Abstract
Gastric cancer (GC) is a malignant disease worldwide. Angiopoietin-like protein 4 (ANGPTL4) plays a role in pathophysiological processes, including metabolic reprogramming, angiogenesis, proliferation, and metastasis. Current evidence shows conflicting findings regarding the role of ANGPTL4 in the progression of GC. ANGPTL4 in GC was confirmed through bioinformatic analysis and immunofluorescence staining. The impact of ANGPTL4 was subsequently validated in GC cell lines using various assays, including 5-ethynyl-2-deoxyuridine (EdU), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), Flow Cytometry (FCM), wound healing, transwell, tube formation, chorioallantoic membrane model, and nude mouse model assays. RNA-seq analysis, polymerase chain reaction (PCR), western blotting (WB), immunofluorescence (IF) and coimmunoprecipitation (co-IP) were conducted to determine the potential downstream mechanism of ANGPTL4. In SNU5 and MKN7 cells, ANGPTL4 was found to augment proliferation, migration, invasion, evasion of apoptosis, and angiogenesis. Conversely, in the AGS cell line, ANGPTL4 was observed to suppress these processes. Notably, the overexpression of ANGPTL4 in AGS cells led to the upregulation of LGALS7, which has emerged as a pivotal factor contributing to the manifestation of an anticancer phenotype induced by ANGPTL4. LGALS7, which is involved in the regulation of the hedgehog pathway and subsequent promotion of GC progression through various processes, such as proliferation, migration, apoptosis evasion, angiogenesis, and lymphangiogenesis, was found to contribute to the contradictory effects of ANGPTL4.
Similar content being viewed by others
Introduction
Gastric cancer (GC) is a serious digestive malignancy and accounts for approximately 700,000 deaths worldwide each year, placing a heavy burden on families and society1. Although neoadjuvant chemotherapy and surgical treatment have improved survival rates in GC patients, lung metastases still result in a survival rate of only 25–30% after 5 years2.
Angiopoietin-like 4 (ANGPTL4), a secreted glycoprotein, plays a key role in regulating carcinogenesis, glucose homeostasis, immune infiltration, lipid metabolism, and angiogenesis3. ANGPTL4 has multiple molecular functions in different cancer types, including ovarian cancer4, GC5, breast cancer6, colorectal cancer7 and lung cancer8. However, in previous studies, ANGPTL4 exhibited completely different effects and contradictory roles, especially in GC5,9 and ovarian cancer4,10. It is possible that these differences arise from the different biological functions of ANGPTL4 in different cancer types via interactions with different proteins.
Galectin-7 (LGALS7) is a member of the galactolectin family that interacts with glycoproteins and plays important roles in various pathological processes11. It can act as an effective double regulator in different types of cancer12. LGALS7 contributes to various events associated with the differentiation and development of pluristratified epithelia, as well as epithelial cell migration, which is crucial for the re-epithelialization process of corneal or epidermal wounds13. Furthermore, LGALS7 is epigenetically regulated by DNA methylation, acts as a tumour suppressor, and its expression is significantly reduced in GC cells14. However, it remains unclear whether the paradoxical role of ANGPTL4 in GC is due to its interaction with LGALS7 or the molecular biological function of LGALS7.
In this study, ANGPTL4 levels were confirmed by immunofluorescence (IF) of a GC tissue microarray. We found that ANGPTL4 was significantly increased in patients with GC. The contradictory effects of ANGPTL4 on proliferation, migration, invasion and angiogenesis in multiple cancer cell lines, including AGS, MKN7 and SNU5. Furthermore, LGALS7 plays a key role in the paradoxical role of ANGPTL4 in GC progression by inactivating the hedgehog pathway.
Methods
GC tissue samples collection
Between 2018 and 2023, 70 GC samples were resected at the Second Affiliated Hospital of the University of South China. Based on the Helsinki Declaration, tissues were collected and used according to ethical standards. The University of South China’s research ethics committee approved the study after receiving written informed consent from each patient. Protocols were approved under University of South China IRB protocols #USC-2023-358.
Cell culture and transfection
The American Type Culture Collection (ATCC, Manassas) provided GC cells (MKN7, MGC803, SNU5, AGS, MKN1), normal gastric cells (GES-1) and human umbilical vein endothelial cells (HUVECs) for our experiment.
