Abstract
Metabolic comorbidities, such as obesity and diabetes, are associated with subclinical alterations in both cardiac structure/function and natriuretic peptides prior to the onset of heart failure (HF). Despite this, the exact metabolic pathways of cardiac dysfunction which precede HF are not well-defined. Among older individuals without HF in the Multi-Ethnic Study of Atherosclerosis (MESA), we evaluated the associations of 47 circulating metabolites measured by 1H-NMR with echocardiographic measures of cardiac structure and function. We then evaluated associations of significant metabolites with circulating N-terminal pro-B-type natriuretic peptide (NT-proBNP). In a separate cohort, we evaluated differences between top metabolites in patients with HF with preserved ejection fraction (HFpEF) and comorbidity-matched controls. Genetic variants associated with top metabolites (mQTLs) were then related to echocardiographic measures and NT-proBNP. Among 3440 individuals with metabolic and echocardiographic data in MESA (62 ± 10 years, 52% female, 38% White), 10 metabolites broadly reflective of glucose and amino acid metabolism were associated with at least 1 measure of cardiac structure or function. Of these 10 metabolites, 4 (myo-inositol, glucose, dimethylsulfone, carnitine) were associated with higher NT-proBNP and 2 (d-mannose, acetone) were associated with lower NT-proBNP. In a separate cohort, patients with HFpEF had higher circulating myo-inositol levels compared with comorbidity-matched controls. Genetic analyses revealed that 1 of 6 known myo-inositol mQTLs conferred risk of higher NT-proBNP. In conclusion, metabolomic profiling identifies several novel metabolites associated with cardiac dysfunction in a cohort at high risk for HF, revealing pathways potentially relevant to future HF risk.
Similar content being viewed by others
Introduction
Heart failure with preserved ejection fraction (HFpEF) is currently the most prevalent heart failure disease subtype1 and is associated with high morbidity and mortality. Certain cardiometabolic risk factors have been associated with the development of HFpEF, including hypertension, obesity, and diabetes2. Numerous mechanisms have been proposed to explain how these comorbidities contribute to HFpEF development, including the induction of systemic inflammation by metabolic stress (termed ‘meta-inflammation’)2,3. Precise metabolic triggers of meta-inflammation, however, remain relatively underexplored.
Metabolomics, the study of the substrates and products of metabolism that drive essential cellular functions, holds promise in improving our understanding of the pathophysiology of meta-inflammation and early HFpEF4. Several metabolites have previously been associated with development of HF, and there are unique metabolomic features of each HF subtype5,6,7,8. Additionally, certain amino acids have been associated with traditional echocardiographic measures of diastolic dysfunction9, suggesting that altered metabolism may be associated with subclinical cardiac dysfunction prior to the onset of clinical HF. There is limited understanding, however, of the precise metabolic pathways associated with both sensitive echocardiographic measures of cardiac structure and function and natriuretic peptides among older individuals at high risk for HF. As subclinical cardiac dysfunction and natriuretic peptide elevation precede development of HFpEF10,11,12,13, identification of corresponding metabolic changes holds promise to elucidate upstream pathways prior to clinical HF. By leveraging the circulating metabolome and its genetic variation, we therefore aimed to identify metabolic pathways associated with early heart failure, as defined by alterations in cardiac mechanics on echocardiography and circulating natriuretic peptides, in a population without prevalent HF.
Methods
Study populations
The Multi-Ethnic Study of Atherosclerosis (MESA) is a prospective cohort of US adults aged 45 to 84 years old at the time of study recruitment. Comprehensive details regarding MESA participants and study design have been described previously14. 6814 participants were recruited from 2000 to 2002 across 6 different field centers (Baltimore, MD; Chicago, IL; St Paul, MN; Forsyth County, NC; New York, NY; and Los Angeles, CA). All participants self-identified as 1 of 4 race/ethnic groups: Black, White, Chinese or Hispanic. At the time of recruitment, participants were free of cardiovascular diseases including coronary artery disease, heart failure (HF), atrial fibrillation (AF), peripheral artery disease, implantable cardiac devices, history of stroke or transient ischemic attack (TIA), and history of cardiac surgery. Participants were initially evaluated in person between 2000 to 2002, and then invited to 5 subsequent in-person evaluations occurring once every 2 to 5 years. They were also contacted once every 9–12 months to assess clinical morbidity and mortality.
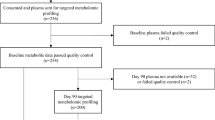
In this analysis, we evaluated participants who (1) underwent measurement of 47 circulating metabolites at Exam 1 by proton nuclear magnetic resonance (1H-NMR), (2) attended the 6th in-person examination (September 2016 to June 2018) with available covariate data, (3) were free of clinical HF prior to Exam 6, and (4) underwent comprehensive echocardiography. History of HF was defined as hospitalization for HF, which was ascertained though independent review of hospitalization medical records of participants by 2 study physicians who were otherwise blinded to participant study data. Designation of HF required both documentation of HF symptoms and either imaging suggestive of HF or medical treatment for HF. Estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI equation15. The MESA study protocol was approved by the institutional review board (IRB) of each field center, and all participants provided written informed consent. All study methods were performed in accordance with relevant guidelines and regulations.
