Abstract
Staphylococci are responsible for a wide range of infections in animals. The most common species infecting animals include Staphylococcus aureus, Staphylococcus epidermidis and Staphylococcus intermedius. Recent increases in antibiotic use and antibiotic resistance in animals highlight the need to understand the potential role of commercial livestock as a reservoir of staphylococci and antibiotic resistance genes. Nasal swabs were collected from 143 apparently healthy pigs and 21 pig farm workers, and 45 environmental swabs of feed and water troughs, from two commercial pig farms in the Western Cape, South Africa. Staphylococci were isolated, identified using mass-spectrometry, and antimicrobial susceptibility testing and Illumina whole genome sequencing were performed. One hundred and eighty-five (185) Staphylococcus spp. isolates were obtained, with Mammalicoccus sciuri (n = 57; 31%) being the most common, followed by S. hyicus (n = 40; 22%) and S. aureus (n = 29; 16%). S. epidermidis was predominantly identified in the farm workers (n = 18; 86%). Tetracycline resistance was observed across all species, with rates ranging from 67 to 100%. Majority of M. sciuri isolates (n = 40; 70%) were methicillin resistant, with 78% (n = 31) harbouring mecA. M. sciuri isolates had genes/elements which were associated with SCCmec_type_III (3A) and SCCmec_type_VIII(4A) and were mostly observed in ST61 strains. ST239 strains were associated with SCCmec_type_III(3A). High rates of tetracycline resistance were identified among staphylococci in the pig farms in Western Cape, South Africa. This highlights the need for policy makers to regulate the use of this antibiotic in pig farming.
Similar content being viewed by others
Introduction
Staphylococci are commensals and opportunistic pathogens in animals, responsible for a wide range of infections such as mastitis in cows and exudative epidermitis in pigs1. The common species colonizing and infecting animals include Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus intermedius, Staphylococcus xylosus, Staphylococcus chromogenes, Staphylococcus hyicus, Staphylococcus hominis, and Staphylococcus haemolyticus2,3.
Much of the research on staphylococci in animals has focused on S. aureus due to its importance as a human pathogen. S. aureus mostly causes mastitis in cattle and pyoderma in dogs and cats4,5, while pigs are mostly asymptomatic carriers6. A review on S. aureus in animals and food in Africa suggests that the prevalence of methicillin susceptible S. aureus (MSSA) in animals ranges from 3 to 58% depending on the type of animal and the source of sample2. The colonization of animals by methicillin resistant S. aureus (MRSA) in Africa was found to be very low, ranging from 0 to 3%2. Livestock-Associated Methicillin-Resistant Staphylococcus aureus (LA-MRSA) is an emerging pathogen globally. The average carriage rate of LA-MRSA in European countries is 14% in pig breeding farms and 26.9% in pig production farms7. A typical LA-MRSA strain is sequence type (ST) 398, which has been reported to cause infections in humans, particularly in individuals with frequent contact with animals8. Recently, ST9 has been reported to be an emerging LA-MRSA strain in Asia9 There have also been reports of human to animal transmission of S. aureus across the globe7,10,11.
Little is known about the prevalence and epidemiology of Staphylococcus other than S. aureus (SOSA), especially in Africa3. A recent systematic review on SOSA in domestic animals and livestock in Africa showed that most research on SOSA in animals has focused on cattle (32/75 studies)3. However, pigs are one of the most common livestock globally and in South Africa12,13. Staphylococci other than S. aureus such as S. haemolyticus have been reported in pigs from Nigeria14 and South Africa15. Only a few cases of SOSA transmission between humans and animals have been reported globally16,17. However, there are few studies describing zoonotic and reverse zoonotic transmission on pig farms in Sub-Saharan Africa9.
In South Africa, the pig population was estimated at 1.357 million for the year 2020, and the Western Cape province accounts for 11% of South Africa’s pig production13. Considering the high usage of antibiotics, especially tetracycline, in pig production in South Africa18, the lack of data on antibiotic resistance in pig-associated SOSA globally and in South Africa is of concern. Similarly, there is little information on the population structure and antibiotic resistance mechanisms in LA-SOSA in South Africa. Therefore, there is a need to understand the potential role of pig farms as a reservoir of staphylococci and antibiotic resistance genes19.
This study describes the species distribution, population structure and antibiotic resistance mechanisms amongst staphylococci from pigs, their caregivers, and the pig farm environment in the Western Cape, South Africa.
Methods
Study setting
The study was conducted at two commercial pig farms (Farm A and Farm B) in the Western Cape Province of South Africa; both farms have herd sizes of between 500 and 1000 pigs. Farm A is a high health pig farm while Farm B is a standard health farm. High health farms have strict biosafety measures in place to limit the introduction and spread of infectious diseases. These include strict access control and regular disease monitoring and surveillance. Standard health farms have less stringent biosafety measures in place.
