Abstract
To explore the favorable factors that help slow the progression of disease in patients with mild Cervical Spondylotic Myelopathy (CSM). A retrospective analysis was conducted, involving the enrollment of 115 CSM patients. The categorization of patients into two groups was based on the duration of symptoms, assessments using the mJOA scale and Health Transition (HT) scores: mild-slow group and severe-rapid group. We found that the patients in both groups had similar degrees of spinal cord compression, but mild-slow group were older and had smaller C2–C7 cobb angle (Flexion) (CL(F)), C2–C7 cobb angle (Range of motion) (CL(ROM)), Transverse area (TA), Normal-TA, Compressive spinal canal area (CSCA), Normal-Spinal canal area (Normal-SCA) and lower Spinal cord increased signal intensity (ISI) Grade than the severe-rapid group. A binary logistic regression analysis showed that CL(ROM) and Normal-TA are favorable factors to help slow the progression of disease patients with mild CSM. Through ROC curves, we found that when CL(ROM) < 39.1° and Normal-TA < 80.5mm2, the progression of disease in CSM patients may be slower. Meanwhile, we obtained a prediction formula by introducing joint prediction factor: L = CL(ROM) + 2.175 * Normal-TA. And found that when L < 213.0, the disease progression of patients may be slower which was superior to calculate CL(ROM) and Normal-TA separately.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Cervical spondylotic myelopathy (CSM) results from compression in the cervical region, including the vertebral bodies, intervertebral discs, ligaments, and facet joints. This compression can cause damage to the cervical spinal cord, leading to functional impairment of nerves. The consequences may manifest as sensory deficits in the limbs and trunk, as well as motor dysfunction1.
The course of CSM's natural history involves a gradual deterioration of both signs and symptoms as time progresses, yet the specific pace and pattern of this decline remain uncertain2,3. CSM progression is typically characterized by two primary patterns: (1) a gradual deterioration of function over time, and (2) a prolonged phase of consistent neurological function followed by a rapid decline3,4. Kadanka et al.5 evaluated the natural history of mild CSM treated either conservatively or surgically, and no distinction was observed in long-term functional outcomes among patients with mild cervical spondylotic myelopathy when comparing these therapies. However, Fehlings et al.6 found that for mild, moderate or severe CSM, surgical treatment can achieve a good prognosis.
Recently released clinical guidelines regarding the management of CSM propose the following recommendations7: (1) Patients exhibiting moderate to severe Cervical Spondylotic Myelopathy (CSM) with a modified Japanese Orthopaedic Association (mJOA) score of 14 or lower are advised to undergo surgical intervention. (2) Those with mild CSM, characterized by an mJOA score of 15 or higher, may be presented with the option of surgical intervention or a supervised trial of structured rehabilitation. Surgical consideration is warranted in the event of neurological decline or a lack of improvement following rehabilitation. However, the reason for the slow disease progression of patients with mild CSM is unclear. Therefore, the purpose of this study is to identify and explore the favorable factors that help slow the progression of disease in patients with mild CSM and determine whether the progression of the disease can be predicted by cervical imaging parameters.
Materials and methods
Ethics
Ethical approval for this study was provided by the Ethics Committee of Fujian Medical University Union Hospital on April 06, 2022. The ethical review number is 2022KY055.
Statement
We declare that we confirm that all methods were performed in accordance with relevant guidelines and regulations and that all experimental protocols have been approved by notified agencies and/or licensing committees. At the same time, we ensure that informed consent has been obtained from all subjects and/or their legal guardians.
Study participants
This retrospective study spanned from January 2017 to January 2020, involving the enrollment of 115 patients diagnosed with Cervical Spondylotic Myelopathy (CSM). Inclusion criteria comprised: (1) patients diagnosed with CSM based on clinical symptoms and imaging data; (2) individuals with fully visible and interpretable lateral cervical radiographs containing all relevant bone markers for accurate measurement; (3) subjects with comprehensive cervical MR images enabling precise measurement of spinal cord compression, muscle/fat volume in the adjacent vertebral body segment and MR T2-weighted spinal cord increased signal intensity (ISI)8; and (4) participants with well-documented records of Visual Analog Scale (VAS), modified Japanese Orthopedic Association (mJOA), Neck Disability Index (NDI) scores, and Health Transition (HT) scores. Exclusion criteria included: (1) a history of trauma or spinal surgery; (2) the presence of infection, tuberculosis, tumors, or other diseases; and (3) incomplete imaging or functional score data. Figure 1 provides a summary of excluded patients based on the inclusion criteria. According to the duration of symptoms, mJOA scores and HT scores, the individuals were separated into two categories: the mild-slow group (duration of symptoms > 1 year, mJOA > 14, HT ≥ 50; n = 48) and the severe-rapid group (duration of symptoms ≤ 1 year, mJOA ≤ 14; n = 67). Definition of duration of symptoms: the patient reported abnormal gait/limb weakness/decreased fine motor function/trunk paresthesia/bladder and intestinal dysfunction for more than 1 year (only pain and numbness were excluded). The Ethics Committee of Fujian Medical University Union Hospital granted approval for this study.
