Abstract
This study aimed to evaluate the impact of contrast media application on CT attenuation of the bone using a novel calcium-only imaging technique (VCa) from dual-layer spectral detector CT (DLCT), which enables CT-based bone mineral density measurement unimpeded by soft tissue components. For this, true non-contrast (TNC) and venous phase images (VP) of n = 97 patients were acquired. CT attenuation of the first lumbar vertebra (L1) was measured in TNC-VCa, VP-VCa, and in virtual non-contrast images (VNC). CT attenuation was significantly higher in VP-VCa than in TNC-VCa (p < 0.001), although regression analyses revealed a strong linear association between these measures (R2 = 0.84). A statistical model for the prediction of TNC-VCa CT attenuation was established (TNC-VCa[HU] = − 6.81 + 0.87 × VP-VCa[HU]-0.55 × body weight[kg]) and yielded good agreement between observed and predicted values. Furthermore, a L1 CT attenuation threshold of 293 HU in VP-VCa showed a sensitivity of 90% and a specificity of 96% for detecting osteoporosis. The application of contrast media leads to an overestimation of L1 CT attenuation in VCa. However, CT attenuation values from VP-VCa can be used within CT-based opportunistic osteoporosis screening eighter by applying a separate threshold of 293 HU or by converting measured data to TNC-VCa CT attenuation with the given regression equation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Osteoporosis, characterized by low bone mass and deterioration of bone microarchitecture, leads to a notable increase in fracture risk1,2. Despite its substantial impact on morbidity, mortality and healthcare expenditures3,4 osteoporosis remains underdiagnosed and undertreated5,6,7. Early detection is crucial for effective treatment and fracture prevention, prompting research into the use of routine computed tomography (CT) for opportunistic osteoporosis screening8,9,10,11. Conventional single-energy CT (SECT) has broad screening potential12,13 in evaluating bone mineral density (BMD), but these estimates are prone to errors due to contributions from both bone and soft tissue. Thus, bone marrow changes (e.g., due to conversion of red bone marrow to bone marrow fat or tumor infiltration) could lead to a systematic over- or underestimation of BMD9,14,15,16,17. Dual-energy CT (DECT) offers a solution by differentiating bone from soft tissue15,18,19,20. The introduction of the Dual-layer spectral detector CT (DLCT) scanner, which uses two separate detector layers to capture different parts of the X-ray spectrum, has advanced the clinical use of energy-resolved imaging without the need for specific dual-energy protocols or increased radiation exposure21. Thus, with DLCT, spectral data can be obtained without pre-selecting a particular dual-energy protocol or increasing radiation exposure.
A recent study utilizing spectral data from DLCT has introduced a calcium-based postprocessing algorithm. This technique facilitates the reconstruction of virtual calcium-only images (VCa), which depict bone structures devoid of soft tissue interference22. In that investigation, CT attenuation values in VCa demonstrated superior concordance with DXA-derived bone mineral density (BMD) and known calcium concentrations compared to conventional CT images. This suggests that VCa could enhance the accuracy of CT-based osteoporosis screening.
Considering that the previous study encompassed only non-contrast examinations and recognizing that iodinated contrast media (CM) significantly influence CT attenuation of bone tissue, the applicability of the findings is confined to non-enhanced DLCT data22,23,24,25. Consequently, this study aimed to assess whether BMD can be accurately measured in VCa reconstructed from contrast-enhanced CT data. Given that the majority of clinical CT scans involve CM administration, evaluating the feasibility of calcium-only imaging for an opportunistic osteoporosis screening necessitates this investigation. Additionally, a secondary objective was to assess the utility of virtual non-contrast reconstructions derived from post-contrast imaging.
Material and methods
Ethics approval and consent
This retrospective exploratory single-center study was approved by the loval review board ethics committee of the medical faculty of Heidelberg University (S-348/2019). The need for written informed consent was waived. The study was performed according to the ethical guidelines of the 1964 Declaration of Helsinki and its later amendments.
Study design and clinical data selection
The primary objectives were:
-
1.
to compare mean CT attenuation values of the first lumbar vertebra (L1) measured in VCa of true non-contrast acquisitions (TNC-VCa) with mean CT attenuation values of L1 measured in VCa of post-contrast venous phase acquisitions (VP-VCa)
-
2.
to establish a statistical model enabling the prediction of TNC-VCa L1 CT attenuation values from VP-VCa L1 CT attenuation values.