AGS and MKN7 cells were maintained in RPMI 1640 medium (Sigma Aldrich; Thermo Fisher Scientific, Inc.) or GES-1, MGC803, SNU5, MKN1 cells in DMEM medium (Sigma Aldrich; Thermo Fisher Scientific, Inc.) with 10% fetal bovine serum, 100 IU/mL penicillin, and 10 µg/mL streptomycin. ANGPTL4 shRNA, ANGPTL4 OE, LGALS7 shRNA, and LGALS7 OE plasmid were synthesized by HonorGene (Changsha, China). Inoculated cells were transfected with either the ANGPTL4/LGALS7 shRNA or scramble control shRNA into six-well plates. A complete medium containing 5.0 g of puromycin per liter of complete medium was used to screen stable cells for two weeks.
Bioinformatic analysis
TCGA database (https://www.cancer.gov/tcga) was used to confirm the expression and prognosis of ANGPTLs in GC patients. Genemania database (http://www.genemania.org) was used to constructed PPI network for ANGPTLs. DAVID database (https://david.ncifcrf.gov/) was utilized to make GO and KEGG15,16,17 (https://www.kegg.jp/kegg/kegg1.htm) enrichment analysis for these genes based from PPI network. GSVA analysis was used to conform the potential functions in AGS cell. The data of ANGPTL4 (6U1U) and LGALS7 (3ZXF) were downloaded from PDB database (https://www.rcsb.org/), which uploaded to the online protein docking ZDOCK online server (http://zdock.umassmed.edu/) for protein–protein docking. The Pymol software was used to analyze the interaction between the binding modes. Please refer to our previous article for more detailed bioinformatic analysis methods18.
Cell function experiment
MTT analysis, EdU analysis, wound healing assay, transwell analysis, tube formation assay, western blot, RT-PCR, and Flow Cytometry (FCM) analysis were performed following the protocols described in our previous study18,19,20. The primary antibodies: ANGPTL4 (Abcam-ab196746), Galectin-7/LGALS7 (Abcam, ab206435), Shh (Abcam-ab53281), Smo (Abcam-ab235183), and Gli-1 (Abcam-ab217326) for western blot. Moreover, the blots were cut prior to hybridisation with antibodies during blotting. Original blots/gels are presented in Supplementary Material 1.
IHC and IF staining
The IHC staining follows the protocol used in our previous study18,19,20. The primary antibodies: ANGPTL4 (Abcam-ab196746), Galectin-7 (Abcam-ab206435), Shh (Abcam-ab53281), Gli-1 (Abcam-ab217326), PCNA (Abcam-ab265609), Cdc25A (Abcam-ab2357), Ki-67 (Abcam-ab16667), Bax (Abcam-ab32503), Bcl-2 (Abcam-ab32124), CCNB1 (Abcam-ab32053), Vimentin (Abcam-ab8978), E-cadherin (Abcam-ab40772).
RNA-sequence
AGS cell samples transfected with ANGPTL4 plasmid were processed according to Beijing Genomics institution (BGI)’s requirements for RNA collection and sequencing. Quality control measures were taken throughout the process. Refer to the genomics company guidelines for more information (https://www.yuque.com/yangyulan-ayaeq/oupzan/lmx609).
Co-immunoprecipitation (Co-IP)
To extract total protein, cell lysates were collected in RIPA buffer, which contained 50 mM Tris–HCl pH 8.0, 150 mM NaCl, 0.02% sodium azide, 0.1% SDS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 1 mM NaF, 1 mM Na3VO4, and a complete protease inhibitor cocktail. Nuclear extracts were obtained following the manufacturer's protocol, utilizing the NE-PER® Kit (Pierce, Rockford, IL). Co-immunoprecipitation was carried out with Protein A/G agarose (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The resulting pellets were washed three times using RIPA buffer. The proteins that were co-immunoprecipitated were subsequently analyzed through Western blotting.
Angiogenesis model
For the chorioallantoic membrane model, Initially, fertilized chicken eggs were maintained under controlled conditions of 80% humidity and 37 °C for 7 days. The eggs were subjected to a window opening on the shell to expose the chorioallantoic membrane at day 8. A gelatin sponge (0.3 cm × 0.3 cm × 0.3 cm) containing PBS or the specified conditioned medium was placed on the exposed chorioallantoic membrane. Subsequently, 2 × 105 GC cells/100 μL with Matrigel were placed onto a single egg of the chick embryo and were allowed to incubate for 4 days. Finally, the window was covered with tape and the eggs were allowed to incubate for a further 2 days. Branches of blood vessels was then quantified to determine angiogenesis.
