Abstract
As an emerging global issue in coastal marine ecosystems, eutrophication may lead to profound ecological consequences or disasters. Six locations in Xiangshan Bay were sampled during 2012–2022 along the eutrophication gradient from the innermost bay with the most eutrophication to the outer bay with the least eutrophication. A trait-based method was adopted to explore the ecological effects of eutrophication on macrobenthic communities. The results showed that the community composition is mostly characterized by deposit feeders and predators with small (1–3 cm) and large (> 10 cm) body sizes, classified as indifferent and tolerant species (AMBI ecological groups), deposit feeders and predators (feeding mode), and a preference for a free living lifestyle. The RLQ and fourth-corner analyses further confirmed that there was a negative correlation between the abundance of small macrobenthic organisms (< 1 cm) and nitrate concentration. Phosphorus was a crucial influencing factor for macrobenthic spatial patterns and was strongly affected by the activities of deposit feeders and the decomposition of macrobenthos. Due to mass organic deposition resulting from increased primary production, long-term eutrophication had led to an increase in the proportion of detritus feeders. In addition, the significant negative correlation between the concentration of dissolved oxygen and first-order opportunistic species represented by the polychaete Capitella capitata indicated tolerance to hypoxia. The macrobenthic community in Xiangshan Bay had been negatively affected but maintains considerable stability in functional diversity and functional redundancy under the influence of long-term eutrophication.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Eutrophication refers to the accumulation of organic matter in water bodies, with nitrogen and phosphorus being the most important elements1, and has been recognized as one of the greatest pollution problems worldwide for most freshwater and coastal marine ecosystems2,3. As an emerging global issue, some ecosystems may be naturally eutrophic due to hydrological conditions4, such as tide-associated accumulation of organic matter, sedimentation5, nutrient flow into enclosed estuaries, and oceanic or coastal upwelling6. However, since the twentieth century, eutrophication has been associated with increasing anthropogenic nutrient loading7,8. Eutrophication of coastal waters may cause subsequent effects on pelagic and benthic community structures, changes in species and biotic community composition9, reductions in diversity, changes in dominant species of smaller body size10, and cascading effects on ecosystem functioning11. In recent decades, many oligotrophic bays or coastal waters, including the Caribbean Sea, the Adriatic Sea, the Baltic Sea, the North Sea, the Bohai Sea, and the Yangtze River estuary, had been found to experience increasing eutrophication12,13. According to the China Marine Ecological Environment Status Bulletin in 2022, the eutrophicated sea area is expected to reach more than 2.8 × 104 km2, and 16 bays with an area of more than 100 km2 will experience severe eutrophication.
At the initial stage of eutrophication, the primary productivity of algae increases greatly. Some macrobenthos, e.g., amphipods, shrimp, filter-feeding bivalves, and phytophagous fishes, benefited from increased primary productivity and suspended organic matter14,15, as indicated by increased abundance, biomass, growth rate and fecundity16,17,18,19. The macrobenthic organisms are subsequently consumed by higher trophic organisms, such as nekton. When the increasing primary productivity of algae cannot be fully consumed7, excessive organic matter deposition exceeds the bacterial remineralization capacity and organic matter oxidation capacity20,21,22, leading to seasonal hypoxia23,24 and decreasing macrobenthic species richness, abundance and biomass25. In addition, the macrobenthic communities will shift from bivalves, polychaetes and crustaceans with high diversity and heterogeneity and more complex trophic levels to many deposit feeders with a short lifespan and strong tolerance to eutrophication and possible seasonal hypoxia26,27, whose biomass will also be greatly reduced28.
Marine macrobenthos are important in the nutrient cycle and energy flow in marine ecosystems29,30. Numerous ecologists had focused on the use of macrobenthos as an indicator species for various disturbances31,32. Macrobenthos have limited motility, a long life span, high diversity, various tolerance abilities and important ecological functions33,34. As a result, biological traits35,36 or macrobenthic composition and community structure can reflect the combined effects of multiple environmental stresses and disturbances on seabeds37,38,39. For long-term cumulative ecological effects, macrobenthos is the best choice for monitoring biological community differences and assessing ecological quality40,41,42. In addition, the growth rate, reproduction and metabolism of macrobenthos are also affected by direct toxic effects from inorganic nitrogen compounds such as NH4+ and NO2–11,43,44. Certain species, including the polychaetes Alkmaria romijni, Capitella capitata, and bivalve Scrobicularia plana, can be used as indicators of eutrophication45,46,47. Consequently, these species can be valuable indicators for monitoring and assessing eutrophic stress in aquatic environments.
Biological traits, such as feeding mode, body size and mobility, that characterize the behavioral, morphological, and physiological attributes of organisms are closely associated with environmental gradients48,49. These traits are important links between community structure and ecosystem function50 and are believed to strongly influence ecosystem properties, including sediment oxygenation, benthic respiration, nutrient flux and denitrification51. Functional trait analysis has been advocated for revealing the global mechanisms behind biodiversity responses to environmental variation52. Functional diversity, which is the range of functions that organisms can perform in a community or ecosystem, is an essential factor in describing the consequences of anthropogenic disturbances and is suggested as a baseline for measuring the recovery of organic enrichment53,54,55.
Xiangshan Bay, a long and narrow bay on the east coast of Zhejiang Province, is one of the largest areas for mariculture on the coastline of the East China Sea56. The rapid development of coastal industry and agriculture has led to the discharge of nutrients and organic matter into Xiangshan Bay, causing eutrophication. This results in harmful algal blooms57 and lower macrobenthic diversity58,59 but increases the biomass of tolerant species60. Thus, several hypotheses were tested in this research, including (1) verifying whether the distribution patterns of taxonomic and functional diversities are associated with eutrophication gradients or durations of eutrophication, (2) elucidating the sequential changes in macrobenthic trait modalities within Xiangshan Bay resulting from exposure to long-term eutrophication, and (3) deciphering whether eutrophication-related environmental factors contribute to structuring macrobenthic biological trait patterns.
Materials and methods
Study area
Xiangshan Bay, a 563 km2 narrow semienclosed typical eutrophic embayment, is in the northwestern region of the East China Sea in northern Zhejiang Province59. It is connected to Hangzhou Bay in the north and Sanmen Bay in the south. Three of the bay's sides are covered with hills, and two waterways (the Fodu and Niubishan waterways) connect the bay to the East China Sea. Xiangshan Bay is a narrow semienclosed bay and is one of the three major bays in Zhejiang Province, along with Sanmen Bay and Taizhou Bay61. Xiangshan Bay has a low water exchange capacity; it takes 125 days to replace 65% of the water volume in the bay and 305 days to replenish 90% of the water volume62. Xiangshan Bay has been eutrophic since the 1980s63, and the eutrophication in Xiangshan Bay showed a numerical improvement between 2010 and 2018, with eutrophication index (EI) values ranging from 7.0 to 15.1 (i.e., 15.1, 10.0, 8.7, 9.0, 10.1, 10.3, 9.0, 8.7, and 7.0)64. Nevertheless, the bay is affected by eutrophication64. The average water depth in Xiangshan Bay is approximately 10–20 m65,66, while that in the central part is approximately 20–55 m. The tidal range is 3.18 m, and the maximum ebb velocity is 183 cm/s. The salinity of Xiangshan Bay ranges from 20.82 to 28.1363. A total of 95 small ephemeral rivers flow into the bay; the longest river is the Dasong River, with a mean annual runoff of 12.89 × 108 m363,67. The surface seawater temperature ranges from 28.08 to 29.09 °C in summer (from July to September)68. The bay's hydrodynamic processes are mostly regulated by the ebb and flow of tides and wind-induced currents. The tidal fluctuations align perpendicular to the mainland shoreline, with average speeds of 50 cm s−1 (for rising tidal current) and 57 cm s−1 (for falling tidal current) and maximum current velocities of 154 cm s−1 (for rising tidal current) and 183 cm s−1 (for falling tidal current). Due to the limited amount of external sediment carried by the Yangtze River and the sediment resuspended in the coastal waters of Zhejiang, the seawater in Xiangshan Bay is clear, with slightly silted waterways and beaches63.
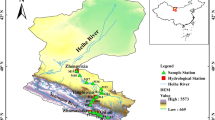
Sampling was carried out annually in Xiangshan Bay from 2012 to 2022 during the summer. The six sampling locations were geographically distant from one another (Fig. 1) and depicted the inner bay, bay entrance, and outer bay, respectively.
