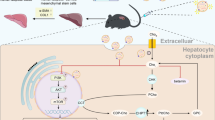
Abstract
Liver fibrosis is a chronic liver disease with progressive wound healing reaction caused by liver injury. Currently, there is no FDA approved drugs for liver fibrosis. Human adipose mesenchymal stem cells (hADSCs) have shown remarkable therapeutic effects in liver diseases. However, few studies have evaluated the therapeutic role of hADSCs in liver fibrosis, and the detailed mechanism of action is unknown. Here, we investigated the in vitro and in vivo anti-fibrosis efficacy of hADSCs and identified important metabolic changes and detailed mechanisms through transcriptomic and metabolomic analyses. We found that hADSCs could inhibit the proliferation of activated hepatic stellate cells (HSCs), promote their apoptosis, and effectively inhibit the expression of pro-fibrotic protein. It can significantly reduce collagen deposition and liver injury, improve liver function and alleviate liver inflammation in cirrhotic mouse models. In addition, transcriptome analysis revealed that the key mechanism of hADSCs against liver fibrosis is the regulation of AGE-RAGE signaling pathway. Metabolic analysis showed that hADSCs influenced changes of metabolites in lipid metabolism. Therefore, our study shows that hADSCs could reduce the activation of hepatic stellate cells and inhibit the progression of liver fibrosis, which has important potential in the treatment of liver fibrosis as well as other refractory chronic liver diseases.
Similar content being viewed by others
Introduction
Liver fibrosis is one of the major causes of global morbidity and mortality, and the global burden of hepatic fibrosis is expected to further increase in the coming years1. It is a chronic liver disease with persistent inflammatory response and progressive wound healing response caused by liver injury2. During the progression of liver fibrosis, further intensification of inflammatory cell recruitment, myofibroblast activation, and excessive extracellular matrix (ECM) deposition may eventually lead to cirrhosis and liver cancer3,4. However, to date, there are no FDA-approved drugs and effective therapies for cirrhosis other than liver transplantation5. Due to cirrhosis related complications, donor shortage, and the need for lifelong immunosuppression, only a small percentage of cirrhotic patients have benefited from remedial liver transplantation to date6. Therefore, liver regeneration therapy has become an attractive treatment strategy.
Mesenchymal stem cells (MSCs), as the main stem cells for cell-based therapy, provide another option for liver organ or cell transplantation. Various stem cells from human bone marrow (BMSCs), umbilical cord (UCSCs) and adipose tissue (ADSCs) have been used in the treatment of liver diseases7,8,9,10. Clinical and experimental studies have shown that MSCs could effectively protect hepatocytes through transdifferentiating into functional hepatocytes, secreting a variety of immunosuppressive factors and nutritional factors, and secrete exosomes through autocrine or paracrine, thus playing an anti-fibrotic role and promoting liver regeneration8,11. In addition, there are several advantages of adipose mesenchymal stem cells12. First, they are relatively easy to source, can be self-obtained, and have relatively few clinical ethical problems. Second, they have strong ability of self-renewal, trans-dermal multiplex differentiation potential (hepatogenic differentiation), proliferation ability, and genetic stability in long-term culture. Finally, the production process is relatively simple, and it has more potential for clinical application. The clinical trial (NCT01062750) showed good efficacy and safety13. Growing evidence suggests that the BMSCs and UCSCs demonstrate an improved therapeutic effect in treating acute and chronic liver injuries, and ADSCs have a particular advantage in the treatment of chronic liver disease14. The previous studies have showed that hADSCs promoted endogenous hepatocyte regeneration and ameliorated hepatic fibrosis by secreting cytokines such as the hepatocyte growth factor (HGF) and fibroblast growth factor (FGF)15. Importantly, it has been reported that human adipose mesenchymal stem cells successfully inhibited the activity of activated HSCs and functionally alleviated liver fibrosis in liver fibrosis mice model16. However, the detailed mechanism remains unclear.
As the largest metabolic organ, the liver plays an important role in maintaining metabolic homeostasis. The process of liver fibrosis is closely related to metabolism. A profound metabolic reprogramming, including a shift to aerobic glycolysis and excessive lipid accumulation, has recently been identified as the fundamental mechanism and hallmark of HSCs activation, leading to pathological liver fibrosis17,18,19. Conversely, improvements in metabolic reprogramming can promote the reversal of liver fibrosis. MSCs regulate metabolic changes and play a major role in HSCs activation and fiber formation20,21. However, it is unclear whether and how hADSCs regulate metabolic reprogramming by improving liver function and inhibiting liver fibrosis.
In this study, the effects of hADSCs on liver fibrosis were investigated using carbon tetrachloride (CCl4)-induced liver cirrhosis mouse model and TGF-β1-induced activated HSCs model, respectively. We demonstrated that hADSCs could improve HSCs activation and inhibit the progression of liver fibrosis. Importantly, we combined transcriptomic and metabolomic studies to identify important metabolic processes and detailed mechanisms. Our findings suggest that hADSCs ameliorate liver fibrosis primarily by restoring abnormal lipid metabolism and inhibiting the AGE-RAGE pathway.
Materials and methods
Characteristics and culture of hADSCs
Abdominal adipose tissue was obtained from 10 young women (aged 20–30 years) who underwent liposuction at the Nanfang Hospital, Southern Medical University from January to May 2023. The study protocol has been approved by the Nanfang Hospital, Southern Medical University Ethics Committee (NO2023-578). Obtain informed consent from each subject and the study has been performed in accordance with the Declaration of Helsinki. All methods in this study were performed in accordance with ARRIVE guidelines. As previously mentioned22, hADSCs were isolated. Simply put, the adipose tissue was washed three to four times with phosphate-buffered saline (PBS) plus penicilline-streptomycin, then mechanically chopped and digested for 30 min with 0.75 mg/ml collagenase I (BioFroxx) in a MaxQ 8000 oscillating incubator at 37 °C, 225 RPM. The digested tissues were washed with Dulbecco Modified Eagle Medium (DMEM) F12 containing 10% fetal bovine serum (FBS) and centrifuged at 300×g for 5 min. The cell particles were resuspended in DMEM F12 supplemented with 10% FBS in an incubator at 37 °C and 5% carbon dioxide. The 3rd–5th generation hADSCs were used in this study.
