Abstract
The aim of this study was to establish and validate the precision of a novel radiomics approach that integrates 18Fluorine-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)-computed tomography (CT) scan data with clinical information to improve the prognostication of survival rates in patients diagnosed with stage III Non-Small Cell Lung Cancer (NSCLC) who are not candidates for surgery. We evaluated pretreatment 18F-FDG PET-CT scans from 156 individuals diagnosed with stage III inoperable NSCLC at Shandong Cancer Hospital. These individuals were divided into two groups: a training set comprising 110 patients and an internal validation set consisting of 46 patients. By employing random forest classifier and cox proportional hazards model , we identified and utilized relevant features to create predictive models and a nomogram. The effectiveness of these models was assessed through the use of the receiver operating characteristics(ROC) curves, Kaplan–Meier (KM) curves, and the application of the nomogram. Our findings showed that the combined model, which integrates both clinical and radiomic data, outperformed those based solely on clinical or radiomic features in predicting 3-year overall survival(OS). Furthermore, calibration plots revealed a high level of agreement between predicted and actual survival times. The research successfully established a predictive radiomics model that integrates 18F-FDG PET/CT imaging with clinical indicators to enhance survival predictions for patients with stage III inoperable NSCLC.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Worldwide, the prevalence of lung cancer as the primary cause of death from oncological diseases continues unabated, placing significant strain on both individuals afflicted by the condition and the healthcare infrastructure1. The bulk of lung cancer incidences are classified as NSCLC, with those diagnosed at stage III forming a notably diverse cohort, marked by an array of tumor sizes and differing outcomes for patients2. Close to one-third of NSCLC diagnoses are confirmed at stage III, which often precludes the possibility of surgical removal of the tumor3. For such cases of inoperable stage III NSCLC, combined chemoradiotherapy is the primary therapeutic protocol, associated with a five-year OS rate of merely 16%4. Post-CRT immunotherapy consolidation has been adopted as the normative treatment following the PACIFIC trial, which demonstrated an improved 5-year survival rate of 42.9%5. Nonetheless, the restrained efficacy of immunotherapy serves as a significant barrier to enhancing survival prospects for patients with advanced NSCLC. Identifying individuals at high risk for poor outcomes and innovating superior therapeutic modalities for NSCLC thus remain imperative.
Currently, the 8th edition TNM staging for NSCLC is highly regarded for its prognostic significance6. However, considerable variability exists among patients within the same stage, including disparities in survival times. Thus, there is an urgent need to identify new non-invasive biomarkers that provide a deeper understanding of tumor diversity before starting treatment. These developments could aid clinicians in customizing treatment plans.
The field of radiomics, which involves mining and interpreting a vast array of sophisticated quantitative features from diagnostic images, offers potential for enhancing the predictive accuracy of patient outcomes in oncology, surpassing the capabilities of conventional imaging indicators. This non-invasive approach transforms visual information into high-dimensional datasets, potentially revealing aspects of diseases that evade direct visual detection. Utilizing CT imaging-derived radiomic profiles, it is possible to anticipate the histopathological classifications of lung cancer, assess the response to targeted and immune therapies, and project the survival outcomes for individuals with NSCLC7,8,9,10.
The integration of anatomical and metabolic insights provided by 18F-FDG PET/CT is pivotal for delineating primary neoplasms, categorizing disease stages, and gauging the efficacy of therapeutic interventions11,12,13. Traditional assessments of PET/CT scans tend to center on the maximum standardized uptake value (SUVmax), an approach that does not fully leverage the extensive data these scans yield. Research has shown that employing machine learning algorithms in conjunction with radiomic analysis of PET/CT images offers a non-invasive technique for ascertaining ALK mutation status in NSCLC patients, as well as identifying those at heightened risk for disease progression following treatment with first and second-generation epidermal growth factor receptor–tyrosine kinase inhibitors14,15. Moreover, PET/CT radiomics may yield additional prognostic information that could enhance the prediction of survival rates among lung cancer patients16,17. However, in the era of big data and within the context of immunotherapy, there is a need for an efficient and straightforward prognostic tool that integrates multi-source data from PET/CT images, clinical information, laboratory findings, and metabolic parameters to predict overall survival in stage III NSCLC patients due to substantial prognostic heterogeneity among this patient population.