Xenograft model and Popliteal lymph node metastasis model
For Xenograft model, the 1 × 106 ANGPTL4-MKN7/AGS cells, and vector-MKN7/AGS cells were injected subcutaneously into athymic BALB/c nude mice (4 weeks old). In each case, the tumor volume (in cm3) was determined by width2 × length × 0.5 every seven days. After 48 days, the mice were humanely sacrificed, and the xenografts were measured. Subsequently, we stained the xenograft samples after dehydrating, embedding, and embedding them in paraffin.
For Popliteal lymph node metastasis model, a total of twenty male nude mice, aged five weeks old were hypodermically injected of ANGPTL4-MKN7/AGS cells, and vector-MKN7/AGS cells in 50 μL cell suspensions (1 × 105 cells), which were administered in the footpads of each group. A weekly tumor assessment was conducted, and after thirty days, the mice were humanely euthanized, with footpad tumors and popliteal lymph nodes dissected and photographed.
All procedures were approved by the research ethics committee of University of South China with Institutional Review Board (IRB) approval (#USC-2023-559). Mice were euthanized using CO2 inhalation. The study is reported in accordance with ARRIVE guidelines. All methods were performed in accordance with relevant guidelines and regulations.
Statistical analysis
All statistical analyses were conducted using R software, which could be download from https://www.r-project.org/. The data presented normal distribution and were expressed as means ± SD obtained from at least three independent experiments. Results were considered statistically significant at P < 0.05.
Results
ANGPTL4 is a key ANGPTL protein in GC patients
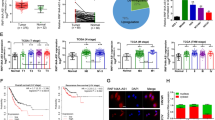
First, the levels of ANGPTL1-7 mRNA in GC patients were measured in the TCGA database, which indicated that ANGPTLs were both dysregulated in GC samples compared to normal gastric tissue samples (Fig. 1A). We further constructed a protein‒protein interaction (PPI) network for ANGPTLs based on the GeneMANIA database (Fig. 1B). These PPI genes were almost significantly correlated with each other in GC patients (Fig. 1C). GO analysis indicated that these genes were enriched in the regulation of endothelial cell apoptotic process, complement activation, lectin pathway, collagen-containing extracellular matrix, and extracellular matrix structural constituent (Fig. 1D,E). KEGG analysis indicated that these genes were enriched in the PI3K-Akt signalling pathway, ECM-receptor interaction, and focal adhesion (Fig. 1F,G). Survival analysis indicated that only ANGPTL4 was significantly correlated with poor prognosis in these ANGPTLs in GC patients (Fig. 1H). Furthermore, the expression of ANGPTL4 in multiple GC cell lines was confirmed by the CCLE database (Fig. 1I). IF staining indicated that ANGPTL4 protein expression was significantly greater in GC samples than in paracancerous tissue samples (Fig. 1J), and ANGPTL4 protein expression was significantly correlated with shorter survival (Fig. 1K), TNM stage and greater tumour size (Table 1). Due to the limited availability of pathological tissue for stages I and IV, necessitated by challenges in early diagnosis and missed surgical opportunities in advanced stages, we amalgamated stage I and II patients into a single group and stage III and IV patients into another group. The tabulated outcomes indicate a significant correlation between ANGPTL4 expression and tumour size among patients with gastric cancer, suggesting a potential role for ANGPTL4 in promoting the progression of the disease through a positive feedback loop involving ANGPTL4 protein expression. Moreover, we found that the expression of ANGPTL4 was also increased in GC samples compared to normal gastric tissue samples based on the HPA database (Fig. 1L).
The expression, function and prognosis of ANGPTLs in GC. (A) The mRNA level of ANGPTLs in GC based on the TCGA database. (B) The PPI network of ANGPTLs based on the GeneMANIA database. (C) The correlation of ANGPTL-related genes in the TCGA database GC dataset. (D) GO enrichment analysis of ANGPTL-related genes. (E) The network constructed for the GO enrichment terms. (F) KEGG enrichment analysis of ANGPTL-related genes. (G) The network constructed for the KEGG enrichment terms. (H) Prognosis analysis for ANGPTLs in GC patients. (I) The mRNA levels of ANGPTL4 in multiple GC cell lines based on the CCLE database. (J) ANGPTL4 protein expression in GC samples by IF. (K) Overall survival analysis according to ANGPTL4 expression in GC patients. (L) ANGPTL4 expression in GC samples based on the HPA database.
