Abstract
This study aims to explore the relationship between the Systemic Immune-Inflammation Index (SII) and Cardiovascular-Kidney-Metabolic (CKM) Syndrome and its components. Data from the National Health and Nutrition Examination Survey (NHANES) from 2001 to 2018 were analyzed. CKM Syndrome is defined as the coexistence of Cardiometabolic Syndrome (CMS) and Chronic Kidney Disease (CKD). The SII is calculated using the formula: SII = (Platelet count × Neutrophil count)/Lymphocyte count. Weighted logistic regression models were used to examine the associations between SII and CKM, as well as its specific components. Restricted cubic splines explored non-linear relationships, and piecewise linear regression models assessed threshold effects. A consistent positive correlation was observed between elevated SII levels and the likelihood of CKM and its related diseases. In the fully adjusted Model 3, an increase of 1000 units in SII was associated with a 1.48-fold increase in the odds of CKM (95% CI 1.20–1.81, p < 0.001). Quartile analysis revealed a dose–response relationship, with the highest quartile of SII (Q4) showing the strongest association with CKM and its components. Nonlinear analyses revealed inflection points for waist circumference, triglycerides, low HDL-C, and cardiometabolic syndrome at specific SII levels, indicating a change in the direction or strength of associations beyond these points. Conversely, a linear relationship was observed between SII and chronic kidney disease. The SII is positively correlated with the risk of CKM Syndrome and its individual components, with evidence of non-linear relationships and threshold effects for some components.
Similar content being viewed by others
Introduction
Cardiovascular-Kidney-Metabolic (CKM) Syndrome, marked by metabolic disorders, chronic kidney disease (CKD), and cardiovascular diseases1, poses significant public health challenges due to their complex interactions and impact on morbidity and mortality2,3. The prevalence of CKM Syndrome necessitates effective biomarkers for early detection and management. The Systemic Immune-Inflammation Index (SII) offers insights into the inflammation underlying these conditions and helps monitor treatment responses4,5,6.
Systemic inflammation is central to many chronic diseases, affecting cardiovascular, renal, and metabolic systems. It is marked by elevated levels of pro-inflammatory cytokines like TNF-α, IL-6, and C-reactive protein (CRP), which promote atherosclerosis, endothelial dysfunction, and vascular stiffness, leading to hypertension and heart failure7,8. In renal diseases, inflammation causes kidney damage through glomerular injury and fibrosis, contributing to CKD progression6. Metabolically, inflammation is linked to insulin resistance, a key feature of type 2 diabetes and metabolic syndrome, exacerbating visceral fat storage and further inflammation9.
The SII, calculated from platelet, neutrophil, and lymphocyte counts, provides a measure of immune response and systemic inflammation10. Studies have validated SII as a marker for inflammation, showing its prognostic value in cardiovascular diseases and oncology, where higher SII levels are associated with worse outcomes11,12. No studies have thoroughly explored the relationship between SII and CKM Syndrome. Our study uses data from the 2001–2018 National Health and Nutrition Examination Survey (NHANES), a United States database, to assess whether SII is associated with the risk of CKM Syndrome and its components.
Methods
Study design and population
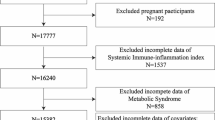
NHANES is a cross-sectional, stratified, multistage probability survey conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC)13. The survey protocol was approved by the NCHS Institutional Review Board, and all participants provided written informed consent. The NHANES data used for this analysis is available at https://www.cdc.gov/nchs/nhanes. This study analyzed the NHANES dataset from 2001 to 2018. Initially, 91,351 participants were included in the study. Exclusions were made for 56,467 participants lacking SII, CKD, and CMS data, and 1468 participants with missing or zero weights. After screening, 23,416 eligible participants were included in the study. Figure 1 illustrates the sample selection process.