Hence, our objective was to develop a predictive model and a nomogram (incorporating clinical features, PET, and CT radiomic features) to forecast the 3-year OS after combined treatment in patients diagnosed with stage III inoperable NSCLC.
Methods
Study design
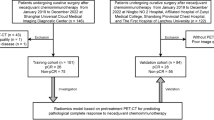
The study protocol received approval from the Ethics Committee of the Cancer Hospital affiliated with Shandong First Medical University, and the stipulation for written informed consent from participants was exempted. An overview of the study's design and methodology is depicted in Fig. 1. Patients diagnosed with NSCLC through pathological assessment and who received combined chemoradiotherapy were recruited for this study. Their PET/CT images were collected to perform radiomic analysis. The extraction and selection of radiomic features were guided by their relevance and predictive utility concerning the 3-year OS rate.
Patients
In the course of this study, we compiled a cohort of 156 patients diagnosed with NSCLC between September 2018 and May 2022. Figure 2 illustrates the patient selection process.
In conclusion, from the patient pool at the Cancer Hospital affiliated with Shandong First Medical University, a total of 156 qualified individuals were selected for the study. In response to our research objectives, we employed the hold-out method by randomly dividing the dataset into non-overlapping training and validation sets at a 7:3 ratio. Specifically, we incorporated this approach into our study in accordance with best practices outlined in recent literature. The resulting training cohort comprised 110 patients, while the validation cohort was composed of 46 patients. Detailed records of the patients' clinical demographics were maintained. In addition, the study involved assessing various metabolic indicators derived from PET imaging, such as the metabolic tumor volume (MTV), the average standardized uptake value (SUVmean), SUVmax, and the lowest standardized uptake value (SUVmin). The total lesion glycolysis (TLG) was also computed using the equation TLG = SUVmean × MTV. Detailed comparisons of patient demographics and PET metabolic indicators for both the training and validation groups are presented in Table 1. Statistical analysis showed that there were no significant differences in the variables studied between the two groups (P > 0.05).
Treatment and follow-up
The majority of participants underwent platinum-based concurrent chemoradiotherapy, adhering to the guidelines set forth by the NCCN and CSCO. The radiation therapy was designed to cover both the primary neoplasm and the metastatic lymph nodes within the irradiation zone. Therapeutic doses of radiation ranged from 60 to 66 Gy, administered in 2 Gy fractions. Patients who were not candidates for concurrent chemoradiotherapy received sequential chemoradiation instead. Treatment regimens were also customized according to the genetic mutation status and levels of immune checkpoint proteins, integrating targeted therapies or immunomodulators when indicated.
Follow-up visits were scheduled at intervals of every 3–6 months for the initial two years post-treatment, biannually for the third to fifth years, and annually thereafter. In this investigation, the primary clinical outcome was mortality. OS was measured from the completion of treatment to the event of death or up to the cutoff date of May 31, 2023.
PET-CT image acquisition
Initial PET/CT scans were scheduled within a two-week window prior to the biopsy and commencement of cancer therapy. For these examinations, Shandong First Medical University Affiliated Cancer Hospital utilized the Philips Gemini TF scanner (Phillips Medical Systems, The Netherlands). Patients observed a fasting period exceeding six hours, after which blood glucose was tested to confirm it was maintained below 140 mg/dL. Administered intravenously, 18F-FDG was dosed at 4.4 MBq/kg. Subsequently, comprehensive whole-body PET and CT imaging took place one hour post-injection. The spiral CT scans were performed with dose modulation using a quality reference of 150 mAs and parameters of 130 kV, a 512 × 512 matrix, 3-mm slice thickness, and a voxel size of 1.17188 × 1.17188 × 5 mm. The PET scans, conducted immediately following the CT scans, had a matrix size of 144 × 144, a pixel spacing of 4 × 4 mm, and a voxel size of 4 × 4 × 4 mm. The imaging coverage extended from the distal femur to the top of the skull.
