Abstract
Although immune checkpoint inhibitors (ICIs) are effective in some patients with salivary gland carcinoma (SGC), biomarkers which predict the efficacy and prognosis of SGC patients treated with pembrolizumab have not been identified. We conducted a multi-institutional retrospective cohort study to evaluate the efficacy and safety of pembrolizumab monotherapy in patients with recurrent and/or metastatic SGC and to determine optimal cut-off values of the combined positive score (CPS) and tumor proportion score (TPS) as numerical expression levels of programmed death-ligand 1 (PD-L1), which predict the efficacy of pembrolizumab. Furthermore, we investigated the association of patient characteristics and hematological markers with clinical outcomes, including overall response rate (ORR), progression-free survival (PFS), and overall survival (OS). From 2016 to 2021, 27 patients were included in the analysis. ORR of SGC was 25.9%. Optimal cut-off values of CPS and TPS were 15 and 25%, respectively. ORRs of CPS-high and TPS-high were 55.6 and 75.0%, respectively, and significantly higher than those of CPS-low and TPS-low. Furthermore, patients with a low platelet-lymphocyte ratio (PLR) had a significantly longer PFS. No grade 4 or greater adverse events were observed. This study demonstrated the efficacy and safety of pembrolizumab monotherapy and identified optimal cut-off values of CPS and TPS.
Similar content being viewed by others
Introduction
Salivary gland carcinoma (SGC) is a relatively rare cancer that accounts for 0.3% of all cancers1. According to a histological classification of the World Health Organization, SGCs are classified into more than 20 histological types, each with different biological characteristics and prognosis2. Cytotoxic chemotherapy has been indicated for recurrent and/or metastatic (R/M) disease as a conventional systemic therapy, regardless of histopathological type3,4. While recent studies have revealed the efficacy of targeted therapy for SGC, including anti-androgen receptor (AR), HER2, and NTRK therapies5,6,7,8, the majority of patients with R/M SGC are not candidates for these targeted therapies.
The use of immune checkpoint inhibitors (ICIs) in SGC has been extensively studied over the past few years9,10,11,12,13,14. The latest NCCN guidelines recommend pembrolizumab, an anti-PD-1 antibody, for SGC with Microsatellite Instability (MSI)-High or Tumor Mutational Burden (TMB)-High8,15. In contrast, other studies note that the efficacy of ICIs is theoretically greater against tumors with high PD-L1 expression16,17,18. For head and neck squamous cell carcinoma, dosing is generally determined based on the results of the combined positive score (CPS) divided by evidence of therapeutic efficacy16. In SGC, the KEYNOTE-158 trial reported that PD-L1-positive patients (CPS ≥ 1) had a better response rate than PD-L1-negative patients, suggesting that CPS may be a biomarker for pembrolizumab in SGC9. However, the impact of PD-L1 expression on the efficacy of pembrolizumab in SGC patients remains controversial.
This study aimed to clarify the efficacy and safety of pembrolizumab monotherapy in R/M SGC in a real-world setting, and to measure CPS and tumor proportion score (TPS) as real values for PD-L1 expression to identify their optimal cut-off values for this treatment. Further, regarding treatment-related biomarkers, we investigated the association of patient background factors and hematological markers with clinical outcomes of pembrolizumab.
Results
Patient characteristics and treatment sequences
Twenty-seven patients with R/M SGC were treated with pembrolizumab monotherapy during the study period. No patients received pembrolizumab with chemotherapy concurrently. Patient characteristics are summarized in Table 1. Data cutoff date was April 30, 2021, and median follow-up period for all patients was 6.9 months (range, 2.1–58.8 months). The most common histopathological type was salivary duct carcinoma (SDC) (n = 15; 56%). Sixteen patients (59%) had received chemotherapy before pembrolizumab, all of whom had disease progression within 6 months prior to pembrolizumab initiation. Median follow-up period of patients with SDC was 6.9 months (range, 2.1–14.0 months). While 4 patients received pembrolizumab as the first-line treatment, 11 of 15 (73%) patients with SDC had received chemotherapy and then had disease progression prior to pembrolizumab monotherapy. Trastuzumab was administered to 6 patients with HER2-positive SGC.