Secondary objectives were:
-
1.
to compare mean CT attenuation values of L1 between the following reconstructions:
-
a.
conventional images reconstructed from true non-contrast acquisitions (TNC) vs. virtual non-contrast images reconstructed from post-contrast venous phase acquisitions (VNC),
-
b.
TNC vs. post-contrast venous phase acquisitions (VP),
-
c.
TNC vs. TNC-VCa
-
d.
VP vs. VP-VCa
-
a.
-
2.
to assess the capability of VP-VCa L1 CT attenuation values in detecting osteoporosis.
Osteoporosis was defined as a L1 CT attenuation value ≤ 110 HU in TNC images as suggested by Pickhardt et al.11.
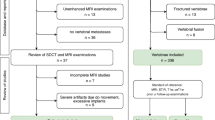
For this purpose, all patients of Heidelberg University Hospital who underwent a DLCT examination including the thoracolumbar junction from May 25, 2021 to June, 18 2021 were considered for study inclusion. In total, n = 108 patients were identified. Inclusion criteria were: (1) availability of a DLCT examination including a true non-contrast and a post-contrast venous phase acquisition of the thoracolumbar junction, (2) availability of spectral base images (SBI) for both acquisitions to enable the reconstruction of virtual non-contrast images from venous phase acquisitions and calcium-suppressed images from both acquisitions, (3) age at least 18 years. Criteria for study exclusion were: (1) severe structural defects of L1 such as fractures (Genant grade 2 and 3) and malignant lesions, (2) presence of dorsal spondylodesis, cement augmentation, or (3) severe imaging artifacts. The final study population consisted of n = 97 patients. The process of study enrollment is depicted in Fig. 1. Clinical information on patient age, sex and body weight were extracted from the hospital information system (I.S.-H.*med., SAP).
Flow chart of study enrollment in line with the STROBE guidelines38. DLCT dual-layer spectral detector CT, L1 first lumbar vertebra, SBI spectral base images.
DLCT acquisition and post-processing
A dual-layer spectral CT (Spectral CT7500, Philips Healthcare) with helical technique was used for the imaging of all patients. All acquisitions were performed with the same parameters: Tube voltage 120 kVp, dose right index 18 (automated, attenuation-based dose modulation), reference tube current–time product 87 mAs, volumetric computed tomography dose index (CTDIvol) 7.5 mGy (32 cm body-phantom), pitch 1.473 and collimation 64 × 0.625 mm. For contrast enhancement, all patients received an individually tailored body weight-adapted CM bolus using a dedicated contrast injection software (Certegra™ P3T, Bayer). The calculation of the amount of contrast media was as followed:
Image reconstruction and analysis
Using the manufacturer’s image post-processing suite (IntelliSpace Portal Version 12, Philips), overlapping sagittal reconstructions of the lumbar spine were computed with 2 mm slice thickness and 1 mm increment. The following reconstructions were created from SBI of true non-contrast acquisitions: (1) conventional images (TNC), (2) virtual non-Calcium images, suppression index 25 (VNCa25), and (3) virtual non-Calcium images, suppression index 100 (VNCa100). Additionally, from SBI of post-contrast venous phase acquisitions, the following reconstructions were created: (1) conventional images (VP), (2) virtual non-contrast images (VNC), (3) virtual non-Calcium images, suppression index 25 (VNCa25), and (4) virtual non-Calcium images, suppression index 100 (VNCa100). For TNC and VP reconstructions, a sharp kernel YA and for VNCa reconstructions kernel B was used. The higher the VNCa index in the reconstruction the less calcium was subtracted and more bony structures were visible. VCa CT numbers were calculated as difference between VNCa100 and VNCa25 CT attenuation values and therefore are considered to be a measure of bone mass which is supposed to be unaffected by soft tissue components.
Using the same software as for image reconstruction, oval ROIs as large as possible were placed in the center of L1, avoiding the inclusion of blood vessels or cortical bone. The focus was set on L1 as (1) it is included on many routine CT examinations (like abdomen and thorax), (2) can be easily identified, and (3) research results suggest that BMD measurements of L1 are as accurate or more accurate than those of other thoracal or lumbar vertebrae or even than combinations of multiple vertebrae11,26,27. For all reconstructions of the true non-contrast and the contrast-enhanced acquisition, respectively, the positions of the ROIs were identical to ensure comparability of measurements. The ROI measurements were performed by two independent readers to increase accuracy and to quantify interreader agreement. For the main statistical analyses, mean CT attenuation values of both reads were calculated.