Comprehensive information pertaining to the Northwestern cohort, including metabolomic profiling, can be found in the Supplemental Methods. In brief, patients diagnosed with HFpEF and control subjects were recruited from outpatient clinics at Northwestern University during the period from November 2013 to May 2017. The diagnostic criteria for HFpEF have been outlined in the Supplemental Methods. Control participants exhibited at least one cardiovascular risk factor but lacked a history of heart failure.
Metabolite profiling
Untargeted 1H-NMR analysis of serum samples obtained at the MESA baseline examination (Exam 1) was performed using a method previously described16. Samples utilized in this study were analyzed as part of the European Union-funded Development of Combinatorial Biomarkers for Subclinical Atherosclerosis project. Specifically, we used both standard 1-dimensional NMR spectra displaying the resonances of all molecules containing protons as well as Carr-Purcell-Meiboom-Gill (CPMG) spectra which attenuate macromolecule peaks and allow for better definition of small molecules. Based upon unique parts-per-million values from the CPMG NMR spectra, representative peaks were identified for a set of 47 annotated metabolites based on previous CPMG NMR studies, peak separation within MESA, and peak quality within MESA. NMR spectra underwent harmonization and alignment, and have been used in prior investigations of the MESA cohort17,18,19.
In the Northwestern cohort, metabolic profiling was conducted using liquid chromatography-mass spectrometry (LC–MS) (Supplemental Methods).
Echocardiographic protocol
Comprehensive resting 2-dimensional, Doppler and speckle-tracking echocardiography was performed at MESA Exam 6. At each study site, one sonographer was designated as the dedicated field center sonographer for all MESA echocardiograms. These designated sonographers each attended centralized training at the Northwestern University Echocardiography Core Lab (NUECL, Chicago, IL). 2D images were obtained at a frame rate of 50–80 frames per second (FPS), and all echocardiograms underwent formal analysis at NUECL using GE EchoPAC software (version 201; GE Healthcare; Waukesha, WI).
Cardiac chamber quantification was performed in accordance with previous societal guideline recommendations20. The biplane method of disks was applied to apical 4- and 2-chamber views to measure LV volumes, LV ejection fraction (EF) and LA volume. The Devereux formula was used to calculate LV mass. Tissue Doppler was performed at the lateral and septal mitral annulus during early diastole (e’ velocities). M-mode was used to measure tricuspid annular plane systolic excursion (TAPSE). The simplified Bernoulli equation was used to estimate pulmonary artery systolic pressure (PASP).
An experienced sonographer blinded to other clinical data used GE EchoPAC software to perform speckle-tracking echocardiography of the RA, RV, LA and LV. All strain curves were independently verified by 2 cardiologists with echocardiographic expertise. The endocardial border of each cardiac chamber was traced and epicardial to endocardial regions of interest were defined. Then the program defined standard anatomic segments of each cardiac chamber prior to performing regional speckle tracking analysis. Speckle-tracking software then generated curvilinear graphs of strain measurements over time corresponding to the anatomic segments of each chamber of interest, along with a curve to represent the average of the segments. The absolute values of all strain measurements are reported, with lower strain values corresponding to worse function. LA strain was calculated using strain measurements from apical 4- and 2-chamber views, and RA strain was calculated using the apical 4-chamber view. LV global longitudinal strain was calculated as the average strain from apical 4-, 3- and 2-chamber views, and LV circumferential strain was obtained from the LV mid-chamber short axis view.
NT-proBNP measurement
N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels were obtained through the Olink Cardiovascular III panel (Uppsala, Sweden) and were subsequently expressed as normalized protein units on a log2 scale. The correlation between NT-proBNP measured by the Olink panel and by a commercially available ELISA immunoassay is strong (r = 0.89), and NT-proBNP measured by Olink correlated closely with log-transformed ELISA NT-proBNP (r = 0.93)21 in a subset of 993 participants in MESA Exam 6.
Genotyping and mQTL identification
The Affymetrix Genome-Wide SNP Array 6.0 (Thermo Fisher Scientific, Waltham, MA) was used to genotype the MESA cohort. Specific genotyping and quality control procedures have been previously described22. MESA principal component (PC) analysis was performed using EIGENSTRAT within each race/ethnic group23.
Metabolite quantitative trait loci (mQTLs) for myo-inositol were identified via query of the publicly available National Human Genome Research Institute Genome Wide Association Studies (GWAS) catalog24. Eight single nucleotide polymorphisms (SNPs) were identified in the GWAS catalog to be associated with myo-inositol levels (Supplemental Table 1). Upon evaluating SNPs for linkage disequilibrium (r2 > 0.30), 2 separate pairs of SNPs were found to be in LD and therefore 6 SNPs were identified for further analysis.
