Abstract
Although the relationship between hypertension and hyperuricemia is widely recognized, there is still a relative lack of research on prehypertensive individuals and the individual associations of systolic and diastolic blood pressure with the risk of hyperuricemia. From 2011 to 2016, we conducted a study on 53,323 individuals at Wuhu City Hospital in China. Based on initial blood pressure readings, participants were categorized into normal, prehypertension, or hypertension groups. We used Cox regression to analyze the associations with baseline factors. In subgroup analyses, systolic and diastolic pressures were treated as continuous variables, and their relationship with the risk of hyperuricemia was examined using restricted cubic spline analysis. The risk increased in the prehypertension and hypertension groups compared to the normal blood pressure group, with hazard ratios of 1.192 and 1.350, respectively. For each unit increase in blood pressure, the risk of hyperuricemia rose by 0.8% (systolic) and 0.9% (diastolic), especially when blood pressure levels exceeded 115/78 mmHg. Additionally, we observed that factors such as gender, alcohol consumption habits, obesity, and dyslipidemia might further influence this association. These findings emphasize the importance of early risk assessment and intervention in these patient populations in clinical practice.
Similar content being viewed by others
Introduction
Hyperuricemia (HUA) results from an imbalance in uric acid metabolism, characterized by elevated serum uric acid (SUA) levels1. This increase often originates from abnormalities in purine metabolism or impaired uric acid excretion. Given its high prevalence and potential association with several non-communicable diseases—including lipid disorders, hypertension, and cardiovascular diseases—HUA has become a significant global health concern2. Current data suggest that approximately 20% of the global population is affected by HUA3. Specifically, in China, the prevalence is 13.3%, impacting nearly 170 million people, and this number is increasing4.
Since the 1870s, researchers have been interested in the association between uric acid levels and hypertension5. In recent years, some prospective studies have provided further evidence for this link. For example, Zhang et al.6 conducted an 8-year follow-up study on a cohort of 6,424 individuals from Tianjin General Hospital in China, finding that the risk of HUA was significantly increased in patients with hypertension. Another large prospective cohort study based on data from the 2001–2018 National Health and Nutrition Examination Survey also found a significant association between hyperuricemia and hypertension7. Hypertension may lead to elevated uric acid levels through various mechanisms, such as decreased glomerular filtration rate, increased renal vascular resistance, tissue ischemia, and oxidative stress8.
However, current research mainly focuses on patients with hypertension, while the relationship between uric acid levels and blood pressure in individuals with prehypertension has not been sufficiently addressed9. The independent association between systolic and diastolic blood pressures, as two important components of blood pressure, and the risk of HUA needs further clarification. While some cohort studies have established a link between elevated systolic pressure and hyperuricemia, the role of diastolic pressure remains controversial10. Therefore, our study aims to further elucidate the relationship between blood pressure and the risk of hyperuricemia, providing more accurate guidance for the prevention and management of hyperuricemia.
Materials and methods
Study population
This study is a large observational cohort study based on a natural population, utilizing electronic health record data from the health examination cohort at Yijiangshan Hospital in Wuhu City, China. The inclusion criteria are as follows: (1) aged 18 years or older at baseline and without HUA; (2) having a unique medical identifier with detailed records of age, gender, marital status, blood pressure, body mass index, and laboratory test results; (3) holding Chinese nationality and residing in Wuhu City for at least six months; (4) undergoing at least one additional full physical examination between 2011 and 2016, apart from the baseline examination. A total of 53,323 participants met these criteria. The baseline period is defined as the time of the participant's first physical examination at Yijiangshan Hospital between 2011 and 2016. The follow-up period ends at the earliest occurrence of hyperuricemia, loss to follow-up, or the end of the study period (December 31, 2016).