Diagnosis of cardiovascular-kidney-metabolic syndrome
Currently, there are no definitive diagnostic criteria for CKM Syndrome. In this study, it is defined as CMS with concurrent CKD based on its characteristics14. CMS was defined according to the NCEP-ATP III report15. A diagnosis of CMS is made if participants meet at least three of the following five criteria: (1) Central obesity: waist circumference ≥ 102 cm for men or ≥ 88 cm for women; (2) Hypertriglyceridemia: serum TG ≥ 150 mg/dL; (3) Low HDL cholesterol: serum HDL-c < 40 mg/dL for men or < 50 mg/dL for women; (4) Hypertension: systolic blood pressure (SBP) ≥ 130 mmHg or diastolic blood pressure (DBP) ≥ 85 mmHg, or undergoing antihypertensive treatment; (5) Hyperglycemia: fasting plasma glucose ≥ 100 mg/dL or undergoing antidiabetic treatment. Waist circumference, weight, and height were collected using standard procedures during the physical examination. SBP and DBP were calculated as the arithmetic mean of up to four repeated measurements for each participant. TG and HDL-c were measured in serum, while fasting glucose was measured in plasma. CKD is defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 or a urine albumin-to-creatinine ratio > 30 mg/g16. The 2009 CKD-EPI creatinine equation by the Chronic Kidney Disease Epidemiology Collaboration was employed for the eGFR computation17.
Definition of systemic immune-inflammation index
The SII is a novel biomarker reflecting local immune response and systemic inflammation, and has been associated with the prognosis of various diseases. The SII for each participant was calculated using the formula10:
Lymphocyte, neutrophil, and platelet counts were measured using an automated hematology analyzer (Coulter® DxH 800 Analyzer), expressed as × 103 cells/ml. In this study, the SII was used as the exposure variable, and CKM Syndrome as the outcome variable. To enhance the effect size, SII was divided by 1000 (SII/1000).
Demographic characteristics and other covariates
Participants in this survey were categorized into racial/ethnic groups: Mexican American, Non-Hispanic Black, Non-Hispanic White, Other Hispanic, and Other Race. Educational levels were divided into two categories: high school and above, and below high school. Marital status was categorized as married and other. The Poverty Income Ratio (PIR), which accounts for economic inflation and family size, was used to measure income relative to the federal poverty line. Self-administered questionnaires collected information on smoking habits, alcohol consumption, physical activity, diabetes, and hypertension history. Smoking status was classified into never smokers, former smokers, and current smokers. Alcohol consumption was divided into five levels: never, former, mild, moderate, and heavy. Specific criteria for alcohol consumption included18: (1) current heavy drinking (more than 3 drinks per day for females, 4 or more for males, or binge drinking of 4 or more drinks for females, 5 or more for males on 5 or more days per month), (2) current moderate drinking (more than 2 drinks per day for females, 3 or more for males, or binge drinking more than 2 days per month), and (3) a history of daily binge drinking. Participants' physical activity levels, including walking, cycling, exercise, and leisure activities, were quantified by weekly Metabolic Equivalent Tasks (METs).
Statistical analysis
NHANES is a multistage, stratified, probability-based survey with oversampling of specific populations13. To adjust for unequal sampling probabilities and non-responses, all participant data were weighted according to NHANES examination weights and fasting subsample weights. Data analysis was performed using R open-source software version 4.3.2, with the “nhanesR” package (R Core Team, 2023). Participants were divided into two groups based on the presence or absence of CKM Syndrome. Continuous variables were presented as mean ± standard deviation (SD) and compared using the weighted T-test. Categorical variables were presented as frequencies (percentages) and compared using the chi-square test.
This study conducted weighted univariate and multivariate logistic regression analyses to investigate the association between SII and CKM and its components. Multivariate logistic regression included demographic characteristics and traditional factors associated with the SII index and CKM Syndrome. Odds ratios (ORs) were calculated through three models: an unadjusted model (Model 1), an age, sex, and race/ethnicity-adjusted model (Model 2), and a fully adjusted model (Model 3) for potential confounders, including age, sex, race, education level, Poverty Income Ratio, smoking status, alcohol consumption, and physical activity measured in total METs per week.