Attenuation-corrected PET data were processed using an iterative reconstruction algorithm, specifically the ordered subset expectation maximization technique. Subsequently, these PET scans were fused with corresponding CT images to generate comprehensive whole-body representations across the transverse, coronal, and sagittal views. Throughout this imaging sequence, respiratory gating methods were employed to enhance the sharpness and resolution of the images.
Tumor segmentation
The tumor segmentation process was carried out using AccuContour software, version 3.2, developed by Manteia Medical Technologies Co., Ltd., based in Xiamen, China. A pair of seasoned nuclear medicine specialists utilized a 40% threshold of the SUVmax to delineate the gross tumor volume (GTV) on the PET scans, reaching a consensus without the influence of prior histopathological information18,19. Simultaneously, the GTV boundaries on the CT images were meticulously outlined, merging metabolic information from PET with anatomical details provided by the CT data. Subsequently, two expert radiologists jointly evaluated the delineated targets.
Feature extraction
AccuContour software version 3.2 (Manteia Medical Technologies Co. Ltd., Xiamen, China) was designed based on Pyradiomics version 3.0.1, an open-source Python library equipped with a user-friendly graphical interface. This enables the extraction of an extensive array of features as outlined by the Image Biomarker Standardization Initiative20. To enhance the images for radiomic analysis, the original PET and CT data underwent filtering through various methods, including wavelet transforms, Laplacian-of-Gaussian (with sigma set at 1.0), as well as square, square root, logarithmic, exponential, and gradient operations. These transformations yielded a set of augmented images. Both the original and enhanced images were then processed to extract the radiomic features. Quantization of absolute intensity was applied with an upper threshold set at 25, and the discretization levels were calibrated to 64 levels. A comprehensive inventory of the extracted radiomic features is presented in Supplementary Table S1. The Pyradiomics configuration utilized in our analysis is detailed in Table S2.
Feature selection
In the training cohort, radiomics features were selected using random forest classifier. The number of generated trees was set to 200, and the minimum number of observations required in a leaf node was 14. The clinical features of the patients were initially selected using univariate analysis, and then the selected features were further screened using multivariate analysis (P < 0.05).
Model development and validation
The selected CT-based features and PET-based features were used to construct a radiomics model. The selected clinical features were used to construct a clinical model. The survival model was built using the Cox proportional hazards model. Then, the radiomics model was combined with the clinical model to create an integrated model. The performance of the models was evaluated using ROC curves and the C-index.
To identify the optimal cutoff value, patients were categorized into high-risk and low-risk groups using the KM method, and KM curves were generated. A p-value of less than 0.05 was considered statistically significant for the difference between the high-risk and low-risk groups. Calibration curves were plotted separately for the training and validation sets to assess the consistency between predicted and actual outcomes. The efficacy of the combined model was assessed by creating decision curve analysis (DCA) graphs. A tailored nomogram, which visually integrates selected clinical parameters with PET/CT radiomic indicators from the training dataset, was crafted to predict the 3-year OS for patients with non-small cell lung cancer.
Statistical analysis
The processes of feature selection, constructing the model, and its subsequent validation were conducted using R software (Version 3.4.0, available at https://www.r-project.org/). The Wilcoxon test, applied through SPSS software version 25.0 from International Business Machines Corporation in New York, US, facilitated the statistical analysis of different groups. A two-tailed test with a p-value threshold below 0.05 was deemed to denote statistical significance.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board of the Shandong First Medical University Affiliated Cancer Hospital (Approval No. SDTHEC2023012025). All methods were carried out in accordance with the Declaration of Helsinki guidelines and regulations. 18F-FDG PET/CT is a common, non-invasive and safe examination method, and it was a retrospective study. The requirement for written informed consent was waived by the Institutional Review Board of the Shandong First Medical University Affiliated Cancer Hospital.
