Abstract
Obesity is often associated with sex-dependent metabolic complications, in which altered intestinal barrier function and gut microbiota contribute. We aimed to characterize in mice the sex-dependent effects of a high fat diet on these parameters. Male and female C57BL/6 mice received a standard (SD) or high fat diet (HFD; 60% kcal from fat) during 14 weeks (W14). Body composition, glucose tolerance, insulin sensitivity, intestinal permeability, colonic expression of 44 genes encoding factors involved in inflammatory response and gut barrier function, cecal microbiota, plasma adipokines and white adipose tissue response have been assessed. Both male and female HFD mice exhibited an increase of body weight and fat mass gain and glucose intolerance compared to SD mice. However, only male HFD mice tended to develop insulin resistance associated to increased Tnfα and Ccl2 mRNA expression in perigonadal adipose tissue. By contrast, only female HFD mice showed significant intestinal hyperpermeability that was associated with more markedly altered colonic inflammatory response. Cecal microbiota richness was markedly reduced in both sexes (Observed species) with sex-dependent modifications at the phyla or family level, e.g. decreased relative abundance of Bacillota and Lachnospiraceae in females, increased of Bacteroidaceae in males. Interestingly, some of these microbiota alterations were correlated with peripheral metabolic and inflammatory markers. In conclusions, male and female mice exhibit different responses to a high fat diet with specific changes of gut microbiota, intestinal barrier function, colonic and white adipose tissue inflammation, metabolic markers and body weight gain. The underlying mechanisms should be deciphered in further investigations.
Similar content being viewed by others
Introduction
Obesity is a major public health issue in industrial and developing countries. Indeed, 50% of the European population is overweight and obese1, and predictive studies suggest that nearly 1 in 2 adults in the United States will have obesity by 20302. Among subjects with obesity, almost 9 in 10 will become insulin-resistant that is associated with an increased risk of mortality3 and precedes the development of type 2 diabetes mellitus (T2DM).
Insulin resistance in obesity is highly related to a low-grade systemic inflammation and the production of pro-inflammatory cytokines in adipose tissue4,5. Several studies highlighted the links between low-grade systemic inflammation, glycemic control related-dysregulations and intestinal barrier function in obesity6,7. An alteration of the intestinal barrier function, and more specifically a disruption of the tight junction (TJ) proteins, has been observed in animal models of obesity7,8,9, whereas the data in humans remain controversial10,11,12. For instance, metabolically-healthy individuals with obesity exhibiting a systemic low-grade inflammation did not show an increased intestinal permeability measured by the lactulose-mannitol-sucralose test, despite an intestinal dysbiosis12. The role of the gut microbiota in the development of obesity and its associated comorbidities have been highlighted both in rodents and humans9,13. Germ-free mice fed with a high fat diet (HFD) gained neither weight nor fat mass compared to control mice, and were immune to the onset of ileal inflammation14. Gut microbiota transfer from lean donors to patients with obesity with metabolic syndrome increased insulin sensitivity and the level of butyrate-producing bacteria15.
Increasing data underline the importance of sex in the pathophysiology and outcomes of many diseases, in particular in T2DM for which obesity is the first risk factor. The prevalence of obesity is higher in women than in men16, whereas diabetes is more widespread in men17. Thus, taking into account differences between male (M) and female (F) animals in experimental studies is increasingly emphasized. Mice fed with a HFD during several weeks developed a prediabetes state, but insulin resistance appeared first in M-HFD mice and then in F-HFD mice18,19. Similarly, white adipose tissue (WAT) that plays an important role in the occurrence of obesity-associated metabolic disorders because of adipokines release (e.g. leptin, adiponectin and resistin), is also influenced by sex. Indeed, female HFD mice developed larger perigonadal fat depots and have higher plasma adiponectin level than male HFD mice18,19. Serum adiponectin and leptin levels were higher in women than in men independently of BMI that can be partly explained by the sex differences in body fat distribution20. In the same way, F-HFD mice gained weight more slowly than M-HFD mice18,19. Independently of the diet, male and female mice exhibited differences in the composition of gut microbiota. F-HFD mice showed a higher relative abundance of Parabacteroides, Lactobacillus, Bacteroides and Bifidobacterium compared to M-HFD mice21. In humans, the relative abundance of Bacteroides was decreased in men compared to women, only when body mass index (BMI) was above 3322. It is also well accepted that gut microbiota contributes to develop a specific profile of bile acids23. A HFD compared with normal fat diets increased the cecal content of both primary and secondary bile acids in M-HFD mice, that was associated to disturbances in gut barrier function and an intestinal hyperpermeability24. To our knowledge, there is little data about sex differences on permeability intestinal and colonic inflammation in HFD mice model since previous studies mainly used male mice.
Thus, we aimed to assess the sex differences induced by a HFD on glycemic control and gut microbiota with a special focus on intestinal barrier function, systemic and adipose tissue inflammation in mice. Thus, our study provides an integrated overview of the sex-dependent response to HFD.
Material and methods
Animal experimentation
Six-week-old male and female C57BL/6J mice were obtained from Janvier Labs (Le Genest-Saint-Isle, France, n = 24/sex) and acclimatized for 4 days in a controlled environment (23 °C with a 12 h light–dark cycle) with free access to food and water (n = 4/cage). During acclimatization, mice were fed with a standard diet (SD, 3.34 kcal/g, 1314 formula, Altromin, Lage, Germany; Table 1). Male and female mice were then randomized into two groups receiving for 14 weeks either SD or high fat diet (HFD) providing 60% kcal as fat, 20% kcal as carbohydrates and 20% kcal as protein (5.21 kcal/g, D12492i, Research Diet, New Brunswick, NJ, US; Table 1). In HFD, fat is from lard (0.316 g/g) and soybean oil (0.032 g/g). Body weight was measured weekly. Body composition was assessed by EchoMRI (EchoMRI, Houston, TX, US). Protocol was approved by the regional ethics committee called CENOMEXA (authorization on APAFIS #29283-2021012114574889 v5) and performed in accordance with the current French and European regulations and the ARRIVE guidelines.
Oral glucose tolerance test (OGTT)
OGTT were performed after 4 and 14 weeks of SD and HFD. Briefly, after a 12 h overnight fasting period with free access to water, mice were given a glucose solution by oral gavage (1 g glucose per kg of body weight). Blood glucose levels were obtained from the tail vein and measured using a glucometer (Accu Check Guide, Roche, Bale, Switzerland) at 0, 15, 30, 45, 60 and 90 min after glucose administration, as previously reported25,26.