The locations of the sampling stations in Xiangshan Bay during the years 2012–2019 and 2022. This map was produced using ArcGIS Desktop 10.5. The coordinate system is GCS_ WGS_ 1984, the source of the base image was downloaded from national geomatic center of China (https://www.ngcc.cn/ngcc/html/1/index.html).
Data collection
Sampling was carried out annually in summer (usually August, the flooding season) during 2012–2022 in Xiangshan Bay, a total of eight years excluding 2020 and 2021. At each site, four samples were collected using a 0.05 m2 Van Veen grab and washed in situ through a 0.5 mm mesh screen. The macrobenthos and other organisms in the mesh were preserved in 75% ethyl alcohol for further analyses in the laboratory. Once in the laboratory, the macrobenthos were stained with 1% rose bengal and readily separated and collected. The macrobenthos were subsequently identified using stereo- and binocular microscopy (Olympus SZ61, Tokyo) at the lowest feasible taxonomic level. The wet weight was determined using an electronic YP6002B balance (Libang Chenxi, Shanghai) with an accuracy of 0.01 g. The collection, preservation, and laboratory processing of macrobenthos were carried out in accordance with the "Specification for Marine Investigation" (GB/T 12763.6-2007)69. Broken nemertea and annelids were counted only when the head was present. The empty shells of Molluska were not counted. Before weighing, the samples were patted dry with absorbent paper, and the outer shells of the tubicolous animals and hermit crabs were removed.
Environmental abiotic parameters, including temperature, salinity, depth and pH, were examined by a conductivity-temperature-depth system (OCEAN SEVEN 308, Idronaut Co., Italy). The CTD and water sampler were deployed from the surface layer down to deeper depths. According to the specification for offshore environmental monitoring HJ 442—2008, only the surface layer was sampled from a depth of 0.1 to 1 m below the surface for stations less than 10 m deep. For stations between 10 and 25 m deep, the bottom layer was also sampled, approximately 2 m above the sea floor. For stations with depths greater than 25 m, the intermediate layer (approximately 10 m below the water surface) was sampled in addition to the surface layer. Three water layers at most were collected from a single sampling location, depending on the water depth. Surface water was used for nutrient-related abiotic parameters, whereas bottom water was used for pH and DO measurements. Aquatic environmental parameters, including nitrate nitrogen (NO3–N) (mg/L), nitrite nitrogen (NO2–N) (mg/L), phosphate (DIP) (mg/L), ammonia nitrogen (NH4–N) (mg/L), dissolved oxygen (DO) (mg/L), chemical oxygen demand (COD) (mg/L) and chlorophyll a (Chl-a) (µg/L) contents, were determined in the laboratory. The NO3–N content was measured by the zinc cadmium reduction method, NO2–N content was measured by naphthalene ethylenediamine hydrochloride spectrophotometry, DIP content was measured by the ascorbic acid reduction phosphomolybdate blue method, DO concentration was measured by iodometry, COD was measured by the alkaline potassium permanganate method, and Chl-a content was measured by fluorescence spectrophotometry. Inorganic nitrogen (DIN)=NO2–N + NO3–N + NH4–N.
The total organic carbon (TOC), total nitrogen (TN) and sulfur contents in the substrate were evaluated using a vario MICRO CUBE (Elementar, Germany).
The eutrophication index (EI) was calculated using the following formula:
where COD is the chemical oxygen demand (mg/L), DIN is the dissolved inorganic nitrogen (mg/L), and DIP is the active phosphorus (mg/L). The trophic status was classified into five levels: oligotrophic (EI < 1.0), light trophic (1.0 ≤ EI < 2.0), mesoeutrophic (2.0 ≤ EI < 5.0), heavy eutrophic (5.0 ≤ EI < 15.0) and hypertrophic (EI ≥ 15.0)70. However, a water body would be designated as eutrophic if its EI value was greater than 1.0.
Biological traits
Five biological trait categories of macrobenthos were selected based on feature availability and the importance of organisms to ecosystem function. These categories included adult body size, living habit, motility, feeding mode, and the AZTI's Marine Biotic Index (AMBI) ecological group. A total of 23 trait modalities were identified (Table 1). The biological trait data were obtained from published monographs71,72,73, peer-reviewed papers15,36,61,74,75 and online databases such as BIOTIC76 and polytraits77. The affinity of a species for different modalities was measured using a fuzzy coding method (range 0–3), where 0 denoted full noncorrespondence and 3 denoted substantial correspondence78. The method of fuzzy coding was reliable because biological traits are not absolute but rather involve spatial–temporal variations79. For species whose information at the species level was unavailable, the traits of congeneric species were used52. If information on certain traits of a species was not available, a value of 0 was assigned for those trait modalities80. Given that some species may exhibit more than one behavioral characteristic—for example, the ability to move by swimming and crawling—values were allocated to these two trait modalities independently, with the dominant motility receiving a value of 3 and the other receiving a value of 175,81.
Data analyses
Macrobenthic community data were standardized (ind. m−2) and then processed using univariate and multivariate statistical analyses. The biological traits were analyzed as community-level weighted means (CWMs) of trait values. Taxonomic diversity, functional diversity and functional redundancy were calculated using the “vegan”82, “FD”83 and “adiv” packages84, respectively, in R85. Overall spatial–temporal variations in both species and functional diversity indices were examined with the Kruskal‒Wallis rank sum test, and pairwise comparisons were subsequently performed for the overall significant indices using the Wilcoxon rank sum test with continuity correction. Two-way permutational multivariate analysis of variance (PERMANOVA) was also used to detect the overall spatial–temporal differences in the species diversity matrix (all species diversity indices) and functional diversity matrix (all functional diversity indices). The data were log(x + 1) transformed, Euclidean distance was calculated before analysis, and subsequent pairwise analysis was performed if a significant difference was detected via PERMANOVA. A distance-based test for homogeneity of multivariate dispersions (PERMDISP) was used to test the overall spatial–temporal differences in dispersion for the species and functional diversity matrixes. Subsequent pairwise analysis was performed if a significant difference was detected in PERMDISP. Both PERMANOVA and PERMDISP were performed in PRIMER 6 & PERMANOVA + software. RLQ and fourth-corner approach analyses were performed in R using the “ade4” package86 to explore correlations between macrobenthic biological traits and long-term eutrophic stress; this approach represents the most comprehensive method for revealing the correlation between biological traits and environmental stress87,88. These two approaches can be produced by three related tables, including an environmental variables table (termed R; sites in rows and environmental variables in columns), a species abundance Table (L; sites in rows and species in columns), and a biological traits Table (Q; species in rows and traits in columns)88,89. Prior to RLQ analysis, these tables were analyzed separately. A correspondence analysis (CA) was performed using the R environmental table, and a principal component analysis (PCA) was applied to the Q trait table. Hill–Smith analysis was used because the R environmental table enables mixed continuous and categorical data90,91. The variance explained (%) by the first two RLQ axes was compared, and a global Monte Carlo test using 9,999 random permutations for Models 2 and 4 was used to further evaluate the overall relevance of the RLQ model. Fourth-corner analysis was employed to examine the significance of the relationships between species traits and environmental factors. To avoid the intrinsic drawbacks of RLQ and fourth-corner analyses, the combined use of these two approaches is recommended88.
Results
Environmental variables and eutrophication disturbance
Six sampling stations were measured in Xiangshan Bay; four inside (D1–D4) and two outside (D5 and D6). The water depths at the stations inside the bay ranged from 9.0 to 43 m, while those beyond the bay ranged from 5.0 to 17.8 m. The investigation was conducted during the summer, and the typical summer water temperature varied between stations. D5 had the lowest temperature of 24.95 °C, and D1 had the highest temperature of 29.01 °C. The temperatures at the other locations were comparable, ranging from 27.15 to 28.26 °C. The salinity ranged from 25.64 to 28.86, with D5 and D6 being significantly greater outside the bay than within the bay. The innermost station, D1, had the lowest salinity of 25.64. The DO concentration ranged from 5.54 to 6.33 mg/L. The COD ranged from 0.97 to 1.14 mg/L. The phosphorus concentration ranged from 0.025 to 0.043 mg/L. Nitrate was the most common type of nitrogen, with concentrations ranging from 0.448 to 0.718 mg/L (Table 2).