Restricted cubic spline (RCS) analysis was used to explore potential nonlinear relationships between SII and CKM Syndrome and its components. Missing covariates were addressed using multiple imputation designed for survey data19. Stratification and interaction analyses were conducted according to sex, age, race, smoking, and drinking habits. Results were considered statistically significant if pvalues were less than 0.05.
Ethics approval and consent to participate
The study involves the use of a publicly available dataset (NHANES), which was collected under ethical standards including informed consent from all participants. All methods were carried out in accordance with the relevant guidelines and regulations.
Results
Baseline characteristics
Table 1 presents the baseline characteristics of 23,416 participants, categorizing them into those with CKM Syndrome (1792 individuals) and participants without (21,624 individuals). The data indicate that those with CKM Syndrome have a higher average age (62.53 vs. 41.52 years). There is no marked difference in the racial/ethnic composition of the two groups. Educational attainment is lower among individuals with CKM Syndrome, as evidenced by fewer having attained a high school education or higher. Marital status also shows variation, with a reduced proportion of married individuals in the CKM Syndrome group. Lifestyle assessments reveal a higher incidence of current smoking and a lower incidence of never smoking within the CKM group. A greater percentage of individuals with CKM Syndrome are former alcohol consumers. Health-wise, this group is characterized by elevated body mass index (BMI), waist circumference, systolic and diastolic blood pressures, fasting plasma glucose, and serum creatinine, along with higher triglyceride levels and reduced high-density lipoprotein cholesterol. Notably, SII levels are increased in those with CKM Syndrome, coinciding with a greater occurrence of hypertension and diabetes mellitus. The disparities in continuous health metrics such as BMI, blood pressure, and serum markers are statistically significant, with p-values less than 0.001, signifying robust differences between those with and without CKM Syndrome.
Association of SII with CKM syndrome and its components
Multivariate logistic regression was used to assess the association between the SII and the occurrence of CKM Syndrome and its components, as delineated in Table 2. Through a tiered analysis involving three models, each incorporating increasing levels of adjustment for potential confounders, a consistent positive association was observed between elevated SII levels and the heightened likelihood of CKM Syndrome, as well as associated conditions including increased waist circumference, elevated triglycerides, reduced HDL-C, heightened fasting plasma glucose, increased blood pressure, cardiometabolic syndrome, and chronic kidney disease.
In Model 1, without any adjustments, a per-1000-unit rise in SII was significantly linked to an increased probability of CKM Syndrome (Odds Ratio [OR] 2.02, 95% Confidence Interval [CI] 1.70–2.40, p < 0.0001). This relationship remained significant, though slightly attenuated, after adjustments for age, sex, and ethnicity in Model 2 (OR 1.58, 95% CI 1.27–1.96, p < 0.0001), and further adjustments in Model 3, which accounted for socioeconomic status, lifestyle factors, and physical activity (OR 1.48, 95% CI 1.20–1.81, p < 0.001). Quartile-based analysis of SII, compared to the first quartile (Q1), across all models revealed a dose–response relationship, with the fourth quartile (Q4) invariably demonstrating the most pronounced association with CKM Syndrome and its components. In Model 3, fully adjusted, the ORs for CKM Syndrome across SII quartiles Q2, Q3, and Q4 were 1.19 (1.04–1.36, p = 0.010), 1.40 (1.21–1.62, p < 0.0001), and 1.60 (1.39–1.83, p < 0.0001), respectively, highlighting a significant trend (p for trend < 0.0001).
This pattern persisted across other conditions, with analyses based on continuous SII values and quartiles consistently indicating an increased risk with higher SII levels. However, the association between SII and elevated triglycerides was somewhat diminished in the fully adjusted Model 3, with the analysis based on continuous SII values yielding a non-significant result (p = 0.220), suggesting the presence of confounders that could potentially attenuate this specific association.