ITT were performed after 4 and 14 weeks of SD and HFD. Briefly, after a 2 h fasting with free access to water, mice received an i.p. injection of human insulin (0.75 U per kg of body weight, I9278, Sigma-Aldrich, Saint-Quentin-Fallavier, France). Then, blood glucose levels were measured in the same way as for the OGTT.
Intestinal permeability
At week 14, after a 2 h fasting period and 3 h before euthanasia, mice received a FITC-dextran solution (4 kDa, 40 mg/mL, Sigma-Aldrich) by oral gavage at 10 µL/g of body weight to evaluate whole intestinal large paracellular permeability in vivo, as previously described27. The fluorescence intensities of FITC-dextran in plasma were measured with the Spark multimode microplate reader (Tecan, UK; excitation at 485 nm, emission at 535 nm).
Tissue sampling
Mice were anesthetized by i.p. injection of ketamine/xylazine solution (100 and 10 mg/kg, respectively). Blood samples were collected, centrifuged at 3000×g, 4 °C for 15 min and plasma was frozen at − 80 °C. Subcutaneous and perigonadal adipose tissue depots and colon were collected, washed with ice-cold PBS, immediately frozen in liquid nitrogen and stored at − 80 °C until analysis. Cecal content was collected to evaluate microbiota and short-chain fatty acids.
Plasma assays: obesity and inflammation markers
Plasma insulin, leptin, resistin, adiponectin, IL-6 and TNFα levels were assessed using Bio-Plex Pro assays (BioRad Laboratories, Marnes-la-Coquette, France) following the manufacturer’s instructions and measured using Luminex technology (BioRad Laboratories). Plasma urea, alanine aminotransferase (ALAT), aspartate aminotransferase (ASAT), and total cholesterol concentrations were determined using a Catalyst One analyzer (IDEXX Laboratories, Westbrook, ME, US).
Colonic RNA extraction and RT-qPCR
Colonic total RNAs were extracted by trizol method (Invitrogen, Fisher Scientific, USA), quantified by using nanoDrop 2000 spectrometer and reverse transcribed as previously described28. Integrity analysis of total RNA was performed by running 1 µL of each sample on an Agilent 2100 Bioanalyzer (Agilent RNA 6000 Nano Kit, Agilent Technologies, Santa Clara, CA, US). Then, qPCR were realized by using the SYBR Green technology on a QuantStudio 12 K Flex real-time PCR system (Life Technologies, Carlsbad, CA, USA) targeting 44 markers of interest mainly involved in inflammatory response and gut barrier function (see Supplemental Table S1 online for details). The mean of ACTB, β2M and GAPDH genes were used as reference. Specific primers are displayed in the Supplemental Table S1 online.
Adipose tissue RNA extraction and RT-qPCR
Perigonadal (pWAT) and Subcutaneous white adipose tissues (scWAT) RNAs were extracted using the RNeasy Lipid Tissue Mini Kit (Qiagen, Courtabœuf, France) following the manufacturer’s instructions. Quantification and reverse transcription were performed as previously described28. Then, qPCR were performed by using the SYBR Green technology on a Bio-Rad CFX96 real-time PCR system (Bio-Rad Laboratories) targeting pro-inflammatory cytokines (Il6, Tnf, Ccl2) and adipokines (leptin, resistin and adiponectin). The mean of β2M and RPS18 was used as reference. Specific primers are displayed in the Supplemental Table S1 online.
Short chain fatty acid analysis (SCFAs)
Twenty to fifty grams of frozen cecal contents were used to analyze the short-chain fatty acids (SCFAs) composition. Briefly, cecal content sample were homogenized in milliQ water to a final concentration of 1 g of content/mL and kept at 4 °C for 2 h. Supernatant was obtained after centrifugation (12,000 rpm for 30 min at 4 °C). Proteins were precipitated overnight at 4 °C with 10% saturated phosphotungstic acid and then centrifuged at 12,000 rpm for 15 min at 4 °C. 2-Ethylbutyrate was added at a 1:4 ratio to supernatants as an internal standard. The SCFA content was determined from a 0.3 µL volume of supernatant by gas chromatography (Agilent 7890B gas chromatograph, Agilent Technologies, Les Ulis, France) equipped with a split-splitless injector, a flame-ionisation detector and a fused silica capillary column (15 m × 0.53 mm × 0.5 µm, Supelco, Saint-Quentin-Fallavier, France). Carrier gas (H2) flow rate was 10 mL/min. Initial oven temperature was 100 °C for 10 min and then increased from 100 to 180 °C at a rate of 20 °C/min and hold 2 min. Detector temperature was 240 °C. 2-Ethylbutyrate was used as internal standard. Samples were analysed in duplicate. The peaks obtained were integrated using OpenLAB Chemstation software (Agilent Technologies, Les Ulis, France)29.
Cecal microbiota analysis
Total DNA was extracted from cecal content samples with PowerFecal Pro DNA Kit (Qiagen, France) and using the mechanical bead-beating disruption method. The V3–V4 hypervariable region of the bacterial 16S rDNA was amplified by PCR (PCR1F_460: CTTTCCCTACACGACGCTCTTCCGATCTACGGRAGGCAGCAG and PCR1R_460: GGAGTTCAGACGTGTGCTCTTCCGATCTTACCAGGGTATCTAATCCT) using Phanta Max Super-Fidelity DNA Polymerase (Vazyme, PRC) (95 °C for 3 min and then 35 cycles at 95 °C for 15 s, 65 °C for 15 s, and 72 °C for 1 min before a final step at 72 °C for 5 min). The purified amplicons were sequenced using Miseq sequencing technology (Illumina) at the GeT-PLaGe platform (Toulouse, France). The resulting sequences were analysed using R (Team, 2019) workflow combining dada2 v.1.2830 and FROGS 4.0.131 software. Reads were filtered, merged and assigned to the amplicon sequence variants (ASV) with dada2 as follows. Adapters were first removed using cutadapt v. 3.5. Reads were then filtered using the dada2 filterAndTrim function, with a truncation length of 200 bp for 16S forward and reverse reads. This truncation reduced the error rate while still allowing the merging of most reads. The error model was then calculated using the learnErrors function and the dada2 core sample inference algorithm was executed. Forward and reverse reads were then merged with a minimum overlap of 20 bp. The resulting sequences were saved in a sequence table using makeSequenceTable. The guidelines of FROGS v4.0.1 were followed to detected and removed chimeras using the vsearch tool. ASVs with global abundance lower than 0.0005% were removed from the following analysis with FROGS filters and were then assigned to species using FROGS affiliation with 16S REFseq Bacteria.