The basic data collection and parameters measured
(1) Basic data: Age, sex, BMI, smoking history, alcohol consumption history, number of lesion segments (Significant cord compression > Grade 2. The details can be found in Fig. 2)9, VAS scores, NDI, mJOA scores, and HT scores.
Smoking background: Individuals who smoked more than one cigarette daily for over six months or those who had a cumulative history of smoking more than 100 cigarettes. Alcohol Consumption History: Participants who engaged in drinking at least once a week for over six months.
The Visual Analog Score (VAS) was documented using an 11-point numeric rating scale, ranging from zero (indicating no pain) to ten (representing the worst imaginable pain). The Neck Disability Index (NDI) was employed to assess neck function. Patients underwent evaluations based on pain intensity, self-care capabilities, lifting capacity, reading proficiency, presence of headaches, concentration level, work performance, sleep quality, driving proficiency, and engagement in recreational activities. The total NDI score was set at 100, with higher scores reflecting poorer neck function.
The modified Japanese Orthopedic Association (mJOA) scale encompassed three categories: exercise, sensation, and bladder function, yielding a total score of 18 points. Health Transition (HT) scores, exclusive to the SF-36, served as the sole indicator for assessing overall health changes over the preceding year. The details of specific items and corresponding HT scores can be found in Fig. 3.
(2) Evaluation of cervical vertebral sagittal parameters in X-rays involved the assessment of C2–C7 Cobb angle (CL), T1 slope (T1S), C2–C7 sagittal vertical axis (SVA), and CL in Flexion (F), Extension (E), and Range of Motion (ROM). Standard radiographic techniques were employed for lateral cervical radiographs, with a tube-to-subject distance of 72 inches. The radiographic tube was centered at the C4–C5 intervertebral disc space without magnification. Patient positioning included a comfortable standing stance with natural placement of upper extremities at the sides and a straight-ahead gaze.
For the flexion (or extension) cervical radiograph, the patient assumed a sideways position in front of the camera frame. The head and neck were maximally flexed (or extended), aligning the long axis of the neck parallel to the film's long axis, while shoulders drooped as far as possible. The same imaging technician captured all images. Range of Motion (ROM) was calculated as the difference between extension and flexion. Measurement methods for cervical sagittal parameters are outlined in Table 1 and Fig. 4.
(3) Assessment of corresponding segmental paravertebral muscle degeneration on MRI included evaluation of up(low)-fat/muscle, up(low)-fat/centrum, and up(low)-muscle/centrum.
(4) Analysis of the compression degree of the cervical spinal cord and spinal canal in MRI involved parameters such as Maximum Spinal Cord Compression (MSCC), Maximum Canal Compromise (MCC), Compression Ratio (CR), Transverse Area (TA), Normal-TA, Transverse Area Ratio (TAR), Compressive Spinal Canal Area (CSCA), Normal-Spinal Canal Area (Normal-SCA), and Spinal Canal Area Ratio (SCAR).
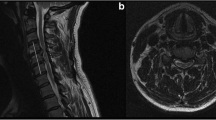
(5) In sagittal MR T2-weighted images, ISI at the narrowest spinal cord level was classified into: Grade 0, none; Grade 1, light (obscure); and Grade 2, intense (bright) (Table 1, Fig. 5), which was adopted by Machino et al.8. And Intense ISI was considered similar to the cerebrospinal fluid signal.
All registered participants underwent a 3.0 T MRI scan using Siemens Medical Solutions in Erlangen, Germany. The cross-sectional area (CSA) at each level was computed by delineating points in a polygonal shape around the outer boundaries of fat tissue, extensor muscles, and the vertebral body on the axial T2-weighted image (refer to Fig. 6). The extensor muscles encompassed multifidus and rotators, semispinalis capitis and cervicis, splenius capitis and cervicis, levator scapulae, and longissimus. Additionally, we determined the CSA ratio among fat, muscle, and centrum.