Then hADSCs were identified with anti-CD90 fluorescein isothiocyanate (FITC) conjugated antibodies, anti-CD73 isophycocyanin (APC) conjugated antibodies, PreCP-CD105, and anti-CD44 phycoglobin (PE) conjugated antibodies (BD, CA, USA). To assess the trilineage differentiation capacity, hADSCs were cultured in osteogenic, lipogenic, and chondrogenic induction media for 20 days. The differentiation of bone cells, chondrocytes and adipocytes was confirmed by alizarin red staining, oil red O staining and alcian blue staining. respectively.
Co-culture of HSCs and hADSCs
Human hepatic stellate cells (LX-2 cells) were purchased from Shanghai Mcellbank Biotechnology Co., Ltd, China, and cultured maintained as described in protocol from the provider. 10 ng/ml TGF‐β1 (PeproTech, CA, USA) was added to induce activation of LX-2 cells for 24 h. LX-2 cells were then incubated with different concentrations of hADSCs (1 × 104, 5 × 104 and 1 × 105 cells/well). The same volume of PBS was added to the control group. The cells were examined after 24 h incubation.
In vitro cell viability and proliferation detection
LX-2 cells were seeded at a density of 5000 cells/well in 96-well plates and stabilized for 24 h at 37 °C in a humidified 5% carbon dioxide atmosphere. LX-2 cells were activated by TGF‐β1 and incubated for 24 h. Then the cells were incubated with hADSCs for 24 h, the viability of LX-2 cells in the presence of different concentrations of hADSCs was quantified by CCK-8 assays (Dojindo, Kumamoto, Japan). The optical density (OD 450 nm) values were measured by a microplate reader.
To measure the proliferation of cells, the Cell Light EdU DNA imaging kit was used for EdU incorporation experiments, which were extensively described elsewhere23. The ratio of EdU-stained cells (with green fluorescence) to Hoechst-stained cells (with blue fluorescence) was used to evaluate the cell proliferation activity.
RNA extraction and quantitative real-time polymerase chain reaction (RT-PCR)
Total RNA from fresh frozen tissues and cells was isolated using Trizol reagent (Invitrogen, NY, USA) according to the manufacturer’s instructions. RNA was quantified using a NanoDrop (Thermo Fisher Scientific, MA, USA). Nest, 1 μg of total RNA was used to produce cDNA using PrimeScript RT reagent (Takara, Kusatsu, Japan), and qRT-PCR was performed using TB Green™ Premix Ex Taq™ II (Takara, Japan) in the CFX96 Real-time System (Bio-Rad, CA, USA). A detailed description of the method is published elsewhere24. mRNA expression was calculated via the standard 2−ΔΔCt method. The sequences of primers were designed and were as follows: IL-10, forward primer-5ʹ-CCCATTCCTCGTCACGATCTC-3ʹ, reverse primer-5ʹ-TCAGACTGGTTTGGGATAGGTTT-3ʹ, IL-6, forward primer-5ʹ-AAGAGCCGGAAATCCACGAAA-3ʹ, reverse primer-5ʹ-GTCTCAAAAGGGTCAGGGTACT-3ʹ, TNF-α, forward primer-5ʹ-CTTTCGGTTGCTCTTTGGTTG-3ʹ, reverse primer-5ʹ-CGACAGCACAAGTCACAGC-3ʹ, IL-2, forward primer-5ʹ-TGAGCAGGATGGAGAATTACAGG-3ʹ, reverse primer-5ʹ-GTCCAAGTTCATCTTCTAGGCAC-3ʹ. IL-1β, forward primer-5ʹ-AGTTGACGGACCCCAAAAG-3ʹ, reverse primer-5ʹ-AGCTGGATGCTCTCATCAGG-3ʹ.
Western blot analysis
The RIPA lysis buffer (Solarbio, Beijing, China) was used for purification of the protein of cells and tissues. Total proteins were extracted, detected by BCA, then isolated by 12% SDS-PAGE and transferred to membranes (Millipore Corp, Billerica, USA). After blocking, the membrane was incubated with primary antibody and horseradish peroxidase coupled with secondary antibody. Using Image Lab software (Bio-Rad, Hercules, CA, USA) and Immobilon Western Chemiluminescent HRP substrate. Detect and quantify signals using the ChemiDoc XRS + system. Antibodies utilized in this study are listed in Supplemental Table S1.
Experimental animals
Male mice (C57BL/6J, 20–25 g, 6 weeks of age) were purchased from Beijing Weitong Lihua Experimental Animal Technology Co. Ltd (syxk2023-0071, Beijing, China). All procedures involving animals were performed in accordance with the ARRIVE guidelines and were approved by the Animal Ethic Committee of Nanfang Hospital, Southern Medical University (Approval number: 2023-578). Procedures for CCl4-induced liver fibrosis were as detailed elsewhere25. After adaptive feeding for a week, animals were randomly divided into normal group and liver fibrosis modeling group, with administered olive oil vehicle-alone 2× week for 8 weeks, or CCl4 (5 ml/kg intraperitoneally; diluted 1:4 in olive oil), respectively. Then Liver Fibrosis Group (LFG, model group) was sacrificed. Regression group (REG, PBS group) are treated with 200 μl PBS, hADSCs group received 1 × 106 hADSCs in 200 μl PBS26,27. All treatments were administered intravenously twice a week for 4 weeks. At the completion of experiments, mice were sacrificed with overdose of isoflurane followed by neck dislocation. Blood was taken from eyeball and immediately centrifuged for serum collection. Liver tissue was excised and snap-frozen in liquid nitrogen.
Shear wave elastrography (SWE)
After 8 weeks of CCl4 treatment, fibroscan was performed (Echo-Sens, Paris, France). Procedures for SWE were as detailed elsewhere28. Ultrasound elastography data were collected at the last scan before treatment began (baseline), during treatment (week 2), and before sacrifice (week 4).
Liver histopathological evaluation, immunohistochemistry analysis
The liver tissue of the same leaf was embedded in paraffin wax and sectioned at 5 µm. Hematoxylin and eosin (H&E), Masson tricolor (MT) and Sirius red were stained using paraffin embedded sections. The inflammatory activity of liver fibrosis and necrosis was assessed semi-quantitatively by two independent pathologists in a blind method and scored according to the Ishak fibrosis scoring system29. MT and Sirius red staining were used quantitative analysis in 5 random fields with Image J. For immunohistochemical staining (IHC), sections were sliced with the primary antibody against α-SMA and Collagen I at 4 °C overnight, and the second antibody at 37 °C overnight for 1 h. The histology of different tissues was analyzed under microscope (BX51, Olympus, Tokyo, Japan). DP2-BSW software (Olympus) was used for image acquisition. Procedure for Enzyme-linked immunosorbent assay (ELISA) was as described elsewhere30.