Baseline data collection
Skilled researchers gathered participants' sociodemographic details, including age, gender, and marital status, using structured questionnaires. Additionally, participants provided information about their lifestyle habits, such as smoking and drinking frequency, and their medical and medication histories. Marital status classifications relied on the participants' current legal standing, such as single or married. Participants self-reported their smoking habits, which were then grouped into: never smoked (no smoking within the past year), occasionally smoked (1 to 3 days a week), and regularly smoked (over 3 days a week). Similarly, drinking habits were categorized as: never drank (no drinking within the past year), occasionally drank (1 to 3 times a week), and regularly drank (over 3 times a week). Using standard techniques, we measured participants' height and weight to calculate their body mass index (BMI) using the formula: weight (kg) divided by height (m) squared. Seasoned technicians assessed the resting blood pressure on participants' right arm with standardized equipment, ensuring a rest period of at least five minutes before measurement. Blood pressure readings were taken twice, averaged, and the systolic (SBP) and diastolic (DBP) values were documented.
In the morning, participants provided fasting venous blood samples, which were subsequently subjected to biochemical analysis in the laboratory. The evaluated parameters encompassed serum uric acid (SUA), blood glucose, serum creatinine (CRE), blood urea nitrogen (BUN), globulin(GLO), alanine aminotransferase (ALT), triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). Additionally, routine hematological assessments, such as white blood cell count (WBC), mean corpuscular hemoglobin concentration (MCHC), platelet count (PLT), and mean platelet volume (MPV) were conducted.
Hyperuricemia was ascertained when SUA levels surpassed 420 mmol/L for males or 360 mmol/L for females11,12. Dyslipidemia was characterized by any of the following: TC level ≥ 5.2 mmol/L, TG ≥ 1.7 mmol/L, HDL-C < 1.0 mmol/L, or LDL-C ≥ 3.4 mmol/L13. Diabetes is identified by a fasting blood glucose (FBG) level of ≥ 7.0 mmol/L, while hypertension is defined by a SBP of ≥ 140 mmHg and/or a DBP of ≥ 90 mmHg, or by being on medication for hypertension or self-reporting as hypertensive14,15. Based on China's BMI standards, a value of ≥ 28 kg/m2 indicated obesity16.
Follow-up assessment
Between 2011 and 2016, participants underwent several physical examinations. For those who subsequently developed hyperuricemia, their follow-up period began from the date of their initial examination until the date of their hyperuricemia diagnosis. For participants without new-onset hyperuricemia, the follow-up spanned from the date of their first examination to their most recent examination.
Statistical analysis
In this study, baseline characteristics are presented in the form of mean ± standard deviation and percentages, and trend p-values are used to explore the linear association between different blood pressure categories. Through the Kaplan–Meier curve, we demonstrate the probability of developing hyperuricemia over time in patients with normal blood pressure, prehypertension, and hypertension. The schoenfeld residual test confirmed the applicability of our model, showing no violation of the proportional hazard assumption. Using the Cox proportional hazards model, we calculated the hazard ratios (HR) and their 95% confidence intervals (CI) for each blood pressure category.
Before multivariate Cox regression analysis, independent variables related to hyperuricemia were identified through univariate analysis. Only variables that showed statistical significance (P value < 0.05) in the preliminary analysis were included in the initial multivariate model. We evaluated multicollinearity for all variables and optimized the model under the guidance of AIC and BIC criteria(see Additional file 1: Table S1). We constructed four multivariate models adjusting for different confounding factors: Model 1 made no adjustments; Model 2 adjusted for age and gender; Model 3 further adjusted for marital status, smoking and drinking habits, BMI, FBG, TG, LDL-C, and HDL-C; Model 4 also considered CRE, ALT, GLO, WBC, MCHC, PLT, and MPV. We also conducted stratified analyses based on gender and age to further investigate their effects on the relationship between hyperuricemia and blood pressure.