The measurement range is within the fan shape enclosed by the bilateral lines connecting the midpoint of the anterior vertebral body and the front vertices of the side blocks. A: Vertebral body; B: Levator scapulae; C: Splenius capitis and cervicis; D: Semispinalis capitus; E: Longissimus; F: Multifidus; G: Fat; Muscle area = B + C + D + E + F.
The measurements were confined to the fan-shaped region enclosed by two lines connecting the midpoint of the anterior vertebral body to the front vertices of the vertebrae's sides. The fat area was delineated by a green line, while the centrum was outlined by a red line. Various colors were employed to identify distinct muscles.
Further details on the methods can be found in Table 1, Figs. 6, and 7. In cases of multilevel cervical spondylotic myelopathy (CSM), we gauged the segment with the most pronounced spinal cord compression and measured the adjacent paravertebral muscles and fat corresponding to that specific segment.
Statistical analysis
DICOM data facilitated the transfer of all images to a computer. Two independent observers utilized imaging software (OsiriX Lite v7.5; Icestar Media Ltd, Essex, UK) to conduct measurements. Agreement between the observers was reached before two orthopedic spine surgeons independently measured each parameter twice. The intraclass correlation coefficient (ICC) was then analyzed. Intra- and interobserver agreement were evaluated using the ICC, where values between 0.8 and 1.0 were considered excellent, 0.6 to 0.79 were deemed good, and less than 0.6 were categorized as poor.
Statistical analyses were performed using SPSS 24.0, with significance determined at P < 0.05. The Shapiro–Wilk normality test was applied to continuous variables, expressing normally distributed ones as mean ± standard deviation and non-normally distributed ones as median (lower quartile ~ upper quartile). Intergroup analyses were conducted as follows: chi-square test for categorical variables, independent-sample t-test for normally distributed continuous variables, and Mann–Whitney U test for non-normally distributed continuous variables. Binary logistic regression analysis and ROC curves were employed to identify independent risk factors and critical values, respectively. And obtaining prediction formulas through joint prediction factors. Pearson and Spearman correlation coefficients were used to calculate the correlation between each parameter.
Reliability analysis
Concerning the parameters of the cervical spine, the reliability results for intraobserver and interobserver assessments demonstrated excellent and good agreement, respectively (ICC ≥ 0.8).
Results
Basic data
Between January 2017 and January 2020, a total of 115 individuals diagnosed with CSM were included in the study. They were divided into two groups according to the duration of symptoms, mJOA scores and HT scores: the mild-slow group (duration of symptoms > 1 year, mJOA > 14, HT ≥ 50; n = 48) and the severe-rapid group (duration of symptoms ≤ 1 year, mJOA ≤ 14; n = 67).
The mild-slow cohort comprised 48 individuals, consisting of 28 males (58.3%) and 20 females (41.7%), with an average age of 56.9 ± 11.3 years. The mean Body Mass Index (BMI) was 22.8 ± 3.0 kg/m2. There were 14 smokers (29.2%) and 12 alcohol drinkers (25.0%). The number of lesion segments: 9 single-level cases (18.8%), 19 two-level cases (39.6%) and 20 multilevel cases (41.6%). There were 22 patients (45.8%) in ISI Grade 0 group, 19 patients (39.6%) in ISI Grade 1 group and 7 patients (14.6%) in ISI Grade 2 group.
The severe-rapid group included 67 patients, 43 males (64.2%) and 24 females (35.8%), with an average age of 52.5 ± 10.2 years. The mean BMI was 23.2 ± 3.0 kg/m2. There were 27 smokers (40.3%) and 18 alcohol drinkers (26.9%). The number of lesion segments was as follows: 16 single-level cases (23.9%), 18 two-level cases (26.9%) and 33 multilevel cases (49.2%). There were 16 patients (23.9%) in ISI Grade 0 group, 37 patients (55.2%) in ISI Grade 1 group and 14 patients (20.9%) in ISI Grade 2 group.
The remaining basic data results are shown in Tables 2 and 3.
Comparison between the mild-slow group and the severe-rapid group
No noteworthy distinctions were observed in the fundamental data between the mild-slow and severe-rapid groups with respect to sex, BMI, smoking history, drinking history, number of lesion segments, VAS, or NDI (P > 0.05). However, a notable difference was found in age (P = 0.029) (Table 2).