Analysis of restricted cubic spline regression
Figure 2 demonstrates the nonlinear relationships between the SII and various health conditions within CKM Syndrome and its components. RCS regression analysis reveals a linear relationship between SII and Chronic Kidney Disease (p = 0.263), whereas nonlinear associations are observed between SII and other components of CKM Syndrome (p < 0.001). Notably, no inflection points were detected for CKM Syndrome, Elevated Fasting Plasma Glucose, and Elevated Blood Pressure, indicating a stable correlation across SII values for these conditions.
Nonlinear relationship between SII and Cardiovascular-Kidney-Metabolic Syndrome (A). Nonlinear relationship between SII and elevated Waist Circumference (B). Nonlinear relationship between SII and Elevated Triglycerides (C). Nonlinear relationship between SII and Low HDL-C (D). Nonlinear relationship between SII and Elevated Fasting Plasma Glucose (E). Nonlinear relationship between SII and Elevated Blood Pressure (F). Nonlinear relationship between SII and Cardiometabolic Syndrome (G). Nonlinear relationship between SII and Chronic Kidney Disease (H).
Inflection points were identified at an SII level of approximately 702 for Elevated Waist Circumference, around 660 for Elevated Triglycerides, and about 804 for both Low HDL-C and Cardiometabolic Syndrome, suggesting changes in the direction or magnitude of associations at these SII levels. The results of two piecewise linear regression models, included in Table 3 with SII/1000 as the regression variable, show that below the inflection points, the risks for elevated waist circumference, triglycerides, low HDL-C, and cardiometabolic syndrome increase with rising SII, with ORs for SII/1000 being 4.99 (3.61, 6.90), 2.24 (1.69, 2.97), 2.24 (1.69, 2.97), and 2.65 (2.03, 3.46), respectively, all significant (p < 0.0001). Beyond the inflection points, the risk for elevated triglycerides decreases with increasing SII (OR = 0.75, 95% CI [0.59, 0.95], p = 0.020), while no significant statistical relationships were observed between SII and elevated waist circumference, low HDL-C, or cardiometabolic syndrome (p > 0.05).
Subgroup analysis and interaction
Multivariate logistic regression analysis, adjusted for potential confounders, was conducted to examine the association between the Systemic Immune-Inflammation Index (SII/1000) and CKM Syndrome across subgroups defined by age, sex, race, smoking status, and alcohol consumption (Table 4). In most subgroups, a significant association was observed between an increase in SII and the odds of CKM Syndrome, with ORs ranging from 1.412 to 2.896 per 1000 unit increase in SII. This association was not significant in the heavy drinking subgroup (p = 0.220). Furthermore, a significant interaction was found between SII and age subgroups (p for interaction = 0.014), but not with other subgroups, suggesting that the effect of SII on CKM Syndrome may vary with age. Regardless of sex, race, smoking, or alcohol consumption, a higher immune-inflammatory state as reflected by SII was identified as a strong independent risk factor for CKM Syndrome.
Discussion
Analysis of data from 23,416 NHANES participants revealed significant associations between elevated SII levels and CKM Syndrome and its components. These associations remained robust after adjusting for confounders. SII was identified as an independent risk factor linked to CKM Syndrome's onset and progression. The relationship between SII and CKM components, except for CKD, was nonlinear (p < 0.001), indicating varying risk patterns with different SII levels. The impact of SII on CKM Syndrome also changes with age. Regardless of gender, race, smoking, or alcohol consumption, a higher SII was a strong risk factor for CKM Syndrome. These findings provide crucial evidence for SII's role in cardiovascular, renal, and metabolic health and suggest new research directions.