Results
Selection of features and construction of predictive models
A total of 1409 radiomic features derived from CT scans and 1409 from PET scans were systematically computed and extracted from each Volume-of-Interest (VOI). Subsequently, a subset of features, comprising two from CT (Fig. S1A) and two from PET (Fig. S1B), was further refined. The radiomics features that were retained for further analysis are detailed in Table S3. After performing feature selection, radiomics models were constructed using the selected attributes and analyzed with the Cox proportional hazards model to forecast the 3-year OS. Univariate and multivariate analyses were conducted to evaluate the clinical factors associated with 3-year OS in the training cohort, and these findings are elaborated in Table 2. The analysis identified immunotherapy and neuron-specific enolase (NSE) as independent prognostic indicators for OS, with statistical significance (P < 0.05). Ultimately, the combined models designed to predict 3-year OS were created, incorporating two selected radiomic features along with two clinical factors.
Prediction performance and clinical utility of prediction models
The KM survival curves of the combined model (Fig. 3A,C) depict the 3-year OS and demonstrate the efficacy of the selected radiomics and clinical factors in stratifying patients into high-risk and low-risk categories. Additionally, the ROC curves (Fig. 3B,D) exhibit the predictive capabilities of the three models for 3-year OS.
The Kaplan–Meier survival curves for the training group (A) and validation group (C). The receiver operating characteristic curves for the 3-year overall survival prediction model in the training group (B) and validation group (D). "OS" refers to overall survival, "KM" to Kaplan–Meier, and "ROC" to receiver operator characteristic.
In the task of predicting 3-year OS, the combined model that merged radiomic features with clinical features demonstrated enhanced predictive power. It achieved an AUC of 0.80 and C-index of 0.76 in the training cohort, while in the validation cohort, it attained an AUC of 0.79 and a C-index of 0.75. Comprehensive results are collated in Table 3.
Establishment and validation of the nomogram
A prognostic nomogram for estimating 3-year OS was developed by amalgamating two clinical features with two features each from CT and PET radiomics (Fig. 4A). The calibration curves for the nomogram, which assess the concordance between the forecasted and observed probabilities for 3-year OS, indicated a high level of agreement in both patient cohorts, as illustrated in Fig. 4B,C.
A prognostic nomogram that integrates radiomic and clinical parameters to forecast the 3-year overall survival (OS) for the training group (A). This tool translates the impact of each predictor into a point value on the topmost “Points” scale of the nomogram. Summing these values yields the predicted 3-year OS probability, displayed at the base of the nomogram. The model’s calibration was assessed using curves for both the training set (B) and an independent validation group (C). On these graphs, the X-axis denotes the nomogram’s estimated probabilities, while the Y-axis reflects the actual observed frequencies. A perfect model prediction would align with the gray dashed line, whereas the pink solid line depicts the nomogram’s performance.
To appraise the nomogram's clinical benefit, the DCA was conducted and presented in Fig. 5. These analyses further corroborated that the comprehensive model, which incorporated the selected radiomic and clinical features, surpassed its counterparts in precision and practical utility.
The DCA for the models that forecast 3-year overall survival within the validation cohort. On the X-axis, the threshold probability is shown, representing a trade-off between the advantages of receiving treatment versus foregoing it. The Y-axis quantifies the net benefit gained from basing treatment choices on the model’s predictions. Within this analysis, the assumption that all patients with NSCLC benefit from treatment is depicted by the gray line, and the presumption that no patients gain considerable benefit from treatment is represented by the black line.
Discussion
In our investigation, we utilized 18F-FDG PET/CT radiomics to probe the prognostic outcomes for individuals with NSCLC. The findings from our research indicate that a multifaceted risk stratification framework, which integrates clinical factors with radiological features obtained from PET/CT scans, can effectively forecast the three-year overall survival likelihood for patients with stage III NSCLC who are not candidates for surgical intervention. Furthermore, this combined model demonstrates superior predictive accuracy when compared to models based solely on clinical or radiomics. Notably, the textural feature known as 'cluster shade' derived from the gray-level co-occurrence matrix exhibited a robust predictive capability.