There were no noteworthy distinctions in imaging parameters between the mild-slow group and severe-rapid group in terms of CL, T1S, C2-7SVA, CL(E), Upfat/muscle, Upfat/centrum, Upmuscle/centrum, Low-fat/muscle, Low-fat/centrum, Low-muscle/centrum, MSCC, MCC, CR, TAR, or SCAR (P > 0.05). However, significant differences were observed in CL(F), CL(ROM), TA, Normal-TA, CSCA, Normal-SCA and ISI Grade groups (P < 0.05) (Table 3).
Conducting binary logistic regression and ROC curve analysis to identify advantageous factors
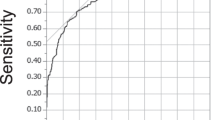
After a binary logistic regression analysis, we found that CL(ROM) and Normal-TA are favorable factors to help slow the progression of disease in patients with mild CSM (P < 0.05) (Table 4 and Fig. 8). To assess the impact of individual risk factors, ROC curves and critical value calculations were employed. Our findings indicate that a CL(ROM) less than 39.1° and a Normal-TA less than 80.5 mm2 may be associated with a slower progression of the disease in patients with CSM. Meanwhile, we obtained a prediction formula by introducing joint prediction factor: L = CL(ROM) + 2.175 * Normal-TA. And by calculating the critical value, we found that when L < 213.0, the disease progression of patients may be slower which was superior to calculate CL(ROM) and Normal-TA separately (Fig. 9).
Intragroup analysis results of the Mild-slow and Severe-rapid groups
The correlation between each parameter was determined using Pearson and Spearman correlation coefficients. Within the mild-slow category, there was a positive correlation observed between smoking history and both VAS and NDI scores. Additionally, CL(ROM) exhibited positive correlations with VAS and NDI scores, while displaying a negative correlation with mJOA scores. TAR was negatively correlated with mJOA scores. The remaining correlations are shown in Tables 5 and 6.
In the severe-rapid group, smoking history was correlated with VAS scores. The correlation analysis revealed a positive association between CL(ROM) and VAS as well as NDI scores, while a negative correlation was observed with mJOA scores. Normal-TA exhibited a negative correlation with mJOA scores, and TAR also showed a negative correlation with mJOA scores. Detailed information on the remaining correlations can be found in Tables 7 and 8.
Discussion
Cervical spondylotic myelopathy results in a gradual deterioration of the spinal cord, leading to the development of severe physical and social disabilities. This condition imposes a significant economic burden on both individuals and society10.
CSM, or cervical spondylotic myelopathy, is a prevalent degenerative condition arising post spinal surgery. It encompasses a range of clinical manifestations characterized by compromised spinal function resulting from prolonged compression and degeneration of the cervical spinal cord. Surgical decompression stands out as the most efficacious method for halting the progression of this disease6,11,12. However, considering the depth of research on CSM, an increasing number of experts believe that mild CSM can be treated conservatively5,7. Therefore, it is particularly important to determine the favorable factors that help slow the progression of mild CSM. In our study, we found that patients in the mild-slow group were elderly. On the one hand, we think that as the cervical spine chronically degenerates and age increases, the cervical spine reconstructs the stability of the cervical spine through self-regulation (such as hyperosteogeny, etc.), and the symptoms become chronic. On the other hand, it may also be that elderly patients have a stronger tolerance to spinal cord compression symptoms. Certainly, this requires additional high-quality research.
Cervical vertebral sagittal parameters, the degree of compression on the cervical spinal cord, MRI intramedullary hyperintensity signal have been widely used to evaluate the preoperative and postoperative conditions of patients with CSM1,8,13,14,15,16. However, these studies target all CSM patients, and studies specifically targeting mild CSM patients are still relatively rare. In order to clarify the potential factors related to disease progression in mild CSM patients more clearly, we grouped CSM patients based on symptom duration, HT score, and mJOA score, and concluded that for mild CSM patients, the small spinal canal and spinal cord may be favorable factors for their long-term stability. We think this may be because a smaller CL(F) and smaller CL(ROM) indicate that the cervical spine is in a more stable state; that is, the relative stability of the cervical spine may be more conducive to the relatively slow progression of symptoms. However, our finding is inconsistent with the report by Nicholson13. Nicholson KJ found that when CL(F) and CL(ROM) were greater, patients with CSM experienced less severe symptoms13. We think this may be because Nicholson's findings included the entire population of patients with CSM and directly analyzed the correlation between mJOA scores and the relevant parameters without considering other additional factors, such as the duration of symptoms and the rate of disease progression.