SII and CKM syndrome components: associations and pathophysiological mechanisms
This study reveals intricate associations between SII and CKM Syndrome components, highlighting systemic inflammation's multifaceted effects. Elevated SII is linked to central obesity due to pro-inflammatory cytokines from adipose tissue impairing insulin signaling, leading to insulin resistance and disrupted lipid metabolism, which contributes to hypertriglyceridemia 20,21. Inflammation also lowers HDL cholesterol by impairing its anti-inflammatory and cholesterol transport functions 22,23. Hypertension is linked to inflammation-induced endothelial dysfunction, reducing nitric oxide bioavailability and increasing vascular resistance 24,25. Hyperglycemia is associated with cytokines like TNF-α and IL-6 impairing insulin receptor signaling, reducing glucose uptake 26,30,28. Lastly, SII is linked to CKD due to inflammation-induced renal damage, promoting fibrosis and inflammatory cell activation in the kidney 29,30. These findings underscore systemic inflammation's critical role in metabolic and renal dysfunctions, advocating for targeted interventions.
Dose–response relationships between SII and CKM syndrome components
In exploring the relationship between the SII and various health outcomes, the nonlinear associations observed with conditions like central obesity, hypertriglyceridemia, low HDL cholesterol, hypertension, and hyperglycemia, compared to the linear association with CKD, are deeply rooted in differential pathophysiological responses to systemic inflammation. Central obesity initially increases with high SII levels due to adipokine imbalance and inflammatory cytokine release but later stabilizes due to compensatory anti-inflammatory responses like increased IL-10 and TGF-β secretion 31,32. Dyslipidemia worsens initially due to impaired lipoprotein lipase activity and altered HDL functionality but may stabilize as adaptive changes in HDL functionality occur 33,34. Hypertension is elevated by inflammation-driven endothelial dysfunction and reduced nitric oxide availability but may stabilize over time with vascular remodeling and adaptive responses35. Hyperglycemia's progression is initially driven by insulin resistance due to TNF-α and IL-6 impairing insulin signaling but may stabilize with beta-cell compensatory mechanisms that enhance insulin secretion or sensitivity34. In contrast, the relationship between SII and CKD is linear, as inflammation consistently contributes to renal endothelial dysfunction, glomerular damage, and fibrosis, leading to progressive renal decline without significant compensatory responses4,36. This lack of effective compensatory mechanisms in the kidneys contrasts with other systems where feedback mechanisms can moderate disease progression under chronic inflammatory conditions4,37, 38.
Utilizing SII for stratified management in CKM syndrome
The SII is a crucial biomarker for identifying and managing CKM Syndrome. It effectively differentiates patients at elevated risk for various CKM components, facilitating early intervention and tailored strategies. The linear association with chronic kidney disease highlights its value in predicting renal impairment progression, allowing timely therapeutic interventions. Nonlinear relationships with central obesity, dyslipidemia, and hyperglycemia enable personalized care based on individual inflammatory profiles. Integrating SII into routine assessments can enhance precision in CKM Syndrome management, optimize treatment outcomes, and support the development of new therapies targeting inflammation in CKM Syndrome.
Strengths and limitations
This study highlights SII as a key biomarker for risk stratification in CKM Syndrome, supported by a large sample size and detailed analysis. It clarifies the complex relationships between systemic inflammation and CKM Syndrome components. However, the cross-sectional design limits causality and potential residual confounding remains due to unmeasured variables. The sample's geographical and demographic specificity may limit generalizability. Also, focusing solely on SII without comparing other biomarkers might overlook more predictive indicators. Despite these limitations, this research underscores systemic inflammation's role in CKM Syndrome and paves the way for future longitudinal studies.
Conclusions
This study elucidates the significant associations between the SII and various components of the CKM Syndrome, affirming the efficacy of SII as a key biomarker for assessing disease risk. Notably, it reveals a consistent linear relationship between SII and CKD, while demonstrating nonlinear associations with other components of CKM Syndrome. These findings suggest that elevated SII levels serve as an effective tool for screening and identifying individuals at high risk for CKM Syndrome.
Data availability
The NHANES data used for this analysis can be found at https://www.cdc.gov/nchs/nhanes.