Blood biochemical assay
To investigate the liver function test in blood, the isolated serum of each mice was analyzed for aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatease (ALP). These parameters of the serum were evaluated by ChemOn Inc (Suwon, Korea).
RNA-seq and data analysis
Different treated RNA was isolated from mouse liver by Trizol Reagent (Invitrogen Life Technologies). The sequencing libraries were constructed using the NEBNext®Ultra RNA library Prep Kit for Illumina® and then sequenced on the NovaSeq 6000 platform (Illumina). Differential expression genes (DEGs) were screened, and the P value after adjustment was < 0.05, and the log-fold change was ≥ 1. Heatmaps and gene expression enrichment analysis were generated using Pheatmap and ggplots2 in R, and pathway analysis was performed using clusterProfler23 software package in R.
LC–MS/MS analysis
Each tissue sample was taken 20 mg, homogenized with 2 chlorophenylalanine (4 ppm) 0.6 ml containing methanol, centrifuged, filtered 0.22 μm membrane, and then analyzed by ultra-high performance liquid chromatography-mass spectrometry. LC conditions and MS conditions were as described previously31. Data-dependent acquisition was used to identify metabolites and remove unnecessary MS/MS information through dynamic elimination. Metabolites were identified by MassBank, human metabolome database, METLIN, lipidmaps, mzCloud and other metabolome databases, as well as BioNovoGene database established by Suzhou PANOMIX Biomedical Technology Co., Lld. Metabolic pathway enrichment analysis was carried out using Metaboanalyst 5.0 (https://www.metaboanalyst.ca/).
Statistical analysis
All data were analyzed by GraphPad Prism13.0 software, Image J and R 3.6.0 (The R Foundation for Statistical Computing, Vienna, Austria). Comparisons between two or more groups were made using one-way ANOVA and T-test. p < 0.05 was considered statistically significant.
Ethics approval and consent to participate
The studies involving human participants and all mouse experimental protocols were reviewed and approved by the Institutional Review Board for Clinical Research and Animal Ethics Committee of Nanfang Hospital, Southern Medical University Ethics Committee (NO2023-578). The participants provided their written informed consent to participate in this study. The study was reported in accordance with the Declaration of Helsinki and ARRIVE guidelines.
Results
Characterization of hADSCs
The isolated primary cells from human adipose tissues were passaged to the third generation (P3) for further identification and source of exosomes. The morphology, immunophenotype and multipotential differentiation ability are the critical factors to characterize human adipose-derived mesenchymal stem cells (hADSCs). The morphology of hADSCs was monolayer adherent and fibroblast-like spindle-shaped (Fig. 1A). For detecting multilineage differentiation potential of hADSCs, we evaluated hADSCs inducing osteogenic, adipogenic and chondrogenic differentiation under different inducing conditioned medium. As showed in Fig. 1B, compact cell layer and intense Alizarin red staining both illustrated calcium deposition (bright red spots) of osteogenically differentiated tissues, which supported the osteogenic differentiation of hADSCs; Lipid droplets (dark red spots) formed during adipogenic differentiation of hADSCs was visualized by Oil Red O staining; After 1 months of chondrogenic differentiation in culture, we observed clear cartilage formation, and production of proteoglycan (blue circle) was visualized separately by Alcian Blue staining. These results suggested that hADSCs have the capacity for multi-lineage differentiation potential. In addition, to better understand the immunophenotype of hADSCs, we employed analysis of characteristic stem-cell surface markers CD44, CD73, CD90, and CD105 utilizing flow cytometry. The result showed that hADSCs were positive for CD44, CD73, CD90 and CD105 with 99.7%, 98%, 99.6% and 88.5%, respectively (Fig. 1C). Collectively, these data suggested that the isolated primary cells from human adipose tissues was identified as hADSCs, which showed readily differentiate along osteogenic, chondrogenic, and adipogenic lineages.
Characterization of and hADSCs. (A) Morphological appearance of cultured hADSCs (bar = 100 μm). (B) The potential of hADSCs on differentiation of adipocytes, osteoblasts and chondrocytes in the specified medium was identified by the Oil Red O staining, Alizarin Red staining and Alcian Blue staining, respectively; Scale bar: 500 μm. (C) Flow cytometry detection of the typical surface markers CD44 CD90, CD105, and CD73 of hADSCs.
hADSCs inhibits hepatic stellate cell proliferation and exhibit antifibrotic effect in vitro
Hepatic stellate cells (HSCs) are the primary cell type responsible for liver fibrosis, and its phenotype can be regulated by TGF-β132. We used TGF-β1 incubated with LX2 cells for 24 h. Subsequently, we evaluated the effects of hADSCs on activated LX-2 cells by the transwell co-culture system (Fig. 2A). As showed in Fig. 2B, compared to that quiescent HSCs, activated HSCs formed pseudopodia with elongated synapses and dense fibrotic stroma. When educated with hADSCs, activated HSCs appeared iridophore shape transitions from dense-to-loose. Moreover, The EdU experiment showed that compared with the PBS group, the green fluorescence gradually decreased in hADSCs group (p < 0.01), indicating that hADSCs exerted significant inhibition on proliferation of the activated HSCs (Fig. 2C). The immunofluorescence assay results revealed that when aHSCs were treated with hADSCs, the fluorescence signals of α-SMA significantly diminished in a dose-dependent manner, indicating excellent antifibrotic activities of hADSCs (Fig. 2D). These results suggested that hADSCs could inactivate the activated HSCs through inhibition of proliferation, liver fibrosis process and ECM degradation.