During 2012–2022, 98.15% of the total number of stations had EI values higher than 1 (EI ≥ 1), except Station D5 in 2016 (EI < 1). During the last decade, the average eutrophication index in Xiangshan Bay was highest at Station D1 (EI = 8.87) and lowest at Station D5 (EI = 2.59). The average eutrophication index for all stations was greater than 2, indicating eutrophication, from mesotrophic to hypertrophic. The average EI in Xiangshan Bay during 2012–2022 decreased gradually from the innermost part to the open sea (Table 3).
Biological traits and functional diversity
The biological traits, trait modalities and trait codes of macrobenthic organisms in Xiangshan Bay are listed in Table 1, and the proportions of macrobenthic trait modalities in Xiangshan Bay across the sampling period and sampling area are presented in Fig. 2. The biological traits of the macrobenthic communities (community-weighted mean traits) were characterized by small (1–3 cm) and large (> 10 cm) body sizes. According to the AMBI ecological groups, the macrobenthic community was mostly composed of indifferent and tolerant species, with sensitive species making up a smaller percentage of the population. With respect to feeding habits, deposit feeders and predators were the most abundant in the communities, and most the species favored free-living habits.
The temporal variations in macrobenthic species number, functional diversity indices and functional redundancy are shown in Figs. 3 and 5. The results of the Kruskal‒Wallis rank sum test indicated that there was no significant overall temporal difference (P > 0.05) in any species or functional diversity indices or functional redundancy. However, the results of two-way PERMANOVA indicated that the species diversity matrix showed a significant overall temporal difference (P < 0.05). A pairwise test indicated that the species diversity matrix significantly differed between 2012 and 2014, between 2012 and 2018, between 2013 and 2018, and between 2017 and 2018. PERMDISP indicated no significant temporal differences in dispersion (P > 0.05) for the species diversity matrix. No significant temporal difference was detected for the functional diversity matrix via two-way PERMANOVA (P < 0.05). PERMDISP indicated significant temporal differences in dispersion (P > 0.05) for the functional diversity matrix. Pairwise tests revealed that the dispersion of functional diversity in 2019 significantly differed from that in 2012, 2013, 2014, 2015, 2016, 2017 and 2022 (P < 0.05); functional dispersion in 2018 significantly differed from that in 2013, 2016 and 2022 (P < 0.05); and functional dispersion in 2014 significantly differed from that in 2016 (P < 0.05).
The spatial variations in the macrobenthic species and functional diversity indices and functional redundancy are shown in Figs. 4 and 5. The results of the Kruskal‒Wallis rank sum test revealed significant overall spatial differences in several taxonomic diversity indices, including the number of species, Margalef richness index, Shannon–Wiener diversity index, Simpson diversity index, Pielou’s evenness index, abundance and biomass. Post hoc pairwise comparisons (pairwise Wilcoxon rank sum test) revealed that the number of species was significantly greater in D1 than in D3, and D6 and was significantly greater in D2 than in D6. The Margalef index was significantly greater in D1 than in D5, and D6 and was significantly greater in D2 than in D3 and D6. The Shannon–Wiener diversity index and Simpson diversity index were both significantly greater in D1 and D2 than in D5 and D6, respectively. The Pielou evenness index was significantly greater in D1, D2 and D4 than in D5. Abundance was significantly greater in D1 than in D3, D4 and D6. Biomass was significantly greater in D1 and D2 than in D4, D5 and D6. The results of two-way PERMANOVA also revealed significant overall spatial differences (P < 0.05) in the species diversity matrix. A pairwise test indicated that D1 and D2 were significantly different from the other sites. PERMDISP indicated significant spatial differences in dispersion (P < 0.05) for the species diversity matrix, and pairwise tests further showed that dispersion in D5 significantly differed from that in D1, D2 and D3 (P < 0.05).
For functional diversity indices, only functional evenness showed significant overall differences according to the Kruskal‒Wallis rank sum test. Post hoc pairwise comparisons further indicated that functional evenness was significantly greater in D1 than in D5 and was significantly greater in D4 than in D2 and D5. No significant overall spatial difference (P > 0.05) in functional redundancy was detected. According to two-way PERMANOVA, no significant spatial differences were detected for the functional diversity matrix (P > 0.05). Significant spatial differences in dispersion (P < 0.05) for the functional diversity matrix were detected. A pairwise test further indicated that functional dispersion in D5 significantly differed from that in D2 and D3 (P < 0.05), and dispersion in D2 and D3 was also significantly different (P < 0.05).
Response of biological traits
The global testing procedure (a multivariate statistic equivalent to the sum of eigenvalues from RLQ analysis) was highly significant (P = 0.0196 for Model 2 and P = 0.0192 for Model 4), suggesting a globally significant correlation between biological traits and environmental variables (Fig. 6). The fourth-corner method was used to identify these significant relationships, and their representation on the RLQ factor map helped to illustrate the major patterns of variation and correlation. The combined RLQ and fourth-corner analyses showed that 12 of the 23 functional trait modalities were significantly correlated (P < 0.05) with environmental factors (Fig. 7). The proportion of small body sizes (< 1 cm, B1) in the community composition was significantly negatively affected by the concentration of surface nitrate. The tubicolous living habit (H3) was positively related to surface pH but negatively correlated with surface phosphate, and the burrow-dwelling living habit (H4) was positively correlated with surface ammonia but negatively related to surface nitrite. Moreover, the free-living habit (H5) was positively correlated with surface phosphate and depth but negatively related to surface pH. The first and second axes represented 66.53 and 15.96% of the variation, respectively. The mobility of the macrobenthic communities was significantly related to the surface COD and phosphate content, with the discretely motile (M2) correlating negatively with phosphate and the motile (M3) correlating positively with phosphate and negatively with COD. In terms of feeding modes, the EI significantly positively influenced detritus feeders (F2). However, deposit feeders (F3) and herbivores (F5) were significantly less abundant when exposed to high concentrations of surface phosphate and nitrite. For the AMBI ecological groups, indifferent species (E2) were significantly positively associated with bottom temperature and surface nitrite content, and tolerant species (E3) were more abundant with higher ammonia and lower nitrite content in surface water. Moreover, first-order opportunistic species (E5) were significantly negatively related to dissolved oxygen (DO) in bottom water.
Results of the RLQ analysis: Coordinates of the sampling sites (top left) and species (top right), the relationships between axes 1 and 2 in the environmental space (bottom left), the environmental variables (the second from the bottom left), the biological traits (the middle from the bottom left), the relationships between axes 1 and 2 in the functional trait space (the second from the bottom right) and the eigenvalues (bottom right). The traits are explained in Table 1.
Significant associations between environmental factors and biological traits identified using the fourth-corner method on the factorial map of the RLQ analysis. Significant positive associations are represented by red cells (A) and red lines (B), and significant negative associations correspond to blue cells (A) and blue lines (B). Nonsignificant associations are shown in gray (A). P values were adjusted for multiple comparisons using the FDR (false discovery rate) method only between levels of a factor. The values of d give the grid size (B). The trait abbreviations are explained in Table 1.
Discussion
Coastal eutrophication and macrobenthic communities
Coastal eutrophication caused by anthropogenic nutrient loading in aquatic ecosystems92 has become a prominent problem and major concern due to the notable deterioration of nearshore ecosystems during the past two decades worldwide93,94,95,96,97,98. Xiangshan Bay, a semienclosed subtropical bay, had experienced severe eutrophication since the 1980s due to unsustainable mariculture activities and the discharge of industrial and agricultural effluent99,100,101. The eutrophication indices were approximately 1 in the 1980s, with Stations D1, D2 and D4 having values higher than 1 in October63, and the eutrophication index rose as high as 19.6 at Station D1 during 2012–2022 in this study, with an average EI value of 5.36. Due to the inflow of land-based pollutants into the sea and the inadequate water exchange capacity of Xiangshan Bay102, the innermost portion of the bay had the highest degree of eutrophication for both the surface and bottom layers, and there was a significant decreasing trend from the inner bay to the estuary outlet100,103.
The initial response of marine macrobenthos to eutrophication involved decreased diversity and individual mean size of macrobenthos37 but an increase in both the biomass and production of macrozoobenthos104. However, high levels of nitrogen and total phosphorus can cause a large decline in macrobenthic species and an increase in the biomass of species that can tolerate these conditions60. During the past few decades, macrobenthic communities had experienced species loss, with fewer species collected and lower diversity58,59,63. The number of species collected was 11063 in the 1980s, 71 in 2000105 and 49 during 2010–2022 in this study. The macrobenthic community in Xiangshan Bay was dominated by one or a few small species because of severe environmental pollution59, especially from eutrophic-related environmental factors. From the 1980s to 2000, the number of macrobenthic species significantly decreased, while the average biomass and average macrobenthic abundance remained relatively unchanged105. During the past decade (2012–2022), the species number continued to decline, while the abundance and biomass in Xiangshan Bay remained constant without significant temporal variation. However, this study differed from that in a eutrophic estuary in Australia in that the abundance of macrobenthos increased, while the diversity decreased35.