At the end of the study period, 10 patients (37.0%) continued to receive pembrolizumab whereas 17 patients (63.0%) discontinued treatment due to progressive disease (PD) (n = 16; 94.1%) or immune-related adverse event (irAE) (n = 1; 5.9%). The median number of cycles of pembrolizumab administered was 4 (range, 1–22) in all patients and 4 (range, 1–14) in patients with SDC. Twelve patients (44%), including 10 SDC patients, received 1 or more sequential chemotherapy regimens after pembrolizumab treatment.
Response and survival outcomes
The efficacy of pembrolizumab therapy is shown in Table 2. In all 27 patients, 1 (4%), 6 (22%), 5 (19%) and 15 (56%) patients showed CR, PR, stable disease (SD) and PD, respectively. Overall response rate (ORR) was 25.9% [95% confidence interval (95%CI), 11.1–46.3], and clinical benefit rate (CBR) and disease control rate (DCR) were 33.3% (95%CI, 16.5–54.0) and 44.4% (95%CI, 25.5–64.7), respectively. The Kaplan–Meier survival curves of progression-free survival (PFS) and overall survival (OS) of all patients are shown in Fig. 1. Median PFS and median OS were 2.6 months (95% CI, 1.9–not estimable [NE]) and not reached (NR) (95% CI, 11.5–NE), and 1-year PFS and OS were 30.3% (95% CI, 11.1–52.3) and 75.1% (95% CI, 33.2–92.8), respectively.
Response and survival outcomes by histological type
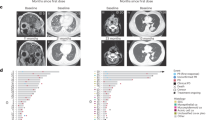
The therapeutic effects observed in 15 SDC patients were as follows: ORR, 26.7% (95%CI, 7.8–55.1); CBR, 33.3% (95%CI, 11.8–61.6); DCR, 40.0% (95%CI, 16.3–67.7); median PFS, 2.2 months (95% CI, 1.3–NE); and median OS, NR (95% CI, NE–NE) (see Supplementary Fig S1 online). None of the six patients with adenoid cystic carcinoma (AdCC) showed a response, and three of these AdCC patients died due to disease progression. Figure 2 shows images of the tumor before and during pembrolizumab monotherapy in a representative patient.
Representative images of the tumor before and during pembrolizumab monotherapy in a patient with pancreatic metastasis of salivary duct carcinoma. (a) Pre-treatment of the patient with pancreatic metastasis. (b) Tumor shrinkage observed 63 days after the initiation of pembrolizumab monotherapy. (c) Tumor disappeared 154 days after the initiation of pembrolizumab monotherapy.
Safety
All AEs reported are listed in Table 3. Nine patients (33.3%) experienced irAE during treatment. One patient (3.7%) showed grade 3 irAE diabetes. No grade 4 or greater irAEs were observed. One patient discontinued treatment due to the irAE of grade 2 uveitis.
PD-L1 status
CPS and TPS are shown in Supplementary Table S1 and S2 online. In all 25 cases with numerical evaluation, median CPS and TPS was 5 (range, 0–180) and < 1% (range, 0–100), respectively. The optimal cut-off value of CPS and TPS against objective response was 15 and 25%, respectively (see Supplementary Fig S2 online). Using these cutoffs to divide patients into 2 groups, numbers of CPS-high (CPS-H, CPS ≥ 15) vs. CPS-low (CPS-L, CPS < 15) SGC, and TPS-high (TPS-H, TPS ≥ 25%) vs. TPS-low (TPS-L, TPS < 25%) SGC were 9 (36%) vs. 16 (64%), and 4 (16%) vs. 21 (84%), respectively. ORR of pembrolizumab monotherapy in the CPS-H SGC group was significantly better than that in the CPS-L SGC group (56% vs. 13%), and ORR in the TPS-H SGC group was also significantly better than that in the TPS-L SGC group (75% vs. 19%; Table 4). Median PFS of CPS-H SGC and CPS-L SGC was not reached and 2.3 months, respectively, while that of TPS-H SGC and TPS-L SGC was not reached and 2.2 months, respectively. There were no significant differences in PFS by CPS or TPS status (Fig. 3).