hADSCs inhibits hepatic stellate cell proliferation and the process of liver fibrosis. (A) Schematic illustration of the cell experiment. (B) In vitro cell morphologies of activated LX-2 cells co-cultured with hADSCs at the indicated concentration in the presence of TGF-β1 (10 ng/ml) for 24 h (bar = 100 μm). (C) Edu assay showed that HSCs proliferation was suppressed by hADSCs in a concentration-dependent manner. EdU% is used as the reference for proliferation rate. (D) Representative images of immunofluorescence staining of α-SMA (bar = 100 mm). Data expressed as the mean ± SEM. ns, not significant, *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
hADSCs could alleviate CCl4-induced mice liver fibrosis in vivo
In order to assess the therapeutic potential of hADSCs in liver fibrosis mice, the modeling and treatment process is depicted in Fig. 3A. We assess liver stiffness measurement (LSM) by SWE after 2 weeks and 4 weeks of treatment. As shown in Fig. 3B, these results demonstrated that liver matrix stiffness of hADSCs group returned to normal levels (6.9 ± 0.87) and were significantly decreased compared to PBS group (9.0 ± 0.67) after 4 weeks of treatments. In addition, compared with sham group and PBS group, the body weight of hADSCs group increased faster. There was significantly difference between hADSCs group and PBS group in the third week (Fig. 3C).
hADSCs could alleviate CCl4-induced mice liver fibrosis in vivo. (A) Diagram of experimental scheme. (B) Elastograms obtained using shear wave elastrography (reliable images were obtained when uniform colour filled > 90% of the sampling area) and liver stiffness quantification. (C) Body weight changes during treatment. (D) Representative photographs of lives from mice in each group and histopathological images of liver sections were evaluated using H&E, Masson trichrome staining and Sirius red staining in each group. Liver histopathology grading was evaluated by Ishak (modified Knodell) scoring system after treatment. (E) Images of immunohistochemistry staining of extracellular matrix (ECM) proteins (collagen I and α-SMA). (F) The protein levels of ECM-related proteins in the liver tissues of the mice with different treatments. Data are expressed as mean ± SEM (n = 4), ns, not significant, *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001. Scale bar, 40 μm.
Mice were sacrificed after 4 weeks treatment, and parameters of liver injury, hepatic inflammation and liver fibrosis were assessed, to verify the effectiveness of hADSCs in treating liver fibrosis. As shown in Fig. 3D, the surface of the liver of the normal group was reddish, soft in texture and smooth, with an evident gloss. On the contrary, the livers in the model group had hard texture, rough surface with a brownish color and nodule formation, indicating serious liver injury. Intriguingly, hADSCs group showed clear morphological restoration along with a smooth surface and bright red color in macroscopic images. The stage hepatic fibrosis and architectural alteration was further assessed by the Ishak (modified Knodell) scoring system using H&E staining33. The results showed that hADSCs group showed reduction of hepatocyte piecemeal, confluent necrosis, ductal proliferation and infiltration of immune cells in histological examinations and their staging score of liver fibrosis, which was dramatically lower than that in model and PBS group (1.75, 5.25, 3.25, p < 0.01). Masson’s trichrome and Sirius Red staining was performed to indicate collagen deposition in liver sections. The Masson-stained area of the hADSCs group was 2.6 times lower than that of the PBS group and was 3.8 times lower than that of the model group. The Sirius Red-stained area of the hADSCs group was 2.1 times lower than that of the PBS group and was 3.8 times lower than that of the model group. These results were consistent with those of α-SMA and collagen I staining, showing that a remarkable reduction was found in the α-SMA and collagen I stained areas of the hADSCs-treated group (Fig. 3E). Furthermore, Western blot experiments verified the above result and confirmed anti-fibrosis effect of hADSCs (Fig. 3F, Fig. S1). Thus, these data substantiated that systemic administration of hADSCs could histologically and functionally alleviate liver fibrosis in CCl4-induced mice liver fibrosis.
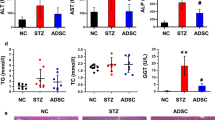
hADSCs treatment improves liver function, reduces liver inflammation and apoptosis
Then, we performed serum biochemical tests to measure the extent of liver function and hepatocellular damage in each group. The serum levels of ALT, AST and ALP were significantly suppressed in the hADSCs-treated mice when compared with model and PBS groups (Fig. 4A), indicating that hADSCs repaired liver function. Furthermore, we detected the expression of inflammatory cytokines including Interleukin-1β (IL-1β), Interleukin-6 (IL-6), Interleukin-10 (IL-10) and tumor necrosis factor-α (TNF-α) in liver tissue of these mice by qRT-PCR (Fig. 4B). The results indicated their expression were significantly decreased in the livers of hADSCs group compared with those of the model and PBS groups, demonstrating the powerful and effective anti-inflammatory effects of hADSCs in the liver fibrosis mice.
hADSCs treatment improves liver function, reduces liver inflammation and apoptosis. (A) Serum levels of ALT, AST, ALP in different groups. (B) The relative inflammatory gene expression for IL-1β, IL-6, IL-10 and TNF-α. (C) Immunohistochemical staining was performed to detect the protein expressions of HNF-α and Caspase 3 in the liver of each group. Scale bar, 40 μm. (D) Western blot assay for apoptosis-related proteins in mouse liver issue. Data are expressed as mean ± SEM (n = 4), ns, not significant, *p < 0.05, **p < 0.01 and ***p < 0.001. AST aspartate aminotransferase, ALT alanine aminotransferase, ALP alkaline phosphatease.
In addition, to evaluate whether hADSCs treatment could reduce hepatocellular injury and apoptosis, improve liver regeneration, we performed IHC to assess caspase 3 and HNF-4α expression levels, respectively. As shown in Fig. 4C, the percentage of Caspase 3+ cells in hADSCs (7.68%) treatment group was decreased significantly when compared to the model (21.65%) and PBS (19.97%). The percentage of HNF-4α+ cells in hADSCs (62.97%) group was increased significantly when compared with the model (38.50%) and PBS (40.38%). Then we tested the degree of apoptosis by western bolt. The results showed that hADSCs remarkably decreased Caspase-3 and Bax expression, and improved Bcl2 expression compared to control, indicating significant inhabitation of hepatocyte apoptosis (Fig. 4D). These data illustrated that hADSCs could reduce hepatocellular injury and apoptosis of CCl4-damaged liver.