Abbreviations
- BMI:
-
Body mass index
- CKD:
-
Chronic kidney disease
- CKM Syndrome:
-
Cardiovascular-kidney-metabolic syndrome
- CMS:
-
Cardiometabolic syndrome
- CI:
-
Confidence interval
- DBP:
-
Diastolic blood pressure
- DM:
-
Diabetes mellitus
- eGFR:
-
Estimated glomerular filtration rate
- FPG:
-
Fasting plasma glucose
- HDL-C:
-
High-density lipoprotein cholesterol
- MET:
-
Metabolic equivalent task
- NCEP-ATP III:
-
National cholesterol education program’s adult treatment panel III
- NHANES:
-
National health and nutrition examination survey
- OR:
-
Odds ratio
- PIR:
-
Poverty income ratio
- RCS:
-
Restricted cubic splines
- SBP:
-
Systolic blood pressure
- SCR:
-
Serum creatinine
- SII:
-
Systemic immune-inflammation index
- TG:
-
Triglycerides
- UACR:
-
Urinary albumin-to-creatinine ratio
References
Ndumele, C. E. et al. A synopsis of the evidence for the science and clinical management of cardiovascular-kidney-metabolic (CKM) syndrome: A scientific statement from the American heart association. Circulation 148(20), 1636–1664 (2023).
Rao Kondapally Seshasai, S. et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 364(9), 829–841 (2011).
Matsushita, K. et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375(9731), 2073–2081 (2010).
Navarro-González, J. F. et al. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 7(6), 327–340 (2011).
Ridker, P. M. et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N. Engl. J. Med. 342(12), 836–843 (2000).
Tonelli, M. et al. Biomarkers of inflammation and progression of chronic kidney disease. Kidney Int. 68(1), 237–245 (2005).
Libby, P., Ridker, P. M. & Hansson, G. K. Inflammation in atherosclerosis: From pathophysiology to practice. J. Am. Coll. Cardiol. 54(23), 2129–2138 (2009).
Attiq, A. et al. Hegemony of inflammation in atherosclerosis and coronary artery disease. Eur. J. Pharmacol. 966, 176338 (2024).
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444(7121), 860–867 (2006).
Zhao, X., Li, J. & Li, X. Association between systemic immune-inflammation index and psoriasis: A population-based study. Front. Immunol. 15, 1305701 (2024).
Xia, Y. et al. Systemic immune inflammation index (SII), system inflammation response index (SIRI) and risk of all-cause mortality and cardiovascular mortality: A 20-year follow-up cohort study of 42,875 US adults. J. Clin. Med. 12(3), 1128 (2023).
Zhang, K. et al. Systemic immune-inflammation index predicts prognosis of patients with advanced pancreatic cancer. J. Transl. Med. 17(1), 30 (2019).
Johnson, C. L. et al. National health and nutrition examination survey: Analytic guidelines, 1999–2010. Vital Health Stat. 2(161), 1–24 (2013).
Ndumele, C. E. et al. Cardiovascular-kidney-metabolic health: A presidential advisory from the American Heart Association. Circulation 148(20), 1606–1635 (2023).
Grundy, S. M. et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 109(3), 433–438 (2004).
Levey, A. S. et al. Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 67(6), 2089–2100 (2005).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150(9), 604–612 (2009).
Rattan, P. et al. Inverse association of telomere length with liver disease and mortality in the US population. Hepatol. Commun. 6(2), 399–410 (2022).
Zhang, Z. Multiple imputation with multivariate imputation by chained equation (MICE) package. Ann. Transl. Med. 4(2), 30 (2016).
Chalmers, L., Kaskel, F. J. & Bamgbola, O. The role of obesity and its bioclinical correlates in the progression of chronic kidney disease. Adv. Chronic Kidney Dis. 13(4), 352–364 (2006).
Wisse, B. E. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J. Am. Soc. Nephrol. 15(11), 2792–2800 (2004).
Mahemuti, N. et al. Association between systemic immunity-inflammation index and hyperlipidemia: A population-based study from the NHANES (2015–2020). Nutrients 15(5), 1177 (2023).
Roever, L. et al. High-density lipoprotein-cholesterol functionality and metabolic syndrome: Protocol for review and meta-analysis. Medicine (Baltimore) 97(24), e11094 (2018).