Biological trait composition, taxonomic diversity and functional diversity
Healthy ecosystems are resilient to external stressors and may exist without human intervention106. Healthy macrobenthic communities typically contain a few abundant species and many rare ones, with individual species often providing benefits than competing107. The dominant feeding types in Xiangshan Bay were predator and deposit feeders, which were adaptability and feedback mechanisms used to prevent the collapse of benthic communities, which was consistent with previous research108. However, the dominant feedint types in Xiangshan Bay differed from those in Waquoit Bay, Massachusetts, where carnivores dominated the oligotrophic estuary and herbivores dominated the eutrophic estuary109. In addition, increasing nutrient concentrations could stimulate benthic primary production, resulting in greater food availability for grazers, whereas increased sedimentation from pelagic production benefits filter-feeding and deposit-feeding macrobenthos110.
The functional structure of ecosystems with higher taxonomic diversity is usually more stable than that of ecosystems with lower taxonomic diversity because of the functional redundancy provided by species with similar functions that can compensate for the absence of other species with ecological functions111, including species number, K-dominant curves and diversity indices112. Macrobenthic communities had simplified since the 1980s, with fewer species collected and decreased diversity58,59,63; nevertheless, macrobenthic diversity in Xiangshan Bay had not recovered and had remained low for the past ten years. Over the past decade, the macrobenthic diversity in Xiangshan Bay had undergone no detectable changes and had remained low. Combined with the constant community abundance for several decades, it could be inferred that the macrobenthic community in Xiangshan Bay had been greatly affected by environmental disturbances such as eutrophication for several decades, but its community structure had good stability against the intensity of environmental disturbance.
Interestingly, the distributions of the biological traits in Xiangshan Bay showed no significant spatial or temporal differences, and functional evenness was the only functional diversity index that exhibited significant spatial differences. Species responded to environmental gradients primarily through their functional characteristics and roles in ecosystems113. Biotic homogenization refers to the process of gradual reductions in biological differences, especially genetic, taxonomic, or functional characteristics, in regional biotic communities at any organizational level over time and involves environmental alterations, species invasions, and extinctions114,115. The environmental homogenization caused by human disturbances may lead to biotic homogenization116 due to the loss of species-specific features117,118. After a change in environmental conditions, sensitive species may become tolerant species, leading to increased similarity in species characteristics, known as functional homogenization114,117.
During the past few decades, functional diversity has been highlighted in marine ecology119,120, as indicated by methods such as biological trait analysis (BTA)52,121 and functional diversity indices122,123. By combining species spatial–temporal distribution patterns with multiple biological traits (life history, morphological, behavioral)124, biological trait analysis (BTA) and functional diversity indices (FRic, FEve, FDis and Rao's Q) are reliable approaches for revealing the laws underlying community change in response to environmental disturbances36,74,125,126. However, in our study, no significant spatial or temporal variations in functional diversity (except for spatial variation in functional evenness) were detected in Xiangshan Bay, which has been subjected to long-term eutrophication, at a decadal time scale. Many species may share similar pathways related to resource use, resulting in levels of functional redundancy in macrobenthic communities127. Nevertheless, competition for resources between functionally similar species is symmetric, and the loss of some resources from a community is unlikely to affect ecosystem processes128. This species redundancy is important for ensuring the stability and reliability of ecosystems. Eutrophication can lead to the homogenization of taxonomic and functional diversity, with taxonomic homogenization being more responsive than functional homogenization due to functional redundancy129,130, which is consistent with our results. Communities with more species tend to have more functional redundancy than communities with fewer species, at least when there is no crucial variation in either functional richness or evenness among communities131.
Response of biological traits revealed by RLQ and fourth-corner analyses
Nitrogen and phosphorus are the focus of ecological eutrophication research, but nitrogen has gained additional attention because it is more widely used in synthetic fertilizers than phosphorus132. Although sediment particle size is considered the most important environmental factor regulating organism size133, our results indicated that the proportion of small individuals (< 1 cm) was negatively correlated with eutrophication-related nitrate content. As the most stable and prevalent inorganic nitrogen form in seawater134, nitrate accounted for 94.5% of the total nitrogen content associated with eutrophication in Xiangshan Bay. The toxicity of nitrate is the result of its ability to convert oxygen-carrying proteins into a form that cannot carry oxygen135. Moreover, its toxicity to aquatic animals increases with increasing concentrations of nitrate and exposure time136,137 and decreases with increasing body size138,139; this toxicity may be the most fundamental trait of an organism and is associated with many biological properties140. The RLQ and fourth corner analyses further confirmed the toxic and inhibitory effects of high concentrations of nitrate on small aquatic organisms (< 1 cm).
Living habits indicate the way of life or behavior that is characteristic of a species or organism and may aid in discerning patterns in how these animals interact with their physical environment, including their feeding, activity patterns, and physical adaptations73. Mobility is also a crucial characteristic that affects the predation method and determines the trophic relationship in the macrobenthic community141. Total phosphorus is a crucial influencing factor for macrobenthic temporal patterns132, such as the loss of macrobenthic species142. Generally, the activity of macrobenthos crucially impacts sediment biogeochemistry, often stimulating processes that prevent eutrophication by increasing the retention of phosphorus in sediments143, especially in areas with higher biomasses of deposit feeders144, which are mainly associated with total phosphorus132. The hypoxia and accumulation of phycotoxins caused by eutrophication can negatively affect the living environment of benthic organisms and may result in massive mortality in macrobenthic individuals145. The decomposition of these dead individuals may worsen anaerobic conditions and help release phosphorus from sediments146,147. This may explain the strong positive correlation between macrobenthic motile (M3) traits and phosphorus concentration and the negative correlation between macrobenthic discretely motile (M2) traits and deposit feeders (F3) and phosphorus concentration.
Eutrophication in the ocean is usually caused by a continuous increase in limiting nutrients, especially nitrogen and phosphorus, carried by river runoff from land sources, leading to an increase in the primary productivity of plankton and benthic algae. The subsequent increase in the amount of organic matter deposited in the sediment will be assimilated by the increase in heterotrophic metabolism of the macrobenthic detritus feeders148. Therefore, the mass development of detritus-feeding species is usually associated with eutrophication and organic enrichment, as reported for the Black Sea and the Romanian Black Sea coast149. As the gradient of organic inputs to the environment increases, the trophic relationships become more simplified150. From oligotrophic to eutrophic extremes, complex food webs gradually simplify communities dominated by detritus feeders151. The long-term eutrophication of Xiangshan Bay had increased the proportion of detritus feeders in the macrobenthic community. However, the highest proportion of detritus feeders in the macrobenthic community of Xiangshan Bay was only 17.55%, corresponding to an average eutrophication index of 8.87 over the past decade, with a maximum value of 19.90 in 2015 and a minimum value of 3.56 in 2013.
DO is one of the most important environmental factors in coastal ecosystems and is essential for the survival and reproduction of aquatic organisms23. When the dissolved oxygen concentration is less than the commonly referred anoxic threshold of 2.0 mg/L, the ecological environment rapidly deteriorates, endangering the survival of many aquatic organisms, such as fish and shrimp152. Even seasonal hypoxia affects benthic faunal composition and promotes the proportion of more tolerant benthos in the community153. Due to the different hypoxia tolerances of macrobenthos in different ocean regions (Sturdivant et al., 2015) and because the use of this threshold is restricted to waters not affected by hypoxia at the successional time scale (Rabalais et al., 2010), some scholars have set a higher threshold of hypoxia at 3 mg/L154,155 or 4 mg/L156. Therefore, although the dissolved oxygen concentration in Xiangshan Bay was 3.78–10.96 mg/L, many macrobenthic taxa were affected by low dissolved oxygen concentrations. Therefore, the significant negative correlation between the concentration of dissolved oxygen and the concentration of first-order opportunistic species (AMBI ecological group: E5) reflected the strong tolerance of the polychaete Capitella capitata to hypoxic stress, which is consistent with previous findings in the Changjiang Estuary157 and Hangzhou Bay158.