Statistical analysis
For all analyses, metabolites were log transformed and standardized to a mean of zero and standard deviation (SD) of 1. Correlation coefficients were calculated for each pair of metabolites. Multivariable general linear models were used to evaluate associations of 47 circulating metabolites with individual measures of cardiac mechanics on echocardiography: septal e’, average E/e’, biplane LV ejection fraction (EF), LV global longitudinal strain (GLS), LA reservoir strain, right ventricular (RV) free wall strain (FWS), tricuspid annular plane systolic excursion (TAPSE), RA area, RA reservoir strain, RV systolic pressure, LV mass, LA volume and LV end diastolic volume (EDV). All models were adjusted for age, sex, race/ethnicity, body mass index, systolic blood pressure, diabetes, smoking, anti-hypertensive medication use, total cholesterol, and estimated glomerular filtration rate (all at time of metabolite measurement). To control for multiple hypothesis testing, a false discovery rate < 5% was applied for statistical significance.
Metabolites significantly associated with a measure of cardiac structure/function were then tested for associations with NT-proBNP measured at Exam 6 in multivariable general linear models adjusting for the same covariates as above.
In sensitivity analyses, we also included individuals in MESA who had developed HF by Exam 6 echocardiography (N = 239) in the analytic cohort and repeated analyses.
In exploratory analyses, we also evaluated for effect modification by gender on the association of metabolites with measures of cardiac structure/function. Specifically, we evaluated the interaction by gender on the associations of the top 10 metabolites with their respective echocardiographic measures. In our primary analyses, there were 10 metabolites that were significantly associated with 5 different echocardiographic measures (19 total tests). Therefore, an interaction P value of 0.05/19 = 0.0026 was considered a statistically significant interaction.
Four metabolites that were associated with cardiac structure and function and/or NT-proBNP in MESA were also measured in the Northwestern HFpEF cohort. These four metabolites were tested for associations with HFpEF (compared with comorbidity control status) in the Northwestern cohort. Linear models were adjusted for age, gender, diabetes status, hypertension, and body mass index.
Metabolites associated with cardiac structure and function or NT-proBNP in MESA and with HFpEF in the Northwestern cohort were then evaluated in mQTL analyses in MESA. Additive models were used for each SNP. Linear models were used to evaluate associations of mQTLs with respective measure of cardiac structure/function and NT-proBNP, adjusting for age, race/ethnicity, gender, and ancestry principal components 1–10. In MESA, principal component (PC) analysis was previously performed using EIGENSTRAT within each race/ethnic group23. For initial models, all race/ethnic groups were pooled, as there has been increasing recommendation to treat race as a social, and not biological, construct25. Race-stratified analyses were performed subsequently among myo-inositol mQTLs that were significantly associated with echocardiographic traits or NT-proBNP.
All statistical analyses were performed using R software Version 4.0.2. This study was approved by the Northwestern University IRB.
Results
Participant characteristics
Among 6450 MESA participants who consented to genotyping, 3440 were included in this analysis who had metabolite profiling at Exam 1, echocardiography at Exam 6, covariate data, and were free of HF at the time of echocardiography. The analytic cohort was comprised of 52% women (mean age 62 years at Exam 1; Table 1).
Metabolomic profile of cardiac structure and function
Correlation coefficients for each pair of the 47 metabolites are represented in Fig. 1. Strongest correlations were noted between branched chain amino acids. Generally, metabolite associations with measures of left-sided chamber structure, including LV mass, LAV and LV EDV, had larger beta coefficients compared to the other echocardiographic traits (i.e., left-sided cardiac function, right-sided structure/function; Fig. 2), although outcome measures were not standardized. In multivariable models, 10 of 47 metabolites were associated with at least 1 measure of cardiac structure and function on echocardiography (Table 2). The echocardiographic traits with at least one significant metabolomic association included e’ septal, LAV, RAA, LVEF and LV EDV (Table 2, Fig. 2). The most common echocardiographic trait to have metabolomic associations was LAV: 6 metabolites were associated with higher LAV (acetate, dimethylsulfone, carnitine, methanol, myo-inositol, glucose) and 2 were associated with lower LAV (acetone, D-mannose). Dimethylsulfone was the only metabolite significantly associated with LVEF (Table 2). There were no significant associations between metabolites and average E/e’, LV GLS, LA reservoir strain, RV FWS, TAPSE, RA reservoir strain, RV systolic pressure, or LV mass after FDR correction (Supplemental Tables 2–14).
Effect sizes of metabolite-echocardiographic trait relationships. These heatmaps display the effect sizes of the associations between each of the 47 circulating metabolites measured at MESA Exam 1 with each echocardiographic outcome measure of cardiac mechanics obtained at MESA Exam 6, including measures of (a) cardiac function and (b) left-sided chamber structure. Positive regression coefficients are represented with blue shading and negative regression coefficients with red shading.
Figure 3 displays volcano plots of the associations between all 47 metabolites and the four outcome measures with the most significant associations. Notably, eight of the metabolites had a significant association with LAV. Three metabolites (acetone, dimethylsulfone, glucose) had significant associations with > 2 echocardiographic measures.
Associations of metabolites with cardiac structure and function. These volcano plots depict the regression coefficients and P values for the linear regression models of each of the 47 circulating metabolites measured at MESA Exam 1 and 4 echocardiographic outcome measures of cardiac mechanics: (a) e′ septal, (b) LAV, (c) RAA and (d) LV EDV.
Of the 10 metabolites associated with echocardiographic traits, 6 had significant associations with circulating NT-proBNP measured at MESA Exam 6 (Table 2). Four metabolites were associated with higher NT-proBNP (myo-inositol, dimethylsulfone, carnitine, glucose). On the other hand, acetone and D-mannose were associated with lower NT-proBNP.
