey from 1999 to 2020, WWI was calculated by dividing waist circumference (WC) by the square root of body weight. Multivariate logistic regression, multivariate Cox regression and restricted cubic spline curves were used to assess the association between WWI and the prevalence of CVD and mortality in patients with diabetes, subgroup and sensitivity analyses were carried out to delve into the stability of the findings. The predictive performance of WWI was evaluated using the area under the receiver operating characteristic curve (ROC). This study included 8,005 individuals with diabetes. With the increase in WWI values, the risk of developing CVD and the likelihood of mortality progressively rise. The fully adjusted continuous model indicated a 28% higher chance of developing CVD and a 25% higher risk of all-cause mortality for each one-unit increase in WWI. When using the lowest quartile of WWI as the reference category, the highest quartile was linked to an increased risk of CVD (OR 1.66; 95% CI 1.10–2.50, p = 0.015) and all-cause mortality (HR 1.53, 95% CI 1.27–1.83, p < 0.001) among patients with diabetes. Subgroup and sensitivity analyses confirmed that these associations were consistent and stable in most different demographics. The ROC analysis indicated that WWI had a higher predictive capacity for CVD and all-cause mortality than WC, waist to hip ratio, and weight to height ratio. The WWI was significantly associated with the prevalence of CVD and all-cause mortality among patients with diabetes in the United States and may serve as a useful tool for identifying individuals at risk.
Similar content being viewed by others
Introduction
The burgeoning elderly population has led to a surge in the incidence and mortality of cardiovascular disease (CVD), making it a critical issue in global public health. In 2019, CVD impacted 252 million people across 204 nations and territories, which is a significant increase from the 271 million affected in 1990. Moreover, the number of deaths attributable to CVD has increased by 6.5 million, solidifying its position as the leading cause of death worldwide1. Despite significant progress in the treatment of cardiac and vascular ailments over the past two decades, CVD remains the primary cause of death, posing a severe threat to human well-being2. It is noteworthy that CVD is prevalent among individuals with diabetes and is associated with poor health outcomes3,4. Hence, the early detection of individuals with elevated cardiovascular risk factors among patients with diabetes is crucial for mitigating the prevalence and mortality rates of these conditions.
Globally recognized as a critical health concern, obesity is affecting more people each day5. A strong association has been identified between obesity and the likelihood of CVD6,7. Obesity, through the increase in adipose tissue, altered the states of inflammation, oxidative stress, and cardiac cell survive, ultimately affecting myocardial function8, for example, in patients with metabolic syndrome who often were identified obesity, the likelihood of arrhythmias and adverse cardiovascular outcomes was significantly increased, regardless of whether there was any structural heart disease9,10. While the body mass index (BMI) is a standard tool for assessing obesity, it falls short as it doesn't accurately indicate how fat is distributed throughout the body11. Waist circumference (WC) offers a straightforward and dependable method to measure obesity in the abdominal area and is sometimes used to estimate total body fat12, though it does not differentiate between fat located just under the skin and fat around the organs13. Evidence suggested that fat around the organs releases more free fatty acids than subcutaneous fat, which could lead to higher risk for developing CVD14,15,16,17,18. In the clinical setting, measuring visceral fat and muscle mass with imaging technology is often not feasible. To overcome this challenge, Park et al. introduced an innovative metric for obesity known as the "weight-adjusted waist circumference index (WWI)"19, which was the result of dividing WC by the square root of an individual's body weight. This index minimized its association with BMI and capitalized on the strengths of WC to target central obesity, regardless of overall weight20. Consequently, the WWI may offer enhanced accuracy in predicting obesity-related risks.
Nevertheless, the relationship between WWI and the risk of CVD and mortality in patients with diabetes remains to be fully elucidated. Consequently, this study leveraged comprehensive and representative data from a national study to conduct an extensive comparative analysis. The goal was to investigate how WWI correlates with the prevalence of CVD and mortality in patients with diabetes among the United States population.
Methods
Study population
This observational research drew on data collected over 11 iterations of the National Health and Nutrition Examination Survey (NHANES) conducted across the United States from 1999 to 2020. Annually, NHANES selected approximately 5,000 individuals from 15 distinct geographical regions for inclusion in its study. For comprehensive insights into the survey's methodology, one should refer to the NHANES Plan and Operations manual. The survey was approved by the National Center for Health Statistics Research Ethics Review Board, adhering to established protocols. Since this investigation was a secondary analysis of anonymized NHANES data, no additional ethical approval was warranted. All methods were performed in accordance with the relevant guidelines and regulations.