Similarly, in 14 patients with SDC, median CPS and TPS was 10% (range, 1 – 100%) and < 1% (range, 0 – 70%), respectively. Numbers of CPS-H vs. CPS-L SDC and TPS-H vs. TPS-L SDC were 7 (50%) vs. 7 (50%), and 2 (14%) vs. 12 (86%), respectively. ORRs for pembrolizumab monotherapy between CPS-H SDC (43%) and CPS-L SDC (14%), and those between TPS-H SDC (50%) and TPS-L SDC (25%) showed no significant differences (Supplementary Table S3). Median PFS of CPS-H SDC and CPS-L SDC was 2.6 months and 2.0 months, respectively, and median PFS of TPS-H SDC and TPS-L SDC was 2.6 months and 2.2 months, respectively (Supplementary Table S3). Neither CPS nor TPS status significantly correlated with PFS in SDC patients (Supplementary Figure S4).
In 6 patients with AdCC, median CPS and TPS was low at 2.5 (range 0–5) and < 1% (range, 0–1%), respectively. SDC patients had significantly higher CPS than AdCC patients (Supplementary Figure S3). In one patient each with lymphoepithelial carcinoma and acinic cell carcinoma, CPS was high at 180 and 70, and TPS was 100% and 30%, respectively. One patient with adenocarcinoma was negative for CPS and TPS.
Impact of other biomarkers on clinical response
In all 27 patients, median neutrophil–lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR) and lymphocyte-monocyte ratio (LMR) was 3.3 (range 1.1–12.7), 194.1 (range 68.3–1331.3) and 3.1 (range 1.1–12.9), respectively (Table 1). The optimal cut-off value of NLR, PLR and LMR against objective response was 3.0, 232.4 and 4.5, respectively. We divided the patients accordingly into high and low groups to clarify the impact of NLR, PLR, and LMR on clinical outcomes. Seventeen (63%), 9 (33%), and 9 (33%) patients were categorized in the NLR-, PLR-, and LMR-high groups, and 10 (37%), 18 (67%), and 18 (67%) patients were categorized in the NLR-, PLR-, and LMR-low groups, respectively. Regarding the modified Glasgow Prognostic Score (mGPS), 24 (89%) and three (11%) patients were assigned scores of 0 and 1, respectively. No patient was assigned a score of 2.
Regarding hematological biomarkers, patients with higher PLR had a significantly worse PFS than those with lower PLR among all patients [Table 4, hazard ratio (HR) = 2.8; 95% CI, 1.0–7.8; p = 0.049].
Discussion
Here, we demonstrated the efficacy of immunotherapy with pembrolizumab in patients with R/M SGC, with an acceptable rate of irAE. Compared to patients with CPS-L and TPS-L SGC, patients with CPS-H and TPS-H SGC had significantly higher ORRs, respectively.
In our cohort, the ORR of pembrolizumab in patients with SGC and SDC was 25.9 and 26.7%, respectively. In previous clinical trials, the ORR of pembrolizumab monotherapy in SGC ranged from 4.6% – 11.5%8,9,13. Nivolumab has shown similar efficacy in R/M SGC10,12. The large differences in ORR between the previous and our present data are likely due to the abundance of SDCs with high PD-L1 expression, despite the difficulty of direct comparison given that many previous reports reported PD-L1 positivity only. We counted numerical scores for CPS and TPS in most patients, and determined optimal cut-off values of 15 and 25%, respectively. The ORRs of pembrolizumab in patients with R/M SGC with CPS-H and TPS-H were 55.6% and 75.0%, all of which were significantly higher than those with CPS-L and TPS-L, respectively. Higher CPS and TPS also tend to be associated with longer PFS in patients with R/M SGC treated with pembrolizumab. No patient with AdCC had high PD-L1 expression, and none responded to pembrolizumab. In contrast, although a good response was observed in two patients with SDC and myoepithelial carcinoma with no or low PD-L1 expression, a poor response was observed in the patient with the highest PD-L1 expression in SDC (Table S2). These results suggest that, despite their limitations, CPS and TPS may be useful biomarkers for the selection of pembrolizumab for SGC.