There were 239 individuals who had developed HF by Exam 6 echocardiography. In sensitivity analyses in which these participants were included (total N = 3679), associations between metabolites and measures of cardiac mechanics and NT-proBNP were consistent (Supplemental Table 15).
Upon evaluation of interaction by gender on the associations of top metabolites with their respective echocardiographic traits, we identified 2 significant interactions by gender on metabolite associations with LAV. The associations between acetate and d-mannose with LAV were stronger among women as compared with men (Supplemental Table 16). There were no other significant interactions by gender on associations of top metabolites with respective echocardiographic measures. Specifically, there was no interaction by gender on the association of myo-inositol with LAV (P-interaction = 0.60).
Associations of top metabolites in Northwestern cohort
Of the 10 metabolites associated with echocardiographic traits and/or NT-proBNP in MESA, 4 were also measured in the Northwestern cohort (glutamine, glucose, myo-inositol, and D-mannose). Characteristics of the Northwestern cohort are shown in Supplemental Table 17. Compared to a control population of patients matched for comorbidities, HFpEF patients had significantly higher levels of circulating myo-inositol (β = 0.51, P = 0.002; Table 3). There were no significant associations between the remaining 3 metabolites and HFpEF status.
Associations of myo-inositol mQTLs with LA volume and NT-proBNP
Having established that higher myo-inositol levels were associated with higher LAV and NT-proBNP in MESA and with HFpEF in the Northwestern cohort, we next evaluated the associations of myo-inositol mQTLs with both LAV and NT-proBNP in MESA. Of the 6 myo-inositol mQTLs identified, all had been previously associated with higher myo-inositol levels based on review of the GWAS catalog. There were no associations of the 6 myo-inositol mQTLs with LAV (Table 4). There was, however, 1 SNP that was significantly associated with higher NT-proBNP after adjustment for multiple comparisons using false-discovery rate (rs10037610; Table 4). The SNP was identified to be intergenic, within long intergenic non-protein coding RNA 01377 (LINC01377). Race-stratified analyses revealed that rs10037610 was most common in African Americans (MAF = 5%) and was absent in Chinese individuals in MESA (Supplemental Table 18). The direction of effect of rs10037610 on NT-proBNP levels was consistent across African American, White, and Hispanic populations, and effect size was largest among White individuals (Supplemental Table 18).
Discussion
In an older cohort at risk for HF, we evaluated the associations of plasma concentrations of 47 circulating metabolites with echocardiographic measures of cardiac structure and function and NT-proBNP over 14 years later. We subsequently investigated the associations between top metabolites and HFpEF prevalence in a separate cohort. Several metabolites broadly reflective of glucose and amino acid metabolism had significant associations with a range of echocardiographic traits, including measures of diastolic and systolic dysfunction, along with chamber size. Of 10 metabolites identified to be associated with cardiac structure and function, 6 carried associations with NT-proBNP levels in MESA. Upon evaluation of these top metabolites in a separate cohort, only circulating myo-inositol was significantly higher among HFpEF patients compared with comorbidity-matched controls. Genetic analyses of variants previously associated with higher circulating myo-inositol (mQTLs) demonstrated that a specific mQTL, rs10037610, was associated with higher circulating NT-proBNP. Taken together, we identify several novel metabolic pathways that may be implicated in subclinical cardiac dysfunction prior to development of HFpEF. Specifically, altered myo-inositol metabolism demonstrated significant metabolomic and genetic associations with subclinical HF, suggesting it may be a particularly relevant metabolic pathway in HF development.
Circulating metabolites have been associated with echocardiographic measures and HF in prior investigations, and our findings build upon these prior metabolomic studies of cardiac function. Specifically, several lipid and amino acid-derived metabolites have been associated with E/e’, suggesting mitochondrial uncoupling may drive many of the metabolic changes preceding clinical HF9. An analysis of the Framingham Heart Study reported a correlation between the vasoactive metabolite kynurenine and left ventricular diastolic dimension26. Prior studies have also identified elevations in metabolites reflecting dysregulated fatty acid oxidation in patients with HFpEF8, and metabolites involved in posttranscriptional RNA modifications have been associated with incident HF7. It should be noted that most previous studies have reported correlations between metabolites and contemporaneous echocardiographic outcome measures. Our study evaluated metabolite levels measured years prior to echocardiography, demonstrating that even in older individuals, alterations in metabolites earlier in life may be associated with subclinical cardiac dysfunction. Additionally, prior studies have primarily focused on White and African American cohorts, but our study includes a more diverse sample of patients, many of whom were Hispanic or Chinese. Finally, the current study evaluated unique echocardiographic outcome measures, including RV and RA chamber size and function and LA and LV strain, which have not been previously interrogated. Furthermore, we leveraged NT-proBNP as an additional outcome measure to corroborate echocardiographic associations. Finally, we validated metabolomic findings in a separate cohort and used genetics to better understand relationships between metabolites and subclinical cardiac dysfunction. Through these techniques, we further define novel metabolic pathways relevant to future HF risk.