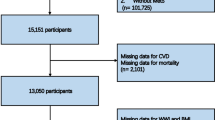
The research sample was derived from NHANES participants spanning the years 1999 to 2020. Initially, there were 116,876 potential candidates. However, 20,014 of these were excluded due to incomplete data necessary for computing the WWI. Additional exclusions were made for the lack of data on CVD (n = 40,238) and mortality (n = 8,041). Participants without diabetes were also excluded, totaling 40,678. Post-exclusions, the final dataset included 8,005 individuals, as illustrated in Fig. 1.
Measurement of WWI
The calculation of the WWI involved dividing the WC in centimeters by the square root of the body weight in kilograms. To ensure accurate measurements, they were conducted by trained medical personnel and dedicated recorders. Participants' body weight was assessed using an electronic scale with individuals wearing their examination clothing and standing barefoot, arms at their sides and looking forward, as per the established protocol. For WC measurement, a measuring tape was positioned at the point where the horizontal line above the top of the right kneecap intersects with the midaxillary line21. This methodical approach ensured that the WWI was calculated based on precise and consistent measurements, which was crucial for the reliability of the index in assessing obesity and related health risks.
Evaluation of diabetes and CVD
According to the 2013 U.S. Diabetes Guidelines22, diabetes was diagnosed if any of the following conditions were met: (1) an individual reported having been diagnosed with diabetes by a physician; (2) the individual was currently on hypoglycemic medications or using insulin injections; (3) a random blood glucose reading of 11.1 mmol/L or higher; (4) a glycated hemoglobin (HbA1c) level of 6.5% or higher; (5) a fasting blood glucose level of 7.0 mmol/L or higher; (6) a 2-h blood glucose reading from an oral glucose tolerance test of 11.1 mmol/L or higher. In this analysis, the occurrence of diabetes was considered the primary outcome of interest.
The identification of CVD was based on self-reported diagnoses by healthcare professionals, which were collected through personal interviews utilizing a standardized medical inquiry form. Survey participants were queried, "Have you ever been told by a doctor or health professional that you have angina, coronary heart disease, heart failure, myocardial infarction, or stroke?" An affirmative response to any of these inquiries classified the individual as having CVD23.
Assessment of mortality
To ascertain the death rates among the participants in our study's subsequent period, we employed the NHANES public-use linked mortality database, updated until December 31, 2019. The National Center for Health Statistics (NCHS) has merged this database with the National Death Index (NDI) using a complex probabilistic matching process. Furthermore, the identification of deaths due to specific diseases was facilitated by the International Statistical Classification of Diseases, 10th Revision (ICD-10). Deaths attributed to cardiovascular causes, such as rheumatic heart disease, hypertensive heart disease, ischemic heart disease, acute myocardial infarction, pericardial conditions, and acute myocarditis along with heart failure, were categorized under the ICD-10 codes ranging from I00 to I09, I11, I13, and I20 through I5124.
Potential confounders
Demographic factors were taken into account as potential confounders, encompassing details such as gender (male or female), age, ethnicity (classified as White, Black, Mexican, or other), education level (categorized as below high school, high school or equivalent, and above high school), and the marriage (married, never married or other). Lifestyle factors like smoking status (dichotomized as non-smoker or smoker), alcohol consumption (distinguished as non-drinker or drinker). Systolic blood pressure (SBP), and diastolic blood pressure (DBP) were also considered.
Furthermore, the study included various blood markers such as triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C), along with liver enzymes like glutamic pyruvic transaminase (ALT), glutamic oxaloacetic transaminase (AST), fast glucose and HbA1c. The estimated Glomerular Filtration Rate (eGFR) was also calculated using the CKD-EPI 2021 creatinine formula to assess kidney function.
Additionally, this study also took into account the patient's medication use, including anti-diabetic and anti-ischemic treatments (including aspirin, beta receptor blocker, and statin). Furthermore, since sodium-glucose transport protein 2 inhibitors (SGLT2i) could improve the clinical outcomes of patients with CVD and diabetes25,26,27, the use of SGLT2i was also considered in this study (including canagliflozin, dapagliflozin and empagliflozin).