hADSCs ameliorated liver fibrosis through AGE-RAGE signaling pathway
After data filtering, there were 45,967,128 average number of clean reads per sample. The high quality of reads comprised the over 93.17% of the total reads. The median per-base sequence quality was more than 34. To further explore the mechanisms underlying the regulation of liver fibrosis by hADSCs, we compared the RNA sequencing gene expression profiles of between the model, PBS, hADSCs group (Fig. 5A). Among the differentially expressed genes (DEGs), we observed a partial intersection of 343 unique DEGs between the hADSCs versus model group, and 133 unique DEGs between the hADSCs versus PBS group (Fig. 5B). The unique DEGs were then analyzed by Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses34,35. The top 10 significantly enriched terms in molecular function (MF), biological process (BP), and cellular component (CC) were shown in Fig. 5C. The results showed that hADSCs was mainly involved in extracellular matrix structural constituent and organization. In GO terms of cellular components, the major genes involved are ECM, collagen-containing extracellular matrix and extracellular region (Fig. 5D). For molecular function, they are consistent with our results showing that hADSCs regulates ECM secretion in HSCs. Furthermore, GO and KEGG pathway analyses showed that ECM and its downstream mediator AGE-RAGE were the top-ranked signaling pathways (Fig. 5E). The GO and KEGG pathway analyses for transcriptomics between hADSCs and REG were showed in Fig. S2. These results suggested that hADSCs might inhibit AGE-RAGE signaling pathway and prevent the formation of ECM to ameliorate the progression of liver fibrosis.
hADSCs ameliorated liver fibrosis through AGE-RAGE signaling pathway. (A) Heatmap of differential expression analysis of RNA-sequencing data from LFG, REG and hADSCs. (B) Venn diagram of LFG vs REG, LFG vs hADSCs and REG vs hADSCs. (C) GO analysis results of the DEGs between REG and hADSCs, including cell component (CC), molecular function (MF) and biological process (BP). Red represents CC, green represents MF, blue represents BP. (D) The top 20 significant GO terms of DEGs. (E) KEGG analysis of significant pathway of DEGs. (F) Western blot assay for AGE and RAGE in mouse liver issue. (G) Quantification of AGE and RAGE by qRT-PCR. (H) Quantification of RAGE by qRT-PCR. Data are presented as means with SEM (n = 3 independent experiments). ns, not significant, *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001. LFG liver fibrosis group, REG regression group.
To determine whether hADSCs regulates AGE-RAGE signaling pathway, we analyzed activities of AGE and it mediated RAGE expression in activated HSCs and liver tissues. Consistent with our analysis on the transcriptome, western blot analysis showed that when comparing the hADSCs group to PBS, AGE induced by hADSCs was significantly inhibited, a statistically significant decrease was accordingly observed in the RAGE expressions in vivo (Fig. 5F). As expected, qRT-PCR also confirmed that decreased mRNA expression of AGE, RAGE in hADSCs group (Fig. 5G,H). All these results confirmed that hADSCs could effectively inhibit the progression of HSCs activation and liver fibrosis by preventing the stimulation of the AGE-RAGE signaling pathways.
hADSCs inhibits liver fibrosis by regulating lipid metabolism
To further reveal mechanisms underlying hADSCs-mediated metabolic activities in inhibiting liver fibrosis, we performed metabolomic analysis of liver tissue in each groups using LC/MS technology. Quality Control (QC) analyses for metabolomics in the positive and negative modes of LC/MS showed that the distribution of QC samples was densely distributed, which also indicated data quality satisfactory and reliable (Fig. S3). Moreover, the metabolites of mouse liver tissue in each group were obviously discriminated to the four different groups by principal components analysis (PCA) (Fig. 6A), and Orthogonal partial least squares discriminant analysis (OPLS-DA) revealed a clear and statistically significant separation between each group individuals (OPLS-DA model: R2X = 0.358, R2Y(cum) = 0.993, Q2(cum) = 0.779 (Fig. 6B). A total of 1026 changed metabolites was identified referred by the variable importance in the projection (VIP) ≥ 1 and p ≦ 0.05, of which 136 differential metabolites between hADSCs-treated group and model group, and 100 differential metabolites between hADSCs-treated group and regression group (Fig. 6C). Name, Variable Importance Projection (VIP), p value and Fold change of the top 20 most significant up and downregulated metabolites in hADSCs-treated group compared to PBS and model group were listed in Table 1 and Table S2, respectively. Among the most significantly up-regulated metabolites were 8-Isoprostane, FMN, Baicalin, 5-Methyl-2-furancarboxaldehyde, N-a-Acetylcitrulline and the most significantly down-regulated metabolites were Docosapentaenoic acid (22n-3), Betaine, 2-Hydroxy-2-ethylsuccinic acid, d-Fructose, Hydroquinone, most of which were classified as lipids (Fig. 6D). Moreover, the top 30 differentially represented metabolites were shown graphically by hierarchical clustering to heatmap among each group (Fig. 6E). Additionally, the content of azelaic acid, 9-cis-Retinoic acid, homocitrulline, sphinganine, l-Alanyl-gamma-d-glutamyl-l-lysine, 20-Carboxy-leukotriene B4 were significantly enriched in hADSCs group compared with PBS group (Fig. 6F).
hADSCs inhibits liver fibrosis by regulating lipid metabolism. (A) Score plots from the PCA model derived from the UPLC-MS profile of mice liver in different groups. (B) Score plots from the OPLS-DA model from metabolic profiles of different groups. (C) The histograms of differential metabolites between LFG, REG and hADSCs-treated group. (D) The histograms of the top 5 downregulated and top 5 upregulated metabolites between different groups. (E) The hierarchical clustering heatmap of the top 30 metabolites between different groups. (F) The hierarchical clustering heatmap of the top 30 metabolites between REG and hADSCs-treated group. (G) Summary of pathway analysis of differential metabolites between REG and hADSCs-treated group. Data expressed as the mean ± SEM. ns, not significant, *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001. LFG liver fibrosis group, REG regression group.
A pathway analysis of differential metabolites between hADSCs-treated group and PBS group (Fig. 6G) revealed that hADSCs‐induced metabolic perturbations were mainly related to intestinal immune network for IgA production, choline metabolism, mTOR signaling pathway, cGMP-PKG signaling pathway, PPAR signaling pathway, whereas the differential metabolites detected among hADSCs-treated group compared to model group (Fig. S4) were notably majoring in lysosome, mTOR signaling pathway, FoxO signaling pathway and choline metabolism, suggesting that hADSCs are indeed involved in lipid and energy metabolism (choline metabolism, PPAR signaling, fatty acid metabolism, sphingolipid metabolism, etc.). hADSCs alter the perturbation of metabolomic profiling in mice with hepatic fibrosis.