Study Population and sample collection
The study population consisted of the pigs on the farms as well as farm workers. Samples were collected from all farm workers who have direct or indirect contact with the pigs and gave consent. Bio-Cult Amies transport swabs with charcoal (Liofilchem, Italy) were used to obtain nasal swabs from farm workers (self-collected from the anterior nares), as well as for sampling the nostrils of the pigs. At least 10 pigs were sampled per pen/holding on each farm. Environmental samples were collected from feed, water troughs and sewage at each farm. Demographic data such as age, sex, antibiotic use and living conditions were collected from the farm workers. The farm managers provided information on antibiotic use and hygiene practices on the farms (Supplementary S1).
Isolation and identification of staphylococci
The swabs were stored at 4 °C for ≤ 4 days before analysis. The swabs were inoculated in 2 ml Lysogeny Broth and incubated aerobically for 6 h at 37 °C to promote the growth of staphylococci. The broth was then sub-cultured onto 5% sheep blood agar plates supplemented with 10 μg/ml each of nalidixic acid and colistin (Thermofisher, USA) to suppress the growth of gram-negative bacteria. Isolates were selected based on a mostly creamy colony morphology consistent with staphylococci. Multiple isolates were collected from each individual, an approach that enabled us to describe the distribution of different staphylococcal species colonizing the pigs and the farm workers.
Presumptive staphylococci were further confirmed based on a negative bile esculin agar test and a positive catalase test (both from NHLS Media Laboratory, Greenpoint, South Africa), and identified using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF/MS; VITEK® MS, BioMérieux, France).
Antibiotic susceptibility testing
Antibiotic susceptibility testing (AST) was performed on S. aureus isolates and the most common SOSA species using Kirby Bauer disc diffusion. The following antibiotic discs (MAST Group, UK) were used: cefoxitin (30 µg), clindamycin (2 µg), erythromycin (15 µg), rifampicin (5 µg), linezolid (30 µg), trimethoprim/sulfamethoxazole (1.25/23.75 µg), ceftaroline (30 µg), chloramphenicol (30 µg), fusidic acid (10 µg), levofloxacin (5 µg), and tetracycline (30 µg). Cefoxitin (30 µg) was used to determine oxacillin susceptibility. Vancomycin susceptibility testing was performed using vancomycin-supplemented (6 µg/ml) brain heart infusion media (NHLS Media Laboratory).
Susceptibility testing results were interpreted using the Clinical and Laboratory Standards Institute (CLSI) guidelines, 2020 for staphylococci; but where available, species specific breakpoints were used, such as for S. aureus and S. epidermidis20. The term non-susceptible used in this study refers to the CLSI guidelines for resistant and intermediately resistant staphylococci. Multidrug resistance (MDR) is defined as non-susceptibility to at least one agent from at least three different antibiotic classes21.
DNA extraction and whole genome sequencing
Whole genome sequencing was performed at the Centre for Epidemic Response and Innovation, Stellenbosch University, using Illumina DNA Library Prep kits and the NextSeq 2000 (Illumina). Raw sequence reads were automatically generated, checked for quality, trimmed, and de novo assembled using FastQC v0.11.7, Trimmomatic v0.39 and SPAdes v3.15.5 respectively22,23,24. The assembled genomes were submitted to rMLST (https://pubmlst.org/species-id) for species identification25. Genomes showing matches to multiple staphylococcal species were further examined using EZBioCloud2 for average nucleotide identity (ANI), with identities assigned based on ≥ 95% ANI26.
Prokka v1.14.5 was used for genome annotation for common staphylococcal species27,28. PubMLST (https://pubmlst.org/organisms) and SCCmecFinder (https://cge.cbs.dtu.dk/services/SCCmecFinder/) were used to predict sequence types (ST) and SCCmec types, respectively. Resistance genes were identified using Resistance Gene Identifier (RGI) v6.0029.
Statistical analysis
The minimum number of samples to be collected (sample size) was estimated to be 163 using a 95% confidence level, an expected methicillin resistance prevalence of 12%19 and desired precision of 5%30 in order to describe the prevalence of methicillin-resistant Staphylococcus spp. Differences in the prevalence of SOSA species and antibiotic resistance rates between the two farms were calculated using Fisher exact and Chi squared tests where appropriate. Microsoft Excel was used for the analysis and p-values of < 0.05 were considered significant.
Ethical considerations
Ethical approval was obtained from the Research Ethics Committee at Stellenbosch University. Informed consent was obtained from the farm workers and managers. The study was approved by the South African Department of Agriculture, Land Reform and Rural Development.
The study was conducted in accordance with the Declaration of Helsinki (2013), as well as South African and ICH GCP Guidelines and the Ethical Guidelines of the Department of Health. The study also observed relevant regulations by the South African Department of Agricultural, Land Reform and Rural development.