Statistical analysis
The statistical analysis was conducted using R software, specifically version 4.1.1. The criterion for statistical significance was set at a P value of less than 0.05, considering both directions of the test (two-tailed). To ensure the generalizability of the study findings and to prevent any bias due to overrepresentation, sampling weights were incorporated into the analysis.
Data that was continuous in nature was reported as either the mean with 95% confidence intervals (CI). Discrete data was summarized by the number of occurrences and their respective percentages. For further analysis, multivariate logistic and Cox regression was utilized to evaluate the impact of the WWI on the prevalence of CVD and mortality in patients with diabetes. The crude model was not adjusted for any covariates. Model 1 incorporated adjustments for factors such as age, gender, ethnicity. Building upon Model 1, Model 2 also considered additional variables, including educational level, marriage, smoking habits, alcohol intake, and several blood markers.
Subgroup analyses were conducted and are reported, stratified using a comprehensive Model 2 that included all adjustments. To ensure the robustness of the association, sensitivity analysis was conducted, we included drug use (including anti-diabetic treatments, anti-ischemic treatments and SGLT2i) in the analysis based on model 2. The area under the curve (AUC) for the receiver operating characteristic (ROC) was determined to evaluate predictive performance of WWI. The potential nonlinear associations were assessed utilizing restricted cubic spline (RCS) curves.
Ethical approval
The studies involving human participants were reviewed and approved by the NCHS Research Ethics Review Board (ERB). All participants provided written informed consent.
Results
Baseline characteristics
After excluding 40,678 patients without diabetes, we included a total of 8,005 individuals with diabetes, accounting for 16.44%. Table 1 presented the baseline demographics of the cohort study's participants, categorized into quartiles based on WWI. The cohort had an average age of 58.74 years, with a slight male predominance at 51.31%. Among them, 62.09% of the patients received anti- diabetic treatment, 55.97% received anti-ischemic treatment, but only 0.83% of the patient received treatment with SGLT2i. The mean WWI score for the participants was 11.58, and the score ranges for the quartiles (Q1-Q4) were 8.85–11.12, 11.12–11.61, 11.61–12.09, and 12.09–15.39, respectively. Table 2 detailed the risks associated with CVD and mortality, stratified by WWI quartiles. Notable variations were observed across the quartiles in terms of age, gender, ethnicity, education, marital status, alcohol consumption, SBP, DBP, eGFR, HDL-C, LDL-C, all of which were statistically significant (P < 0.05). When comparing the lowest WWI quartile to the higher ones, there was a progressive increase in the likelihood of experiencing CVD (Q1: 338 cases, 14.29%; Q2: 467 cases, 22.55%; Q3: 518 cases, 24.48%; Q4: 588 cases, 29.74%; p < 0.0001), CVD mortality (Q1: 111 deaths, 4.38%; Q2: 137 deaths, 6.09%; Q3: 175 deaths, 7.53%; Q4: 173 deaths, 7.74%; p < 0.001), and all-cause mortality (Q1: 407 deaths, 15.83%; Q2: 508 deaths, 21.98%; Q3: 569 deaths, 25.21%; Q4: 646 deaths, 28.59%; p < 0.0001). This indicated a clear trend of escalating health risks with increasing WWI scores.
Association between WWI with CVD and mortality
As shown in Table 3, the fully adjusted continuous model indicated that for each one-unit increase in WWI, patients with diabetes experienced a 28% higher chance of developing CVD, with a 95% CI ranging from 1.04 to 1.58. In the fully adjusted categorical model, using the lowest quartile of WWI as a benchmark, the second and fourth quartiles were associated with a significant increase in CVD risk, by odds ratio (OR) 1.59 (95% CI 1.10–2.29) and OR 1.66 (95% CI 1.10–2.50) respectively. These findings underscored a strong positive correlation between elevated WWI levels and the likelihood of CVD among individuals with diabetes.