Contradictory effect of ANGPTL4 on the proliferation and migration of GC cell lines
To confirm the effect of ANGPTL4 on GC proliferation, apoptosis escape, migration and invasion. We confirmed ANGPTL4 expression in immortal gastric cells and multiple GC cell lines, including GES-1, MKN7, MGC803, SNU5, AGS and MKN1 (Fig. 2A). Moreover, we enhanced the expression of ANGPTL4 in MKN7 and AGS cells and repressed ANGPTL4 in SNU5 cells (Fig. 2B). MTT assays indicated that ANGPTL4 overexpression (OE) significantly enhanced the viability of MKN7 cells; similarly, ANGPTL4 knockdown (KD) decreased the viability of SNU5 cells, but ANGPTL4 OE contradictorily reduced the viability of AGS cells (Fig. 2C). EdU assays showed that ANGPTL4 OE enhanced MKN7 cell proliferation and paradoxically reduced AGS cell proliferation, and ANGPTL4 KD significantly repressed SNU5 cell proliferation (Fig. 2D). An apoptosis assay indicated that ANGPTL4 KD significantly increased apoptosis in SNU5 cell lines and that ANGPTL4 OE increased apoptosis in AGS cell lines but decreased apoptosis in MKN7 cell lines (Fig. 2E). Although ANGPTL4 was not significantly associated with lymphatic metastasis in our GC tissue sample set, this may be attributed to our small sample size. In the study of Toshiyuki et al.21, ANGPTL4 and GC in patients with venous invasion, lymphatic invasion or lymph node metastasis were closely related. We therefore decided to examine the molecular biological role of ANGPTL4 in different GC cell lines using molecular biology techniques. A wound healing assay and transwell assay showed that ANGPTL4 acted as an oncogene in MKN7 and SNU5 cells but acted as a tumour suppressor gene in AGS cells (Fig. 2F,G).
The effects of ANGPTL4 on GC cell proliferation, apoptosis, migration, invasion and angiogenesis. (A) The expression of ANGPTL4 protein in multiple GC cell lines by western blot. (B) The expression of ANGPTL4 in ANGPTL4 KD-SNU5 cells, ANGPTL4 OE-MKN7 cells, and ANGPTL4 OE-AGS cells. The effects of ANGPTL4 on GC cell proliferation by (C) MTT analysis and (D) EdU analysis (a representative image is shown at ×200 magnification). (E) FCM analysis was used to confirm the apoptosis ability of ANGPTL4 KD-SNU5 cells, ANGPTL4 OE-MKN7 cells, and ANGPTL4 OE-AGS cells. Wound healing analysis (a representative image is displayed at 40× magnification) (F) and Transwell invasion assays (a representative image is displayed at 200× magnification) (G) were used to confirm the migration and invasion ability of ANGPTL4 KD-SNU5 cells, ANGPTL4 OE-MKN7 cells, and ANGPTL4 OE-AGS cells. (H) Chorioallantoic membrane assays and (I) tube formation assays were used to confirm the angiogenesis ability of ANGPTL4 KD-SNU5 cells, ANGPTL4 OE-MKN7 cells, and ANGPTL4 OE-AGS cells in vivo and in vitro, respectively (a representative image is displayed at ×100 magnification). The effect of ANGPTL4 on HUVEC migration and proliferation in the conditioned medium of ANGPTL4 KD/Vector-SNU5 cells, ANGPTL4 OE/Vector-MKN7 cells, and ANGPTL4 OE/Vector-AGS cells, as determined by a wound healing assay (a representative image is displayed at 40× magnification) (J) or an EdU analysis (a representative image is displayed at 200× magnification) (K). *p < 0.05, **p < 0.01, ***p < 0.001.