HF is regulated in part by alterations in cardiac energy metabolism secondary to a decrease in mitochondrial oxidative capacity. Utilization of different fuels for mitochondrial ATP production also change. Specifically, glucose and amino acid oxidation are decreased and the failing heart becomes less efficient. This pathophysiology is related to transcriptional changes in key metabolic enzymes, as well as alterations in metabolite signaling that contribute to post-translational epigenetic changes in the control of expression of genes encoding energy metabolic enzymes27. These mechanisms have been thoroughly studied in HFrEF, but a consensus on metabolic changes associated with HFpEF has not yet emerged. We provide hypotheses for potential mechanisms linking certain metabolites with the development of HFpEF below.
Myo-inositol is a stereoisomer of the carbocyclic sugar polyalcohol inositol and is necessary for many critical biologic functions including signal transduction, metabolic flux, insulin signaling, ion channel permeability regulation and stress response28,29. Inositol requirements are met through a combination of dietary intake and endogenous synthesis. It is synthesized de novo from glucose and is a product of phosphatidylinositol metabolism, as well as a participant in the diacylglycerol pathway where it generates new phosphoinositide30. Myo-inositol is synthesized from glucose-6-phosphate (G6P) in two steps, each catalyzed by a different enzyme. First myo-inositol-3-phosphate synthase (MIPS) converts G6P to myo-inositol-1-phosphate, and then inositol monophosphatase (IMPase) dephosphorylates myo-inositol-1-phosphate to produce free myo-inositol. This free myo-inositol can then be utilized in glycogen storage via the action of epimerase or converted to D-glucuronic acid by myo-inositol oxygenase (MIOX)29.
Prior studies have found associations between elevated circulating myo-inositol levels and the prevalence of HFrEF, as well as between myo-inositol levels and HFrEF severity31,32,33,34. Our results expand on prior knowledge by showing that elevated levels of myo-inositol actually precede myocardial dysfunction and are seen in clinical HFpEF. It is possible that myo-inositol levels could be upregulated in response to disorders in glucose control which also contribute to future development of HF, as myo-inositol and its downstream metabolites are important in glucose uptake and glycogen storage, as well as insulin sensitization35. These interactions are displayed in Supplemental Fig. 1. Additionally, endogenous synthesis of myo-inositol takes place primarily in the kidneys36. It is possible that renal changes related to common metabolic comorbidities such as diabetes and obesity, which are known to be associated with HFpEF, may play a role in upregulated myo-inositol production. Our genetic analyses suggest that higher myo-inositol levels may be causally related to higher NT-proBNP. The genetic variant associated with higher natriuretic peptide is a SNP within a long non-coding RNA (lncRNA), LINC01377. The function of LINC01377 is not well known, but other genetic variants within this lncRNA have been associated with various traits, including glycerophospholipid and dimethylglycine levels37. Based on summary data from the GTEx database, LINC01377 is expressed in brain tissue and testicular tissue. How LINC01377 regulates myo-inositol is currently unknown. Further investigations are required to understand how genetic variation in LINC01377 may lead to higher myo-inositol levels to better understand potential mechanisms by which higher myo-inositol may lead to cardiac dysfunction.
Our study demonstrated associations of other metabolites, including carnitine, with alterations in cardiac structure. Prior studies have identified higher levels of carnitine and its acyl derivatives in patients with HFpEF and HFrEF38, and specifically higher concentrations of medium and long-chain acylcarnitines in HFpEF patients compared to both HFrEF patients and non-HF controls39. Biosynthesis of carnitine is primarily controlled by four enzymes: 6-N-trimethyllysine dioxygenase (TMLD), 3-hydroxy-6-N-trimethyllysine aldolase (HTMLA), 4-N-trimethylaminobutyraldehyde dehydrogenase (TMABADH), and γ-butyrobetaine dioxygenase (BBD)40. Carnitine is synthesized primarily in the liver and kidneys and then concentrated in tissues which metabolize fatty acids as an energy source, such as skeletal muscle and cardiac muscle. Since patients with severe HF have been shown to have lower levels of myocardial carnitine41, it is possible that serum levels may be higher due to release into the circulation from the myocardium. In our study, carnitine had the largest effect size of all metabolites on LAV and NT-proBNP. Carnitine plays a critical role in fatty acid oxidation and energy metabolism and also regulates important cellular functions, such as apoptosis42. It is intrinsically involved in mitochondrial function and aerobic metabolism, contributing to a healthy energy balance in all body tissue.
Two other metabolites with significant associations with echocardiographic traits were glucose and glutamine, which have been noted to be associated with worse LV diastolic function43. In the MESA cohort, worse cardiac function and higher NT-proBNP levels were seen in patients with elevated serum glucose, although higher glucose levels were not seen in HFpEF patient in the Northwestern cohort. Glucose is involved in the transition to a more glycolytic state of energy production from a fatty acid bio-energetic state, a known compensatory response and metabolic signature of HF44. On the other hand, higher glutamine was associated with a higher e’ tissue Doppler velocity in MESA. The enzyme glutamine synthetase is responsible for the conversion of glutamate to glutamine, while the opposite reaction is catalyzed by glutaminase. It is also known that glutamine contributes to aerobic oxidation and energy production by acting as an alternative substrate in replenishing the tricarboxylic acid cycle45. Furthermore, altered glutamine homeostasis occurs in failing human cardiomyocytes46. Our findings further substantiate that glutamine metabolism may be relevant in early HF.