Furthermore, we independently assessed systolic and diastolic blood pressures as potential risk factors for hyperuricemia. Through analysis of different subgroups (age, gender, BMI, etc.), we deepened our understanding of the connection between blood pressure and hyperuricemia. Using restricted cubic spline analysis, we explored the dose–response relationship between systolic and diastolic blood pressures and the risk of hyperuricemia. When evaluating hazard ratios, we considered all adjustment variables in Model 4. All statistical analyses were conducted using R software version 4.3, and P values below 0.05 were considered statistically significant.
Results
Baseline characteristics of participants
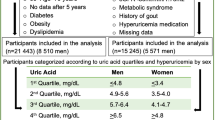
In this study, a total of 53,323 participants were included in the analysis, with an average age of 42.24 ± 13.22 years, of which 56.22% were male. The median follow-up time for the entire cohort was 3.01 years. Among all participants, 8320 (15.6% of the total) were diagnosed with hyperuricemia. Based on blood pressure levels, participants were categorized into three major groups: normal blood pressure, prehypertension, and hypertension. Table 1 presents the baseline characteristics for each of these categories.
Association between prehypertension, hypertension, and hyperuricemia
Participants were categorized into three groups based on their blood pressure readings: normal blood pressure, prehypertension, and hypertension. The Kaplan–Meier curve illustrated the changes in hyperuricemia occurrence probability over time among the different blood pressure groups (Fig. 1). Cox regression analysis indicated that, compared to the normal blood pressure group, the risk of developing hyperuricemia was significantly higher in the prehypertension and hypertension groups (P < 0.01). After adjusting for relevant confounders, this elevated risk persisted with hazard ratios (HR) of 1.192 (95% CI 1.128–1.260) for the prehypertension group and 1.350 (95% CI 1.263–1.443) for the hypertension group. Subsequent analyses by gender and age showed that, relative to the normal blood pressure group, the risk notably increased for females and those aged 65 and above in the prehypertension and hypertension groups. However, this heightened risk was not statistically significant among participants aged 65 and older (Table 2).
Impact of systolic and diastolic blood pressure on hyperuricemia
We investigated the association between systolic and diastolic blood pressures and the risk of hyperuricemia. Our analysis revealed that, after adjusting for various confounders, each unit increase in either systolic or diastolic pressure was associated with a 0.8% and 0.9% increase in the risk of hyperuricemia, respectively (P < 0.05). This correlation was more pronounced in certain subgroups, particularly among females, non-drinkers, individuals who are not obese, and those with dyslipidemia (Table 3). A similar trend was observed with diastolic pressure; however, its interaction with age was more notable: in participants under 65, the link between diastolic pressure and the risk of hyperuricemia was stronger (see Additional file 1: Table S2). Restricted cubic splines clearly depicted the dose–response relationship between both systolic and diastolic pressures and the risk of hyperuricemia. Overall, the risk of hyperuricemia progressively increased across the entire blood pressure spectrum when pressures exceeded 115/78 mmHg (Figs. 2 and 3).
Cumulative systolic blood pressure and hyperuricemia risk. The plot was generated using restricted cubic splines with an automatic selection of 4 knots. Results were adjusted for age, sex, marital status, smoking status, alcohol consumption, BMI, FBG, TG, LDL, HDL, CRE, ALT, GLO, WBC, MCHC, PLT, and MPV.
Discussion
In our study, we categorized participants into three groups based on their blood pressure levels: normal, prehypertension, and hypertension. It was evident that individuals in the hypertension category were generally older, reinforcing prior research linking increased age to a higher risk of hypertension17. Our findings strongly validate the association between hypertension and particular lifestyle determinants18. Specifically, the proportion of individuals who smoke and consume alcohol is higher in the hypertension group. Elevated measures of physiological markers like BMI, GLU, TG, LDL, CRE, WBC, MCHC, and MPV were more common in the hypertensive group, whereas HDL and PLT levels were reduced. These insights offer a foundation for future exploration into the mechanisms driving hypertension.