In addition to PD-L1, other factors in the tumor immune microenvironment (TIME) in SGC are important and can be targeted. The baseline TIME is heterogeneous among different histological types of SGC19,20,21: AdCC and myoepithelial carcinomas generally possess a low tumor mutational burden while SDC is reported to possess a high mutational burden and a disproportionately higher degree of immune infiltration, which is greater indeed than even those of lung adenocarcinoma and melanoma19. Similarly, heterogeneity exists in PD-L1 expression by histological type of SGCs. In particular, SDC express higher levels of PD-L1 than other subtypes of SGC20. In our recent analysis, 93 (53.1%) of 175 SDCs were positive for CPS (≥ 1), and elevated expression levels of PD-L1, CD8, FOXP3, PD1, CTLA4, and LAG3 in TIME were associated with poor prognosis21. Taken together with the treatment results of our present and previous studies10,14, SDC might respond better to ICIs than other types of SGC. Nevertheless, given that the response rate of ICIs in SGC patients is still unsatisfactory, the development of combination treatment with multiple ICIs and with an ICI and other types of antitumor drugs is warranted. In a phase 2 trial of nivolumab plus ipilimumab14, the ORR for non-AdCC SGC patients was 16%, suggesting promising efficacy in this cohort, especially for SDC. Results from ongoing trials of pembrolizumab plus docetaxel (NCT03360890) and pembrolizumab plus pemetrexed (NCT04895735) in R/M SGCs are awaited.
Hematological, inflammatory, and nutritional biomarkers predict prognosis in patients with diverse histological types of neoplasia treated with ICI22,23,24,25,26. These are standardized markers which are routinely available in daily practice. Among them, increased PLR was associated with worse prognosis in patients with non-small-cell lung cancer, urothelial carcinoma, and upper gastrointestinal cancer treated with pembrolizumab27,28,29,30, as the decreased number of lymphocytes reflects attenuated antitumor immunity while activated platelets protect tumor cells from immune elimination within the circulatory system and support the establishment of secondary lesions31. In the present study, the ORR of pembrolizumab in patients with R/M SGC with low PLR was 38.9%, which was higher than the 0% in those with high PLR. Patients with low PLR also had a significantly longer PFS. These results suggest that PLR might be a predictive marker of clinical outcome in patients with R/M SGC treated with pembrolizumab.
The frequency of irAE in patients with SGC in our cohort was consistent with previous reports and no unexpected toxicity was observed. Almost all patients were able to continue treatment without discontinuation due to irAEs, except for one patient with grade 2 uveitis.
Several limitations of this study should be addressed. First, the study was retrospective and the sample size was modest, which resulted in low statistical power and might have produced a degree of selection bias. This should be considered against the fact that markedly few patients with SGC are treated with pembrolizumab, and that we nevertheless successfully included 27 patients from multiple institutions in Japan. Second, the impact of HER2 status and HER2-targeted therapies on pembrolizumab therapy has not been fully investigated. Third, the follow-up period after pembrolizumab treatment was not long enough to assess OS. However, this study mainly addressed ORR and PFS, since these outcomes directly reflect the efficacy of pembrolizumab in SGC patients. Our future goal is to assess pembrolizumab’s impact on subsequent chemotherapy, which might affect time to second disease progression (PFS2), and OS32.
In conclusion, this study demonstrated the efficacy and safety of pembrolizumab monotherapy in patients with R/M SGC. We identified optimal cut-off values of CPS and TPS in predicting the clinical outcomes of this treatment, where CPS-H and TPS-H SGC had significantly better ORR than CPS-L and TPS-L SGC. We also showed that PLR was significantly associated with PFS in patients with SGC.