In this study, the use of biological traits to illustrate the long-term effects of eutrophication on macrobenthic communities was attempted. To properly comprehend benthic ecosystem responses, more studies are needed, including research on indicator species, biotic indices, secondary production, and dominant macrobenthic species, to establish solid theoretical underpinnings for protecting marine biodiversity and assessing the availability of fishery resources in the nearshore marine environment.
Conclusion
This work reported the response of macrobenthic functional trait characteristics to long-term eutrophication in Xiangshan Bay using weighted means (CWMs), RLQs and fourth-corner analyses. Based on our results, the macrobenthic community in eutrophic Xiangshan Bay had remained constant in terms of functional diversity indices, functional redundancy, abundance, biomass and detritus feeders. However, taxonomic homogenization is more responsive than functional homogenization due to large spatial differences in taxonomic diversity when exposed to varying levels of eutrophication stress. The macrobenthic biological traits in Xiangshan Bay were characterized by small (1–3 cm) and large (> 10 cm) body sizes, indifferent and tolerant species (AMBI ecological groups), deposit feeders and predators (feeding mode), and a preference for a free living lifestyle. The relationships between eutrophication-related variables and macrobenthic biological traits were further investigated using RLQ and fourth-corner analyses. High concentrations of nitrate can exert toxic and inhibitory effects on small aquatic organisms (< 1 cm), and phosphorus was a crucial influencing factor for macrobenthic temporal distribution patterns and was strongly affected by the activities of deposit feeders and the decomposition of macrobenthos. Moreover, the significant negative correlation between the concentration of dissolved oxygen and first-order opportunistic species represented by the polychaete Capitella capitata indicates tolerance to hypoxic disturbance. This work revealed important insights into the probable functional succession of the macrobenthic community, which diminishes taxonomic diversity while maintaining a stable state of community function.
Data availability
The data that support the findings of this study are available from Marine Environmental Monitoring Center of Ningbo, Ministry of Natural Resources, Ningbo, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Marine Environmental Monitoring Center of Ningbo, Ministry of Natural Resources, Ningbo.
References
Wurtsbaugh, W. A., Paerl, H. W. & Dodds, W. K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. Wiley Interdiscip. Rev. Water 6(5), e1373 (2019).
Howarth, R. W., Sharpley, A. & Walker, D. Sources of nutrient pollution to coastal waters in the United States: Implications for achieving coastal water quality goals. Estuaries 25, 656–676 (2002).
Smith, V. H. & Schindler, D. W. Eutrophication science: Where do we go from here?. Trends Ecol. Evol. 24(4), 201–207 (2009).
Carpenter, S. R. Submersed vegetation: An internal factor in lake ecosystem succession. Am. Nat. 118(3), 372–383 (1981).
de Jonge, V. N., Elliott, M. & Orive, E. Causes, historical development, effects and future challenges of a common environmental problem: Eutrophication. Hydrobiologia 475(476), 1–19 (2002).
Retnamma, J. et al. Ecological responses of autotrophic microplankton to the eutrophication of the coastal upwelling along the Southwest coast of India. Environ. Sci. Pollut. Res. 28(9), 11401–11414 (2021).
Rabalais, N. N., Turner, R. E., Díaz, R. J. & Justić, D. Global change and eutrophication of coastal waters. ICES J. Mar. Sci. 66(7), 1528–1537 (2009).
Maúre, E. D. R., Terauchi, G., Ishizaka, J., Clinton, N. & Dewitt, M. Globally consistent assessment of coastal eutrophication. Nat. Commun. 12(1), 6142 (2021).
Rast, W. & Thornton, J. A. Trends in eutrophication research and control. Hydrol. Process. 10(2), 295–313 (1996).
Xin, M. et al. Long-term changes in nutrient regimes and their ecological effects in the Bohai Sea, China. Mar. Pollut. Bull. 146, 562–573 (2019).
Jessen, C., Bednarz, V. N., Rix, L., Teichberg, M., Wild, C. Marine Eutrophication. Environmental Indicators 177–203 (Springer, 2015).
Yao, Y. & Shen, Z. A review on eutrophication research of coastal waters. Mar. Sci. 29, 53–57 (2005).
Oelsner, G. P. & Stets, E. G. Recent trends in nutrient and sediment loading to coastal areas of the conterminous US: Insights and global context. Sci. Total Environ. 654, 1225–1240 (2019).
Gaudêncio, M. J. & Cabral, H. N. Trophic structure of macrobenthos in the Tagus estuary and adjacent coastal shelf. Hydrobiologia 587(1), 241–251 (2007).
Jumars, P. A., Dorgan, K. M. & Lindsay, S. M. Diet of worms emended: An update of polychaete feeding guilds. Annu. Rev. Mar. Sci. 7(1), 497–520 (2015).
Weiss, E. T., Carmichael, R. H. & Valiela, I. The effect of nitrogen loading on the growth rates of quahogs (Mercenaria mercenaria) and soft-shell clams (Mya arenaria) through changes in food supply. Aquaculture 211(1), 275–289 (2002).
Kirby, M. X. & Miller, H. M. Response of a benthic suspension feeder (Crassostrea virginica Gmelin) to three centuries of anthropogenic eutrophication in Chesapeake Bay. Estuar. Coast. Shelf Sci. 62(4), 679–689 (2005).
Kraufvelin, P. et al. Eutrophication-induced changes in benthic algae affect the behaviour and fitness of the marine amphipod Gammarus locusta. Aquat. Bot. 84(3), 199–209 (2006).
Martinetto, P. et al. High abundance and diversity of consumers associated with eutrophic areas in a semi-desert macrotidal coastal ecosystem in Patagonia, Argentina. Estuar. Coast. Shelf Sci. 88(3), 357–364 (2010).
Rabalais, N. N. et al. Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences 7(2), 585–619 (2010).
Turner, R. E., Rabalais, N. N. & Justić, D. Predicting summer hypoxia in the northern Gulf of Mexico: Redux. Mar. Pollut. Bull. 64(2), 319–324 (2012).
Pacheco, A. S. et al. Functional diversity of marine macrobenthic communities from sublittoral soft-sediment habitats off northern Chile. Helgol. Mar. Res. 65(3), 413–424 (2011).
Diaz, R. J. Overview of hypoxia around the world. J. Environ. Qual. 30(2), 275–281 (2001).
Lake, S. J. & Brush, M. J. Contribution of nutrient and organic matter sources to the development of periodic hypoxia in a tributary estuary. Estuar. Coasts 38(6), 2149–2171 (2015).
Kodama, K. & Horiguchi, T. Effects of hypoxia on benthic organisms in Tokyo Bay, Japan: A review. Mar. Pollut. Bull. 63(5), 215–220 (2011).
Briggs, K. B. et al. Influence of hypoxia on biogenic structure in sediments on the Louisiana continental shelf. Estuar. Coast. Shelf Sci. 164, 147–160 (2015).
Tomasovych, A. et al. Tracing the effects of eutrophication on molluscan communities in sediment cores; outbreaks of an opportunistic species coincide with reduced bioturbation and high frequency of hypoxia in the Adriatic Sea. Paleobiology 44(4), 575–602 (2018).
Kemp, W. M. et al. Eutrophication of Chesapeake Bay: Historical trends and ecological interactions. Mar. Ecol. Prog. Ser. 303, 1–29 (2005).
Brey, T. A multi-parameter artificial neural network model to estimate macrobenthic invertebrate productivity and production. Limnol. Oceanogr. Methods 10(8), 581–589 (2012).
Delfan, N., Shojaei, M. G. & Naderloo, R. Patterns of structural and functional diversity of macrofaunal communities in a subtropical mangrove ecosystem. Estuar. Coast. Shelf Sci. 252, 107288 (2021).
Calle, P. et al. Mercury assessment, macrobenthos diversity and environmental quality conditions in the Salado Estuary (Gulf of Guayaquil, Ecuador) impacted by anthropogenic influences. Mar. Pollut. Bull. 136, 365–373 (2018).
Bustos-Baez, S., Frid, C. Using indicator species to assess the state of macrobenthic communities. In Advances in Polychaete Research. Developments in Hydrobiology, vol. 170 (eds. Sigvaldadóttir, E., et al.) (SpringerNetherlands, 2003) 299–309 (2003).