Results
On farm A, nasal swabs were collected from nine (9) farm workers and 73 pigs, and 22 environmental samples were taken; while on farm B, nasal swabs were taken from 12 farm workers and 69 pigs, and 23 environmental swabs were collected.
In total, 142 pigs were sampled, with ages ranging from 7 to 35 weeks. All pigs on farm A were finishers aged 25–30 weeks while in farm B, 45 (34.5%) pigs were finishers, 10 weaners aged 7–14 weeks, and nine piglets aged 3–5 weeks (Supplementary S2). Both farms have concrete flooring and engage in the following practices: use commercial food, use antibiotics to prevent infections, health check animals at least every three months, diagnose the animals using farm workers and a veterinarian, and impregnate pigs using artificial insemination. Twenty-one (21) farm workers were included of whom 71% (n = 15) were male. The time workers spent on the farms ranged from 6 to 12 h per day (Supplementary S3).
Distribution of Staphylococci
Ninety six of the 142 pigs (68%) were colonised with any Staphylococcus species. Mammaliicoccus sciuri (previously Staphylococcus sciuri) was the commonest (n = 47; 33%), followed by S. hyicus (n = 37; 26%) and S. aureus (n = 15; 11%) (Table 1). Additionally, 20 of the 21 farm workers (95%) were colonised with any Staphylococcus spp., with S. aureus (n = 6; 29%) and S. epidermidis (n = 6; 29%) being the commonest. One or more staphylococcal species were isolated from 24 of the 45 environmental samples (53%): M. sciuri (n = 9; 20%), S. aureus (n = 8; 18%), S. hyicus (n = 7; 16%), S. haemolyticus (n = 3; 7%), S. chromogenes (n = 2; 4%), and S. epidermidis (n = 2; 4%).
The prevalence of any staphylococcus was higher on farm A compared to farm B (p = 0.008), however there were no statistically significant differences in the distribution of specific staphylococcal species between farms. This might be due to the small sample size. S. epidermidis was most common amongst farm workers (86%; n = 18) while M. sciuri (33%; n = 47) and S. hyicus (26%; n = 37) were common in pigs. S. aureus, and M. sciuri were common species isolated in the pigs, the farm workers and the environment.
Antibiotic Susceptibility of SOSA
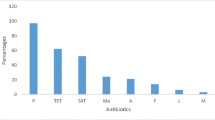
In total, 185 isolates were subjected to AST, and all (100%) were susceptible to chloramphenicol, linezolid, rifampicin, and vancomycin. All S. chromogenes, S. hyicus, and S. haemolyticus isolates were susceptible to oxacillin. All M. sciuri and S. chromogenes isolates were non-susceptible to clindamycin, and all S. chromogenes isolates were non-susceptible to tetracycline.
In isolates from the pigs, the rate of non-susceptibility to erythromycin ranged from 51 to 100%, to clindamycin from 59 to 100%, and to tetracycline from 93 to 100% between species (Table 2).
A high proportion of the farm workers (n = 12; 57%) were also colonized by tetracycline non-susceptible S. epidermidis. Although only a small number of staphylococcal isolates were isolated from the environment, all isolates of all species were non-susceptible to tetracycline. Supplementary S4 shows the proportion of antibiotic resistance in SOSA isolated from farm workers and the environment.
Whole genome sequence analysis of Staphylococcus spp.
The WGS of four S. aureus and one S. hyicus isolates had insufficient data and were excluded from further analysis, leaving 181 analysable isolates. Speciation of all isolates was confirmed by WGS analysis, except 3 of the 15 S. haemolyticus isolates which were identified as Staphylococcus borealis by WGS. The average nucleotide identity (ANI) of the three isolates was ≥ 95% when compared to S. borealis and ≤ 91% when compared to S. haemolyticus.
Identification of antibiotic resistance genes
The penicillin resistance gene blaZ was common in both S. aureus (83%) and SOSA: S. epidermidis (90%), S. chromogenes (78%), S. hyicus (45%), and S. haemolyticus (20%) (Supplementary S5). The blaZ gene was absent in all M. sciuri, however, the methicillin resistance gene mecA was more frequent in M. sciuri (54%) than other SOSA (20%). Of the 40-methicillin resistant M. sciuri isolates, 31 (78%) harboured mecA. Only one S. epidermidis was methicillin resistant and harboured the mecA gene. Only one methicillin susceptible S. aureus harboured mecA. A single MRSA isolate had neither mecA or mecC.