In recent times, a growing number of academics have come to recognize the significance of cervical paraspinal muscles in the biomechanics of the cervical spine1,17,18,19. In our study, we found that the degree of paravertebral muscle degeneration at the corresponding segment was not correlated with the severity of spinal cord compression symptoms or the progression of the disease, which was consistent with our previous studies1.
Observing the compression degree of the spinal cord by magnetic resonance imaging (MRI) is a vital way to determine the state of CSM patients20. In addition to the routine measurement of MSCC, MCC, TA and CR, we also proposed Normal-TA and TAR, and to determine the occupied space of the spinal canal, we proposed Normal-SCA and SCAR. In our study, we found that there was no significant difference in the degree of spinal cord compression between the two groups, which means that both the mild-slow group and the severe-rapid group had similar degrees of spinal cord compression. However, there are anatomical differences between the two groups; that is, for patients with mild CSM, a small spinal canal and small spinal cord may be favorable factors for their long-term stability. Finally, a binary logistic regression analysis showed that CL(ROM) and Normal-TA are favorable factors to help slow the progression of disease in patients with mild CSM. Through ROC curves and the calculation of critical values, we found that when CL(ROM) < 39.1° and Normal-TA < 80.5 mm2, the progression of disease in CSM patients may be slower. Meanwhile, we obtained a prediction formula by introducing joint prediction factor: L = CL(ROM) + 2.175 * Normal-TA. And found that when L < 213.0, the disease progression of patients may be slower which was superior to calculate CL(ROM) and Normal-TA separately.
Spinal cord increased signal intensity (ISI) on T2-weighted is considered to be significantly correlated with the prognosis of CSM patients. Myelopathic changes often appear as hyperintense areas on T2-weighted images (T2-WI). When these changes have well-defined borders, they are suggested to represent necrosis, microcavities, or spongiform alterations. Conversely, when the signal changes are diffuse, they may indicate edema, demyelination, or Wallerian degeneration15. Machino et al.8 and Kato et al.16 found that the relief and resolution of postoperative ISI in CSM patients reflect better relief of postoperative symptoms and more satisfactory surgical outcomes. In our study, we used the grouping method adopted by Machino et al. to classify the ISI on T2-weighted into three groups and found that the patients of the mild-slow group had lower ISI Grade than the severe-rapid group (P < 0.05). This is consistent with the research of the aforementioned scholars, further demonstrating the important predictive significance of ISI in patients with CSM.
The Health Transition (HT) scores within the SF-36 serve as the exclusive metric for evaluating health changes, providing an assessment of overall health alterations over the preceding year. HT has been applied to the disease progression of patients with CSM in previous studies21. To determine the rate of disease progression in patients more objectively, we also used the HT score for grouping. Nevertheless, our investigation is accompanied by certain limitations and unresolved issues that warrant additional exploration. Initially, the study's scope is constrained by a limited number of cases, all originating from a singular center. This insufficient case pool prevents the formulation of conclusive findings. Additionally, the retrospective nature of this analysis highlights the necessity for prospective studies to elucidate the association between a diminutive cervical spinal cord or spinal canal and decelerated disease progression. Furthermore, exploring the cervical sagittal parameters in patients with mild CSM requires dedicated investigation through prospective studies.
Conclusion
For patients with mild CSM, a small spinal canal and small spinal cord may be favorable factors for their long-term stability. Meanwhile, smaller CL(F) and CL(ROM) are also conducive to the long-term stability of the disease. And the prediction formula: L = CL(ROM) + 2.175 * Normal-TA can be obtained. When L < 213.0, the disease progression of patients may be slower.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Lin, T. et al. Predictive effect of cervical spinal cord compression and corresponding segmental paravertebral muscle degeneration on the severity of symptoms in patients with cervical spondylotic myelopathy. Spine J. 21(7), 1099–1109 (2021).
Lebl, D. R. et al. Cervical spondylotic myelopathy: Pathophysiology, clinical presentation, and treatment. HSS J. 7(2), 170–178 (2011).
Aljuboori, Z. & Boakye, M. The natural history of cervical spondylotic myelopathy and ossification of the posterior longitudinal ligament: A review article. Cureus. 11(7), e5074 (2019).
Matz, P. G. et al. The natural history of cervical spondylotic myelopathy. J Neurosurg Spine. 11(2), 104–111 (2009).
Kadanka, Z. et al. Approaches to spondylotic cervical myelopathy: Conservative versus surgical results in a 3-year follow-up study. Spine (Phila Pa 1976). 27(20), 2205–2210 (2002).