Diaz, R. J., Solan, M. & Valente, R. M. A review of approaches for classifying benthic habitats and evaluating habitat quality. J. Environ. Manag. 73(3), 165–181 (2004).
Ghodrati Shojaei, M. et al. Common trends in German Bight benthic macrofaunal communities: Assessing temporal variability and the relative importance of environmental variables. J. Sea Res. 107, 25–33 (2016).
Tweedley, J. R., Warwick, R. M., Valesini, F. J., Platell, M. E. & Potter, I. C. The use of benthic macroinvertebrates to establish a benchmark for evaluating the environmental quality of microtidal, temperate southern hemisphere estuaries. Mar. Pollut. Bull. 64(6), 1210–1221 (2012).
Dong, J. et al. Response of macrobenthic communities to heavy metal pollution in Laoshan Bay, China: A trait-based method. Mar. Pollut. Bull. 167, 112292 (2021).
Pearson, T. H. & Rosenberg, R. Macrobenthic succession in relation to organic enrichment and pollution of the marine environment. Oceanogr. Mar. Biol. Annu. Rev. 16, 229–311 (1978).
Chen, Q., Yuan, H. & Chen, P. Integrated response in taxonomic diversity and eco-exergy of macrobenthic faunal community to artificial reef construction in Daya Bay, China. Ecol. Indic. 101, 512–521 (2019).
Lu, X., Xu, J., Xu, Z. & Liu, X. Assessment of benthic ecological quality status using multi-biotic indices based on macrofaunal assemblages in a semi-enclosed bay. Front. Mar. Sci. 8, 734710 (2021).
Warwick, R. A new method for detecting pollution effects on marine macrobenthic communities. Mar. Biol. 92(4), 557–562 (1986).
Edgar, G. J. & Barrett, N. S. Benthic macrofauna in Tasmanian estuaries: Scales of distribution and relationships with environmental variables. J. Exp. Mar. Biol. Ecol. 270(1), 1–24 (2002).
Kanaya, G., Nakamura, Y. & Koizumi, T. Ecological thresholds of hypoxia and sedimentary H2S in coastal soft-bottom habitats: A macroinvertebrate-based assessment. Mar. Environ. Res. 136, 27–37 (2018).
Camargo, J. A. & Alonso, Á. Ecological and toxicological effects of inorganic nitrogen pollution in aquatic ecosystems: A global assessment. Environ. Int. 32(6), 831–849 (2006).
Souza, F. M. et al. Macrobenthic community responses to multiple environmental stressors in a subtropical estuary. PeerJ 9, e12427 (2021).
Prena, J. Effects of eutrophication on macrobenthos zonation in Wismar Bay (Western Baltic Sea). Arch. Fur. Hydrobiol. 133(2), 245–257 (1995).
Verdelhos, T., Neto, J. M., Marques, J. C. & Pardal, M. A. The effect of eutrophication abatement on the bivalve Scrobicularia plana. Estuar. Coast. Shelf Sci. 63(1), 261–268 (2005).
Cardoso, P. G., Bankovic, M., Raffaelli, D. & Pardal, M. A. Polychaete assemblages as indicators of habitat recovery in a temperate estuary under eutrophication. Estuar. Coast. Shelf Sci. 71(1), 301–308 (2007).
Kenny, A. J. et al. Assessing cumulative human activities, pressures, and impacts on North Sea benthic habitats using a biological traits approach. ICES J. Mar. Sci. 75(3), 1080–1092 (2018).
Bon, M. et al. Functional changes in benthic macrofaunal communities along a natural gradient of hypoxia in an upwelling system. Mar. Pollut. Bull. 164, 112056 (2021).
Wong, M. C. & Dowd, M. Patterns in taxonomic and functional diversity of macrobenthic invertebrates across seagrass habitats: A case study in Atlantic Canada. Estuar. Coasts 38(6), 2323–2336 (2015).
Braeckman, U. et al. Role of macrofauna functional traits and density in biogeochemical fluxes and bioturbation. Mar. Ecol. Prog. Ser. 399, 173–186 (2010).
Liu, K. et al. Functional trait composition and diversity patterns of marine macrobenthos across the Arctic Bering Sea. Ecol. Indic. 102, 673–685 (2019).
Lavorel, S. & Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 16(5), 545–556 (2002).
Villnäs, A., Perus, J. & Bonsdorff, E. Structural and functional shifts in zoobenthos induced by organic enrichment—Implications for community recovery potential. J. Sea Res. 65(1), 8–18 (2011).
Petchey, O. L. & Gaston, K. J. Functional diversity: Back to basics and looking forward. Ecol. Lett. 9(6), 741–758 (2006).
Wu, Z., Zhou, H., Zhang, S. & Liu, Y. Using 222Rn to estimate submarine groundwater discharge (SGD) and the associated nutrient fluxes into Xiangshan Bay, East China Sea. Mar. Pollut. Bull. 73(1), 183–191 (2013).
Yang, Z. et al. The behavior of phosphate in the Xiangshan Bay and its potential impact on harmful algal blooms. Haiyang Xuebao 40(10), 61–70 (2018).
Wang, J., Yang, C., Sun, Y. & Yang, Y. Secondary productivity and biodiversity of macrobenthos in Xiangshan Bay. J. Tianjin Agric. Univ. 013(02), 24–28 (2006).
Gu, X. et al. The macrobenthic community of the Xiangshan Bay. Oceanol. Limnol. Sin. 41(02), 208–213 (2010).
Cai, W. et al. Assessing benthic health under multiple human pressures in Bohai Bay (China), using density and biomass in calculating AMBI and M-AMBI. Mar. Ecol. 35(2), 180–192 (2014).
Li, Y., Wang, L., Wang, X. & Ning, J. Effects of the sediment type on ecological functions of macrobenthos in the intertidal zones of Sanya Bay. J. Fish. China 42(10), 1559–1571 (2018).
Lin, Z., Yan, X. & Lencart E Silva, J. D. Study on the features of water exchange in Xiangshan Bay. J. Ningbo Univ. 27(1), 1–7 (2014).
China Gulf Chronicles Compilation Committee. China Gulf Chronicles vol(5): Bays in Shanghai and northern Zhejiang Province (China Ocean Press, 1992).
Qi, J. Study on the Spatio-temporal Changes of Eutrophication in Zhejiang Coastal Sea (Zhejiang University, 2022).
Cai, W., Chen, G. & Ding, J. A discussion of the features of the tide and tidal current in the Xiangshan Harbour and their cause of formation. Mar. Sci. Bull. 4(03), 8–12 (1985).
Li, Q., You, X., Zhang, Y., Mao, Y. & Jiao, H. Changes in the characteristics of the bacterial community structure during habitat restoration of aquaculture sites in Xiangshan Bay. J. Fish. Sci. China 20(06), 1234–1246 (2013).
Yuan, Q., Li, J., Xu, L., Chen, P. & Wang, M. Quantitative analysis of river morphological features in Xiangshan Bay Basin. J. Mar. Sci. 32(2), 50–57 (2014).
Dai, M., Zhu, Y., Lin, X. & Mao, S. Interpretation of environmental factors affecting zooplanktonic beta diversity and its components in Xiangshan Bay. Acta Ecol. Sin. 37(17), 5780–5789 (2017).
General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China. Specification for Oceanographic Survey, Part 6: Marine Biological Investigation (Standards Press of China, 2007).
Wang, Y. et al. Current status and changing tendency of eutrophication of Jiaozhou Bay. Trans. Oceanol. Limnol. 45(4), 146–150 (2023).
Yang, D. & Sun, R. The Polychaetes in Coastal Waters of China (China Agriculture Press, 1988).
Yang, D. & Wang, Y. Marine Invertebrates in Northern China (Higher Education Press, 1996).
Macdonald, T. A., Burd, B. J., Macdonald, V. I. & Roodselaar, A. V. Taxonomic and feeding guild classification for the marine enthic macroinvertebrates of the Strait of Georgia. Br. Columbia Can. Tech. Rep. Fish. Aquat. Sci. 2874, 1–63 (2010).
Hu, C. et al. Macrobenthos functional trait responses to heavy metal pollution gradients in a temperate lagoon. Environ. Pollut. 253, 1107–1116 (2019).
Dong, J., Sun, X., Zhan, Q., Zhang, Y. & Zhang, X. Patterns and drivers of beta diversity of subtidal macrobenthos community on the eastern coast of Laizhou Bay. Biodivers. Sci. 30(3), 21388 (2022).