Although the majority of isolates across all species were non-susceptible to clindamycin, only seven M. sciuri and two S. hyicus harboured either inuA or inuG. The erythromycin resistance gene ermC was observed in S. aureus (23%) and across all SOSA species: S. hyicus (33%), M. sciuiri (33%), S. chromogenes (26%), S. epidermidis (14%), and S. haemolyticus (13%). The tetracycline resistance gene tet(K) was also observed in S. aureus (46%) and across all SOSA species: S. epidermidis (67%), S. haemolyticus (67%), S. chromogenes (39%), S. hyicus (33%) and M. sciuiri (12%). Other tetracycline resistance genes such as tet(L), tet(M), tet(T) and tet(45) were identified in the other isolates that were phenotypically tetracycline non-susceptible.
Sequence typing and staphylococcal cassette chromosome mec typing
ST1 was the most common sequence type amongst S. aureus (n = 10; 61%) in farm A, while ST9 and ST398 (n = 3; 27% each) and one ST508 were identified in farm B (Supplementary S6). Six additional S. aureus sequence types were identified on the farms, including a novel ST, ST8620 (n = 3; 27%) on farm B (Supplementary S6).
ST61 (n = 11; 31%) was the most common sequence type amongst M. sciuri in farm A and ST239 (n = 15; 68%) in farm B. Other sequence types identified in M. sciuri included eight novel sequence types. All ST61 and ST239 strains harboured mecA. Among the other SOSA species, there was a diversity of STs, with a number of novel STs identified in all species; specifically, all the STs amongst the S. chromogenes isolates were novel. In total, 29 novel sequence types were identified in this study (Supplementary S6). There is no MLST scheme for S. hyicus.
Only SCCmec_type_II(2A) and SCCmec_type_V(5C2) were identified in S. aureus (farm A) and S. epidermidis (farm B) respectively. On farm A, 29% of M. sciuri isolates had genes/elements which were associated with SCCmec_type_III (3A) & SCCmec_type_VIII(4A) and were mostly observed in ST61 strains. On both farms all ST239 strains were associated with SCCmec_type_III(3A) only.
Discussion
This study describes the species distribution, antibiotic resistance profiles and strain types in Staphylococcus spp. from two commercial pig farms in the Western Cape, South Africa. The staphylococcal isolates from this study are carriage isolates from asymptomatic animals, farm workers and environmental isolates.
There was high diversity of staphylococcal species identified, with M. sciuri and S. hyicus as the most frequently isolated species from pigs. Certain species of Staphylococcus, most commonly S. hyicus, are known to cause exudative epidermitis in pigs31. Although only a small number of staphylococci were isolated from farm workers and the environment, 18 of the 21 S. epidermidis were from humans. This is similar to a reports in literature that S. epidermidis is the most common SOSA colonizing healthy individuals32. This might be an indication that farm workers were colonized outside of the farm setting and that there is a limited transmission of S. epidermidis from workers to the pigs.
The rate of colonisation with S. aureus in the pigs (11%) is similar to what has been reported elsewhere in South Africa (12%) but higher than reports from Nigeria (5%) and Tanzania (4%)19,33,34. The rate of S. aureus identified in the farm workers (28%), the environment (17%) and the pigs (11%) confirms what has been described in literature that up to 30% of the human population are asymptomatic carriers of S. aureus and are permanently colonised35. The proportion of S. aureus isolates identified in this study also agrees with a systematic review conducted in Africa which reported that the rate of S. aureus in pigs ranges from 0 to 55%36. However, data on the environment is lacking.
The most common SOSA identified in pigs were M. scuiri (33%) and S. hyicus (26%). The prevalence of M. sciuri was comparable to a study from Denmark conducted using nasal samples from pigs (40%)37. A recent study in Italy also identified M. sciuri as a predominant SOSA species in five pig farms38. In contrast, prevalence of S. hyicus in this study was higher than in pigs in studies from Denmark (16%) and Uganda (6%)37,39. This is concerning since S. hyicus is known to cause exudative epidermidis in pigs40. However, further research is required to understand the high rate of S. hyicus colonisation in the pigs in our setting. Although, no S. hyicus was identified in humans, the three isolated from the environment could be an indication of dissemination from pigs into the environment.
The prevalence of S. aureus including MRSA in pigs in Africa ranges from 0 to 55%41. In this study, only one S. aureus (7%) isolate was methicillin resistant. The rate observed in this study is surprisingly lower than a previous report from South Africa by Van Lochem et al.,19. The high rate of resistance to erythromycin (80%), clindamycin (100%) and tetracycline (93%) in S. aureus in this study and the high erythromycin (89%), clindamycin (89%) and tetracycline (89%) resistance rates seen in isolates from the environment might be indicative of spread from pigs to the environment. The rate of erythromycin, clindamycin and tetracycline resistance among pigs in this study is higher than that described in a review conducted by Katakweba et al., describing antibiotic-resistant S. aureus in pigs in Africa and a nationwide pig study in Switzerland42. Interestingly, only one S. aureus isolate from the farm workers was non-susceptible to the above antibiotics, which suggests that transfer of resistant organisms from animals to humans is uncommon. It is also not clear what factors may influence the risk of zoonotic transfer.