Fehlings, M. G. et al. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: Results of the AOSpine North America prospective multi-center study. J. Bone Joint Surg. Am. 95(18), 1651–1658 (2013).
Fehlings, M. G. et al. A clinical practice guideline for the management of patients with degenerative cervical myelopathy: Recommendations for patients with mild, moderate, and severe disease and nonmyelopathic patients with evidence of cord compression. Global Spine J. 7(3 Suppl), 70S-83S (2017).
Machino, M. et al. Postoperative resolution of MR T2 increased signal intensity in cervical spondylotic myelopathy: The impact of signal change resolution on the outcomes. Spine (Phila Pa 1976). 44(21), E1241–E1247 (2019).
Lee, S. H., Kim, K. T., & Suk, K. S., et al. Asymptomatic cervical cord compression in lumbar spinal stenosis patients: A whole spine magnetic resonance imaging study. Spine (Phila Pa 1976). 35(23), 2057–2063 (2010).
Nouri, A., Tetreault, L., Singh, A., et al. Degenerative cervical myelopathy: Epidemiology, genetics, and pathogenesis. Spine (Phila Pa 1976). 40(12), E675–E693 (2015).
Fehlings, M. G. & Arvin, B. Surgical management of cervical degenerative disease: The evidence related to indications, impact, and outcome. J. Neurosurg. Spine. 11(2), 97–100 (2009).
Moussellard, H. P. et al. Early neurological recovery course after surgical treatment of cervical spondylotic myelopathy: A prospective study with 2-year follow-up using three different functional assessment tests. Eur. Spine J. 23(7), 1508–1514 (2014).
Nicholson, K. J. et al. Cervical sagittal range of motion as a predictor of symptom severity in cervical spondylotic myelopathy. Spine. 43(13), 883–889 (2018).
Tang, J. A. et al. The impact of standing regional cervical sagittal alignment on outcomes in posterior cervical fusion surgery. Neurosurgery. 71(3), 662–669 (2012).
Nouri, A. et al. Role of magnetic resonance imaging in predicting surgical outcome in patients with cervical spondylotic myelopathy. Spine. 40(3), 171–178 (2015).
Kato, S. et al. Postoperative resolution of magnetic resonance imaging signal intensity changes and the associated impact on outcomes in degenerative cervical myelopathy: Analysis of a global cohort of patients. Spine. 43(12), 824–831 (2018).
Elliott, J. M. et al. The rapid and progressive degeneration of the cervical multifidus in whiplash: An MRI study of fatty infiltration. Spine. 40, E694-700 (2015).
Tamai, K. et al. The impact of cervical spinal muscle degeneration on cervical sagittal balance and spinal degenerative disorders. Clin. Spine Surg. 32, E206–E213 (2019).
Snodgrass, S. J. et al. Cervical muscle volume in individuals with idiopathic neck pain compared to asymptomatic controls: A cross-sectional magnetic resonance imaging study. Musculoskelet. Sci. Pract. 44, 102050 (2019).
Nouri, A. et al. The relationship between preoperative clinical presentation and quantitative magnetic resonance imaging features in patients with degenerative cervical myelopathy. Neurosurgery. 80(1), 121–128 (2017).
Lin, T. et al. Is cervical sagittal balance related to the progression of patients with cervical spondylotic myelopathy?. World Neurosurg. 137, e52–e67 (2020).
Author information
Authors and Affiliations
Contributions
TL: Lead the writing of the original draft of the project and responsible for overall project management, including resource allocation and progress tracking. ZY: Offer innovative suggestions and strategic guidance during the conceptualization phase and contribute to the initial drafting of project content. ZX: Responsible for collecting and organizing relevant data to provide reliable research support. RW, YZ and DC: Assist in developing formal analysis methods and standards to ensure accuracy and completeness. LZ and ZW: Lead the investigation phase, ensuring the project is based on thorough facts and information and responsible for reviewing and editing written content. WL: Lead the overall conceptualization of the project, defining its core ideas and goals, provide professional guidance and support in methodology to ensure the team works according to specified methods and participate in the review and editing process, ensuring the quality and coherence of the final written material. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lin, T., Yao, Z., Xiao, Z. et al. Identifying and exploring the favorable factors that help to slow the progression of disease in patients with mild cervical spondylotic myelopathy. Sci Rep 14, 18986 (2024). https://doi.org/10.1038/s41598-024-69899-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-69899-y
- Springer Nature Limited