Subsequent analysis were done using the phyloseq R packages32. Samples were rarefied to even sampling depths before computing within-samples compositional diversities (observed richness and Shannon index) and between-samples compositional diversity (Bray–Curtis) Raw, unrarefied ASV counts were used to produce relative abundance graphs and to find ASVs with significantly different abundances between male and female.
Statistical analysis
Data were analyzed with GraphPad Prism 8.3 (GraphPad Software Inc., San Diego, CA, US) and expressed as the mean ± standard error of the mean (SEM). To compare two groups, t-test or Mann–Whitney test was used, as appropriate. To compare body weight or glycaemia changes, two-way ANOVA were performed (Time × HFD) followed by Bonferroni post-tests. Alpha diversity index (Observed species and Shannon) were analyzed using Kruskal–Wallis test. A permutational multivariate analysis of variance (PERMANOVA) test was performed on the Bray–Curtis matrices using 9999 random permutations and at a significance level of 0.05. Phylum and family relative abundances were compared using a Mann–Whitney test. DESeq2 was used to estimate abundance fold changes between F-SD/M-SD or F-HFD/M-HFD. The ASVs were selected based on effect size (1/8 < fold change < 8), adjusted p-value (< 0.001) and prevalence (more than 50 copies per sample in at least 25% of the samples). The Spearman’s rank correlation coefficients between the relative abundance of gut microbiome at family level and obesity-related indicators were determined using R software for correlational statistical analysis. For all, a value of p < 0.05 was considered significant. A trend was considered when p value was between 0.1 and 0.05.
Ethics statement
Authors confirm that all experiments were performed in accordance with relevant guidelines and French and European regulations (Official Journal of the European Community L 358, 18/12/1986). Furthermore, the protocol was approved by the local ethical committee named CENOMEXA (authorization on on APAFIS #29283-2021012114574889 v5). We also followed the ARRIVE guidelines. Mice were anesthetized by i.p. injection of ketamine/xylazine solution (see “Material and methods” section).
Results
M-HFD mice gained weight and body fat more significantly and rapidly than F-HFD mice
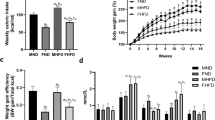
Body weight was significantly higher in M-HFD and F-HFD mice compared to SD mice from W5 and W7, respectively (p < 0.01, Fig. 1a). At W14, the difference in body weight between HFD and SD mice was 10.24 g in males and 7.45 g in females. Weight gain in HFD mice was related to an increase in fat mass by 5.6-fold in M-HFD vs M-SD mice and 4.3-fold in F-HFD vs F-SD mice (p < 0.001, Fig. 1b). Consequently, M-HFD and F-HFD mice exhibited 29.3 and 34.0% of fat mass, respectively.
Body weight (BW) and fat mass gain in male (M) and female (F) mice fed with a standard (SD) or high-fat-diet (HFD). (a) Both M-HFD and F-HFD mice showed a progressive gain in the BW. The BW of M-HFD and F-HFD mice increased significantly from week 5 and week 7, respectively (p < 0.01). (b) At week 14, M-HFD and F-HFD mice showed an increase in fat mass compared to SD mice (p < 0.01). **p < 0.01, ***p < 0.001.
Sex-dependent effects of a HFD on glycemic control and hepatic function
The development of glucose intolerance and insulin resistance was evaluated by performing OGTT and ITT at W4 and W14 (Fig. 2) and revealed disturbances in glucose metabolism in response to HFD. Glucose intolerance appeared from W4 in all HFD mice [(p(AUC) < 0.05, Fig. 2a], but only M-HFD mice tended to develop insulin resistance from W14 [p(AUC) = 0.0780, Fig. 2b] along with a significant increase in insulin plasma level (Fig. 2c). In addition, at the end of the feeding protocol, HFD mice presented an increase in fasting glycaemia and HOMA-IR (p < 0.05, Fig. 2c) emphasizing the alteration of glycemic regulation, however the increase of HOMA-IR was higher in M-HFD mice (2.4 fold change) than in F-HFD mice (1.8-fold change) emphasizing a greater alteration of insulin sensitivity in M-HFD than in F-HFD.
Evolution of glycemic control in male (M) and female (F) mice fed with a standard (SD) or a high fat diet (HFD). Oral glucose tolerance test (OGTT) and insulin tolerance test (ITT) were performed on weeks (W) 4 and 14 [(a) and (b)]. Glucose intolerance is present in all HFD mice from W4 [p(AUC) < 0.05]. However, only M-HFD mice showed an insulin resistance at W14 (p(AUC) = 0.0780). (c) The disturbances in glucose metabolism were supported by an increase in fasting glycemia (p < 0.01), plasma insulin (p < 0.01 in male and p = 0.0860 in female) and HOMA-IR (p < 0.05) at W14 in all HFD mice. *p < 0.05, **p < 0.01, ***p < 0.001.
To deeply assess the sex-dependent metabolic disorders in response to HFD, we performed multiple plasma assays (Fig. 3). At W14, plasma leptin and adiponectin concentrations were increased in both male and female HFD mice (p < 0.01, Fig. 3a), but not plasma resistin concentrations. Interestingly, the leptin/adiponectin ratio was increased in both male and female HFD mice (p < 0.001, Fig. 3a) but in more marked manner in M-HFD (9.1-fold change) compared to F-HFD mice (4.6-fold change) suggesting a major dysfunction of the adipose tissue in M-HFD. Plasma cytokine levels (IL-6, IL-1β and TNFα) remained under the sensibility threshold of used kits in both male and female mice (data not shown).
Plasma markers of glycemic control and hepatic function in male (M) and female (F) mice fed with a standard (SD) or high fat diet (HFD). (a) Plasma leptin (p < 0.001) and adiponectin (p < 0.01) were increased in all HFD mice compared to control, but no changes were observed for plasma resistin concentrations. Interestingly, plasma leptin/adiponectin ratio was more higher in M-HFD mice than in F-HFD mice (p < 0.001). (b) Plasma urea and cholesterol levels were respectively decreased and increased in HFD mice compared to control (p < 0.001). Plasma hepatic enzyme asparate aminotransferase (ASAT) concentration was increased only in M-HFD mice (p < 0.05) while plasma alanine aminotransferase (ALAT) concentration was increased only in F-HFD mice (p < 0.01). *p < 0.05, **p < 0.01, ***p < 0.001.