Dimethylsulfone levels were associated with several adverse echocardiographic outcome measures (lower LVEF, higher LAV, higher LVEDV) and elevation in NT-proBNP. This metabolite is an organic sulfur compound derived from dietary sources as well as endogenous methanethiol metabolism and intestinal bacterial metabolism47,48. The cause of this association is unclear, but the significance of diet and gut bacteria in the accumulation of this metabolite may support the significance of certain dietary patterns in HF risk. Acetate was also associated with worse echocardiographic outcome measures and elevated BNP, despite its previously hypothesized benefit in the prevention of hypertension and heart failure in certain animal models49. To our knowledge, these two metabolites have not been previously identified to be associated with HF or cardiac dysfunction.
Despite the strengths and novelty discussed above, certain limitations exist. Although we adjusted for several covariates in multivariable models, we cannot rule out residual confounding that may be present in metabolite-outcome relationships. However, our use of genetic analyses mitigates this limitation. Our study did not identify significant metabolomic associations of several novel echocardiographic measures (strain, RV function) after adjustment for multiple comparisons. Larger studies that capture these measures will be important to identify metabolite associations in future investigations. While our metabolomic footprint was smaller than other studies (47 metabolites), our study benefits from a validation cohort and genetic validation of results.
Metabolomic profiling in elderly individuals identifies several metabolic pathways that may be implicated in early HF, and in particular, HFpEF. We demonstrate several novel associations between metabolites and several measures of cardiac dysfunction and NT-proBNP, therefore identifying potential therapeutic targets requiring further investigation. Specifically, these data demonstrate a role for altered myo-inositol metabolism in driving early HF among at-risk individuals. Therefore, myo-inositol may turn out to be a useful early marker for risk of developing HFpEF, and has the potential to be utilized in clinical risk prediction tools, but future studies are required to better understand biological mechanisms behind myo-inositol metabolism and cardiac dysfunction.
Data availability
All data generated or analyzed during this study is included in the published article’s supplemental files.
Abbreviations
- GLS:
-
Global longitudinal strain
- 1H-NMR:
-
Proton nuclear magnetic resonance
- MESA:
-
Multi-Ethnic Study of Atherosclerosis
- NT-proBNP:
-
N-terminal pro-B-type natriuretic peptide
References
Dunlay, S. M., Roger, V. L. & Redfield, M. M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 14(10), 591–602. https://doi.org/10.1038/nrcardio.2017.65 (2017).
Shah, S. J. et al. Research priorities for heart failure with preserved ejection fraction: National Heart, Lung, and Blood Institute Working Group Summary. Circulation. 141(12), 1001–1026. https://doi.org/10.1161/CIRCULATIONAHA.119.041886 (2020).
Schiattarella, G. G. et al. Immunometabolic mechanisms of heart failure with preserved ejection fraction. Nat. Cardiovasc. Res. 1(3), 211–222. https://doi.org/10.1038/s44161-022-00032-w (2022).
Schrimpe-Rutledge, A. C., Codreanu, S. G., Sherrod, S. D. & McLean, J. A. Untargeted metabolomics strategies-challenges and emerging directions. J. Am. Soc. Mass Spectrom. 27(12), 1897–1905. https://doi.org/10.1007/s13361-016-1469-y (2016).
Zheng, Y. et al. Associations between metabolomic compounds and incident heart failure among African Americans: The ARIC Study. Am. J. Epidemiol. 178(4), 534–542. https://doi.org/10.1093/aje/kwt004 (2013).
Andersson, C. et al. Metabolomic signatures of cardiac remodelling and heart failure risk in the community. ESC Heart Failure. 7(6), 3707–3715. https://doi.org/10.1002/ehf2.12923 (2020).
Tahir, U. A. et al. Metabolomic profiles and heart failure risk in black adults: Insights from the Jackson Heart Study. Circ. Heart Fail. 14(1), e007275. https://doi.org/10.1161/CIRCHEARTFAILURE.120.007275 (2021).
Hunter, W. G. et al. Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: Evidence for shared metabolic impairments in clinical heart failure. J. Am. Heart Assoc. https://doi.org/10.1161/JAHA.115.003190 (2016).
Razavi, A. C. et al. Novel findings from a metabolomics study of left ventricular diastolic function: The Bogalusa Heart Study. J. Am. Heart Assoc. 9(3), e015118. https://doi.org/10.1161/JAHA.119.015118 (2020).
Choi, E. Y. et al. N-terminal pro-B-type natriuretic peptide, left ventricular mass, and incident heart failure: Multi-Ethnic Study of Atherosclerosis. Circ. Heart Fail. 5(6), 727–734. https://doi.org/10.1161/circheartfailure.112.968701 (2012).
Agarwal, S. K. et al. Prediction of incident heart failure in general practice: The Atherosclerosis Risk in Communities (ARIC) Study. Circ. Heart Fail. 5(4), 422–429. https://doi.org/10.1161/circheartfailure.111.964841 (2012).