Patients and methods
This study was conducted under a retrospective cohort design in 27 patients with R/M SGC treated with pembrolizumab from March 2016 to April 2021 at seven facilities in Japan, following approval from the ethics committee of the participating institutions (Approval number of each institution: International University of Health and Welfare, Mita Hospital, 5–21-17; Nagoya City University Hospital, 70–00-0145; Tokyo Medical University Hospital, T2021-0142; Kitasato University Hospital, C21-092; Tokai University Hospital, 21R105; Niigata University Hospital, 2021–0086; Yokohama City University Hospital, B210500037). This study was also performed in accordance with the Declaration of Helsinki. Regarding consent, informed consent was waived by the Institutional Review Boards of all participating institutions. We provided information regarding the research plan via a web-based public release, and patients and their families were provided an opportunity to opt-out of the study under the policy of the Japanese government.
The charts of all 27 patients who received pembrolizumab monotherapy were retrospectively reviewed, and information on patient characteristics, treatment modality and clinical outcomes was collected. Twenty-five tumor samples were pathologically reviewed and evaluated by a pathologist with expertise in SGCs (T.N.). Carcinoma ex pleomorphic adenoma was classified according to individual carcinoma components. We excluded patients for whom therapeutic effect could not be evaluated.
Treatment and follow-up
Pembrolizumab was administered at 200 mg/body every 3 weeks. After the start of treatment, imaging examination by CT or MRI was performed every 6–9 weeks. The efficacy of pembrolizumab was evaluated with regard to ORR, CBR (defined as the percentage of patients who achieved CR, PR, or SD for at least 24 weeks), DCR (defined as the percentage of patients who achieved CR, PR, or SD regardless of duration), median PFS, and median OS. Treatment efficacy was evaluated according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1). PFS was defined as the time from the start of pembrolizumab to the diagnosis of PD. OS was defined as the period from the day of initiation of pembrolizumab to the day of death regardless of cause or the day of last follow-up. AEs were assessed according to CTCAE version 5.0 up to 30 days after the last administration of pembrolizumab. Administration of pembrolizumab was terminated when PD was confirmed, the patient experienced unacceptable adverse events, or at patient request. After pembrolizumab discontinuation, additional treatment with other agents was permitted.
Evaluation of biomarkers
To clarify the impact of PD-L1 expression on survival and response, we evaluated CPS and TPS, each categorized into two groups by optimum cut-off value. PD-L1 expression in samples was assessed using the PD-L1 IHC 22C3 pharmDx assay (Agilent Technologies, Carpinteria, CA, USA) and characterized by CPS and TPS through central pathological review (H.H.). CPS and TPS were defined as the number of PD-L1-positive cells (tumor cells, lymphocytes, and macrophages) divided by the total number of tumor cells × 100 and as the number of PD-L1-positive tumor cells divided by the total number of tumor cells × 100, respectively. Optimum cut-off values of CPS and TPS were evaluated using area under the receiver operating characteristic curve (AUROC) against objective response.
Additionally, we also examined associations between survival or response and age, sex, Eastern Cooperative Oncology Group (ECOG) performance status (PS), irAE, histopathological type (SDC or non-SDC), previous therapies, and hematological markers. As hematological markers, we evaluated NLR, PLR, LMR and mGPS as examined in peripheral blood just before the start of pembrolizumab treatment. These markers were categorized into two groups by optimum cut-off value evaluated using AUROC against objective response. Regarding mGPS, patients with both elevated C-reactive protein (CRP; > 1.0 mg/dL) and decreased albumin (Alb; < 3.5 g/dL) were assigned a score of 2; those with an elevated CRP (> 1.0 mg/dl) and non-decreased Alb (≥ 3.5 g/dl) were assigned a score of 1; and those with a non-elevated CRP (≤ 1.0 mg/dl) were assigned a score of 0.