Among hepatic markers (Fig. 3b), plasma urea concentration was significantly decreased in M- and F-HFD mice compared to SD mice (p < 0.001), whereas plasma total cholesterol levels were increased (p < 0.001). ALAT concentration was increased in F-HFD mice (p < 0.01), and a similar trend was observed in M-HFD mice (p = 0.0784), whereas ASAT concentration was only increased in M-HFD mice (p < 0.05).
Sex-dependent effects of a HFD on white adipose tissue (WAT) inflammation
Perigonadal (pWAT) and subcutaneous white adipose tissues (scWAT) were studied by RT-qPCR (Fig. 4a,b). In pWAT, we observed that the mRNA levels of Tnfα and leptin were upregulated in M-HFD mice and F-HFD mice, whereas the mRNA level of resistin was downregulated. Interestingly, the mRNA level of Ccl2 was only increased in M-HFD mice while adiponectin mRNA was only upregulated in F-HFD mice (Fig. 4a). In scWAT, the mRNA expressions of Il6, Tnfα and Ccl2 were not significantly affected by HFD in both male and female mice, even if a trend was observed for Tnfα and Ccl2 in M-HFD (Fig. 4b). In scWAT of male HFD mice, the mRNA expression of leptin was not significantly affected by HFD, whereas mRNA expressions of resistin and adiponectin were significantly decreased (p < 0.05). By contrast, in female HFD mice, the mRNA level of adiponectin was not modified whereas leptin mRNA expression increased and resistin mRNA level was reduced (Fig. 4b, p < 0.05). These data suggest that the adipose tissue response to HFD was affected in a sex- and localization-dependent manner.
Inflammation of white adipose tissue (WAT) at 14 weeks in male (M) and female (F) mice fed with a standard (SD) or high fat diet (HFD). (a) and (b) mRNA levels of the inflammatory markers Tnf and Ccl2 were increased in perigonadal white adipose tissue (pWAT) of M-HFD mice, as well as Tnf in F-HFD mice (p < 0.05). By contrast, the expression of these genes was not modified in subcutaneous white adipose tissue (scWAT). mRNA levels of leptin and resistin were increased and decreased in pWAT of M-HFD mice and F-HFD mice, respectively (p < 0.05). Only F-HFD mice showed in increase in mRNA level of adiponectin (p < 0.05). In scWAT, mRNA level of leptin was not changed in M-HFD mice, whereas mRNA levels of resistin and adiponectin were significantly decreased (p < 0.05). In F-HFD mice, mRNA levels of leptin and resistin had a similar expression profile in scWAT than in pWAT. Data are presented as ratio of SD group (dashed line = 1) and significant changes (p < 0.05 versus SD) are shown by color bars.
Sex-dependent effects of a HFD on intestinal barrier function and colonic inflammation.
Intestinal permeability was assessed by dosage of plasma FITC-dextran administered by oral gavage. F-HFD mice showed a significant increase in intestinal permeability at W14 (p < 0.001, Fig. 5a) that was not observed in M-HFD mice. We then studied the colonic mRNA levels factors involved in the regulation of intestinal permeability. The mRNA expression of 9 tight junction (TJ) protein markers (Ocln, Marveld2, Tjp1, Tjp2, Igsf5, Cldn1, Cldn3, Cldn5 and Cgn) were significantly increased in F-HFD mice compared to F-SD mice (p < 0.05, Fig. 5b), while only the F11r mRNA level, encoding JAM-A protein, was reduced. In male mice, HFD induced an increase in the colonic mRNA levels of Marveld2 and Igsf5, and a decrease in the colonic mRNA levels of Cldn4 and Cgn (p < 0.05, Fig. 5b). These results suggest that gut barrier function is more markedly impaired in F-HFD mice compared to M-HFD mice. We also observed several sex-dependent differences in the colonic mRNA levels of pro-inflammatory cytokines (Tnfα, Il1b, Il6, Cxcr3 and Tgfb1) and TLR-pathway related genes (Tlr4, Myd88, Nfkb1, Ticam1, Irf3, Cd14 and Tlr2). In F-FHD mice, the colonic mRNA levels of Tnfα, Il1b, Il6, Cxcr3, Tlr4, Myd88, Nfkb1, Ticam1, Irf3 and Cd14 were upregulated (p < 0.05, Fig. 5c), while Ccl2 mRNA level was reduced. By contrast, only the mRNA levels of Tlr2, Myd88 and Nfkb1 was increased in the colon of M-HFD mice, while the colonic gene expression of Tgfb1 was downregulated (p < 0.05, Fig. 5c). These data suggest that F-HFD mice exhibited more pronounced colonic inflammation than M-HFD mice that may be associated to the impairment of intestinal barrier function.
Intestinal permeability and colonic inflammation at 14 weeks in male (M) and female (F) mice fed with a standard (SD) or high fat diet (HFD). (a) FITC-dextran (4 kDa) was administred by oral gavage and assayed in plasma for evaluation of global intestinal permeability. Intestinal permeability was only increased in F-HFD mice compared to SD mice (p < 0.001). (b) and (c) qPCR analysis showed that colonic mRNA levels of numerous tight junction protein markers were significantly increased in F-HFD mice (p < 0.05) unlike male HFD mice. Moreover, F-HFD mice showed an increase in the colonic mRNA expression of several pro-inflammatory markers (p < 0.05), which was much less pronounced in M-HFD. Data are presented as ratio of SD group (dashed line = 1) and significant changes (p < 0.05 versus SD) are shown by color bars. ***p < 0.001.