Reimer Jensen, A. M. et al. Association of left ventricular systolic function with incident heart failure in late life. JAMA Cardiol. 6(5), 509–520. https://doi.org/10.1001/jamacardio.2021.0131 (2021).
de Simone, G., Gottdiener, J. S., Chinali, M. & Maurer, M. S. Left ventricular mass predicts heart failure not related to previous myocardial infarction: The Cardiovascular Health Study. Eur. Heart J. 29(6), 741–747. https://doi.org/10.1093/eurheartj/ehm605 (2008).
Bild, D. E. et al. Multi-ethnic study of atherosclerosis: Objectives and design. Am. J. Epidemiol. 156(9), 871–881. https://doi.org/10.1093/aje/kwf113 (2002).
Inker, L. A. et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 367(1), 20–29. https://doi.org/10.1056/NEJMoa1114248 (2012).
Dona, A. C. et al. Precision high-throughput proton NMR spectroscopy of human urine, serum, and plasma for large-scale metabolic phenotyping. Anal. Chem. 86(19), 9887–9894. https://doi.org/10.1021/ac5025039 (2014).
Hunter, W. G. et al. Metabolomic profiling of cholesterol efflux capacity in a multiethnic population: Insights from MESA. Arterioscler. Thromb. Vasc. Biol. 43(10), 2030–2041. https://doi.org/10.1161/atvbaha.122.318222 (2023).
Neeland, I. J. et al. Metabolomics profiling of visceral adipose tissue: Results from MESA and the NEO study. J. Am. Heart Assoc. 8(9), e010810. https://doi.org/10.1161/jaha.118.010810 (2019).
Tzoulaki, I. et al. Serum metabolic signatures of coronary and carotid atherosclerosis and subsequent cardiovascular disease. Eur. Heart J. 40(34), 2883–2896. https://doi.org/10.1093/eurheartj/ehz235 (2019).
Lang, R. M. et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 28(1), 1-39.e14. https://doi.org/10.1016/j.echo.2014.10.003 (2015).
Sanders-van Wijk, S. et al. Proteomic evaluation of the comorbidity-inflammation paradigm in heart failure with preserved ejection fraction: Results from the PROMIS-HFpEF study. Circulation. 142(21), 2029–2044. https://doi.org/10.1161/circulationaha.120.045810 (2020).
Purcell, S. et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81(3), 559–575. https://doi.org/10.1086/519795 (2007).
Giro, P. et al. Missense genetic variation of ICAM1 and incident heart failure. J. Card. Fail. 29(8), 1163–1172. https://doi.org/10.1016/j.cardfail.2023.02.003 (2023).
Buniello, A. et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics 2019. Nucleic Acids Res. 47(D1), D1005–D1012 (2019).
Wojcik, G. L. et al. Genetic analyses of diverse populations improves discovery for complex traits. Nature. 570(7762), 514–518. https://doi.org/10.1038/s41586-019-1310-4 (2019).
Andersson, C. et al. Metabolomic signatures of cardiac remodelling and heart failure risk in the community. ESC Heart Fail. 7(6), 3707–3715. https://doi.org/10.1002/ehf2.12923 (2020).
Lopaschuk, G. D., Karwi, Q. G., Tian, R., Wende, A. R. & Abel, E. D. Cardiac energy metabolism in heart failure. Circ. Res. 128(10), 1487–1513. https://doi.org/10.1161/circresaha.121.318241 (2021).
Kiani, A. K. et al. From Myo-inositol to D-chiro-inositol molecular pathways. Eur. Rev. Med. Pharmacol. Sci. 25(5), 2390–2402. https://doi.org/10.26355/eurrev_202103_25279 (2021).
Croze, M. L. & Soulage, C. O. Potential role and therapeutic interests of myo-inositol in metabolic diseases. Biochimie. 95(10), 1811–1827. https://doi.org/10.1016/j.biochi.2013.05.011 (2013).
Thomas, M. P., Mills, S. J. & Potter, B. V. The “other” inositols and their phosphates: Synthesis, biology, and medicine (with recent advances in myo-inositol chemistry). Angew. Chem. Int. Ed. Engl. 55(5), 1614–1650. https://doi.org/10.1002/anie.201502227 (2016).
Mueller-Hennessen, M. et al. A novel lipid biomarker panel for the detection of heart failure with reduced ejection fraction. Clin. Chem. 63(1), 267–277. https://doi.org/10.1373/clinchem.2016.257279 (2017).
Deidda, M. et al. Metabolomic approach to profile functional and metabolic changes in heart failure. J. Transl. Med. 13(1), 297. https://doi.org/10.1186/s12967-015-0661-3 (2015).
Alexander, D., Lombardi, R., Rodriguez, G., Mitchell, M. M. & Marian, A. J. Metabolomic distinction and insights into the pathogenesis of human primary dilated cardiomyopathy. Eur. J. Clin. Investig. 41(5), 527–538. https://doi.org/10.1111/j.1365-2362.2010.02441.x (2011).
McKirnan, M. D. et al. Metabolomic analysis of serum and myocardium in compensated heart failure after myocardial infarction. Life Sci. 221, 212–223. https://doi.org/10.1016/j.lfs.2019.01.040 (2019).