In addition, we performed association analysis of transcriptomic and metabolomics data between hADSCs treatment group and liver fibrosis group (Table S3, Fig. S5), we found that palmitic acid and docosapentaenoic acid (22n-3) were downregulated in mouse with hADSCs treatment because the genes Elovl3 and Scd1 involved in biosynthesis of unsaturated fatty acids were all downregulated. Likely, hADSCs regulated fatty acid metabolism by mediating the down-regulation of palmitic acid, which was caused by decreased genes Cyp4a12a and Cyp4a12b expression. These results suggested that the mechanisms of hADSCs against liver fibrosis might involve in the regulation of lipid metabolism.
Discussion
In summary, this study evaluated the effects of hADSCs on hepatic fibrosis, transcriptional regulation and metabolic changes in CCl4-induced liver fibrosis mouses. The main findings of the study are as following: (1) hADSCs could alleviate liver fibrosis progression, including decrease in liver stiffness, the recovery of liver function, inhibition of inflammation and lipid peroxidation, and reduced hepatocyte apoptosis and damage. (2) The main mechanism of anti-fibrosis of hADSCs is regulated via inhibition of AGEs-RAGE signaling pathway in vivo. (3) Through metabonomic analysis, hADSCs mainly regulates hepatic fibrosis through lipid metabolism. Collectively, these data show that regulation of lipid metabolism and inhibition of AGE-RAGE signaling may be important mechanisms underlying the anti-liver fibrosis effects of hADSCs.
MSCs have emerged as a promising candidate for therapeutic application in a variety of liver diseases. Recent studies demonstrated various MSCs could restore liver homeostasis and enable hepatocytes to recover, repair and regenerate through their paracrine activity to transfer secretions to injured cells, indicating a great therapeutic potential in treatments of liver fibrosis36,37. Growing evidence indicates that hADSCs exhibited a safe and effective therapeutic effect in liver fibrosis38. In our study, it had been verified that hADSCs were capable to regulate phenotypes of activated hepatic stellate cells. Furthermore, we found that hADSCs could inhibit the proliferation of activated HSCs at relatively high concentrations. Importantly, hADSCs also inhibited the expression of profibrogenic proteins and progression of liver fibrosis. In CCl4-induced liver fibrosis mice, our results demonstrated that hADSCs treatment improved fibrosis reversal, with liver stiffness back towards normal. This conclusion was also supported by the significant decreased collagen formation and recovery of liver function. Moreover, hADSCs appears comparable the effect of reducing hepatocellular injury. Previous studies showed that hADSCs attenuate liver injury by inhibiting mitochondrial stress (apoptosis and dysfunctional energy metabolism) and alleviating inflammation (inflammasome expression and immune disorder)39. We found that hADSCs significantly decreased inflammatory cytokines to inhibit inflammatory response. This is consistent with reports previously showing that BMSCs and UCSCs could promote liver tissue repair by reducing the inflammatory response8. Therefore, together with our results, these findings further prove that hADSCs have potential as a potent antifibrotic agents for the treatment of liver fibrosis.
Emerging evidence suggested that MSCs could inhibit liver fibrosis by autophagy, TGF-β/smad, Wnt/β-catenin, LPS/TLR4, EMT/ERK1, PPARγ, NF-κB. However, the possible mechanisms of liver repair induced by hADSCs remain unclear. AGE-RAGE signaling is an important pathway in liver fibrosis formation and progression40. Several studies showed that RAGE expression in activated HSCs was increased41. Inhibition of RAGE signaling via different approaches attenuated liver injury and fibrosis42. In this study, our results showed that molecular mechanism underlying hADSCs-mediated hepatoprotection against CCl4-induced liver fibrosis is related to the inactivation of the AGE-RAGE pathway. Next, we investigate the effects of hADSCs on metabolic profile and the relevant pathways.
Clinical and experimental evidences have showed that Baicalin regulate choline metabolism via TFAM to attenuate cadmium-induced liver fibrosis43. In present study, hADSCs might increase the Baicalin content and further regulate choline metabolism. The previous reports also showed that CCl4 exposure easily causes the disorder of choline metabolism and can strongly induce degradation of lipid peroxides and oxidative damage due to excessive free radicals (ROS), resulting in the destruction of cell membrane structure44,45. In our study, the level of phosphocholine decreased significantly in hADSCs group, suggesting that hADSCs regulated phospholipid metabolism and was essential for the maintenance of normal function and properties of cell membrane, including its fluidity and permeability. In the present study, the taurine, 25-Hydroxycholesterol, palmitic acid and Docosapentaenoic acid (22n-3) were significantly decreased in hADSCs treatment group by enriched unsaturated fatty acids metabolism and primary bile acid biosynthesis pathways.
Recently, it is important that exosomes derived from MSCs were shown to contribute to intercellular communications mediating fibrogenic signaling46,47. Therefore, further detailed explorations are required to determine the key internal contents of hADSCs-Exosomes and specific molecular mechanisms on antifibrotic function. There are some other limitations should be acknowledged in our study. Our finding suggested that hADSCs might regulate lipid metabolism, which contributed to alleviating hepatocyte damage and fibrosis. However, how metabolic process is precisely regulated by hADSCs needs further elucidation. We suggested that the clinical efficacy of antifibrotic drugs related to lipid metabolites (aubercholic acid, polyene phosphatidylcholine, PPAR synthetic ligand thiazolidinediones) and whether lipid metabolites can be used as biomarkers for predicting the severity of hepatic fibrosis should be further explored. Far more than this, we predict that when combined with choline or antifibrotic candidate agents48, hADSCs would exhibit an increased antifibrotic effect.
In conclusion, our study identified the ability of hADSCs in ameliorating liver fibrosis progression. Improvements in lipid metabolism and inhibition of AGE-RAGE signaling pathway appeared to be the underlying mechanism that attenuated stellate cell activation and suppressed the progression of liver fibrosis. Furthermore, we also found the therapeutic effect of hADSCs were dose-dependent. Our findings provide important insights into the molecular mechanisms underlying the antifibrotic effects of hADSCs with a focus on metabolic homeostasis, and help inform the development of a safe and effective therapeutic. The current study is, therefore, an important step before the clinical usage of hADSCs for chronic liver fibrosis.