While S. aureus is the most studied staphylococcal species, there is limited data on antibiotic resistance in SOSA globally and in Africa. The rate of methicillin resistance in M. sciuri isolates in this study (72%) is lower than what has been reported in Italy (88%)38 and in broilers in Turkey (100%)43. Clindamycin, erythromycin and tetracycline resistance rates of greater than 90% were observed in M. sciuri, consistent with the 99% clindamycin resistance rate reported in M. sciuri from Belgium but higher than the rates of erythromycin (52%) and tetracycline (62%) resistance reported by the same Belgian study44. The tetracycline resistance rate was also higher than the 29% reported in Brazilian pigs in 201245. All M. sciuri isolates from the environment were resistant to clindamycin, erythromycin, and tetracycline. This suggests that M. sciuri might be a reservoir of antibiotic resistance genes, and may also indicate movement of isolates from pig to environment (or vice versa).
The high tetracycline resistance rates seen in this study may reflect the use of this antibiotic in pigs in South Africa46. Although we could not obtain data on antibiotic usage from the farms in this study, national surveillance data shows that tetracycline is used in large amounts in animals in South Africa; 45% of antimicrobial sales in livestock industry in 2020 were tetracyclines47. A recent study by Ocloo et al. showed that 39% of S. haemolyticus carriage isolates were resistant to tetracycline, suggesting potential spillage from pigs or livestock48.
A study including three countries in Europe showed that 59% of S. hyicus from Danish pigs were erythromycin resistant which was the highest in Europe49, and is similar to the 51% reported in this study. The rate of clindamycin resistance was 59% among S. hyicus isolates, a similar proportion (53%) was reported in diseased pigs in Europe49. Clindamycin is used in both clinical and veterinary medicine, making the 100% resistance rate among M. sciuri isolates particularly concerning. The primary cause of clindamycin resistance in M. sciuri is the presence of the sal(A) gene. This gene has been demonstrated to provide intrinsic resistance to both lincosamides and streptogramin A in M. sciuri50. In South Africa, fusidic acid is an antistaphylococcal agent used in the treatment of infections due to staphylococci, it is worrying that 91% of M. sciuri isolates were resistant to the antibiotic.
There was only a 60% correlation between phenotypic methicillin resistance and the presence of the mecA gene in SOSA. Additionally, the mecA gene was observed in only one methicillin susceptible S. aureus. No other mec genes were detected in the one MRSA isolate. Similarly, none of the methicillin resistant S. aureus isolated from pigs in Tanzania harboured mecA33. In S. epidermidis methicillin resistance was only seen in one isolate and it harboured mecA. In our study 78% of methicillin resistant M. sciuri isolates harboured mecA. The poor correlation observed in our study between phenotypic methicillin resistance and the presence of mecA in SOSA confirms challenges in using cefoxitin for screening for methicillin resistance in SOSA, as described by Yang et al.51 and Humphries et al.52.
The tetracycline resistance gene tetK is the most common gene identified in tetracycline resistant staphylococcal species isolated from pigs globally53, and this study also identified tetK as the common gene across all staphylococcal species. The tetK gene has been reported to be plasmid-mediated43, and this increases the risk of spread into clinical settings. The erythromycin resistance gene ermC is the most common gene identified in erythromycin resistant staphylococcal species isolated from pigs globally, and was also identified across all staphylococcal species in this study54. The high clindamycin resistance rate seen in this study, in the absence of clindamycin resistance genes, might be due to the expression of erm genes55.
Some typical human-associated S. aureus strains are also found in animals; an example is the ST1 strain56. In this study, 53% of S. aureus isolates from pigs belonged to ST1, this shows a possible human-animal contamination on the farms or that ST1 has become animal-adapted. This strain has been reported to cause infections in cattle and pigs in Europe57,58. The identification of ST398 which is the most common livestock associated strain and a novel ST8620 in pigs, humans and the environment might be an indication of a possible transmission between the selected ecological niches on the farm.
M. sciuri and S. hyicus are the two most common SOSA identified in this study. The predominance of M. sciuri ST61 and ST239 on farm A and farm B respectively, is evidence of clonal spread of different STs within the two farms. ST239 and ST61 are the most common sequence types identified amongst M. sciuri, and although these strains are not newly assigned in this study, there are no reports in literature. This demonstrates the lack of published data on the strain typing of SOSA. There is no MLST scheme available for S. hyicus, restricting their characterisation.
M. sciuri was the only species in this study with diversity of SCCmec types; S. aureus and S. epidermidis harboured one SCCmec type each. SCCmec type III was the commonest among the methicillin resistant M. sciuri isolates. This is consistent with numerous studies where SCCmec type III has been the most commonly identified SCCmec type in M. sciuri from both animals and humans44,59,60. It has been hypothesized that SCCmec type III originated in M. sciuri, and may have been the first SCCmec cassette to be assembled, possibly by a recombination event in a human host or a human-created environment, and later was transferred to S. aureus61.