Sex-dependent effects of a HFD on cecal microbiota composition and metabolism
Numerous studies indicate that HFD-induced metabolic disorders are associated with changes in gut microbiota composition. We investigated whether the sex-dependent effects of HFD on metabolic disorders was associated with a microbiota dimorphic response to HFD. To address this possibility, we examined the cecal microbial composition by sequencing of V3–V4 regions of microbial 16S rDNA. At the ASV level, no change in richness (observed species) or α-diversity (Shannon index) was observed between M-SD mice and F-SD mice (Fig. 6a). HFD induced a marked decrease in cecal microbial richness for both sexes, while α-diversity was significantly reduced in F-HFD mice only. In addition, PCoA of Bray–Curtis compositional dissimilarity between cecum samples showed a significant separation between the communities in response to HFD when compared to SD for male and female mice (p < 0.001 for both sexes, Fig. 6b). The cecal microbiota composition measured by Bray–Curtis β-diversity index differed significantly between not only M-SD and F-SD (p < 0.001) but also between M-HFD and F-HFD (p < 0.001). In males and females, the relative abundance of Bacteroidota decreased significantly in response to 14 weeks of HFD while Thermodesulfobacteriota increased (Fig. 6c,d, Table 2). Less abundant phyla such as Mycoplasmatota and Pseudomonadota also decreased in both males and females in response to 14 weeks of HFD. However, the relative abundance of Bacillota and Actinomycetota was lower in F-HFD mice than in F-SD mice that was not observed in M-HFD mice compared to M-SD. Analysis of family relative abundance variations in response to HFD revealed a marked increase in Desulfovibrionaceae (Thermodesulfobacteriota phylum) in both male and female mice. Within the Bacteroidota phylum, the relative abundance of Rikenellaceae increased and that of Muribaculaceae decreased in both male and female HFD mice, whereas Bacteroidaceae abundance increased and Prevotellaceae abundance decreased only in male mice exposed to HFD. Within the Bacillota phylum, the relative abundance of Oscillospiraceae increased in F-HFD and M-HFD mice when compared with F-SD and M-SD mice, respectively, as well as the abundance of Eubacteriales incertae sedis. To the opposite, HFD strongly reduced the abundance of Clostridiaceae in both sexes. The relative abundance of Lachnospiraceae and of the beneficial Lactobacillaceae decreased in F-HFD mice but remained unchanged in M-HFD mice compared with respective SD mice. Finally, within the phylum Pseudomonadota, Sutterellaceae relative abundance decreased in F-HFD and M-HFD mice when compared with their respective SD controls. In addition to their distinct adaptation to HFD, males and females displayed different initial bacterial composition while fed with a SD as revealed by the differential abundance analysis done at the genus level (Fig. 6e). This analysis identified 23 ASVs that were more abundant (p < 0.001) in females compared to males fed with a SD. Among 23 ASVs, 12 belong to the Lachnospiraceae and 2 to the Lactobacillaceae families, respectively (Fig. 6e). Under SD, differential abundance analysis also identified 18 ASVs with a lower abundance (p < 0.001) in female compared to male mice. After 14-weeks of HFD, 12 and 22 ASVs were, respectively, identified as more or less abundant in females compared to males (Fig. 6f). Interestingly, only one of the differences observed between females and males under SD persisted under HFD, the ASV(239) (identified as C. disporicum) belonging to the Clostridium that remained more abundant in F-HFD mice than M-HFD mice (Fig. 6f).
Microbiota composition at 14 weeks in male (M) and female (F) mice fed with a standard (SD) or high fat diet (HFD). Analysis was based on 16S rDNA sequencing of the cecum content. (a) Observed species richness and Inverse Simpson Index as indicator of α-diversity. (b) Principal coordinates analysis (PCoA) of Bray–Curtis compositional dissimilarity at the amplicon sequence variant (ASV) level. Each dot represents of one mice. (c) and (d) Average relative abundance at the phylum and family levels in caecum content of each group in male (c) and female (d). (e) and (f) Graphic representation of differentially abundant ASVs and the genera to which they belong between female and male mice under SD (e) or HFD (f) dietary conditions with a logarithmic scale (log-2) used for the x-axis. Positive values represent ASVs increased in female compared to male mice. For richness or α-diversity index values are means ± sem. Stars indicate a significant difference after Kruskal–Wallis test followed by Dunn’s multiple comparisons test (with *p < 0.05, ** p < 0.01 and **** p < 0.0001). For figures C and D, the average of each group is represented along the X-axis and the Y-axis refers to relative normalized abundances. Relative abundance data were compared using Mann–Whitney test with $ indicating significantly different phyla or family abundances between SD and HFD in male (with $p < 0.05, $$p < 0.01, $$$p < 0.001 and $$$$p < 0.0001) and * indicating significantly phyla or family abundances between SD and HFD in female (with *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001).
The changes in the composition of the bacteria community were associated with changes in the metabolic activity of the gut microbiota assessed using gas chromatography. Among the major SCFA detected, the cecal amount of acetate and propionate significantly decreased by ~ 50% and a 6.5-fold reduction was measured in F-HFD mice when compared with the F-SD mice leading to a marked reduction of the total SCFA in the female mice fed HFD (Fig. 7). In male, only the levels of butyrate were reduced by 2 in response to HFD.
Short chain fatty acids quantification in the cecum at 14 weeks in male (M) and female (F) mice fed with a standard (SD) or high fat diet (HFD). SCFA quantification in cecum content by gas chromatography in male (left panel) and female (right panel) mice fed SD or HFD. All values are means ± sem; means were compared with Student’s t test. *P < 0.05, **P < 0.001 and ***P < 0.001.
Correlation between the gut microbiome and obesity-related indicators
The correlations between the relative abundances of the gut microbial community at the family level and obesity-related indicators (Fig. 8, left panels) and intestinal gene expression levels (Fig. 8, right panels) were determined by Spearman’s correlation analysis in male (Fig. 8, top panels) and female (Fig. 8, bottom panels and supplemental Table S2 online). Obesity-related indicators included blood markers concentration (leptin, adiponectin, resistin, cholesterol, fasting glycaemia, glucose tolerance [AUC_OGTT, insulin tolerance (AUC_ITT), Il6 and urea], genes expression in pWAT and scWAT (Retn, Adipoq, Lep, Tnf, Ccl2 and Il6), hepatic parameters (ALAT and ASAT), body parameters (fat mass, lean mass and body weight gain) and intestinal permeability. In male, the relative abundances of Desulfovibrionaceae, Rikenellaceae, Oscillospiraceae, Streptococcaceae, Eubacterriales_incertae_sedis, Christensenellaceae, Peptostreptococcaceae, Eggerthellaceae, Bacteroidaceae and Tannerellaceae (Cluster I) were positively correlated with markers of adiposity (body weight gain, fat mass, cholesterol and leptin circulating levels and Lep expression in pWAT), of altered glycaemia (insulin level, fasting glucose level, glucose and insulin tolerance tests) and of inflammation in WATs (Ccl2, Il6 and Tnf). The cluster I also negatively correlated with Resistin and adiponectin expression in both pWAT and scWAt as well as to lean mass and circulating levels of urea. To the opposite, these later 6 parameters were correlated positively with the relative abundance of Clostridiaceae Muribaculaceae, Sutterellaceaa, Eubactriaceae, Anaeroplasmataceae and Corynebacteriaceae (Cluster II). This second cluster of bacteria was also correlated negatively with markers of adiposity, hyperglycemia and pWAT inflammation. Similarly, in females, a significant pattern was detected that was also associated to the markers of altered adiposity, glycaemia, fatty liver and intestinal permeability (body weight gain, fat mass, cholesterol, adiponectin and leptin circulating levels, Lep expression in WATs, fasting glucose level, glucose tolerance, ALAT and intestinal permeability) with members of the family Desulfovibrionaceae, Eubacterriales_incertae_sedis, Rikenellaceae, Tannerellaceae, Streptococcaceae and Oscillospiraceae (Cluster III). This cluster III was also negatively correlated to the markers of fitness (lean mass), Resistin expression in WATs and urea circulating levels. A cluster IV (Lactobacillaceae, Clostridiaceae, Sutterellaceaa, Muribaculaceae, Lachnospiraceae, and Corynebacteriaceae) was correlated (i) negatively to the main markers of adiposity, glycaemia, fatty liver as well as intestinal permeability, and (ii) positively to lean mass, Resistin expression in WATs and urea circulating levels. When the intestinal gene expression (gene of the Table S1 targeting inflammatory response and gut barrier function) was correlated with microbiota, two clusters were identified within the bacterial community in male (cluster V and VI) and in female (cluster VII and VIII) mice. Cluster V and VI correlated positively and negatively with markers of immunity (Il4, Tlr2, Myd88) as well as tight junction (Marveld2), respectively. Bacteria of the Lachnospiraceae family also positively correlated with Il4. In female mice, the cluster VII and VIII correlated positively and negatively with markers of immunity (Tlr4, Myd88, Cxcr3, Il1b and Il6) and marker of tight junctions (Cldn5, Cldn1, Marveld2, Ocln and Cng), respectively. Most of the bacteria of the cluster VII and VIII are also, respectively, negatively and positively correlated to F11r and Ccl2 expression that participate to the tight junction formation and inflammation.