Our results show that relative to individuals with normal blood pressure, those at the prehypertensive stage and those diagnosed with hypertension are at a heightened risk of hyperuricemia. This vulnerability is more pronounced in women and individuals aged 65 and older. While several studies have underscored the link between hypertension and hyperuricemia, research on prehypertension remains scant6. Our study addresses this knowledge gap, solidifying the connection between prehypertension and increased hyperuricemia risk. Potential underlying mechanisms include the possibility that elevated blood pressure damages the renal microvasculature, compromising uric acid excretion efficiency19. Previous research has also identified a relationship between renal impairment and rising uric acid levels20. Furthermore, high blood pressure may precipitate endocrine disturbances, such as insulin resistance, known to influence uric acid metabolism21. Elevated blood pressure could also intensify bodily inflammation and oxidative stress, both of which impact uric acid metabolism22. It is worth noting that some studies have shown that uric acid levels increase before the onset of hypertension, suggesting that hyperuricemia may be a predictor of hypertension8. This finding indicates that elevated uric acid levels may, in turn, promote the development of hypertension, suggesting a bidirectional relationship between the two conditions. For women, hormonal shifts during menopause might alter renal uric acid management, heightening hyperuricemia risk23. While the link between raised blood pressure and hyperuricemia does not vary notably in individuals over 65, this age group is naturally predisposed to hyperuricemia due to factors like diminished kidney function and medication usage24.
Our findings underscore that elevated systolic and diastolic blood pressures markedly amplify the risk of hyperuricemia, a trend that holds true even when accounting for potential confounders. Importantly, our subgroup analysis corroborates these initial observations, further solidifying our confidence in the conclusions drawn. We identified notable interactions between systolic blood pressure and various factors, namely gender, alcohol intake, obesity, and dyslipidemia. The association between systolic blood pressure and hyperuricemia was especially evident in women, non-drinkers, non-obese individuals, and those with dyslipidemia. A Taiwanese prospective cohort study emphasized gender disparities in uric acid concentrations, largely attributed to estrogen's role in facilitating uric acid excretion25. With declining estrogen post-menopause, women might experience diminished uric acid clearance, elevating hyperuricemia risk26,27. Generally, individuals who do not consume alcohol and are not obese tend to have healthier lifestyles and more stable blood pressure28,29. If an increase in blood pressure is observed in this group, it may indicate the presence of other undiscovered health issues, which could affect the metabolism and clearance of uric acid. A cohort research from Tianjin General Hospital, encompassing 6,424 participants aged 20–94 without hyperuricemia, determined that the combined effects of hypertension and elevated triglycerides on hyperuricemia risk surpassed their individual impacts6. Consistent with this, our study accentuates the pronounced interaction between systolic blood pressure and dyslipidemia. Specifically, among those with dyslipidemia, the link between systolic blood pressure and hyperuricemia is exceptionally robust.
Pertaining to diastolic blood pressure, our conclusions align similarly. Yet, a pronounced interaction between diastolic blood pressure and age emerges. For those below 65, an upsurge in diastolic blood pressure significantly amplifies hyperuricemia risk. With aging, vascular elasticity diminishes, often resulting in reduced diastolic pressure30. Consequently, for younger individuals below 65, a minor elevation in diastolic pressure could indicate an augmented risk of hyperuricemia. Collectively, these insights stress the importance of factoring in blood pressure and its interplay with other determinants when evaluating hyperuricemia risk. This equips healthcare professionals with a more comprehensive perspective on hyperuricemia prevention and management.