Conclusion
The study identified a diversity of staphylococcal species in pigs and high rates of tetracycline resistance in pig farms in South Africa, with some evidence of possible sharing of strains and antibiotic resistance genes between pigs and humans. This highlights the need for policy makers to regulate the use antibiotics, and specifically tetracycline in pig farming to reduce the burden of AMR infections in the clinical sector in South Africa.
The study highlights the need for further research on antibiotic resistance and strain typing in SOSA in animals globally and in South Africa, which can help in designing effective antibiotic resistance surveillance and control strategies in animal husbandry.
Data availability
Sequence data that support the findings of this study have been deposited in the National Centre for Biotechnology Information with the primary accession code Bioproject; PRJNA1018240.
References
Phophi, L., Petzer, I. M. & Qekwana, D. N. Antimicrobial resistance patterns and biofilm formation of coagulase-negative Staphylococcus species isolated from subclinical mastitis cow milk samples submitted to the Onderstepoort Milk Laboratory. BMC Vet. Res. 15, 1–9 (2019).
Lozano, C., Gharsa, H., Ben Slama, K., Zarazaga, M. & Torres, C. Staphylococcus aureus in animals and food: Methicillin resistance, prevalence and population structure. A review in the African continent. Microorganisms 4, 12 (2016).
Ocloo, R. et al. Epidemiology and antimicrobial resistance of staphylococci other than Staphylococcus aureus from domestic animals and livestock in Africa: a systematic review. Front. Vet. Sci. 9, 1. https://doi.org/10.3389/fvets.2022.1059054 (2022).
Faires, M. C., Traverse, M., Tater, K. C., Pearl, D. L. & Weese, J. S. Methicillin-resistant and -susceptible Staphylococcus aureus infections in dogs. Emerg. Infect. Dis. 16, 69 (2010).
Khairullah, A. R., Ramandinianto, S. C. & Effendi, M. H. A review of livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) on Bovine Mastitis. Syst. Rev. Pharm. 11, 172–183 (2020).
Back, S. H. et al. Livestock-associated methicillin-resistant Staphylococcus aureus in Korea: Antimicrobial resistance and molecular characteristics of LA-MRSA strains isolated from pigs, pig farmers, and farm environment. J. Vet. Sci. 21, 1–14 (2020).
Khairullah, A. R. et al. A review of new emerging livestock-associated methicillin-resistant Staphylococcus aureus from pig farms. https://doi.org/10.14202/vetworld.2023.46-58 (2023).
Crespo-Piazuelo, D. & Lawlor, P. G. Livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) prevalence in humans in close contact with animals and measures to reduce on-farm colonisation. Ir. Vet. J. 74, 21 (2021).
Yu, F. et al. Molecular evolution and adaptation of livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) sequence type 9. mSystems 6, (2021).
Seguin, J. C. et al. Methicillin-resistant Staphylococcus aureus outbreak in a veterinary teaching hospital: Potential human-to-animal transmission. J. Clin. Microbiol. 37, 1459–1463 (1999).
Lowder, B. V. et al. Recent human-to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 106, 19545–19550 (2009).
Racewicz, P. et al. Welfare health and productivity in commercial pig herds. Animals 11, 1176. https://doi.org/10.3390/ani11041176 (2021).
DALRRD. A profile of the South African pork market value chain. 1–36 (2021).
Momoh, A. H., Kwaga, J. K. P., Bello, M. & Sackey, A. K. B. Prevalence and antimicrobial resistance pattern of coagulase negative. 37, (2016).
Adegoke, A. A. & Okoh, A. I. Species diversity and antibiotic resistance properties of Staphylococcus of farm animal origin in Nkonkobe Municipality South Africa. Folia Microbiol. (Praha) 59, 133–140 (2014).
Werckenthin, C., Cardoso, M., Martel, J. L. & Schwarz, S. Antimicrobial resistance in staphylococci from animals with particular reference to bovine Staphylococcus aureus, porcine Staphylococcus hyicus, and canine Staphylococcus intermedius. Vet. Res. 32, 341–362 (2001).
Cuny, C., Layer-Nicolaou, F., Weber, R., Köck, R. & Witte, W. Colonization of Dogs and Their Owners with Staphylococcus aureus and Staphylococcus pseudintermedius in Households, Veterinary Practices, and Healthcare Facilities. Microorganisms 10, (2022).
Jonker, A. & Picard, J. A. Antimicrobial susceptibility in thermophilic Campylobacter species isolated from pigs and chickens in South Africa. J. S. Afr. Vet. Assoc. 81, 228–236 (2010).