Table 3 also outlined the incidence of 2130 deaths from all causes and 596 CVD-related deaths among individuals with diabetes. To investigate the independent association between WWI and the risk of mortality from all causes and CVD, the Cox regression models were developed. In the initial analysis, WWI was significantly and positively associated with the risk of mortality from all causes (hazard ratio [HR] 1.14, 95% CI 1.07–1.21), and this association persisted in the minimally adjusted model (HR 1.15, 95% CI 1.07–1.23). The positive link between WWI and all-cause mortality risk remained significant even after full covariate adjustment (HR 1.25, 95% CI 1.14–1.37). When participants were categorized into quartiles based on WWI levels, a similar trend was observed. In both the unadjusted and the first adjusted models, those in the highest quartile of WWI had an increased risk of mortality from all causes compared to those in the lowest quartile (unadjusted model: HR 1.16, 95% CI 1.04–1.28; Model 1: HR 1.30, 95% CI 1.13–1.49). In the fully adjusted Model 2, the HR for quartiles 3 and 4 of WWI, in relation to the risk of mortality from all causes, were 1.35 (95% CI 1.15–1.59) and 1.53 (95% CI 1.27–1.83), respectively. However, while there was an initial positive correlation between WWI and CVD mortality in the unadjusted and first adjusted models, this relationship became statistically insignificant after accounting for all confounding factors. Additionally, as shown in Fig. 2, the analysis of RCS revealed no non-linear relationships between WWI and the risks of CVD and all-cause mortality.
The restricted cubic spline regression analyses for the association between weight-adjusted waist circumference index (WWI) and the risk of cardiovascular disease (CVD) and all-cause mortality. All covariates from model 2 were adjusted. (A) Non-linearity of WWI and CVD; (B) Non-linearity of WWI and all-cause mortality.
Subgroup analysis and sensitivity analysis
The study's findings indicated a positive correlation between the WWI and CVD risk among patients with diabetes, which was pronounced in several demographic groups (Table 4). Specifically, younger individuals, males, and those of other races exhibited a heightened risk (OR 3.16, 95% CI 1.57–6.35 for age; OR 2.03, 95% CI 1.11–3.68 for gender; OR 8.62, 95% CI 2.93–25.35 for race). This association was also observed in those without chronic kidney disease (OR 1.84, 95% CI 1.12–3.00), current smokers (OR 1.91, 95% CI 1.06–3.42), and alcohol consumers (OR 1.69, 95% CI 1.05–2.70), with the fourth quartile of WWI showing a greater risk compared to the first quartile.
Similarly, when WWI was in its fourth quartile, there was a significant rise in all-cause mortality across various segments of the population (Table 5). This included individuals of all ages (HR 1.55, 95% CI 1.23–1.95 for those under 65 years; HR 1.65, 95% CI 1.21–2.23 for those 65 and older), females (HR 1.98, 95% CI 1.57–2.52), and different racial groups (whites: HR 1.52, 95% CI 1.16–1.99; blacks: HR 1.75, 95% CI 1.27–2.40, others: HR 1.53, 95% CI 1.03–2.27)). The impact was also felt among smokers (HR 1.94, 95% CI 1.51–2.47) and across all levels of renal function (eGFR < 60: HR 1.85, 95% CI 1.08–3.15; eGFR > = 60: HR 1.53, 95% CI 1.26–1.85).
To confirm the reliability of the findings, a sensitivity analysis was performed. Incorporating medication usage into model 2, the correlation between the WWI and CVD as well as all-cause mortality was found to be statistically robust (Supplementary Table 1).
Receiver operating characteristic of the WWI to CVD and all-cause mortality
The ROC curve analysis was conducted to assess the efficacy of various obesity parameters in detecting CVD and all-cause mortality, as outlined in Fig. 3. Notably, the AUC for all four obesity indicators was greater than 0.5, signifying their potential in identifying CVD and all-cause mortality. In comparison, the WWI demonstrated a higher predictive capacity for CVD and all-cause mortality than WC, waist to hip ratio, and weight to height ratio.
Discussion
This study was the first time to provide compelling evidence that the WWI was significantly associated with the prevalence of CVD and all-cause mortality among individuals with diabetes in the United States. The findings revealed a dose–response relationship, with higher WWI scores correlating to an increased risk of CVD and mortality. Notably, the association remained robust even after adjusting for various confounding factors. Furthermore, The WWI's superiority in predicting CVD and mortality risk, as demonstrated through the ROC curve analysis, suggested that it may be a more effective tool for assessing obesity-related health risks. Overall, the WWI offered a promising metric for enhancing the accuracy of such assessments and could play a pivotal role in shaping future clinical guidelines and public health policies aimed at addressing the growing burden of CVD and mortality in patients with diabetes.