Statistical analysis
The statistical analyses were performed in the statistical environment R (version 4.1.1)28. In case of multiple comparisons, the Bonferroni-Holm method was used for p-value adjustment29. The level of significance was set at p < 0.05. The intra-class correlation coefficient (ICC) with corresponding 95% confidence intervals was calculated as a measure of interrater reliability basing on single-rater [k = 2], absolute agreement, 2-way random effects models30. Wilcoxon Signed-Rank tests were performed to compare CT attenuation values of L1 in TNC-VCa, VP-VCa, TNC, VP and VNC images; corresponding effect sizes are reported as r = Z/\(\sqrt{N}\)31. In order to establish a statistical model for the prediction of TNC-VCa CT numbers from VP-VCa CT numbers, linear regression analysis was conducted with TNC-VCa CT numbers as dependent variable. In a first step, a univariate regression model was fitted using VP-VCa CT attenuation values as predictor to determine the linear association between TNC-VCa and VP-VCa CT attenuation. In a second step, multiple regression analysis with backwards elimination of predictors was performed including age, sex and body weight as further potential predictors of TNC-VCa CT numbers. Assessing the regression models according to standard procedures32 revealed no evidence for violations of model assumptions. A Bland–Altman plot was used for visual evaluation of the agreement between predicted TNC-VCa CT numbers and empirical TNC-VCa CT numbers. ROC curve analysis was conducted to assess the diagnostic performance of VP-VCa CT attenuation values in detecting osteoporosis. The area under the ROC curve (AUC) and corresponding 95% confidence intervals were calculated using a non-parametric, bootstrapping-based approach33. The cut-off value showing the best diagnostic performance was determined and the corresponding sensitivity and specificity were reported.
Results
Study population
The total sample included 42 women and 55 men with a mean age of 63.2 (SD 14.1) years. Further descriptive information on the sample is summarized in Table 1. Most of the CT examinations were performed for oncological reasons (see Table 2 for further details).
Interrater reliability
The ICCs for the two readers’ measurements ranged between 0.95 and 0.96 for all reconstructions indicating an excellent interrater reliability. A detailed listing of ICCs with corresponding confidence intervals is (Table 3).
Primary objectives: comparison of VCa L1 CT attenuation values from non-contrast and contrast-enhanced acquisitions
Mean, median (Mdn) and corresponding measures of dispersion of L1 CT attenuation values from all reconstructions are summarized in Table 4. CT attenuation values were significantly higher in VP-VCa compared to TNC-VCa (Mdn = 345.70 HU vs. Mdn = 236.55 HU, p < 0.001, r = 0.61) (Fig. 2). Univariate regression analysis revealed a strong linear association between TNC-VCa and VP-VCa CT attenuation values (R2 = 0.84; Fig. 3). In multiple regression analysis patients’ body weight was shown to significantly improve the prediction of TNC-VCa CT attenuation values. Sex and age were eliminated from the model. The final regression equation for the prediction of TNC-VCa CT attenuation values was: pTNC-VCa [HU] = − 6.81 + 0.87 × VP-VCa [HU] − 0.55 × body weight [kg]. Visual assessment of the Bland–Altman plot indicated a good agreement between predicted and observed TNC-VCa CT attenuation values when applying the final regression model with most of the differences ranging within the 95% limits of agreement (− 55.85 HU to 55.85 HU) (Fig. 4).
Boxplots of mean L1 CT attenuation values for different reconstructions. The brackets indicate the results of Wilcoxon Signed-Rank tests for the comparison of mean L1 CT attenuation values of TNC, VP, VNC, TNC-VCa and VP-VCa (black bracket = primary objective; grey brackets = secondary objectives). TNC-VCa virtual calcium-only images of true non-contrast acquisitions, TNC conventional images reconstructed from non-contrast acquisitions, VNC virtual non-contrast images reconstructed from post-contrast venous phase acquisitions, VP conventional images reconstructed from post-contrast venous phase acquisitions, VP-VCa virtual calcium-only images of post-contrast venous phase acquisitions. ***p < 0.001.