Van Lochem, S., Thompson, P. N. & Annandale, C. H. Prevalence of methicillin-resistant Staphylococcus aureus among large commercial pig herds in South Africa. Onderstepoort J. Vet. Res. 85, 1–4 (2018).
CLSI. CLSI M100-ED29: 2021 Performance Standards for Antimicrobial Susceptibility Testing, 30th Edition. CLSI vol. 40 (2020).
Magiorakos, A. P. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18, 268–281 (2012).
Andrews, S. A Quality Control Tool for High Throughput Sequence Data [Online]. Available online at: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (2010).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Bankevich, A. et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 19, 455–477 (2012).
Jolley, K. A. et al. Ribosomal multilocus sequence typing: Universal characterization of bacteria from domain to strain. Microbiology 158, 1005–1015 (2012).
Pain, M. et al. Staphylococcus borealis sp. nov., isolated from human skin and blood. 6067–6078 (2020). https://doi.org/10.1099/ijsem.0.004499.
Page, A. J. et al. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 31, 3691–3693 (2015).
Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 30, 2068–2069 (2014).
Alcock, B. P. et al. CARD 2023: expanded curation, support for machine learning, and resistome prediction at the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 51, (2023).
Mamfe, L. M., Akwuobu, C. A. & Ngbede, E. O. Phenotypic detection, antimicrobial susceptibility and virulence profile of staphylococci in the pig production setting, Makurdi, Nigeria. Access Microbiol. 3, (2021).
Lu, L., He, K., Ni, Y., Yu, Z. & Mao, A. Exudative epidermitis of piglets caused by non-toxigenic Staphylococcus sciuri. Vet. Microbiol. 199, 79–84 (2017).
Kateete, D. P., Asiimwe, B. B., Mayanja, R., Najjuka, C. F. & Rutebemberwa, E. Species and drug susceptibility profiles of staphylococci isolated from healthy children in Eastern Uganda. PLoS One 15, e0229026 (2020).
Katakweba, A. S. et al. spa typing and antimicrobial resistance of Staphylococcus aureus from healthy humans, pigs and dogs in Tanzania. J. Infect. Dev. Ctries. 10, 143–148 (2016).
Momoh, A. H., Kwaga, J. K. P., Bello, M., Sackey, A. K. B. & Larsen, A. R. Antibiotic resistance and molecular characteristics of Staphylococcus aureus isolated from backyard-raised pigs and pig workers. Trop. Anim. Health Prod. 50, 1565–1571 (2018).
Sakr, A., Brégeon, F., Mège, J. L., Rolain, J. M. & Blin, O. Staphylococcus aureus nasal colonization: An update on mechanisms, epidemiology, risk factors, and subsequent infections. Front. Microbiol. 9, 1 (2018).
Tillika Samutela, M. et al. Pigs as a potential source of emerging livestock-associated Staphylococcus aureus in Africa: A systematic review. Int. J. Infect. Dis. 109, 38–49 (2021).
Peng, P. et al. Effect of co-inhabiting coagulase negative staphylococci on s. Aureus agr quorum sensing, host factor binding, and biofilm formation. Front. Microbiol. 10, 457508 (2019).
Bonvegna, M. et al. Occurrence of methicillin-resistant coagulase-negative staphylococci (Mrcons) and methicillin-resistant staphylococcus aureus (mrsa) from pigs and farm environment in northwestern italy. Antibiotics 10, (2021).
Ikwap, K. et al. The presence of antibiotic-resistant Staphylococcus spp. and Escherichia coli in smallholder pig farms in Uganda. BMC Vet. Res. 17 (2021).
Park, J., Friendship, R. M., Poljak, Z., Weese, J. S. & Dewey, C. E. An investigation of exudative epidermitis (greasy pig disease) and antimicrobial resistance patterns of Staphylococcus hyicus and Staphylococcus aureus isolated from clinical cases. Can. Vet. J. 54, 139 (2013).
Samutela, M. T. et al. Pigs as a potential source of emerging livestock-associated Staphylococcus aureus in Africa: A systematic review. Int. J. Infect. Dis. 109, 38–49. https://doi.org/10.1016/j.ijid.2021.06.023 (2021).
Oppliger, A. et al. Antimicrobial resistance of Staphylococcus aureus strains acquired by pig farmers from pigs. Appl. Environ. Microbiol. 78, 8010–8014 (2012).
Taher, E. M., Hemmatzadeh, F., Aly, S. A., Elesswy, H. A. & Petrovski, K. R. Molecular characterization of antimicrobial resistance genes on farms and in commercial milk with emphasis on the effect of currently practiced heat treatments on viable but nonculturable formation. J. Dairy Sci. 103, 9936–9945 (2020).
Nemeghaire, S., Vanderhaeghen, W., Angeles Argudín, M., Haesebrouck, F. & Butaye, P. Characterization of methicillin-resistant Staphylococcus sciuri isolates from industrially raised pigs, cattle and broiler chickens. J. Antimicrob. Chemother. 69, 2928–2934 (2014).