Lung cancer is one of the malignancies with the poorest prognosis and highest mortality rate worldwide. Enhancing the prognosis of lung cancer patients represents a critical objective in clinical research21. The prerequisite for improving the prognosis of lung cancer patients is to comprehend the risk factors that affect their outcomes. To this end, we initially explored prognostic indicators associated with non-small cell lung cancer by examining clinical parameters, metabolic variables, and laboratory indices.
TNM staging is a well-established and user-friendly clinical prognosis prediction model. Building on this foundation, researchers included age, pre-treatment hemoglobin level, and other indicators to develop an enhanced prognosis prediction model22. The univariate Cox regression analysis in this study did not indicate T stage, N stage, and PET metabolic parameters could impact prognosis. This may be due to the inclusion of all stage III inoperable patients, resulting in factors such as tumor maximum diameter and lymph node status holding minimal prognostic value at this stage. Wang et al.23 demonstrated that the concurrent measurement of CEA and NSE markedly enhances the diagnostic accuracy for lung cancer, both in terms of sensitivity and specificity, and also aids in the histopathological categorization of the disease. In this study, NSE levels were found to have independent prognostic value, whereas CEA measurements did not show prognostic significance. This discrepancy may be attributed to the differential expression of tumor markers among NSCLC patients with varying pathological types. Notably, the study cohort comprised a substantially higher proportion of patients with squamous cell carcinoma compared to those with adenocarcinoma, all of whom were diagnosed at an advanced stage of the disease. And the hazard ratio (HR) for NSE was 1.0182, suggesting that elevated levels of this marker serve as a prognostic indicator for increased mortality risk; higher NSE levels were associated with poorer survival outcomes. The adoption of immunotherapy, in addition to NSE, also demonstrated an independent prognostic effect. In our study, the HR for immunotherapy was 0.3815, suggesting that it is a favorable prognostic factor. In the meta-analysis conducted by Yao et al.24, it is evident that East Asian patients with non-small cell lung cancer who receive treatment based on immune checkpoint inhibitors (ICIs) experience a significantly increased survival benefit compared to non-East Asian patients. For East Asian patients, early intervention and combination therapies are more strongly recommended. Moreover, PD-1 inhibitors are associated with prolonged survival. This is also why immunotherapy consolidation after chemoradiotherapy for locally advanced NSCLC patients has been established as a standard treatment approach. However, regardless of whether sequential immune consolidation therapy is administered to stage III NSCLC patients after CRT, some patients still experience tumor progression, underscoring the inherent heterogeneity of the tumor. In this study, no association with prognosis of NSCLC was found for indicators such as gender, clinical staging, and Karnofsky Performance Status (KPS) index. This may be related to the sample size and the inclusion criteria selected for the study population.
The study25 on lung cancer utilized a conventional model devoid of radiomic information exhibited significant efficacy in assessing progression-free survival with a C-index of 0.75. Subsequent investigations26,27 indicated that compared to traditional data, radiomics offers enhanced prognostic insights customized for specific patient subgroups. Recently, PET/CT-based radiomics has displayed potential in evaluating treatment response and forecasting associated with immunotherapy. Mu et al.28 extracted radiomic features from baseline PET scans and CT images along with fused PET/CT images to develop a multiparametric radiomic model for predicting outcomes post-immunotherapy. The findings underscored the superior ability of this comprehensive approach in foreseeing long-term clinical benefits among patients, yielding AUC scores of 0·86 during training assessment and 0·83 and 081 respectively during retrospective and prospective testing phases. Given the abundance of similar studies, our focus in this research was not on directly comparing the predictive capabilities of PET/CT multi-parameter models with those of PET or CT alone. Yang et al.'s research16 suggests that radiomic scores from CT, PET, or PET/CT have good predictive ability for survival. Among the three models, the PET/CT model performed the best. Combining clinical factors (such as CEA, TNM staging, targeted therapy) can improve the predictive performance of the PET/CT model. Our study is in line with previous research, demonstrating that combining PET/CT with clinical characteristics yields enhanced predictive efficacy for 3-year overall survival compared to using standalone clinical or radiomics models. In both our training and validation cohorts, the AUCs were 0.80 and 0.79, respectively, while the C-index for predicting overall survival using the nomogram was 0.698 and 0.724, respectively. Furthermore, the predictive value of clinical characteristics was shown to surpass that of radiomic features. This emphasizes the increasing relevance of immunotherapy in predicting outcomes for NSCLC patients. Overall, these results underscore the significance of integrating diverse data types, such as imaging and clinical information, to develop more accurate prognostic models in oncology.