Secondary objectives: comparison of L1 CT attenuation values between further reconstructions
TNC CT attenuation values were significantly higher than VNC CT attenuation values (Mdn: 131.55 HU vs. 67.95 HU, p < 0.001, r = 0.61) and significantly lower than VP L1 CT attenuation values (Mdn: 131.55 HU vs. 156.9 HU, p < 0.001, r = 0.61) and TNC-VCa L1 CT attenuation values (Mdn: 131.55 HU vs. 236.55 HU, p < 0.001, r = 0.61). Accordingly, VP CT attenuation values were significantly lower than VP-VCa CT attenuation values (Mdn: 156.90 HU vs. 345.70 HU, p < 0.001, r = 0.61) (Fig. 2).
Secondary objectives: detecting osteoporosis using L1 CT attenuation values from VP-VCa
ROC analysis revealed an AUC of 0.97 [95% CI 0.93–0.99] for the detection of osteoporosis using VP-VCa L1 CT attenuation values. A threshold of 293 HU was identified showing the best overall diagnostic performance and yielded a sensitivity of 90.3% and a specificity of 95.5% (Fig. 5).
Discussion
The results of the present study showed that the injection of contrast media is accompanied by higher VCa CT attenuation values of the lumbar spine compared to CT attenuation values obtained from VCa of true non-contrast acquisitions. When not taken into account, this effect might lead to a systematic overestimation of bone density within DLCT-based osteoporosis screening with VCa. However, using the formula derived from multiple regression analysis, L1 CT attenuation values from VP-VCa could be used to predict L1 CT attenuation values from TNC-VCa. Furthermore, a L1 CT attenuation threshold of 293 HU was 90% sensitive and 96% specific for diagnosing osteoporosis in VP-VCa and could be used to identify high-risk patients with low bone mass directly, using VCa from venous phase post-contrast acquisitions.
The opportunity to reliably predict TNC-VCa CT numbers from VP-VCa CT numbers enables the application of the former proposed cut-off value by Do et al. of 126 HU in TNC-VCa to identify osteoporosis or osteopenia in the setting of contrast-enhanced CT acquisitions. However, a comparison of diagnostic performance markers between the threshold proposed by Do et al. for TNC-VCa and the threshold we found for VP-VCa in the present study, implies that VP-VCa might be as appropriate as TNC-VCa for a CT-based osteoporosis screening. According to the study by Do et al., a threshold of 126 HU was 90% sensitive and 47% specific in detecting osteoporosis in TNC-VCa. The threshold we found for VP-VCa also yielded a sensitivity of 90%, but a specificity of more than 95%. Therefore, since sensitivity was equivalently high for both thresholds, TNC-VCa and VP-VCa might perform well in clinical settings, in which patients with high risk for osteoporosis must be detected reliably and false-negative rate is supposed to be minimized. Moreover, considering the high specificity and therefore, low false-positive rate, the threshold we propose for VP-VCa L1 CT attenuation might also be well suited for patient cohorts with low risk for osteoporosis and might help avoiding unnecessary further diagnostic examinations of patients with normal BMD.