Utilizing restricted cubic spline functions, we delineated the dose–response association between both diastolic and systolic blood pressures and hyperuricemia risk. After accounting for potential confounders, we determined that a systolic pressure surpassing 115 mmHg correlates with a heightened hyperuricemia risk. Setting 78 mmHg as a reference, diastolic pressure exhibited a congruent trend. This observation partially elucidates our earlier finding that individuals in the pre-hypertensive stage face a greater propensity for hyperuricemia compared to those with normal blood pressure. The American Heart Association's statement on dietary strategies for preventing and managing hypertension highlights a consistent rise in cardiovascular disease risk across the blood pressure spectrum, commencing from 115/75 mmHg31. Our study resonates with this, suggesting that from 115/78 mmHg onward, hyperuricemia risk incrementally grows across the blood pressure range. This parallel might stem from overlapping lifestyle or genetic risk determinants for cardiovascular ailments and hyperuricemia, such as dietary habits, weight, alcohol intake, and other behavioral factors32,33,34. Furthermore, both conditions share ties to renal functionality. Elevated blood pressures can compromise kidney efficiency, potentially instigating these disorders19,35. These pivotal revelations bear significant clinical implications, furnishing practitioners with a valuable benchmark for averting hyperuricemia and related complications in standard care. Additionally, it serves as a vital advisory for the wider community, particularly those nearing or surpassing the 115/78 mmHg threshold, underlining the necessity of vigilant blood pressure supervision and management.
This study surpasses previous research in several key areas. Firstly, our larger sample size provides enhanced statistical power and allows for more rigorous adjustment for confounders, leading to a more accurate understanding of the relationship between blood pressure and hyperuricemia. Secondly, we extend beyond just examining the direct link between hypertension and hyperuricemia by also exploring prehypertension and its effects. This approach offers a more comprehensive view, considering various factors like gender, alcohol consumption, obesity, and lipid profiles. Using restricted cubic splines, we have clearly illustrated the dose–response relationship between blood pressure and hyperuricemia risk, offering valuable insights for healthcare professionals to develop better treatment and prevention strategies.
Although our study provides valuable insights, it has certain limitations. As an observational study, it identifies correlations between blood pressure and hyperuricemia but cannot establish direct causation. Future prospective intervention studies are needed for more definitive conclusions. Additionally, our data is drawn from health records in a Wuhu city hospital, which may limit the generalizability of our findings to similar populations or regions. Furthermore, the limitations of the hospital database prevented us from obtaining important information such as participants' past medical history and medication use, which may have influenced the interpretation of the results. Therefore, while we have accounted for various variables, complete elimination of bias cannot be assured.
Conclusion
In conclusion, this study demonstrates a significantly increased risk of hyperuricemia in individuals with prehypertension and hypertension compared to those with normal blood pressure. The risk is further accentuated with the elevation of both systolic and diastolic blood pressures, especially when levels exceed 115/78 mmHg. Notably, this risk is modulated by factors such as gender, alcohol consumption, obesity, and dyslipidemia. These findings suggest that in preventing hyperuricemia through blood pressure management, a comprehensive approach is needed. This approach should consider multiple risk factors, including prehypertension, and develop interventions tailored to the characteristics of different populations.
Data availability
The authors collected participant data and uploaded it to a database. This system conveniently shields irrelevant data and effectively protects participant privacy. The data supporting this study's findings are available from the Health Management Center at the First Affiliated Hospital of Wannan Medical College, Wuhu, China. However, access to these data is restricted as they were used under license for this study and are not publicly available. Data can be provided upon reasonable request and with the Health Management Center's permission. For data requests, please contact Yufeng Wen, the corresponding author.
Abbreviations
- HUA:
-
Hyperuricemia
- SBP:
-
Systolic blood presure
- DBP:
-
Diastolic blood pressure
- FBG:
-
Fasting blood glucose
- BUN:
-
Blood urea nitrogen
- CRE:
-
Creatinine
- ALT:
-
Alanine aminotransferase
- GLO:
-
Globulin
- WBC:
-
White blood cells
- MPV:
-
Mean platelet volume
- MCHC:
-
Mean corpuscular hemoglobin concentration
- PLT:
-
Platelets
- HR:
-
Hazard ratios
- CI:
-
Confidence intervals
- TG:
-
Triglycerides
- TC:
-
Total cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
References
Maiuolo, J., Oppedisano, F., Gratteri, S., Muscoli, C. & Mollace, V. Regulation of uric acid metabolism and excretion. Int. J. Cardiol. 213, 8–14 (2016).