A previous study29 employed PET/CT-based radiomics to construct prognostic prediction models for NSCLC by integrating two CT radiomic features, two PET radiomic features, and two clinical features. The combined model exhibited superior predictive performance compared to the clinical model in both the training and internal validation datasets, consistent with our own findings. In our research, specific radiomic features comprising of two PET features and two CT features were successfully identified. All selected attributes are derived from wavelet-transformed characteristics, with "wavelet-LHH-firstorder-RootMeanSquared" (WLFR) representing a first-order feature, while "wavelet-LLH-glcm-SumSquares" (WLGS) and "wavelet-HLL-glcm-ClusterShade" (WHGC) represent texture features. Wavelet is a higher-order statistical method that applies filter grids to images for multi-scale analysis aimed at capturing intricate details and characteristics effectively. Additionally, both PET and CT imaging identified the WHGC feature as the ClusterShade attribute from the gray-level co-occurrence matrix; a higher cluster shade indicates increased asymmetry around the mean value. A study30 aligned with our conclusions analyzed radiological features from chest CT scans to predict lingering lung abnormalities in COVID-19-recovered individuals post-hospital discharge. Among six predictive models, three radiological features, including HLL_glcm_ClusterShade, were consistently identified. It suggests that WHGC can attests to the robust predictive performance of this biomarker, and more reliably forecast the three-year overall survival for stage III inoperable non-small cell lung cancer patients.
While the study has noted encouraging outcomes from PET/CT radiomic analysis, it is not without its limitations. This study is a single-center study lacking external validation, and we implemented the hold-out method to randomly divide the dataset into non-overlapping training and validation sets at a 7:3 ratio, rather than utilizing the K-fold cross-validation strategy. This limitation may impact stability and generalizability of the model31,32. In the future, our objective is to explore the integration of advanced deep learning methodologies, along with potential incorporation of multi-center and multi-omics datasets, to further refine and optimize our classification models.
Conclusion
To encapsulate, our research successfully established and independently verified a potent integrative model that combines pre-treatment PET/CT radiomic data with clinical features. From this combined model, we created a non-invasive predictive tool—a nomogram—that can autonomously forecast the 3-year OS of patients with stage III inoperable non-small cell lung cancer who have undergone concurrent chemoradiotherapy. This tool offers additional guidance for medical professionals in their decision-making processes.
Data availability
The data are available from the corresponding author on reasonable request.
Abbreviations
- 18F-FDG:
-
18Fluorine-fluorodeoxyglucose
- PET:
-
Positron emission tomography
- CT:
-
Computed tomography
- NSCLC:
-
Non-small cell lung cancer
- ROC:
-
Receiver operating characteristics
- KM:
-
Kaplan–Meier
- AUC:
-
Area under curve
- CRT:
-
Combined radiochemotherapy
- OS:
-
Overall survival
- VOI:
-
Volume-of -Interest
- SUVmax:
-
Maximum standardized uptake value
- SUVmean:
-
Mean standardized uptake value
- SUVmin:
-
Minimum standardized uptake value.