Micke Moreno, A. et al. Antimicrobial resistance profile of Staphylococcus hyicus strains isolated from Brazilian Swine Herds. https://doi.org/10.3390/antibiotics11020205 (2022).
Strasheim, W., Etter, E. M. C., Lowe, M. & Perovic, O. Method to assess farm-level vaccine and antibiotic usage utilizing financial documentation: A pilot study in a commercial pig farm in South Africa from 2016 to 2018. Front. Vet. Sci. 9, 856729 (2022).
National Department of Health. Surveillance for antimicrobial resistance and Consumption of Antimicrobials in South Africa, 2021. Natl. Dep. Heal. 1, 1–61 (2021).
Ocloo, R., Newton-Foot, M., Ziebuhr, W. & Whitelaw, A. C. Molecular epidemiology and antibiotic resistance of staphylococci other than Staphylococcus aureus in children in Cape Town. South Africa. Front. Microbiol. 14, 1 (2023).
Wegener, H. C. & Schwarz, S. Antibiotic-resistance and plasmids in Staphylococcus hyicus isolated from pigs with exudative epidermitis and from healthy pigs. Vet. Microbiol. 34, 363–372 (1993).
Hot, C., Berthet, N. & Chesneau, O. Characterization of sal(A), a novel gene responsible for Lincosamide and Streptogramin a resistance in staphylococcus sciuri. Antimicrob. Agents Chemother. 58, 3335–3341 (2014).
Yang, C., Anahtar, M. N. & Pierce, V. M. It’s not you, it’s SOSA: A case study on breaking up with an FDA-cleared susceptibility testing system’s oxacillin results for Staphylococcus spp. other than S. aureus and S. lugdunensis. Open Forum Infect. Dis. 9, (2022).
Humphries, R. M. et al. Evaluation of surrogate tests for the presence of mecA- mediated methicillin resistance in Staphylococcus capitis, Staphylococcus haemolyticus, Staphylococcus hominis, and Staphylococcus warneri. J. Clin. Microbiol. 59, 1–11 (2021).
Abdullahi, I. N. et al. Nasal staphylococci community of healthy pigs and pig-farmers in Aragon (Spain). Predominance and within-host resistome diversity in MRSA-CC398 and MSSA-CC9 lineages. One Heal. 16, 100505 (2023).
Samutela, M. T. et al. Pigs as a potential source of emerging livestock-associated Staphylococcus aureus in Africa: A systematic review. Int. J. Infect. Dis. 109, 38–49 (2021).
Fasih, N., Irfan, S., Zafar, A., Khan, E. & Hasan, R. Inducible clindamycin resistance due to expression of erm genes in Staphylococcus aureus: report from a tertiary care Hospital Karachi, Pakistan (2010).
Pantosti, A. Methicillin-resistant Staphylococcus aureus associated with animals and its relevance to human health. Front. Microbiol. 3, 20606 (2012).
EFSA. Analysis of the baseline survey on the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs, in the EU, 2008—Part A: MRSA prevalence estimates. EFSA J. 7 (2009).
Juhász-Kaszanyitzky, É. et al. MRSA transmission between cows and humans. Emerg. Infect. Dis. 13, 630 (2007).
Cirkovic, I. et al. Nasal and pharyngeal carriage of methicillin-resistant Staphylococcus sciuri among hospitalised patients and healthcare workers in a Serbian university hospital. PLoS One 12, 1–11 (2017).
Nemeghaire, S. et al. The ecological importance of the Staphylococcus sciuri species group as a reservoir for resistance and virulence genes. Vet. Microbiol. 171, 342–356 (2014).
Rolo, J. et al. Evolutionary origin of the staphylococcal cassette chromosome mec (SCCmec). Antimicrob. Agents Chemother. 61, 1–16 (2017).
Acknowledgements
Thanks to the pig farms for allowing us to sample the pigs and the farm workers.
Funding
This study was supported by the German Research Council (DFG) through grant ZI665/3-1 and the German Federal Ministry of Education and Research (BMBF) through grant number 01KI2009E and the Harry Crossley foundation.
Author information
Authors and Affiliations
Contributions
R.O contributed to the conceptualisation and design of the study, performed laboratory work, whole genome sequence data analysis and interpretation and wrote the manuscript. M.N, A.W, and W.Z contributed to the conceptualisation and design of the study and provided critical feedback on the manuscript. L.C performed the whole genome sequencing and provided critical feedback on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ocloo, R., Newton-Foot, M., Chabuka, L. et al. Epidemiology and antibiotic resistance of staphylococci on commercial pig farms in Cape Town, South Africa. Sci Rep 14, 19747 (2024). https://doi.org/10.1038/s41598-024-70183-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70183-2
- Springer Nature Limited