Xu, Y. et al. Association between dyslipidaemia and the risk of hyperuricaemia: A six-year longitudinal cohort study of elderly individuals in China. Ann. Med. 54, 2402–2410 (2022).
Zhu, Y., Pandya, B. J. & Choi, H. K. Prevalence of gout and hyperuricemia in the US general population: The National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 63, 3136–3141 (2011).
Neogi, T. Clinical practice. Gout. N. Engl. J. Med. 364, 443–452 (2011).
Mazzali, M. et al. Uric acid and hypertension: Cause or effect?. Curr. Rheumatol. Rep. 12, 108–117 (2010).
Zhang, Y. et al. Association of hypertension and hypertriglyceridemia on incident hyperuricemia: An 8-year prospective cohort study. J. Transl. Med. 18, 409 (2020).
Yin, Y., Zhou, E. & Wu, J. Association between hyperuricemia and long-term mortality in patients with hypertension: Results from the NHANES 2001–2018. Front. Cardiovasc. Med. 11, 1306026 (2024).
Vareldzis, R., Perez, A. & Reisin, E. Hyperuricemia: An intriguing connection to metabolic syndrome, diabetes, kidney disease, and hypertension. Curr. Hypertens. Rep. 26, 237–245 (2024).
Liu, L. et al. Serum uric acid and risk of prehypertension: A dose-response meta-analysis of 17 observational studies of approximately 79 thousand participants. Acta Cardiol. 77, 136–145 (2022).
Cao, J. et al. Incidence and simple prediction model of hyperuricemia for urban Han Chinese adults: A prospective cohort study. Int. J. Environ. Res. Public Health 14, 67 (2017).
Fang, J. & Alderman, M. H. Serum uric acid and cardiovascular mortality the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA 283, 2404–2410 (2000).
Zhang, Y. Y., Qiu, H. B. & Tian, J. W. Association between vitamin D and hyperuricemia among adults in the United States. Front. Nutr. 7, 592777 (2020).
Jacobson, T. A. et al. National lipid association recommendations for patient-centered management of dyslipidemia: Part 2. J. Clin. Lipidol. 9(S1–122), e121 (2015).
Dehghan, A., van Hoek, M., Sijbrands, E. J., Hofman, A. & Witteman, J. C. High serum uric acid as a novel risk factor for type 2 diabetes. Diabetes Care 31, 361–362 (2008).
Kotchen, T. A. Developing hypertension guidelines: An evolving process. Am. J. Hypertens. 27, 765–772 (2014).
Zhou, B. F. Cooperative Meta-Analysis Group of the Working Group on Obesity in C: Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults–study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed. Environ. Sci. 15, 83–96 (2002).
Duncan, M. S. et al. Association between HIV and incident pulmonary hypertension in US Veterans: A retrospective cohort study. Lancet Healthy Longev. 2, e417–e425 (2021).
Goma, F. M. et al. Prevalence of hypertension and its correlates in Lusaka urban district of Zambia: A population based survey. Int. Arch. Med. 4, 34 (2011).
Mennuni, S. et al. Hypertension and kidneys: Unraveling complex molecular mechanisms underlying hypertensive renal damage. J. Hum. Hypertens. 28, 74–79 (2014).
Fathallah-Shaykh, S. A. & Cramer, M. T. Uric acid and the kidney. Pediatr. Nephrol. 29, 999–1008 (2014).
Williams, P. T. Walking and running produce similar reductions in cause-specific disease mortality in hypertensives. Hypertension 62, 485–491 (2013).
Carranza-Leon, D. A. et al. Ambulatory blood pressure in patients with systemic lupus erythematosus: Association with markers of immune activation. Lupus 29, 1683–1690 (2020).
Kim, W. et al. Age and sex dependent association of uric acid and incident hypertension. Nutr. Metab. Cardiovasc. Dis. 31, 1200–1208 (2021).