The findings of this study are in line with previous studies suggesting that the application of CM results in a systematic overestimation of CT-derived BMD23,24,25. Hence, CT attenuation values of osseous structures obtained from non-contrast and contrast-enhanced acquisitions cannot be readily compared and, most importantly, thresholds suggested for non-contrast acquisitions might not be applicable to contrast-enhanced acquisitions, and vice versa. To overcome this issue a recent study by Ding et al. investigated whether L1 CT attenuation values measured in DLCT-derived VNC are comparable to L1 CT attenuation values measured in TNC25. In line with the results from our study, they found L1 CT attenuation values to be significantly lower in VNC compared with TNC. The reconstruction of VNC from spectral CT data is based on material decomposition whereby the specific spectral properties of iodine are utilized to select and virtually remove the amount of attenuation which is due to iodinated material like contrast media. Since the spectral properties of iodine and bone minerals like calcium are similar, the algorithm for VNC reconstruction might not only remove iodinated material but also some of the bone minerals25. This line of reasoning could also explain the difference we found between L1 CT attenuation values obtained from TNC-VCa and VP-VCa. The reconstruction of VNCa, on which VCa are based on, follows the same principles as the reconstruction of VNC, but with calcium being subtracted instead of iodine. Considering the similar spectral properties of calcium and iodine, it seems reasonable that CT attenuation values of osseous structures obtained from VP-VCa are higher than those obtained from TNC-VCa since VP-VCa CT numbers might also include attenuation from iodinated contrast media. In support of these explanations, we found patients body weight to be relevant when predicting TNC-VCa CT attenuation values from VP-VCa CT attenuation values. In our study the participants received an individually tailored CM bolus which was proportional to their body weight. Since body weight is included in the regression equation as a negative term, for patients with higher body weight, i.e., patients who received a greater amount of contrast media, there is a bigger difference between VP-VCa and predicted TNC-VCa CT attenuation values. To conclude, it might be possible that the difference between TNC-VCa and VP-VCa CT attenuation values that we observed in this study was caused by a confounding of iodine- and calcium-containing structures within the reconstruction of VCa images from spectral CT data. Further studies are needed to investigate the role of different CM injection parameters on calcium-based imaging in greater detail.
Lastly, CT attenuation values in VCa of non-contrast and contrast-enhanced acquisitions was found to be higher than CT numbers from corresponding conventional reconstructions (TNC and VP). These results are in line with previous study findings that SECT-based measures of BMD are constantly lower than BMD-measures obtained from DECT and therefore support the assumption that bone mass might be underestimated in conventional SECT in a systematic manner15,19. Measuring CT attenuation of trabecular bone using SECT data, bone and soft tissue are confounded which increases the risk of measurement errors, especially due to changes in bone marrow composition. Therefore, DECT-based methods for BMD-quantification like virtual calcium-only imaging, allowing to control for soft tissue components, might be more suitable for a CT-based opportunistic osteoporosis screening than conventional SECT-based approaches. The present study has some limitations. Firstly, as former research results suggest that CT-derived BMD can vary systematically for different scanner types and acquisition protocols, the generalizability of our findings might be limited34,35,36,37. This might especially apply to different CM protocols since our data point out that the amount of CM that is injected might play a crucial role for the absolute difference between CT attenuation values of osseous structures from non-contrast and contrast-enhanced acquisitions. Furthermore, since DXA results were not available for our study cohort, we defined the presence of osteoporosis according to a CT attenuation threshold from the literature11 that is still controversial. Lastly, the analyses in this study were confined to the L1 vertebra, precluding comparisons with other vertebrae or combinations thereof. Further studies should incorporate various scanner types and contrast media protocols, utilize DXA results as the reference standard, and include BMD measurements of additional target regions to address these limitations.
In conclusion, this study showed that VCa can be used for an accurate assessment of bone mass in the context of contrast-enhanced DLCT acquisitions. Therefore, the results complement former research findings on virtual calcium-only imaging and support the assumption that the implementation of VCa reconstructions within CT-based osteoporosis screenings might help to improve their diagnostic accuracy. To facilitate the interpretation of BMD-measurements in VP-VCa a regression model for the reliable prediction of CT attenuation values from VCa of true non-contrast acquisitions was established. Furthermore, a separate L1 CT attenuation threshold of 293 HU for the detection of osteoporosis in VP-VCa is suggested.
Data availability
The datasets generated and/or analysed during the current study are not publicly available due to patient data protection but are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under the ROC curve
- BMD:
-
Bone mineral density
- CM:
-
Contrast media
- CTDIvol :
-
Volumetric computed tomography dose index
- DECT:
-
Dual-energy CT
- DLCT:
-
Dual-layer spectral detector CT
- DXA:
-
Dual-energy X-ray absorptiometry
- ICC:
-
Intra-class correlation coefficient
- L1:
-
First lumbar vertebra
- Mdn:
-
Median
- TNC-VCa:
-
VCa reconstructed of true non-contrast acquisitions
- ROC:
-
Receiver operating characteristic
- SBI:
-
Spectral base images
- SECT:
-
Single-energy CT
- SD:
-
Standard deviation
- TNC:
-
True non-contrast acquisitions
- VCa:
-
Virtual calcium-only images
- VNC:
-
Virtual non-contrast image data reconstructed from post-contrast venous phase acquisitions
- VNCa100:
-
Virtual non-calcium images, suppression index 100
- VNCa25:
-
Virtual non-calcium images, suppression index 25
- VP:
-
Conventional images reconstructed from post-contrast venous phase acquisitions
- VP-VCa:
-
VCa of post-contrast venous phase acquisitions
References
WHO. Guidelines for preclinical evaluation and clinical trials in osteoporosis (1998). https://apps.who.int/iris/bitstream/handle/10665/42088/9241545224_eng.pdf?sequence=1&isAllowed=y (accessed 17 Dec 2020).