Statistical analyses
OS and PFS were estimated by the Kaplan–Meier product-limit method and tested by the log-rank test in both the SGC and SDC groups. To evaluate the impact of clinical factors, including CPS and TPS, on PFS we estimated HRs and 95% CIs using univariate Cox proportional hazards models. Additionally, to evaluate the impact of these factors on objective response, we performed Fisher's exact test. All statistical analyses were performed using STATA version 16 (Stata Corp., College Station, TX, USA). All tests were two-sided, and p-values < 0.05 were considered statistically significant.
Data availability
The datasets generated in the current study are available from the corresponding author on request.
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 71, 209249 (2021).
El-Naggar, A., Chan, J. K. C. & Grandis, J. WHO classification of head neck tumours, 4th edition, volume 9; 7 tumours of salivary glands. World Health Organization 2017: pp 159. (2017).
Geiger, J. L. et al. Management of salivary gland malignancy: ASCO guideline. J. Clin. Oncol. Official J Am. Soc. Clin. Oncol. 39, 1909–1941 (2021).
van Herpen, C. et al. Salivary gland cancer: ESMO-European reference network on rare adult solid cancers (EURACAN) clinical practice guideline for diagnosis, treatment and follow-up. ESMO Open. 7, 100602 (2022).
Takahashi, H. et al. Phase II trial of trastuzumab and docetaxel in patients with human epidermal growth factor receptor 2-positive salivary duct carcinoma. J. Clin. Oncol. Official J. Am. Soc. Clin. Oncol. 37, 125–134 (2019).
Fushimi, C. et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann. Oncol. Official J. Eur. Soc. Med. Oncol. 29, 979–984 (2018).
Le, X. et al. Larotrectinib treatment for patients with TRK fusion-positive salivary gland cancers. Oncol. https://doi.org/10.1093/oncolo/oyac080 (2022).
Marabelle, A. et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21, 1353–1365 (2020).
Even, C. et al. Evaluation of pembrolizumab monotherapy in patients with previously treated advanced salivary gland carcinoma in the phase 2 KEYNOTE-158 study. Eur. J.Cancer 171, 259–268 (2022).
Nagatani, Y. et al. A phase II trial of nivolumab for patients with platinum-refractory recurrent or metastatic salivary gland cancer. J. Clin. Oncol. Official J. Am. Soc. Clin. Oncol. 41, 6092 (2023).
Niwa, K. et al. Multicentre, retrospective study of the efficacy and safety of nivolumab for recurrent and metastatic salivary gland carcinoma. Sci Rep. 10, 16988 (2020).
Fayette, J., Even, C., Digue, L., Geoffrois, L. & Rolland, F. NISCAHN: A phase II trial of nivolumab in patients with salivary gland carcinoma (Unicancer ORL-08). BMJ Oncol. https://doi.org/10.1136/bmjonc-2023-000065 (2023).
Cohen, R. B. et al. Pembrolizumab for the treatment of advanced salivary gland carcinoma: findings of the phase 1b KEYNOTE-028 study. Am. J. Clin. Oncol. 41, 1083–1088 (2018).
Vos, J. L. et al. Nivolumab plus ipilimumab in advanced salivary gland cancer: A phase 2 trial. Nat. Med. https://doi.org/10.1038/s41591-023-02518-x (2023).
. NCCN Clinical Practice Guidelines in Oncology, Head and Neck Cancers Version 2.2024 https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed February, 29 2024.
Burtness, B. et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet (London, England). 394, 1915–1928 (2019).
Cortes, J. et al. Pembrolizumab plus chemotherapy in advanced triple-negative breast cancer. N. Engl. J. Med. 387, 217–226 (2022).
Hui, R. et al. Pembrolizumab as first-line therapy for patients with PD-L1-positive advanced non-small cell lung cancer: A phase 1 trial. Ann. Oncol. Official J. Eur. Soc. Med. Oncol. 28, 874–881 (2017).
Linxweiler, M. et al. The immune microenvironment and neoantigen landscape of aggressive salivary gland carcinomas differ by subtype. Clin. Cancer Res. Official J. Am. Assoc. Cancer Res. 26, 2859–2870 (2020).