WWI was linked to various cardiovascular conditions and negative outcomes. A thorough analysis of 21,040 individuals revealed a significant correlation between elevated WWI and the likelihood of CVD, particularly in younger adults under 5028. A long-term study of 26,822 participants showed that higher WWI levels were associated with increased risks of cardiovascular and overall mortality29. Another study found that WWI was a significant prognostic indicator for heart failure30. A cross-sectional study involving 23,389 subjects also indicated that higher WWI levels might independently predict stroke31. Additionally, higher WWI was found to be associated with wicked left ventricular function32. These studies collectively underscored the importance of WWI as a key indicator for anticipating a range of cardiovascular events across different populations and age groups. Furthermore, there was a strong association between WWI and diabetes. In the U.S. adult population, for every one-unit increase in WWI, there was a 14% increase in the risk of diabetes33. WWI could predict newly diagnosed diabetes in specific populations, such as Chinese rural adults34. Importantly, WWI may be an independent factor in identifying patients at higher risk of diabetic kidney disease35. Our study provided novel insights into the relationship between WWI and CVD and mortality in individuals with diabetes, enhancing the utility of WWI and offering new metrics for assessing and monitoring risk in this patient group.
Obesity is a well-known risk factor for CVD in diabetes36. Yet, the "obesity paradox" suggested that current measures of obesity, like BMI, may not fully capture the risks. BMI's inability to accurately reflect central fat distribution, which was crucial for cardiovascular and cerebrovascular health, made it a less reliable predictor37. The WWI provided a more accurate way to identify central obesity20. Studies showed that higher WWI values were linked to negative changes in body composition, including higher fat levels, lower muscle mass, and decreased bone density38. WWI had emerged as a superior predictor of health issues compared to BMI, demonstrating greater consistency and reliability in predicting diseases across different populations and settings, especially in multiethnic or multicenter studies39,40.
Several mechanisms could explain the link between the WWI and CVD in patients with diabetes. A high WWI may signal adipose tissue dysfunction, particularly an increase in visceral fat. This fat buildup could spur the release of inflammatory cytokines like interleukin-6 and tumor necrosis factor, which were known to incite inflammation—a risk factor for CVD41. Additionally, adipose tissue in obesity produced more reactive oxygen species (ROS). High ROS levels could deplete nitric oxide (NO), leading to the formation of harmful hydrogen peroxide through ROS-NO interactions. This could impair endothelial cell function42,43. Endothelial dysfunction was directly linked to CVD progression44,45,46. This complex biological interplay highlighted the nuanced relationship between adipose tissue health, as indicated by WWI, and the risk of CVD in patients with diabetes.
This study benefited from using NHANES data, renowned for its stratified, multi-stage probability sampling. This method bolstered the study's credibility and ensured broad population representation. However, the research had some limitations. The cross-sectional design precluded causal inferences, necessitating further prospective studies to confirm the impact of WWI on CVD in patients with diabetes. Also, relying on a single WWI measurement restricted insights into how changes in this index could influence CVD incidence and mortality over time. Finally, while controlling for known variables, we recognized potential oversight of other confounders, such as environmental factors.
Conclusion
Our research indicated that the WWI served as a cost-effective and significant indicator of the risk for CVD and all-cause mortality among patients with diabetes in the United States. Consequently, we recommend the regular assessment of WWI in individuals with diabetes as a potentially advantageous method for evaluating their risk of CVD and the likelihood of mortality. This approach could be integral to enhancing preventive and treatment strategies tailored to the needs of this patient population.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/.
References
Roth, G. A. et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 76(25), 2982–3021 (2020).
Virani, S. S. et al. Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation 141(9), e139–e596 (2020).
Gronda, E., Caldarola, P., Iacoviello, M. & Gabrielli, D. 2019 ESC Guidelines on diabetes, pre-diabetes and cardiovascular diseases: What’s new? The cardiologist’s point of view. G Ital. Cardiol. (Rome) 21(4), 246–251 (2020).
Ma, C. X. et al. Cardiovascular disease in type 2 diabetes mellitus: Progress toward personalized management. Cardiovasc. Diabetol. 21(1), 74 (2022).
Collaboration NCDRF: Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387(10026):1377–1396.
Powell-Wiley, T. M. et al. Obesity and cardiovascular disease: A scientific statement from the American Heart Association. Circulation 143(21), e984–e1010 (2021).