Ungar, A. et al. The association of uric acid with mortality modifies at old age: Data from the uric acid right for heart health (URRAH) study. J. Hypertens. 40, 704–711 (2022).
Chen, J. H., Yeh, W. T., Chuang, S. Y., Wu, Y. Y. & Pan, W. H. Gender-specific risk factors for incident gout: A prospective cohort study. Clin. Rheumatol. 31, 239–245 (2012).
Stockl, D. et al. Reproductive factors and serum uric acid levels in females from the general population: The KORA F4 study. PLoS One 7, e32668 (2012).
Sumino, H., Ichikawa, S., Kanda, T., Nakamura, T. & Sakamaki, T. Reduction of serum uric acid by hormone replacement therapy in postmenopausal women with hyperuricaemia. Lancet 354, 650 (1999).
Fuchs, F. D. & Fuchs, S. C. The effect of alcohol on blood pressure and hypertension. Curr. Hypertens. Rep. 23, 42 (2021).
Cohen, J. B. & Gadde, K. M. Weight loss medications in the treatment of obesity and hypertension. Curr. Hypertens. Rep. 21, 16 (2019).
Touyz, R. M. et al. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 114, 529–539 (2018).
Appel, L. J. et al. Dietary approaches to prevent and treat hypertension: A scientific statement from the American Heart Association. Hypertension 47, 296–308 (2006).
Voruganti, V. S. et al. Genetics of variation in serum uric acid and cardiovascular risk factors in Mexican Americans. J. Clin. Endocrinol. Metab. 94, 632–638 (2009).
Wang, J. et al. Prevalence and risk factors of hyperuricaemia in non-obese Chinese: A single-centre cross-sectional study. BMJ Open 12, e048574 (2022).
Moore, M. N. et al. Exploring the direct and indirect effects of cardiovascular disease risk factors on exercise blood pressure. Scand. J. Med. Sci. Sports 33, 2509–2515 (2023).
Kim, J. Y., Seo, C., Pak, H., Lim, H. & Chang, T. I. Uric acid and risk of cardiovascular disease and mortality: A longitudinal cohort study. J. Korean Med. Sci. 38, e302 (2023).
Acknowledgements
The authors express their gratitude to the staff of the First Affiliated Hospital of Wannan Medical College for their invaluable contributions to this research. We also extend our thanks to the study participants for their collaboration and commitment.
Funding
This work was supported by several initiatives from the Education Department of Anhui Province: The Teaching Reform and Quality Improvement Plan for the Teaching Team (grant number 2018jxtd153), the Famous Teacher segment of the Teaching Reform and Quality Improvement Plan (grant number 2019jxms066), and the Public Health Collaborative Innovation Project of Provincial Undergraduate Medical Colleges (grant number GXXT-2020-22).
Author information
Authors and Affiliations
Contributions
F.Y., T.G., and M.W. wrote the main text of the manuscript; J.C., W.C., and R.X. prepared figures; H.J. and S.F. conducted the experiments; W.H. analyzed the data; S.C., X.M., T.W., and Y.W. provided additional input and support; W.Y. supervised the entire project. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study's protocol has been approved by the Ethics Committee of Wannan Medical College, with data sourced from the Health Management Center at the First Affiliated Hospital of Wannan Medical College in Wuhu, China. Given that participants were hospital visitors for routine medical check-ups, the Medical Ethics Committee deemed verbal informed consent as sufficient. Therefore, necessary verbal informed consent was obtained from all subjects and/or their legal guardian(s). All methods in this study were conducted in accordance with the guidelines and regulations of the Declaration of Helsinki.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Fang, Y., Tavengana, G., Wu, H. et al. Elevated blood pressure and hyperuricemia risk: a retrospective cohort study from Wuhu, China. Sci Rep 14, 19994 (2024). https://doi.org/10.1038/s41598-024-71087-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71087-x
- Springer Nature Limited