Mukaigawa, T. et al. Programmed death ligand-1 expression is associated with poor disease free survival in salivary gland carcinomas. J. Surg. Oncol. 114, 36–43 (2016).
Hirai, H. et al. Prognostic value and clinicopathological roles of the tumor immune microenvironment in salivary duct carcinoma. Virchows Arch. 483, 367–379 (2023).
Liu, C. et al. Prognostic value of nutritional and inflammatory markers in patients with hepatocellular carcinoma who receive immune checkpoint inhibitors. Oncol. Lett. 26, 437 (2023).
Oka, T. et al. Prognostic values of systemic inflammation and nutrition-based prognostic indices in oropharyngeal carcinoma. Laryngoscope Investing. Oto. 8, 675–685 (2023).
Jiang, Y. et al. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: A systematic review and meta-analysis. BMJ Open 11, e048324 (2021).
Portale, G., Bartolotta, P., Azzolina, D., Gregori, D. & Fiscon, V. Prognostic role of platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte, and lymphocyte-to-monocyte ratio in operated rectal cancer patients: Systematic review and meta-analysis. Langenbeck Arch. Surg. 408, 85 (2023).
Matsuki, T. et al. Hematological predictive markers for recurrent or metastatic squamous cell carcinomas of the head and neck treated with nivolumab: A multicenter study of 88 patients. Cancer Medi. 9, 5015–5024 (2020).
Stares, M. et al. Biomarkers of systemic inflammation predict survival with first-line immune checkpoint inhibitors in non-small-cell lung cancer. ESMO Open 7, 100445 (2022).
Zhao, X. et al. Prognostic value of hematologic parameters in advanced non-small cell lung cancer patients receiving anti-PD-1 inhibitors. Front. immunol. 13, 1003581 (2022).
Kadono, Y. et al. Blood cell count biomarkers predicting efficacy of pembrolizumab as second-line therapy for advanced urothelial carcinoma. Anticancer Res. 41, 1599–1606 (2021).
Booka, E. et al. Neutrophil-to-lymphocyte ratio to predict the efficacy of immune checkpoint inhibitor in upper gastrointestinal cancer. Anticancer Res. 42, 2977–2987 (2022).
Gay, L. J. & Felding-Habermann, B. Contribution of platelets to tumour metastasis. Nat. Rev. Cancer. 11, 123–134 (2011).
Harrington, K. J. et al. Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: Updated results of the phase III KEYNOTE-048 study. J. Clin. Oncol. Official J. Am. Soc. Clin. Oncol. 41, 790–802 (2023).
Acknowledgements
This work was supported by a JSPS Grant-in-Aid for Scientific Research (C) to Dr. Yuichiro Tada [Grant number 21K09616], Dr. Daisuke Kawakita [Grant number 20K10508], Dr. Toshitaka Nagao [Grant number 23K06432], Dr. Hideaki Hirai [Grant number 22K06969], Dr. Hideaki Takahashi [Grant number 19K09873], and Dr. Takashi Matsuki [Grant number 20K07597]. The authors thank Guy Harris of Dmed (https://dmed.co.jp) for the English language review of this paper.
Author information
Authors and Affiliations
Contributions
Study concept and design: Y.T. and T.M.; acquisition of data: T.M., H.T., T.O., A.S., Y.U., H.T., K.H., K.M., R.S., M.Y. and Y.A.; analysis and interpretation of data: Y.T., T.M., D.K. and H.T.; statistical analysis: D.K., T.M. and Y.T.; drafting of the manuscript: T.M., Y.T., D.K. and H.T.; review and approval of manuscript: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Matsuki, T., Kawakita, D., Takahashi, H. et al. PD-L1 expression as a predictive biomarker in patients with recurrent or metastatic salivary gland carcinoma treated with pembrolizumab. Sci Rep 14, 19794 (2024). https://doi.org/10.1038/s41598-024-70779-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70779-8
- Springer Nature Limited