- MTV:
-
Metabolic active tumor volume
- TLG:
-
Total lesion glycolysis
- DCA:
-
Decision curve analysis
- WBC:
-
White blood cell count
- NEU:
-
Neutrophil
- LYM:
-
Lymphocyte
- EOS:
-
Eosinophil
- BAS:
-
Basophil
- PLT:
-
Platelet
- CEA:
-
Carcinoembryonic antigen
- LDH:
-
Lactatedehydrogenase
- ALB:
-
Albumin
- HGB:
-
Hemoglobin
- RBC:
-
Red blood cell
- BMI:
-
Body mass index
- KPS:
-
Karnofsky Performance Status
- NSE:
-
Neuron-specific enolase
- Cyfra21-1:
-
Recombinant cytokeratin fragment antigen 21–1
- ADC:
-
Adenocarcinoma
- SCC:
-
Squamous cell carcinoma
- C-index:
-
A concordance index
- HR:
-
Hazard ratio
- LS-SCLC:
-
Limited-stage small cell lung cancer
- ICIs:
-
Immune checkpoint inhibitors
- WLGS:
-
Wavelet-LLH-glcm-SumSquares
- WLFR:
-
Wavelet-LHH-firstorder-RootMeanSquared
- WHGC:
-
Wavelet-HLL-glcm-ClusterShade
- GLCM:
-
Gray-level co-occurrence matrix
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin. 71, 209 (2021).
Hirsch, F. R. et al. New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet 388, 1012 (2016).
Bryan, S. et al. Cancer in Canada: Stage at diagnosis. Health Rep. 29, 21 (2018).
Auperin, A. et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J. Clin. Oncol. 28, 2181 (2010).
Spigel, D. R. et al. Five-year survival outcomes from the PACIFIC trial: Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J. Clin. Oncol. 40, 1301 (2022).
Goldstraw, P. et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J. Thorac. Oncol. 11, 39 (2016).
Ferreira-Junior, J. R. et al. CT-based radiomics for prediction of histologic subtype and metastatic disease in primary malignant lung neoplasms. Int J. Comput. Assist. Radiol. Surg. 15, 163 (2020).
Yang, F. et al. CT-based radiomics signatures can predict the tumor response of non-small cell lung cancer patients treated with first-line chemotherapy and targeted therapy. Eur. Radiol. 32, 1538 (2022).
Li, J. et al. Evaluation of PD-L1 expression level in patients with non-small cell lung cancer by (18)F-FDG PET/CT radiomics and clinicopathological characteristics. Front. Oncol. 11, 789014 (2021).
He, B. et al. Predicting response to immunotherapy in advanced non-small-cell lung cancer using tumor mutational burden radiomic biomarker. J. Immunother. Cancer 8, e000550 (2020).
Kirienko, M. et al. FDG PET/CT as theranostic imaging in diagnosis of non-small cell lung cancer. Front. Biosci. 22, 1713 (2017).
Grootjans, W. et al. PET in the management of locally advanced and metastatic NSCLC. Nat. Rev. Clin. Oncol. 12, 395 (2015).
Hatt, M. et al. 18F-FDG PET uptake characterization through texture analysis: Investigating the complementary nature of heterogeneity and functional tumor volume in a multi-cancer site patient cohort. J. Nucl. Med. 56, 38 (2015).
Chang, C. et al. A machine learning model based on PET/CT radiomics and clinical characteristics predicts ALK rearrangement status in lung adenocarcinoma. Front. Oncol. 11, 603882 (2021).
Shao, D. et al. Identification of stage IIIC/IV EGFR-mutated non-small cell lung cancer populations sensitive to targeted therapy based on a PET/CT radiomics risk model. Front. Oncol. 11, 721318 (2021).
Yang, B. et al. Development and validation of a radiomics nomogram based on (18)F-fluorodeoxyglucose positron emission tomography/computed tomography and clinicopathological factors to predict the survival outcomes of patients with non-small cell lung cancer. Front. Oncol. 10, 1042 (2020).
Huang, B. et al. Prediction of lung malignancy progression and survival with machine learning based on pre-treatment FDG-PET/CT. Ebiomedicine 82, 104127 (2022).