Welty, F. K. How do elevated triglycerides and low HDL-cholesterol affect inflammation and atherothrombosis?. Curr. Cardiol. Rep. 15(9), 400 (2013).
Monteiro, R. & Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010, 289645 (2010).
Jonkers, I. J. et al. Severe hypertriglyceridemia with insulin resistance is associated with systemic inflammation: Reversal with bezafibrate therapy in a randomized controlled trial. Am. J. Med. 112(4), 275–280 (2002).
Esser, N. et al. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 105(2), 141–150 (2014).
Yi, Q. et al. Associations of dietary inflammatory index with metabolic syndrome and its components: A systematic review and meta-analysis. Public Health Nutr. 24(16), 5463–5470 (2021).
Guo, W. et al. Systemic immune-inflammation index is associated with diabetic kidney disease in Type 2 diabetes mellitus patients: Evidence from NHANES 2011–2018. Front. Endocrinol. (Lausanne) 13, 1071465 (2022).
Putnam, K. et al. The renin-angiotensin system: A target of and contributor to dyslipidemias, altered glucose homeostasis, and hypertension of the metabolic syndrome. Am. J. Physiol. Heart Circ. Physiol. 302(6), H1219–H1230 (2012).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 112(12), 1821–1830 (2003).
Zhang, J. M. & An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 45(2), 27–37 (2007).
Feingold, K.R., Introduction to Lipids and Lipoproteins, in Endotext, K.R. Feingold, et al., Editors. 2000, MDText.com, Inc. Copyright © 2000–2024, MDText.com, Inc.: South Dartmouth (MA).
Feingold, K.R. and C. Grunfeld, The Effect of Inflammation and Infection on Lipids and Lipoproteins, in Endotext, K.R. Feingold, et al., Editors. 2000, MDText.com, Inc.
Koga, M. et al. Clinical evaluation of palliative chemotherapy with S-1 for oral cancer patients. Gan To Kagaku Ryoho 34(5), 719–723 (2007).
Donath, M. Y. & Shoelson, S. E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 11(2), 98–107 (2011).
Latif, W. et al. Uric acid levels and all-cause and cardiovascular mortality in the hemodialysis population. Clin. J. Am. Soc. Nephrol. 6(10), 2470–2477 (2011).
McNeil, C. J., Vandervoort, A. A. & Rice, C. L. Peripheral impairments cause a progressive age-related loss of strength and velocity-dependent power in the dorsiflexors. J. Appl. Physiol. (1985) 102(5), 1962–8 (2007).
Acknowledgements
We are grateful for the contributions of Zhang Jing from the Second Department of Infectious Disease, Shanghai Fifth People's Hospital, affiliated with Fudan University, for his dedication to the NHANES database. His exceptional efforts in developing the nhanesR package and accompanying website have significantly simplified our navigation and research within the NHANES database.
Author information
Authors and Affiliations
Contributions
C.G., S.G., R.Z., and P.S. were pivotal in conceptualizing the research and overseeing the design. Y.Y., X.Z., C.D., and C.G. spearheaded the data analysis and interpretation. H.N., L.Z., and Y.X. took on the responsibility of creating the figures and tables. M.L. and Z.X. drafted the initial version of the manuscript. Y.W., along with other contributors, reviewed and approved the final manuscript draft. C.Z. and H.Y., as the corresponding authors, ensured the integrity and accuracy of the work. The data used in this study are derived from the National Health and Nutrition Examination Survey (NHANES), which is publicly available and includes anonymized information intended for scientific research. Consent for publication of these data was obtained by the NHANES program, and all personal identifiers have been removed to protect individual privacy. No additional individual person’s data were used in any form (including individual details, images, or videos) in this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Gao, C., Gao, S., Zhao, R. et al. Association between systemic immune-inflammation index and cardiovascular-kidney-metabolic syndrome. Sci Rep 14, 19151 (2024). https://doi.org/10.1038/s41598-024-69819-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69819-0
- Springer Nature Limited