Heatmap of Spearman’s correlation between the relative abundance of gut microbiome at the family level and obesity-related indicators and intestinal inflammatory markers in male (M) and female (F) mice fed with a standard (SD) or high fat diet (HFD). Heatmap values are shaded from red (> 0) to blue (< 0) according to correlation scores. The correlation scores have been calculated by using R software version 4.3.3 and heatmaps were created with Pheatmap software version 1.0.12. Exact p values are displayed in Supplemental Table S2.
Discussion
In the present study, we report the sex-dependent response to high-fat diet in mice by the assessment of the evolution of gut microbiota, intestinal barrier function, WAT inflammatory characteristics and circulating metabolic parameters in female and male mice throughout the duration of HFD feeding. Our study reveals that in response to HFD, male mice developed more marked alterations of adipose inflammatory and endocrine functions in association with the onset of insulin resistance than the female mice. To the opposite, female fed HFD showed more pronounced alterations of intestinal barrier function and of inflammation than the male mice. Since gut microbiota also showed sex-dependent modifications in response to HFD, its contribution to the host response to HFD will be discussed.
Male are more susceptible to adipose inflammation and glucose homeostasis alteration than female mice
In our study, male mice exhibited higher and earlier body weight and fat mass gain than females in accordance with the literature19,33,34,35,36,37. This higher susceptibility to obesity observed in males was also associated with sex specific WAT inflammatory and endocrine alterations. Indeed, the inflammatory markers in perigonadal WAT are more elevated in male than in female mice in response to HFD. Particularly, Ccl2, encoding for MCP-1, expression levels increased in male but not in female mice after 14 weeks of HFD. MCP-1 is known to favor monocyte migration and previous studies reported a greater monocyte migration and recruitment in lean and obese male mice respectively that in their female counterparts38 suggesting a higher susceptibility of male than female mice to adipose inflammation as observed in our study. In the present study, adiponectin mRNA levels were upregulated by HFD in perigonadal fat of females and reduced in subcutaneous WAT of males. Moreover, male mice fed HFD also displayed more elevated leptin/adiponectin ratio than HFD-fed females. In human, leptin/adiponectin ratio parallels increased body mass index (BMI), but also correlates positively with insulin resistance39 and the circulating pro-inflammatory markers40 suggesting that leptin/adiponectin is a marker of obesity-associated metabolic risk than female mice. This is confirmed in our study in which high leptin/adiponectin ratio observed in male is associated with a more pronounced insulin resistance than in female mice after 14 weeks of HFD. These sex-dependent regulations of endocrine and inflammatory characteristics of adipose tissue may contribute to the delayed metabolic disturbances observed in females, as previously reported in both rodent models19,33 and humans20.
Role of sex-specific bacterial communities in the metabolic dimorphism in response to HFD
Since previous studies underlined the role of gut microbiota and gut barrier function in the occurrence of metabolic complications related to obesity41, we investigated the adaptation of bacterial composition and intestinal epithelium to HFD in both male and female mice. Indeed, some studies previously suggested that gut microbiota may mediated a sex-dependent response to different interventions42,43. Both sexes develop major changes in gut microbiota in response to 14 weeks of HFD, including a significant decrease in alpha-diversity and common changes in microbial composition such as a reduction in relative abundance of Bacteroidota and an increase in relative abundance of Thermodesulfobacterota, which are all markers of HFD-induced dysbiosis44. Indeed, the abundance of Bacteroidota phylum was markedly reduced while Desulfovibrionaceae increased in male mice fed HFD for 4 weeks45, as observed in the present study. Colonization of obese mice with Bacteroidota can also prevent HFD-induced obese phenotype46 emphasizing the role of Bacteroidota in the alteration of metabolic alterations associated to HFD-induced obesity. However, male and female mice also exhibited specific differences in their gut microbiota composition in response to HFD exposure that may result from an initial dimorphism in the microbiota composition under standard diet. These initial and persistence sex-specificities of the microbial composition may, at least partially explained the metabolic dimorphism in response to HFD observed in the present study. In the present study, female mice displayed specific bacterial enrichment in Lachnospiraceae and Lactobacillaceae in SD when compared to their male counterparts as previously reported21. In response to HFD, female mice showed a reduction of Lachnospiraceae and Lactobacillaceae and in our study, both families correlated positively with female lean mass but negatively with fat mass, glucose intolerance, plasma leptin concentration or intestinal permeability (Cluster IV). In male mice, increased abundance of Lachnospiraceae and Lactobacillaceae have also been previously both associated positively to lean phenotype and resistant to HFD-induced obesity in male mice47. Recently, Lactobacillus-derived metabolite has been shown to regulate intestinal lipids metabolism in male mice therefore limiting the metabolic alteration in HFD-induced obesity48. However, in the present study, Lachnospiraceae and Lactobacillaceae abundances were not affected by HFD in males. In addition, the marked decrease of total SCFA in female HFD mice may be related to the reduced abundance of Lachnospiraceae that are SCFA-producing bacteria. Even if carbohydrate diet’s composition differed between SD and HFD, the SCFA cecal content was differentially affected between male and female mice that should be further investigated. In male mice, we also observed an increase of Bacteroidaceae and a decrease of Prevotellaceae abundances that were not present in females. Enrichment of Prevotellaceae family by inulin supplementation in male ob/ob mice had been associated with beneficial effects49. Overall, analysis of the microbiota data suggests that rather than a specific bacterium, a consortium of bacteria is probably involved in the dimorphism of HFD response observed in our study.