Tibdewal, A. et al. Optimal standardized uptake value threshold for auto contouring of gross tumor volume using positron emission tomography/computed tomography in patients with operable nonsmall-cell lung cancer: Comparison with pathological tumor size. Indian J. Nucl. Med. 36, 7 (2021).
Zhang, Y. et al. The utility of PET/CT metabolic parameters measured based on fixed percentage threshold of SUVmax and Adaptive iterative algorithm in the new revised FIGO staging system for stage III cervical cancer. Front Med. -Lausanne 8, 680072 (2021).
Zwanenburg, A. et al. The image biomarker standardization initiative: Standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology 295, 328 (2020).
Zhao, T. et al. Radiotherapy prognosis-associated gene GCNT3 promotes the proliferation, migration and invasion of lung adenocarcinoma cells. Heliyon 8, e12100 (2022).
Zhang, Y. H. et al. Development of a survival prognostic model for non-small cell lung cancer. Front. Oncol. 10, 362 (2020).
Wang, B. et al. Clinical utility of haptoglobin in combination with CEA, NSE and CYFRA21-1 for diagnosis of lung cancer. Asian Pac. J. Cancer Prev. 15, 9611 (2014).
Yao, Y. et al. East Asian patients who received immunotherapy-based therapy associated with improved survival benefit in advanced non-small cell lung cancer: An updated meta-analysis. Cancer Med. 13, e7080 (2024).
Luna, J. M. et al. Radiomic phenotypes for improving early prediction of survival in stage III non-small cell lung cancer adenocarcinoma after chemoradiation. Cancers 14, 700 (2022).
Tankyevych, O. et al. Development of radiomic-based model to predict clinical outcomes in non-small cell lung cancer patients treated with immunotherapy. Cancers 14, 5931 (2022).
Dissaux, G. et al. Pretreatment (18)F-FDG PET/CT radiomics predict local recurrence in patients treated with stereotactic body radiotherapy for early-stage non-small cell lung cancer: A multicentric study. J. Nucl. Med. 61, 814 (2020).
Mu, W. et al. Radiomics of (18)F-FDG PET/CT images predicts clinical benefit of advanced NSCLC patients to checkpoint blockade immunotherapy. Eur. J. Nucl. Med. Mol. Imaging 47, 1168 (2020).
Hannequin, P. et al. FDG PET and CT radiomics in diagnosis and prognosis of non-small-cell lung cancer. Transl. Lung Cancer Res. 11, 2051 (2022).
Huang, J. et al. CT-based radiomics helps to predict residual lung lesions in COVID-19 patients at three months after discharge. Diagnostics 11, 1814 (2021).
Pasini, G. et al. A critical analysis of the robustness of radiomics to variations in segmentation methods in (18)F-PSMA-1007 PET images of patients affected by prostate cancer. Diagnostics 13, 3640 (2023).
Pasini, G. et al. Phenotyping the histopathological subtypes of non-small-cell lung carcinoma: How beneficial is radiomics?. Diagnostics 13, 1167 (2023).
Funding
This work was supported by funds from the Science and Technology Foundation of Xinjiang Uygur AutonomousRegion(No.2022E02050).Cheng Chang ,as a sponsor,play an important role in the study design, data collection andanalysis.
Author information
Authors and Affiliations
Contributions
Yalin Zhang: Conceptualization, Methodology, Formal analysis, Writing-original draft; Yongbin Cui: Methodology, Software, Validation, Formal analysis; Huiling Liu: Data curation; Cheng Chang: Resources, Data curation; YY: Conceptualization, Funding acquisition, Project administration, Writing-review and editing; RW: Conceptualization, Supervision, Funding acquisition, Project administration, Writing-review and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Cui, Y., Liu, H. et al. Prognostic nomogram combining 18F-FDG PET/CT radiomics and clinical data for stage III NSCLC survival prediction. Sci Rep 14, 20557 (2024). https://doi.org/10.1038/s41598-024-71003-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71003-3
- Springer Nature Limited