Marlin. BIOTIC-Biological Traits Information Catalogue. Marine Life Information Network (Marine Biological Association of the United Kingdom, 2006).
Faulwetter, M. S. et al. Polytraits: A database on biological traits of marine polychaetes. Biodivers. Data J. 2, e1024 (2014).
Chevene, F., Doleadec, S. & Chessel, D. A fuzzy coding approach for the analysis of long-term ecological data. Freshw. Biol. 31(3), 295–309 (1994).
Tillin, H. M., Hiddink, J. G., Jennings, S. & Kaiser, M. J. Chronic bottom trawling alters the functional composition of benthic invertebrate communities on a sea-basin scale. Mar. Ecol. Prog. Ser. 318, 31–45 (2006).
Boyé, A. et al. Trait-based approach to monitoring marine benthic data along 500 km of coastline. Divers. Distrib. 25(12), 1879–1896 (2019).
Zhulay, I., Bluhm, B. A., Renaud, P. E., Degen, R. & Iken, K. Functional pattern of benthic epifauna in the Chukchi Borderland, Arctic deep sea. Front. Mar. Sci. 8, 609956 (2021).
Oksanen, J., Blanchet, F. G., Friendly, M., Kindt, R., Legendre, P., Mcglinn, D., Minchin, P. R., O Hara, R. B., Simpson, G. L., Solymos, P. Vegan: Community Ecology Package. R package version 2.5-7. 2020 (2022).
Laliberté, E. & Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 91(1), 299–305 (2010).
Pavoine, S. adiv: An r package to analyse biodiversity in ecology. Methods Ecol. Evol. 11(9), 1106–1112 (2020).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2012).
Dray, S. & Dufour, A. The ade4 package: Implementing the duality diagram for ecologists. J. Stat. Softw. 22(4), 1–20 (2007).
Kleyer, M. et al. Assessing species and community functional responses to environmental gradients: Which multivariate methods?. J. Veg. Sci. 23(5), 805–821 (2012).
Dray, S. et al. Combining the fourth-corner and the RLQ methods for assessing trait responses to environmental variation. Ecology 95(1), 14–21 (2014).
Dolédec, S., Chessel, D., ter Braak, C. J. F. & Champely, S. Matching species traits to environmental variables: A new three-table ordination method. Environ. Ecol. Stat. 3(2), 143–166 (1996).
Dray, S. A tutorial to perform fourth-corner and RLQ analyses in R. 1(75), 82 (2013).
Farneda, F. Z. et al. Trait-related responses to habitat fragmentation in Amazonian bats. J. Appl. Ecol. 52(5), 1381–1391 (2015).
Shi, Y. et al. Species and functional diversity of marine macrobenthic community and benthic habitat quality assessment in semi-enclosed waters upon recovering from eutrophication, Bohai Bay, China. Mar. Pollut. Bull. 181, 113918 (2022).
Cloern, J. E. Our evolving conceptual model of the coastal eutrophication problem. Mar. Ecol. Prog. Ser. 210, 223–253 (2001).
Dolbeth, M., Pardal, M. A., Lillebø, A. I., Azeiteiro, U. & Marques, J. C. Short- and long-term effects of eutrophication on the secondary production of an intertidal macrobenthic community. Mar. Biol. 143(6), 1229–1238 (2003).
Andersen, J. H. & Conley, D. J. Eutrophication in coastal marine ecosystems: Towards better understanding and management strategies. Hydrobiologia 629, 1–4 (2009).
Kanaya, G., Nakamura, Y., Koizumi, T. & Yamada, K. Seasonal changes in infaunal community structure in a hypertrophic brackish canal: Effects of hypoxia, sulfide, and predator–prey interaction. Mar. Environ. Res. 108, 14–23 (2015).
Caswell, B. A., Paine, M. & Frid, C. L. J. Seafloor ecological functioning over two decades of organic enrichment. Mar. Pollut. Bull. 136, 212–229 (2018).
Frigstad, H. et al. Three decades of change in the Skagerrak coastal ecosystem, shaped by eutrophication and coastal darkening. Estuar. Coast. Shelf Sci. 283, 108193 (2023).
Ning, X. & Hu, X. Aquacultural Ecology and Carrying Capacity Assessment of Fish Cages in Xiangshan Bay (Ocean Press, 2002).
Zhang, L., Jiang, X. & Cai, Y. Characteristics of nutrient distributions and eutrophication in seawater of the Xiangshan Harbor. Mar. Environ. Sci. 27(05), 488–491 (2008).
Jiang, Z. et al. Phytoplankton biomass and size structure in Xiangshan Bay, China: Current state and historical comparison under accelerated eutrophication and warming. Mar. Pollut. Bull. 142, 119–128 (2019).
Gu, Y. & Xiang, Y. Relationship of red tide and eutrophication in Xiangshan Harbour area. Mar. Environ. Sci. 21(03), 67–69 (2002).
Liu, J., Pan, J., Xue, B. & Liu, X. Evaluation and analysis of eutrophication in Xiangshan Bay. Environ. Study Monit. 25(1), 15–18 (2012).
Beukema, J. J. Changes in composition of bottom fauna of a tidal-flat area during a period of eutrophication. Mar. Biol. 111(2), 293–301 (1991).
Gao, A. et al. Comparative studies on macrobenthos between cultured and non-cultured areas in Xiangshan Bay. J. Fish. China 27(01), 25–31 (2003).
Tett, P. et al. Framework for understanding marine ecosystem health. Mar. Ecol. Prog. Ser. 494, 1–27 (2013).
Rice, J. et al. Indicators for sea-floor integrity under the European Marine Strategy Framework Directive. Ecol. Indic. 12(1), 174–184 (2012).
Dimitriou, P. D., Papageorgiou, N. & Karakassis, I. Response of benthic macrofauna to eutrophication in a mesocosm experiment: Ecosystem resilience prevents hypoxic conditions. Front. Mar. Sci. 4, 391 (2017).
Fox, S. E., Teichberg, M., Olsen, Y. S., Heffner, L. & Valiela, I. Restructuring of benthic communities in eutrophic estuaries: Lower abundance of prey leads to trophic shifts from omnivory to grazing. Mar. Ecol. Prog. Ser. 380, 43–57 (2009).
Grall, J. & Chauvaud, L. Marine eutrophication and benthos: The need for new approaches and concepts. Glob. Change Biol. 8(9), 813–830 (2002).
Barnes, R. S. K. & Hendy, I. W. Seagrass-associated macrobenthic functional diversity and functional structure along an estuarine gradient. Estuar. Coast. Shelf Sci. 164, 233–243 (2015).
Gong, Z., Xie, P., Tang, H. & Wang, S. The influence of eutrophication upon community structure and biodiversity of macrozoobenthos. Acta Hydrobiol. Sin. 25(03), 210–216 (2001).
Carmona, C. P., De Bello, F., Mason, N. W. & Lepš, J. Traits without borders: Integrating functional diversity across scales. Trends Ecol. Evol. 31(5), 382–394 (2016).
Olden, J. D. & Rooney, T. P. On defining and quantifying biotic homogenization. Glob. Ecol. Biogeogr. 15(2), 113–120 (2006).
Petsch, D. K. Causes and consequences of biotic homogenization in freshwater ecosystems. Int. Rev. Hydrobiol. 101(3–4), 113–122 (2016).
Zorzal-Almeida, S., Bini, L. M. & Bicudo, D. C. Beta diversity of diatoms is driven by environmental heterogeneity, spatial extent and productivity. Hydrobiologia 800, 7–16 (2017).
Mckinney, M. L. & Lockwood, J. L. Biotic homogenization: A few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 14(11), 450–453 (1999).
Brice, M. H., Pellerin, S. & Poulin, M. Does urbanization lead to taxonomic and functional homogenization in riparian forests?. Divers. Distrib. 23(7), 828–840 (2017).
Morais, G. C., Gusmao, J. B., Oliveira, V. M. & Lana, P. Macrobenthic functional trait diversity at multiple scales along a subtropical estuarine gradient. Mar. Ecol. Prog. Ser. 624, 23–37 (2019).
Llanos, E. N., Bottero, M. A. S., Jaubet, M. L., Elías, R. & Garaffo, G. V. Functional diversity in the intertidal macrobenthic community at sewage-affected shores from Southwestern Atlantic. Mar. Pollut. Bull. 157, 111365 (2020).