Hadji, P. et al. The epidemiology of osteoporosis—Bone Evaluation Study (BEST): An analysis of routine health insurance data. Dtsch. Arztebl. Int. 110, 52–57 (2013).
Svedbom, A. et al. Osteoporosis in the European Union: A compendium of country-specific reports. Arch. Osteoporos. 8, 137–137 (2013).
Johnell, O. & Kanis, J. Epidemiology of osteoporotic fractures. Osteoporos Int. 16(Suppl 2), S3-7 (2005).
Lafata, J. E. et al. Improving osteoporosis screening: Results from a randomized cluster trial. J. Gen. Intern. Med. 22, 346–351 (2007).
Morris, C. A. et al. Patterns of bone mineral density testing. J. Gen. Intern. Med. 19, 783–790 (2004).
Compston, J. E., McClung, M. R. & Leslie, W. D. Osteoporosis. Lancet 393, 364–379 (2019).
Gausden, E. B., Nwachukwu, B. U., Schreiber, J. J., Lorich, D. G. & Lane, J. M. Opportunistic use of CT imaging for osteoporosis screening and bone density assessment: A qualitative systematic review. J. Bone Jt Surg. 99, 1580–1590 (2017).
Adams, J. E. Quantitative computed tomography. Eur. J. Radiol. 71, 415–424 (2009).
Murray, T. É., Williams, D. & Lee, M. J. Osteoporosis, obesity, and sarcopenia on abdominal CT: A review of epidemiology, diagnostic criteria, and management strategies for the reporting radiologist. Abdom. Radiol. 42, 2376–2386 (2017).
Pickhardt, P. J. et al. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann. Intern. Med. 158, 588–595 (2013).
Drinka, P. J., DeSmet, A. A., Bauwens, S. F. & Rogot, A. The effect of overlying calcification on lumbar bone densitometry. Calcif. Tissue Int. 50, 507–510 (1992).
Link, T. M. Osteoporosis imaging: State of the art and advanced imaging. Radiology 263, 3–17 (2012).
Peña, J. A. et al. Bone-marrow densitometry: Assessment of marrow space of human vertebrae by single energy high resolution-quantitative computed tomography. Med. Phys. 43, 4174–4183 (2016).
Sfeir, J. G. et al. Evaluation of cross-sectional and longitudinal changes in volumetric bone mineral density in postmenopausal women using single- versus dual-energy quantitative computed tomography. Bone 112, 145–152 (2018).
Borggrefe, J. et al. Association of osteolytic lesions, bone mineral loss and trabecular sclerosis with prevalent vertebral fractures in patients with multiple myeloma. Eur. J. Radiol. 84, 2269–2274 (2015).
Wait, J. M. S. et al. Performance evaluation of material decomposition with rapid-kilovoltage-switching dual-energy CT and implications for assessing bone mineral density. Am. J. Roentgenol. 204, 1234–1241 (2015).
Arentsen, L. et al. Use of dual-energy computed tomography to measure skeletal-wide marrow composition and cancellous bone mineral density. J. Bone Mineral Metab. 35, 428–436 (2017).
Cataño Jimenez, S., Saldarriaga, S., Chaput, C. D. & Giambini, H. Dual-energy estimates of volumetric bone mineral densities in the lumbar spine using quantitative computed tomography better correlate with fracture properties when compared to single-energy BMD outcomes. Bone 130, 115100 (2020).
Adams, J. E., Chen, S. Z., Adams, P. H. & Isherwood, I. Measurement of trabecular bone mineral by dual energy computed tomography. J. Comput. Assist. Tomogr. 6, 601–607 (1982).
Fredenberg, E. Spectral and dual-energy X-ray imaging for medical applications. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 878, 74–87 (2018).