Conclusions
In this study, hADSCs could attenuate hepatic stellate cell activation and suppressed the progression of liver fibrosis in a dose-dependent manner. Though integrative analysis of transcriptomic and metabolomic data, we found that hADSCs could inhibit AGE-RAGE signaling pathway and improve lipid metabolism to against liver fibrosis. This study is the first to use metabolome-wide association with transcriptomics to interrogate underlying mechanisms of hADSCs, suggesting that hADSCs may have potential roles in the treatment of liver fibrosis or cirrhosis, and providing new ideas and theoretical foundations for the treatment and prevention of liver fibrosis.
Data availability
Raw RNA-seq data was deposited in Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) database with the data set identifier GSE199732. Other inquiries can be directed at the corresponding author.
References
Asrani, S. K., Devarbhavi, H., Eaton, J. & Kamath, P. S. Burden of liver diseases in the world. J. Hepatol. 70, 151–171. https://doi.org/10.1016/j.jhep.2018.09.014 (2019).
Bataller, R. & Brenner, D. A. Liver fibrosis. J. Clin. Investig. 115, 209–218. https://doi.org/10.1172/jci200524282 (2005).
Jiang, J. X. & Torok, N. J. Liver injury and the activation of the hepatic myofibroblasts. Curr. Pathobiol. Rep. 1, 215–223. https://doi.org/10.1007/s40139-013-0019-6 (2013).
Mazza, G., Al-Akkad, W. & Rombouts, K. Engineering in vitro models of hepatofibrogenesis. Adv. Drug Deliv. Rev. 121, 147–157. https://doi.org/10.1016/j.addr.2017.05.018 (2017).
Asmani, M. et al. Fibrotic microtissue array to predict anti-fibrosis drug efficacy. Nat. Commun. 9, 2066. https://doi.org/10.1038/s41467-018-04336-z (2018).
Dutkowski, P. et al. The model for end-stage liver disease allocation system for liver transplantation saves lives, but increases morbidity and cost: A prospective outcome analysis. Liver Transplant. 17, 674–684. https://doi.org/10.1002/lt.22228 (2011).
Sabry, D., Mohamed, A., Monir, M. & Ibrahim, H. A. The effect of mesenchymal stem cells derived microvesicles on the treatment of experimental CCL4 induced liver fibrosis in rats. Int. J. Stem Cells 12, 400–409. https://doi.org/10.15283/ijsc18143 (2019).
Tsuchiya, A. et al. Mesenchymal stem cell therapies for liver cirrhosis: MSCs as “conducting cells” for improvement of liver fibrosis and regeneration. Inflamm. Regen. 39, 18. https://doi.org/10.1186/s41232-019-0107-z (2019).
Cao, Y., Ji, C. & Lu, L. Mesenchymal stem cell therapy for liver fibrosis/cirrhosis. Ann. Transl. Med. 8, 562. https://doi.org/10.21037/atm.2020.02.119 (2020).
Shi, M. et al. Mesenchymal stem cell therapy in decompensated liver cirrhosis: A long-term follow-up analysis of the randomized controlled clinical trial. Hepatol. Int. 15, 1431–1441. https://doi.org/10.1007/s12072-021-10199-2 (2021).
Volarevic, V. et al. Mesenchymal stem cell-derived factors: Immuno-modulatory effects and therapeutic potential. Biofactors 43, 633–644. https://doi.org/10.1002/biof.1374 (2017).
Koch, M. et al. Isogeneic MSC application in a rat model of acute renal allograft rejection modulates immune response but does not prolong allograft survival. Transplant Immunol. 29, 43–50. https://doi.org/10.1016/j.trim.2013.08.004 (2013).
Huang, K.-C. et al. Transplantation with GXHPC1 for liver cirrhosis: Phase 1 trial. Cell Transplant. 28, 100–111. https://doi.org/10.1177/0963689719884885 (2019).
Yadav, P. et al. Therapeutic potential of stem cells in regeneration of liver in chronic liver diseases: Current perspectives and future challenges. Pharmacol. Therap. 253, 563. https://doi.org/10.1016/j.pharmthera.2023.108563 (2024).
Rodriguez-Ortigosa, C. M. et al. Hepatocyte growth factor gene-modified adipose-derived mesenchymal stem cells ameliorate radiation induced liver damage in a rat model. PLoS ONE 9, 4670. https://doi.org/10.1371/journal.pone.0114670 (2014).
Han, H. S. et al. Human adipose stem cell-derived extracellular nanovesicles for treatment of chronic liver fibrosis. J. Control Release 320, 328–336. https://doi.org/10.1016/j.jconrel.2020.01.042 (2020).
Hou, W. & Syn, W. K. Role of metabolism in hepatic stellate cell activation and fibrogenesis. Front. Cell Dev. Biol. 6, 150. https://doi.org/10.3389/fcell.2018.00150 (2018).
Khomich, O., Ivanov, A. V. & Bartosch, B. Metabolic hallmarks of hepatic stellate cells in liver fibrosis. Cells 9, 24. https://doi.org/10.3390/cells9010024 (2019).
Barcena-Varela, M. et al. Epigenetic mechanisms and metabolic reprogramming in fibrogenesis: Dual targeting of G9a and DNMT1 for the inhibition of liver fibrosis. Gut 70, 388–400. https://doi.org/10.1136/gutjnl-2019-320205 (2021).
Liu, R. et al. Cholangiocyte-derived exosomal long noncoding RNA H19 promotes hepatic stellate cell activation and cholestatic liver fibrosis. Hepatology 70, 1317–1335. https://doi.org/10.1002/hep.30662 (2019).
Thomou, T. et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542, 450–455. https://doi.org/10.1038/nature21365 (2017).
Ahmadian Kia, N. et al. Comparative analysis of chemokine receptor’s expression in mesenchymal stem cells derived from human bone marrow and adipose tissue. J. Mol. Neurosci. 44, 178–185. https://doi.org/10.1007/s12031-010-9446-6 (2011).
Ye, L. et al. The regulation of acetylation and stability of HMGA2 via the HBXIP-activated Akt–PCAF pathway in promotion of esophageal squamous cell carcinoma growth. Nucleic Acids Res. 48, 4858–4876. https://doi.org/10.1093/nar/gkaa232 (2020).
Zhang, C. et al. Foundation and clinical evaluation of a new method for detecting SARS-CoV-2 antigen by fluorescent microsphere immunochromatography. Front. Cell. Infect. Microbiol. 10, 837. https://doi.org/10.3389/fcimb.2020.553837 (2020).