Van, A. & Gümüş, A. Determination of functional structure of soft-bottom marine macrobenthic communities of the Samsun Shelf Area using biological traits analysis. Oceanol. Hydrobiol. Stud. 50(4), 473–487 (2021).
Lacson, A. Z. et al. A multimetric approach to evaluate offshore mussel aquaculture effects on the taxonomical and functional diversity of macrobenthic communities. Mar. Environ. Res. 151, 104774 (2019).
Fu, X., Yang, W., Zheng, L., Liu, D. & Li, X. Spatial patterns of macrobenthos taxonomic and functional diversity throughout the ecotones from river to lake: A case study in Northern China. Front. Ecol. Evol. 10, 922539 (2022).
Bremner, J. Species’ traits and ecological functioning in marine conservation and management. J. Exp. Mar. Biol. Ecol. 366(1), 37–47 (2008).
Gusmao, J. B., Brauko, K. M., Eriksson, B. K. & Lana, P. C. Functional diversity of macrobenthic assemblages decreases in response to sewage discharges. Ecol. Indic. 66, 65–75 (2016).
Vinagre, P. A. et al. Do structural and functional attributes show concordant responses to disturbance? Evidence from rocky shore macroinvertebrate communities. Ecol. Indic. 75, 57–72 (2017).
Cowling, R. M. P. L. Species diversity; functional diversity and functional redundancy in fynbos communities. S. Afr. J. Sci. 90(6), 333–337 (1994).
Walker, B. H. Biodiversity and ecological redundancy. Conserv. Biol. 6(1), 18–23 (1992).
Zhang, Y. et al. Nutrient enrichment homogenizes taxonomic and functional diversity of benthic macroinvertebrate assemblages in shallow lakes. Limnol. Oceanogr. 64(3), 1047–1058 (2019).
Pei, W. et al. Functional redundancy as an indicator for evaluating functional diversity of macrobenthos under the mussel raft farm near Gouqi Island. Aquaculture 578, 740024 (2024).
Fonseca, C. R. & Ganade, G. Species functional redundancy, random extinctions and the stability of ecosystems. J. Ecol. 89(1), 118–125 (2001).
Carvalho, S. et al. Factors structuring temporal and spatial dynamics of macrobenthic communities in a eutrophic coastal lagoon (Óbidos lagoon, Portugal). Mar. Environ. Res. 71(2), 97–110 (2011).
Bremner, J., Rogers, S. I. & Frid, C. L. J. Methods for describing ecological functioning of marine benthic assemblages using biological traits analysis (BTA). Ecol. Indic. 6(3), 609–622 (2006).
Mutaz, A., Clivia, H. Â. S., Max, M. T. & Boaz, L. Phytoplankton drives nitrite dynamics in the Gulf of Aqaba, Red Sea. Mar. Ecol. Prog. Ser. 239, 233–239 (2002).
Cheng, S. & Chen, J. Study on the oxyhemocyanin, deoxyhemocyanin, oxygen affinity and acid–base balance of Marsupenaeus japonicus following exposure to combined elevated nitrite and nitrate. Aquat. Toxicol. 61(3), 181–193 (2002).
Camargo, J. A. & Ward, J. V. Short-term toxicity of sodium nitrate (NaNO3) to non-target freshwater invertebrates. Chemosphere 24(1), 23–28 (1992).
Alonso, A. & Camargo, J. A. Short-term toxicity of ammonia, nitrite, and nitrate to the aquatic snail Potamopyrgus antipodarum (Hydrobiidae, Mollusca). Bull. Environ. Contam. Toxicol. 70(5), 1006–1012 (2003).
Tsai, S. & Chen, J. Acute toxicity of nitrate on Penaeus monodon juveniles at different salinity levels. Aquaculture 213(1–4), 163–170 (2002).
Camargo, J. A., Alonso, A. & Salamanca, A. Nitrate toxicity to aquatic animals: A review with new data for freshwater invertebrates. Chemosphere 58(9), 1255–1267 (2005).
White, E. P., Ernest, S. M., Kerkhoff, A. J. & Enquist, B. J. Relationships between body size and abundance in ecology. Trends Ecol. Evol. 22(6), 323–330 (2007).
Sigala, K., Reizopoulou, S., Basset, A. & Nicolaidou, A. Functional diversity in three Mediterranean transitional water ecosystems. Estuar. Coast. Shelf Sci. 110, 202–209 (2012).
Ponti, M. & Abbiati, M. Quality assessment of transitional waters using a benthic biotic index: The case study of the Pialassa Baiona (northern Adriatic Sea). Aquat. Conserv. Mar. Freshw. Ecosyst. 14(S1), S31–S41 (2004).
Karin, K., Erik, B. & Rutger, R. The impact of benthic macrofauna for nutrient fluxes from Baltic Sea sediments. AMBIO J. Hum. Environ. 36(2), 161–167 (2007).
Lloret, J. & Marín, A. The contribution of benthic macrofauna to the nutrient filter in coastal lagoons. Mar. Pollut. Bull. 62(12), 2732–2740 (2011).
Gu, P. et al. Effects of cyanobacteria on phosphorus cycling and other aquatic organisms in simulated eutrophic ecosystems. Water 12, 2265 (2020).
Nędzarek, A., Tórz, A., Rakusa-Suszczewski, S. & Bonisławska, M. Nitrogen and phosphorus release during fish decomposition and implications for the ecosystem of maritime Antarctica. Polar Biol. 38(5), 733–740 (2015).
Xing, X., Chen, M., Wu, Y., Tang, Y. & Li, C. The decomposition of macrozoobenthos induces large releases of phosphorus from sediments. Environ. Pollut. 283, 117104 (2021).
Heip, C. Eutrophication and zoobenthos dynamics. Ophelia 41(1), 113–136 (1995).
Surugiu, V. The use of polychaetes as indicators of eutrophication and organic enrichment of coastal waters: A study case–Romanian Black Sea coast. Analele Ştiinţifice Ale Universităţii “Al. I. Cuza” Iaşi, S. Biologie Animală. 51, 55–62 (2005).
Lindeman, R. L. The trophic-dynamic aspect of ecology. Bull. Math. Biol. 53(1), 167–191 (1991).
Tagliapietra, D., Pavan, M. & Wagner, C. Macrobenthic community changes related to eutrophication in Palude della Rosa (Venetian Lagoon, Italy). Estuar. Coast. Shelf Sci. 47(2), 217–226 (1998).
Gray, J. S., Wu, R. S. & Or, Y. Y. Effects of hypoxia and organic enrichment on the coastal marine environment. Mar. Ecol. Prog. Ser. 238, 249–279 (2002).
Seo, J., Park, S., Lee, J. & Choi, J. Structural changes in macrozoobenthic communities due to summer hypoxia in Gamak Bay, Korea. Ocean Sci. J. 47(1), 27–40 (2012).
Chen, C., Gong, G. & Shiah, F. Hypoxia in the East China Sea: One of the largest coastal low-oxygen areas in the world. Mar. Environ. Res. 64(4), 399–408 (2007).
Zhou, F. et al. Hydrographic analysis on the multi-time scale variability of hypoxia adjacent to the Changjiang River Estuary. Acta Ecol. Sin. 30(17), 4728–4740 (2010).
Paerl, H. W. Assessing and managing nutrient-enhanced eutrophication in estuarine and coastal waters: Interactive effects of human and climatic perturbations. Ecol. Eng. 26(1), 40–54 (2006).
Wang, Y., Li, D., Fang, T., Liu, Z. & He, S. Study on relation of distribution of benthos and hypoxia in Yangtze River Estuary and adjacent sea. Mar. Environ. Sci. 27(02), 139–143+164 (2008).
Meng, Z., Han, Q. & Wang, X. Distribution pattern of macrobenthic composition, diversity and secondary production in Hangzhou Bay, northern East China Sea. Reg. Stud. Mar. Sci. 47, 101956 (2021).
Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (NSFC) (No. 42076156; 42006078) and was also sponsored by K.C. Wong Magna Fund in Ningbo University. This study involved laborious field work and careful specimen identification and was conducted by dozens of colleagues.
Author information
Authors and Affiliations
Contributions
Q.H. and Y.X. wrote the main manuscript text and X.W. collected the biological and environmental data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Han, Q., Wang, X. & Xu, Y. Deciphering macrobenthic biological traits in response to long-term eutrophication in Xiangshan Bay, China. Sci Rep 14, 20209 (2024). https://doi.org/10.1038/s41598-024-71239-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71239-z
- Springer Nature Limited