Female susceptibility to intestinal inflammation in response to HFD
In our study, we observed a strong decrease of all SCFA in females while only butyrate was reduced in males. In addition, this decrease in SCFA cecal content observed in females was concomitant with an increased intestinal permeability and altered colonic inflammatory marker expression. In males, we25 and others7 previously described a disruption of gut barrier function that did not reach significance in the present study. These data suggest that, in our study, 14 weeks of HFD induced more changes in female mice at the colonic level than in males that is inversely associated to metabolic alterations. A sexual dimorphism of intestinal immunity has already been underlined in other rodent models50,51 and healthy volunteers52. Interestingly this sex-dependent immune intestinal response was abolished in germ-free mice51. A recent review speculates that increased intestinal immune and inflammatory response to HFD may contribute to explain the slower occurrence of metabolic complications in females by promoting capacity to eliminate pathogenic and opportunistic bacteria53. In our study, the enhanced inflammation and TLR pathway activation measured in the colonic mucosa in females (increase in expression of Tlr4, Tlr2, Cd14, Myd88, Ticam1, Irf3, Tnf, Il1b, Il6) might thus alleviate the impact of gram-negative bacteria endotoxins, LPS. The increase of intestinal permeability and macrophage response in female HFD mice was not abolished by TNFα knock-out54, suggesting that the increase of Tnfα observed in our study maybe not detrimental. Further studies should thus investigate the role of intestinal inflammation in the onset of obesity-associated complications according to the sex.
In the present study, we did not evaluate the mechanisms involved in the sex dependent response. It has been proposed that sex steroid hormones could participate to the sexual dimorphism of gut microbiota. Indeed, antibiotics-treated female mice that received by oral gavage the gut microbiota from male mice became more insulin resistant33. In addition, castration of male mice caused alteration in the gut microbiome that became more similar to the gut microbiome of female mice and improved glucose metabolism33. The interaction between sex steroid hormones and gut microbiota therefore appears to be an important factor in the development of metabolic disorders and should be further investigated.
Perspectives and significance
In conclusion, we report a sex-dependent response to a high fat diet in mice at different levels: gut microbiota, colonic mucosa, white adipose tissue, metabolic markers, body weight and composition. Interestingly, while male mice exhibit faster alterations of glycemic control, female mice show a more marked colonic inflammatory response. The impact of these sex differences and the underlying mechanisms remain to decipher in further experiments.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Marques, A., Peralta, M., Naia, A., Loureiro, N. & de Matos, M. G. Prevalence of adult overweight and obesity in 20 European countries, 2014. Eur. J. Public Health 28, 295–300 (2018).
Ward, Z. J. et al. Projected U.S. state-level prevalence of adult obesity and severe obesity. N. Engl. J. Med. 381, 2440–2450 (2019).
Calori, G. et al. Prevalence, metabolic features, and prognosis of metabolically healthy obese Italian individuals: The Cremona Study. Diabetes Care 34, 210–215 (2011).
He, M.-Q. et al. High-fat diet-induced adipose tissue expansion occurs prior to insulin resistance in C57BL/6J mice. Chronic Dis. Transl. Med. 6, 198–207 (2020).
Yaribeygi, H., Farrokhi, F. R., Butler, A. E. & Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell Physiol. 234, 8152–8161 (2019).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007).
Cani, P. D. et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 (2008).
Nascimento, J. C., Matheus, V. A., Oliveira, R. B., Tada, S. F. S. & Collares-Buzato, C. B. High-fat diet induces disruption of the tight junction-mediated paracellular barrier in the proximal small intestine before the onset of type 2 diabetes and endotoxemia. Dig. Dis. Sci. 66, 3359–3374 (2021).
de La Serre, C. B. et al. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G440-448 (2010).
Bona, M. D. et al. Intestinal barrier permeability in obese individuals with or without metabolic syndrome: A systematic review. Nutrients 14, 3649 (2022).
Teixeira, T. F. S. et al. Intestinal permeability parameters in obese patients are correlated with metabolic syndrome risk factors. Clin. Nutr. 31, 735–740 (2012).
Brignardello, J. et al. Pilot study: Alterations of intestinal microbiota in obese humans are not associated with colonic inflammation or disturbances of barrier function. Aliment. Pharmacol. Ther. 32, 1307–1314 (2010).
Liu, R. et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 23, 859–868 (2017).
Ding, S. et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE 5, e12191 (2010).
Vrieze, A. et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143, 913-916.e7 (2012).
Ng, M. et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781 (2014).
Kautzky-Willer, A., Harreiter, J. & Pacini, G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr. Rev. 37, 278–316 (2016).
Medrikova, D. et al. Sex differences during the course of diet-induced obesity in mice: adipose tissue expandability and glycemic control. Int. J. Obes. 36, 262–272 (2012).
Elzinga, S. E. et al. Sex differences in insulin resistance, but not peripheral neuropathy, in a diet-induced prediabetes mouse model. Dis. Model. Mech. 14, dmm048909 (2021).
Christen, T. et al. Sex differences in body fat distribution are related to sex differences in serum leptin and adiponectin. Peptides 107, 25–31 (2018).
Peng, C. et al. Sex-specific association between the gut microbiome and high-fat diet-induced metabolic disorders in mice. Biol. Sex Differ. 11, 5 (2020).
Haro, C. et al. Intestinal microbiota is influenced by gender and body mass index. PLoS ONE 11, e0154090 (2016).
Ridlon, J. M., Kang, D.-J. & Hylemon, P. B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 47, 241–259 (2006).
Murakami, Y., Tanabe, S. & Suzuki, T. High-fat diet-induced intestinal hyperpermeability is associated with increased bile acids in the large intestine of mice. J. Food Sci. 81, H216-222 (2016).
Bahlouli, W. et al. Stress-induced intestinal barrier dysfunction is exacerbated during diet-induced obesity. J. Nutr. Biochem. 81, 108382 (2020).