Massey, V. L. et al. The hepatic “matrisome” responds dynamically to injury: Characterization of transitional changes to the extracellular matrix in mice. Hepatology 65, 969–982. https://doi.org/10.1002/hep.28918 (2017).
Li, T. et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 22, 845–854. https://doi.org/10.1089/scd.2012.0395 (2013).
Jiang, L. et al. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate acute liver failure by reducing the activity of the NLRP3 inflammasome in macrophages. Biochem. Biophys. Res. Commun. 508, 735–741. https://doi.org/10.1016/j.bbrc.2018.11.189 (2019).
Xie, Z. et al. Exosome-delivered CD44v6/C1QBP complex drives pancreatic cancer liver metastasis by promoting fibrotic liver microenvironment. Gut 71, 568–579. https://doi.org/10.1136/gutjnl-2020-323014 (2022).
Bruden, D. J. T. et al. Risk of end-stage liver disease, hepatocellular carcinoma, and liver-related death by fibrosis stage in the hepatitis C Alaska Cohort. Hepatology 66, 37–45. https://doi.org/10.1002/hep.29115 (2017).
Deng, L. et al. Double-layered protein nanoparticles induce broad protection against divergent influenza A viruses. Nat. Commun. 9, 359. https://doi.org/10.1038/s41467-017-02725-4 (2018).
Huang, Q. et al. Changes in volatile flavor of yak meat during oxidation based on multi-omics. Food Chem. 371, 131103. https://doi.org/10.1016/j.foodchem.2021.131103 (2022).
Puche, J. E., Saiman, Y. & Friedman, S. L. Hepatic stellate cells and liver fibrosis. Compr. Physiol. 3, 1473–1492. https://doi.org/10.1002/cphy.c120035 (2013).
Wilson, E. M., Rosenthal, E. S., Kattakuzhy, S., Tang, L. & Kottilil, S. Clinical laboratory testing in the era of directly acting antiviral therapies for hepatitis C. Clin. Microbiol. Rev. 30, 23–42. https://doi.org/10.1128/CMR.00037-16 (2017).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951. https://doi.org/10.1002/pro.3715 (2019).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30. https://doi.org/10.1093/nar/28.1.27 (2000).
Chen, L., Brenner, D. A. & Kisseleva, T. Combatting fibrosis: Exosome-based therapies in the regression of liver fibrosis. Hepatol. Commun. 3, 180–192. https://doi.org/10.1002/hep4.1290 (2019).
Lou, G., Chen, Z., Zheng, M. & Liu, Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp. Mol. Med. 49, e346. https://doi.org/10.1038/emm.2017.63 (2017).
Hu, C., Zhao, L. & Li, L. Current understanding of adipose-derived mesenchymal stem cell-based therapies in liver diseases. Stem Cell Res. Ther. 10, 199. https://doi.org/10.1186/s13287-019-1310-1 (2019).
Hou, Y. et al. Adipose-derived stem cells alleviate liver injury induced by type 1 diabetes mellitus by inhibiting mitochondrial stress and attenuating inflammation. Stem Cell Res. Ther. 13, 132. https://doi.org/10.1186/s13287-022-02760-z (2022).
Urtasun, R. et al. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin alpha(V)beta(3) engagement and PI3K/pAkt/NFkappaB signaling. Hepatology 55, 594–608. https://doi.org/10.1002/hep.24701 (2012).
Guimarães, E. L., Empsen, C., Geerts, A. & van Grunsven, L. A. Advanced glycation end products induce production of reactive oxygen species via the activation of NADPH oxidase in murine hepatic stellate cells. J. Hepatol. 52, 389–397 (2010).
Park, J.-W. et al. Increased expression of S100B and RAGE in a mouse model of bile duct ligation-induced liver fibrosis. J. Korean Med. Sci. 36, 90. https://doi.org/10.3346/jkms.2021.36.e90 (2021).
Sun, J. et al. Baicalin and N-acetylcysteine regulate choline metabolism via TFAM to attenuate cadmium-induced liver fibrosis. Phytomedicine 125, 337. https://doi.org/10.1016/j.phymed.2024.155337 (2024).
Lin, B. et al. Studies of single-walled carbon nanotubes-induced hepatotoxicity by NMR-based metabonomics of rat blood plasma and liver extracts. Nanoscale Res. Lett. 8, 236. https://doi.org/10.1186/1556-276X-8-236 (2013).
Zeng, B. et al. Anoectochilus roxburghii polysaccharide prevents carbon tetrachloride-induced liver injury in mice by metabolomic analysis. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1152, 122202 (2020).
Qu, Y. et al. Exosomes derived from miR-181-5p-modified adipose-derived mesenchymal stem cells prevent liver fibrosis via autophagy activation. J. Cell. Mol. Med. 21, 2491–2502. https://doi.org/10.1111/jcmm.13170 (2017).
Lou, G. et al. MiR-122 modification enhances the therapeutic efficacy of adipose tissue-derived mesenchymal stem cells against liver fibrosis. J. Cell. Mol. Med. 21, 2963–2973. https://doi.org/10.1111/jcmm.13208 (2017).
Roehlen, N., Crouchet, E. & Baumert, T. F. Liver fibrosis: Mechanistic concepts and therapeutic perspectives. Cells 9, 875. https://doi.org/10.3390/cells9040875 (2020).
Acknowledgements
The authors are grateful to everyone who helped us in this study. They are grateful to Professor Zhang for correcting the English in the manuscript. They acknowledge the highly skilled technical help of Suzhou PANOMIX Biomedical Technology Co., Ltd.
Funding
This work was supported by the Scientific Research Projects from Wuhan Municipal Health Commission (WX23Q27).
Author information
Authors and Affiliations
Contributions
GJ, EZ and CL contributed to the study conception and design. GJ, ZZ, XW and QG performed material preparation, experiments, data collection and analysis. ZZ and XW analyzed data. QG and EZ provided experimental materials. The first draft of the manuscript was written by GJ. EZ and CL reviewed and revised manuscript. ZZ, XW and QG reviewed the paper and gave suggestions on the revision of the article. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ji, G., Zhang, Z., Wang, X. et al. Comprehensive evaluation of the mechanism of human adipose mesenchymal stem cells ameliorating liver fibrosis by transcriptomics and metabolomics analysis. Sci Rep 14, 20035 (2024). https://doi.org/10.1038/s41598-024-70281-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70281-1
- Springer Nature Limited