Dwivedi, A. K., Dubey, P., Cistola, D. P. & Reddy, S. Y. Association between obesity and cardiovascular outcomes: Updated evidence from meta-analysis studies. Curr. Cardiol. Rep. 22(4), 25 (2020).
Sardu, C. et al. Inflammatory cytokines and SIRT1 levels in subcutaneous abdominal fat: Relationship with cardiac performance in overweight pre-diabetics patients. Front. Physiol. 9, 1030 (2018).
Sardu, C. et al. Metabolic syndrome is associated with a poor outcome in patients affected by outflow tract premature ventricular contractions treated by catheter ablation. BMC Cardiovasc. Disord. 14, 176 (2014).
Sardu, C. et al. Stretch, injury and inflammation markers evaluation to predict clinical outcomes after implantable cardioverter defibrillator therapy in heart failure patients with metabolic syndrome. Front. Physiol. 9, 758 (2018).
Lavie, C. J., De Schutter, A., Patel, D., Artham, S. M. & Milani, R. V. Body composition and coronary heart disease mortality–an obesity or a lean paradox?. Mayo Clin. Proc. 86(9), 857–864 (2011).
Ford, E. S., Maynard, L. M. & Li, C. Trends in mean waist circumference and abdominal obesity among US adults, 1999–2012. JAMA 312(11), 1151–1153 (2014).
Haslam, D. W. & James, W. P. Obesity. Lancet 366(9492), 1197–1209 (2005).
Despres, J. P. Body fat distribution and risk of cardiovascular disease: An update. Circulation 126(10), 1301–1313 (2012).
Skidmore, P. M. et al. Plasma free fatty acid patterns and their relationship with CVD risk in a male middle-aged population. Eur. J. Clin. Nutr. 64(3), 239–244 (2010).
Nomura, S. O., Karger, A. B., Weir, N. L., Duprez, D. A. & Tsai, M. Y. Free fatty acids, cardiovascular disease, and mortality in the Multi-Ethnic Study of Atherosclerosis. J. Clin. Lipidol. 14(4), 531–541 (2020).
Sardu, C. et al. SGLT2 breast expression could affect the cardiovascular performance in pre-menopausal women with fatty vs. non fatty breast via over-inflammation and sirtuins’ down regulation. Eur. J. Intern. Med. 113, 57–68 (2023).
Sardu, C. et al. Pre-menopausal breast fat density might predict MACE during 10 years of follow-up: The BRECARD study. JACC Cardiovasc. Imaging 14(2), 426–438 (2021).
Park, Y., Kim, N. H., Kwon, T. Y. & Kim, S. G. A novel adiposity index as an integrated predictor of cardiometabolic disease morbidity and mortality. Sci. Rep. 8(1), 16753 (2018).
Kim, N. H., Park, Y., Kim, N. H. & Kim, S. G. Weight-adjusted waist index reflects fat and muscle mass in the opposite direction in older adults. Age Ageing 50(3), 780–786 (2021).
Tao, J., Zhang, Y., Tan, C. & Tan, W. Associations between weight-adjusted waist index and fractures: A population-based study. J. Orthop. Surg. Res. 18(1), 290 (2023).
American Diabetes A. Standards of medical care in diabetes–2013. Diabetes Care 36(Suppl 1), S11-66 (2013).
Zhang, Q., Xiao, S., Jiao, X. & Shen, Y. The triglyceride-glucose index is a predictor for cardiovascular and all-cause mortality in CVD patients with diabetes or pre-diabetes: Evidence from NHANES 2001–2018. Cardiovasc. Diabetol. 22(1), 279 (2023).
Liu, C., Liang, D., Xiao, K. & Xie, L. Association between the triglyceride-glucose index and all-cause and CVD mortality in the young population with diabetes. Cardiovasc. Diabetol. 23(1), 171 (2024).
Sardu, C. et al. SGLT2-inhibitors effects on the coronary fibrous cap thickness and MACEs in diabetic patients with inducible myocardial ischemia and multi vessels non-obstructive coronary artery stenosis. Cardiovasc. Diabetol. 22(1), 80 (2023).
Sardu, C. et al. Effects of sodium-glucose transporter 2 inhibitors (SGLT2-I) in patients with Ischemic Heart Disease (IHD) treated by coronary artery bypass grafting via MiECC: Inflammatory burden, and clinical outcomes at 5 years of follow-up. Front. Pharmacol. 12, 777083 (2021).