Toubal, A. et al. Mucosal-associated invariant T cells promote inflammation and intestinal dysbiosis leading to metabolic dysfunction during obesity. Nat. Commun. 11, 3755 (2020).
Ghouzali, I. et al. Targeting immunoproteasome and glutamine supplementation prevent intestinal hyperpermeability. Biochim. Biophys. Acta Gen. Subj. 1861, 3278–3288 (2017).
Coëffier, M. et al. Increased proteasome-mediated degradation of occludin in irritable bowel syndrome. Am. J. Gastroenterol. 105, 1181–1188 (2010).
Fréville, M. et al. Chronic dietary exposure to a glyphosate-based herbicide results in total or partial reversibility of plasma oxidative stress, cecal microbiota abundance and short-chain fatty acid composition in broiler hens. Front. Physiol. 13, 974688 (2022).
Callahan, B. J., Sankaran, K., Fukuyama, J. A., McMurdie, P. J. & Holmes, S. P. Bioconductor workflow for microbiome data analysis: From raw reads to community analyses. F1000Res 5, 1492 (2016).
Escudié, F. et al. FROGS: Find, rapidly, OTUs with galaxy solution. Bioinformatics 34, 1287–1294 (2018).
McMurdie, P. J. & Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8, e61217 (2013).
Gao, A. et al. Sexual dimorphism in glucose metabolism is shaped by androgen-driven gut microbiome. Nat. Commun. 12, 7080 (2021).
Bridgewater, L. C. et al. Gender-based differences in host behavior and gut microbiota composition in response to high fat diet and stress in a mouse model. Sci. Rep. 7, 10776 (2017).
Tran, V. et al. Sex-specific effects of a high fat diet on aortic inflammation and dysfunction. Sci. Rep. 13, 21644 (2023).
Lainez, N. M. et al. Diet-induced obesity elicits macrophage infiltration and reduction in spine density in the hypothalami of male but not female mice. Front. Immunol. 9, 1992 (2018).
Kaliannan, K. et al. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome 6, 205 (2018).
Varghese, M. et al. Monocyte trafficking and polarization contribute to sex differences in meta-inflammation. Front. Endocrinol. 13, 826320 (2022).
Inoue, S. et al. The effect of proteasome inhibitor MG132 on experimental inflammatory bowel disease. Clin. Exp. Immunol. 156, 172–182 (2009).
Frühbeck, G. et al. Involvement of the leptin-adiponectin axis in inflammation and oxidative stress in the metabolic syndrome. Sci. Rep. 7, 6619 (2017).
Cani, P. D. Human gut microbiome: Hopes, threats and promises. Gut 67, 1716–1725 (2018).
Sharma, P. et al. Sex-dependent effects of 7,8-dihydroxyflavone on metabolic health are associated with alterations in the host gut microbiome. Nutrients 13, 637 (2021).
Wen, L. & Duffy, A. Factors influencing the gut microbiota, inflammation, and type 2 diabetes. J. Nutr. 147, 1468S-1475S (2017).
Murphy, E. A., Velazquez, K. T. & Herbert, K. M. Influence of high-fat diet on gut microbiota: A driving force for chronic disease risk. Curr. Opin. Clin. Nutr. Metab. Care 18, 515–520 (2015).
Kong, B. et al. Gut microbiota dysbiosis induced by a high-fat diet increases susceptibility to atrial fibrillation. Can. J. Cardiol. 38, 1962–1975 (2022).
Ridaura, V. K. et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341, 1241214 (2013).
Wang, J. et al. Modulation of gut microbiota during probiotic-mediated attenuation of metabolic syndrome in high fat diet-fed mice. ISME J. 9, 1–15 (2015).
Shelton, C. D. et al. An early-life microbiota metabolite protects against obesity by regulating intestinal lipid metabolism. Cell Host Microbe 31, 1604-1619.e10 (2023).
Song, X. et al. Inulin can alleviate metabolism disorders in ob/ob mice by partially restoring leptin-related pathways mediated by gut microbiota. Genomics Proteomics Bioinformatics 17, 64–75 (2019).
Yurkovetskiy, L. et al. Gender bias in autoimmunity is influenced by microbiota. Immunity 39, 400–412 (2013).
Markle, J. G. M., Frank, D. N., Adeli, K., von Bergen, M. & Danska, J. S. Microbiome manipulation modifies sex-specific risk for autoimmunity. Gut Microbes 5, 485–493 (2014).
Sankaran-Walters, S. et al. Sex differences matter in the gut: effect on mucosal immune activation and inflammation. Biol. Sex Differ. 4, 10 (2013).
Brettle, H. et al. Sex hormones, intestinal inflammation, and the gut microbiome: Major influencers of the sexual dimorphisms in obesity. Front. Immunol. 13, 971048 (2022).
Breznik, J. A., Jury, J., Verdú, E. F., Sloboda, D. M. & Bowdish, D. M. E. Diet-induced obesity alters intestinal monocyte-derived and tissue-resident macrophages and increases intestinal permeability in female mice independent of tumor necrosis factor. Am. J. Physiol. Gastrointest. Liver Physiol. 324, G305–G321 (2023).
Acknowledgements
We thank Pamela Lecras and Dr David Vaudry from the PRIMACEN platform (HeRaCleS Inserm US51, CNRS UAR 2026, Université de Rouen Normandie, France) and the staff of GenoToul platform (Toulouse, France).
Funding
The present study was supported by the French Agency for Research (OBEGLU, ANR-20-CE17-0012), the European Society for Clinical Nutrition and Metabolism (ESPEN Fellowship), by the Nutricia Research Foundation and by European Union and Normandie Regional Council. Europe gets involved in Normandie with European Regional Development Fund (ERDF). CL received the support of the university of Rouen Normandy during her PhD and VD from the Inserm and Normandie Regional council. These funders did not participate in the design, implementation, analysis, and interpretation of the data.
Author information
Authors and Affiliations
Contributions
Conceptualization, CL, AG, PD, VDo and MC; CL, AT, EM, MM, AG and MC, formal analysis; CL, AT, CE-B, VDr, CBF, CG, JB, EM, MM, VDo and AG, investigation; CL, VDo, AG and MC, original draft preparation; VDr, JB and PD, writing review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Lefebvre, C., Tiffay, A., Breemeersch, CE. et al. Sex-dependent effects of a high fat diet on metabolic disorders, intestinal barrier function and gut microbiota in mouse. Sci Rep 14, 19835 (2024). https://doi.org/10.1038/s41598-024-70931-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-70931-4
- Springer Nature Limited