Dinicola, S. et al. Inositols: From established knowledge to novel approaches. Int. J. Mol. Sci. https://doi.org/10.3390/ijms221910575 (2021).
DiNicolantonio, J. J. & Okeefe, J. H. Myo-inositol for insulin resistance, metabolic syndrome, polycystic ovary syndrome and gestational diabetes. Open Heart. https://doi.org/10.1136/openhrt-2022-001989 (2022).
Al-Khelaifi, F. et al. Metabolic GWAS of elite athletes reveals novel genetically-influenced metabolites associated with athletic performance. Sci. Rep. 9(1), 19889. https://doi.org/10.1038/s41598-019-56496-7 (2019).
El-Aroussy, W. et al. Plasma carnitine levels as a marker of impaired left ventricular functions. Mol. Cell Biochem. 213(1–2), 37–41. https://doi.org/10.1023/a:1007142919941 (2000).
Zordoky, B. N. et al. Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS ONE. 10(5), e0124844. https://doi.org/10.1371/journal.pone.0124844 (2015).
Almannai, M., Alfadhel, M. & El-Hattab, A. W. Carnitine inborn errors of metabolism. Molecules. https://doi.org/10.3390/molecules24183251 (2019).
Hahn, V. S. et al. Myocardial metabolomics of human heart failure with preserved ejection fraction. Circulation. 147(15), 1147–1161. https://doi.org/10.1161/circulationaha.122.061846 (2023).
Marcovina, S. M. et al. Translating the basic knowledge of mitochondrial functions to metabolic therapy: Role of l-carnitine. Transl. Res. 161(2), 73–84. https://doi.org/10.1016/j.trsl.2012.10.006 (2013).
Zhang, Z. Y. et al. Diastolic left ventricular function in relation to circulating metabolic biomarkers in a population study. Eur. J. Prev. Cardiol. 26(1), 22–32. https://doi.org/10.1177/2047487318797395 (2019).
Brown, D. A. et al. Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 14(4), 238–250 (2017).
Reitzer, L. J., Wice, B. M. & Kennell, D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J. Biol. Chem. 254(8), 2669–2676 (1979).
Kennel, P. J. et al. Impairment of myocardial glutamine homeostasis induced by suppression of the amino acid carrier SLC1A5 in failing myocardium. Circ. Heart Fail. 12(12), e006336. https://doi.org/10.1161/circheartfailure.119.006336 (2019).
He, X. & Slupsky, C. M. Metabolic fingerprint of dimethyl sulfone (DMSO2) in microbial-mammalian co-metabolism. J. Proteome Res. 13(12), 5281–5292. https://doi.org/10.1021/pr500629t (2014).
Engelke, U. F. H. et al. Dimethyl sulfone in human cerebrospinal fluid and blood plasma confirmed by one-dimensional 1H and two-dimensional 1H–13C NMR. NMR Biomed. 18(5), 331–336. https://doi.org/10.1002/nbm.966 (2005).
Marques, F. Z. et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 135(10), 964–977. https://doi.org/10.1161/circulationaha.116.024545 (2017).
Acknowledgements
MESA and the MESA SHARe project are conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support for MESA is provided by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC95169, UL1-TR-000040, UL1-TR-001079, UL1-TR-001420, UL1-TR-001881, and DK063491. Funding for SHARe genotyping was provided by NHLBI Contract N02-HL-64278. Genotyping was performed at Affymetrix (Santa Clara, California, USA) and the Broad Institute of Harvard and MIT (Boston, Massachusetts, USA) using the Affymetrix Genome-Wide Human SNP Array 6.0. The MESA CARe data used for the analyses described in this manuscript were obtained through Genetics (accession numbers). Funding for CARe genotyping was provided by NHLBI Contract N01-HC-65226. We would like to thank the other investigators, the staff, and the participants of the MESA for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org
Funding
This work was supported by grant R01 HL167986 from NIH/NHLBI.
Author information
Authors and Affiliations
Contributions
K.L.C. contributed to the formal analysis, investigation, methodology and writing of the manuscript. A.S., M.F., P.G., N.B.A., K.D.T., X.G., E.T., B.H.F., P.G., W.S.P., A.B., D.H., C.G., Y.W., and S.J.S. contributed to the critical review and editing of the manuscript. R.B.P contributed to the conceptualization, supervision, data curation, formal analysis, and critical review and editing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
S.J.S. has received research grants from AstraZeneca, Corvia, and Pfizer, and consulting fees from Abbott, Alleviant, AstraZeneca, Amgen, Aria CV, Axon Therapies, Bayer, Boehringer-Ingelheim, Boston Scientific, Bristol Myers Squibb, Cyclerion, Cytokinetics, Edwards Lifesciences, Eidos, Imara, Impulse Dynamics, Intellia, Ionis, Lilly, Merck, MyoKardia, Novartis, Novo Nordisk, Pfizer, Prothena, Regeneron, Rivus, Sardocor, Shifamed, Tenax, Tenaya, and Ultromics. The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Culler, K.L., Sinha, A., Filipp, M. et al. Metabolomic profiling identifies novel metabolites associated with cardiac dysfunction. Sci Rep 14, 20694 (2024). https://doi.org/10.1038/s41598-024-71329-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71329-y
- Springer Nature Limited