Do, T. D. et al. Quantitative calcium-based assessment of osteoporosis in dual-layer spectral CT. Eur. J. Radiol. 178, 111606 (2024).
Pompe, E. et al. Intravenous contrast injection significantly affects bone mineral density measured on CT. Eur. Radiol. 25, 283–289 (2015).
Toelly, A. et al. Influence of contrast media on bone mineral density (BMD) measurements from routine contrast-enhanced MDCT datasets using a phantom-less BMD measurement tool. Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren (RöFo) 189, 537–543 (2017).
Ding, Y., Richter, A., Stiller, W., Kauczor, H.-U. & Weber, T. F. Association between true non-contrast and virtual non-contrast vertebral bone CT attenuation values determined using dual-layer spectral detector CT. Eur. J. Radiol. 121, 108740 (2019).
Romme, E. A. P. M. et al. Bone attenuation on routine chest CT correlates with bone mineral density on DXA in patients with COPD. J. Bone Mineral Res. 27, 2338–2343 (2012).
Ohara, T. et al. Relationship between pulmonary emphysema and osteoporosis assessed by CT in patients with COPD. Chest 134, 1244–1249 (2008).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2021). https://www.r-project.org/ (accessed 30 Oct 2021).
Holm, S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 6, 65–70 (1979).
McGraw, K. O. & Wong, S. P. Forming inferences about some intraclass correlation coefficients. Psychol. Methods 1, 30–46 (1996).
Rosenthal, R. Meta-analytic procedures for social research. In Applied Social Research Methods Series, vol. 6 (Sage Publications, 1991).
Field, A., Miles, J. & Field, Z. Discovering Statistics Using R (Sage, 2012).
Carpenter, J. & Bithell, J. Bootstrap confidence intervals: When, which, what? A practical guide for medical statisticians. Stat. Med. 19, 1141–1164 (2000).
Garner, H. W., Paturzo, M. M., Gaudier, G., Pickhardt, P. J. & Wessell, D. E. Variation in attenuation in L1 trabecular bone at different tube voltages: Caution is warranted when screening for osteoporosis with the use of opportunistic CT. Am. J. Roentgenol. 208, 165–170 (2016).
Gerety, E.-L. & Bearcroft, P. W. P. L1 vertebral density on CT is too variable with different scanning protocols to be a useful screening tool for osteoporosis in everyday practice. Br. J. Radiol. 91, 20170395 (2018).
Cropp, R. J., Seslija, P., Tso, D. & Thakur, Y. Scanner and kVp dependence of measured CT numbers in the ACR CT phantom. J. Appl. Clin. Med. Phys. 14, 12 (2013).
Giambini, H. et al. The effect of quantitative computed tomography acquisition protocols on bone mineral density estimation. J. Biomech. Eng. 137, 114502–114502 (2015).
Vandenbroucke, J. P. et al. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. PLoS Med. 4, e297 (2007).
Acknowledgements
PD Dr. Thuy Duong Do was supported by the Olympia-Morata research grant by the Medical Faculty of the University of Heidelberg. Dr. Stephan Skornitzke has ownership interests in investment funds containing stock of healthcare companies and is, at the time of submission, employee of Philips Medicals, Germany, but the work presented herein dates to his previous employment at Heidelberg University Hospital. Prof. Hans-Ulrich Kauczor reported research grant from Siemens, Philips and Boehringer Ingelheim, he is a member of the advisory board of Median, and received honoraria from Siemens, Philips and Sanofi.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
R.S. contributed to analysis, interpretation of the data, and manuscript draft. S.S. contributed to software in the work, and draft of the manuscript. M.C. contributed to conception, and draft of the manuscript. R.T. contributed to conception, and draft of the manuscript. S.W. contributed to software in the work, and draft of the manuscript. H.C.P. contributed to conception, and draft of the manuscript. K.H.U. contributed to conception, and draft of the manuscript. W.T.F. contributed to conception, and draft of the manuscript. D.T.D. contributed to conception, analysis, interpretation of the data, and manuscript draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahn, S., Skornitzke, S., Melzig, C. et al. The influence of contrast media on calcium-based imaging of the spine in dual-layer CT. Sci Rep 14, 18898 (2024). https://doi.org/10.1038/s41598-024-69743-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69743-3
- Springer Nature Limited