Fitchett, D. et al. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME trial. Circulation 139(11), 1384–1395 (2019).
Fang, H., Xie, F., Li, K., Li, M. & Wu, Y. Association between weight-adjusted-waist index and risk of cardiovascular diseases in United States adults: a cross-sectional study. BMC Cardiovasc. Disord. 23(1), 435 (2023).
Han, Y. et al. The weight-adjusted-waist index predicts all-cause and cardiovascular mortality in general US adults. Clinics (Sao Paulo) 78, 100248 (2023).
Zhang, D. et al. Association between weight-adjusted-waist index and heart failure: Results from National Health and Nutrition Examination Survey 1999–2018. Front. Cardiovasc. Med. 9, 1069146 (2022).
Ye, J. et al. Association between the weight-adjusted waist index and stroke: a cross-sectional study. BMC Public Health 23(1), 1689 (2023).
Cai, S. et al. The relationship between the weight-adjusted-waist index and left ventricular hypertrophy in Chinese hypertension adults. Hypertens. Res. 46(1), 253–260 (2023).
Zheng, D. et al. Association between the weight-adjusted waist index and the odds of type 2 diabetes mellitus in United States adults: A cross-sectional study. Front. Endocrinol. (Lausanne) 14, 1325454 (2023).
Yu, S. et al. Weight-adjusted-waist index predicts newly diagnosed diabetes in Chinese rural adults. J. Clin. Med. 12(4), 1620 (2023).
Wang, Z. et al. The relationship between weight-adjusted-waist index and diabetic kidney disease in patients with type 2 diabetes mellitus. Front. Endocrinol. (Lausanne) 15, 1345411 (2024).
Balakumar, P., Maung, U. K. & Jagadeesh, G. Prevalence and prevention of cardiovascular disease and diabetes mellitus. Pharmacol. Res. 113(Pt A), 600–609 (2016).
Neeland, I. J. et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 7(9), 715–725 (2019).
Kim, K. J., Son, S., Kim, K. J., Kim, S. G. & Kim, N. H. Weight-adjusted waist as an integrated index for fat, muscle and bone health in adults. J. Cachexia Sarcopenia Muscle 14(5), 2196–2203 (2023).
Xie, F., Xiao, Y., Li, X. & Wu, Y. Association between the weight-adjusted-waist index and abdominal aortic calcification in United States adults: Results from the national health and nutrition examination survey 2013–2014. Front. Cardiovasc. Med. 9, 948194 (2022).
Kim, J. Y. et al. Associations between weight-adjusted waist index and abdominal fat and muscle mass: Multi-ethnic study of atherosclerosis. Diabetes Metab. J. 46(5), 747–755 (2022).
Huby, A. C. et al. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 132(22), 2134–2145 (2015).
Heitzer, T., Schlinzig, T., Krohn, K., Meinertz, T. & Munzel, T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 104(22), 2673–2678 (2001).
Furukawa, S. et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Invest. 114(12), 1752–1761 (2004).
Shah, M. S. & Brownlee, M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ. Res. 118(11), 1808–1829 (2016).
Yuan, T. et al. New insights into oxidative stress and inflammation during diabetes mellitus-accelerated atherosclerosis. Redox Biol. 20, 247–260 (2019).
Iacobini, C., Vitale, M., Pesce, C., Pugliese, G. & Menini, S. Diabetic complications and oxidative stress: A 20-year voyage back in time and back to the future. Antioxidants (Basel) 10(5), 727 (2021).
Acknowledgements
The authors thank the staff and the participants of the NHANES study for their valuable contributions.
Funding
The National Natural Science Foundation of China [82370346] and Nanjing City Development Project of Medical Science and Technology (No. YKK23267) provided funding for this work.
Author information
Authors and Affiliations
Contributions
Z.T. conceived and designed the study and wrote the manuscript. P.Z. analyzed the data. G.M. took the quality control of data and critically revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Tao, Z., Zuo, P. & Ma, G. The association between weight-adjusted waist circumference index and cardiovascular disease and mortality in patients with diabetes. Sci Rep 14, 18973 (2024). https://doi.org/10.1038/s41598-024-69712-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69712-w
- Springer Nature Limited