The paradoxical role of ANGPTL4 in GC angiogenesis
Next, we detected the effect of ANGPTL4 on angiogenesis in SNU5, MKN7 and AGS cells. Chorioallantoic membrane assays showed that ANGPTL4 KD significantly decreased the microvessel density in SUN5 cells, and ANGPTL4 OE significantly increased the microvessel density in MKN7 cells but decreased the microvessel density in AGS cells (Fig. 2H). Tube formation assays indicated that ANGPTL4 was a proangiogenic factor in SNU5 and MKN7 cells but was an antiangiogenic factor in AGS cells (Fig. 2I). Wound healing analysis indicated that HUVEC migration was decreased in the conditioned medium of SNU5 ANGPTL4-KD cells compared to that in the conditioned medium of vector-treated cells. The conditioned medium of MKN7 ANGPTL4-OE cells increased HUVEC migration, but the conditioned medium of AGS ANGPTL4-OE cells decreased HUVEC migration (Fig. 2J). EdU assays also indicated that the proliferation ability of HUVECs increased in the conditioned medium of MKN7 cells with ANGPTL4 OE but decreased in the conditioned medium of AGS cells with ANGPTL4 OE and SNU5 cells with ANGPTL4 KD (Fig. 2K). However, the level of ANGPTL4 in AGS cells is quite high compared with that in normal cells and other GC cell lines. We further confirmed the function of ANGPTL4 in AGS cells by inhibiting ANGPTL4 (Fig. S1A), and the results showed that the proliferation, migration and invasion abilities were significantly enhanced when ANGPTL4 was knocked down in AGS cells (Fig. S1B–E). Moreover, ANGPTL4 knockdown further enhanced the angiogenesis, proliferation and migration ability of endothelial cells in the microenvironment (Fig. S1F–H).
ANGPTL4-driven Hedgehog pathway activation depends on LGALS7 expression in GC cells
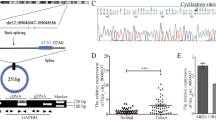
Then, we used RNA sequencing to analyse the potential molecular mechanism underlying the paradoxical role of ANGPTL4 in GC. Volcano plots and heatmaps showed that six genes, namely, TSTD3, COMMD3-BMI1, H4C5, LGALS7B, RARRES2 and LGALS7, were significantly dysregulated in AGS cell lines (Fig. S2A,B). GSVA indicated that TSTD3 was significantly enriched in the TNFA signalling pathway and oxidative phosphorylation, COMMD3-BMI1 was significantly enriched in the oxygen species pathway and protein secretion, H4C5 was obviously enriched in the interferon alpha response and hedgehog signalling, LGALS7B was enriched in kRAS signalling and protein secretion, RARRES2 was significantly enriched in TNFA signalling via NFKB and oxidative phosphorylation, and LGALS7 was enriched in oxidative phosphorylation, hedgehog signalling and TNFA signalling via NFKB (Fig. S2C). Moreover, these six genes were both significantly dysregulated in gastric cancer samples in the TCGA database (Fig. S2D). Moreover, the expression of LGALS7 was obviously greater in the AGS cell line than in the MKN7 and SNU5 cell lines according to the CCLE database (Fig. S2E).
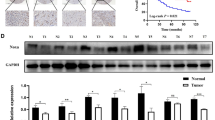
Based on the findings of GSVA enrichment analysis, the enriched components of ANGPTL4 and LGALS7 exhibited a high degree of correlation (Fig. S2C), while the Hedgehog pathway is recognized as a canonical signalling pathway implicated in the pathogenesis of GC22. Consequently, we investigated the impact of the ANGPTL4-LGALGS7 signalling axis on the functionality of this pathway. We further confirmed the effect of ANGPTL4 OE on LGALS7 expression in the MKN7 and AGS cell lines. ANGPTL4 OE upregulated the mRNA and protein levels of LGALS7 in AGS cells but did not change the expression of LGALS7 in MKN7 cells. Moreover, ANGPTL4 activated the hedgehog pathway by upregulating SHH, Smo and Gli-1, which was reversed by LGALS7 OE in MKN7 cells. However, ANGPTL4 inhibited the activation of the hedgehog pathway by inhibiting SHH, Smo and Gli-1, which was reversed by LGALS7 KD in AGS cells (Fig. 3A,B). Moreover, LGALS7 was significantly negatively associated with SHH and SMO mRNA levels in GC patients according to the TCGA database (Fig. 3C). IF staining showed that the colocalization of ANGPTL4 and LGALS7 was obvious in the AGS cell line (Fig. 3D). Co-IP analysis revealed that ANGPTL4 interacted with LGALS7 in AGS cells (Fig. 3E). Molecular docking experiments revealed that ANGPTL4 and LGALS7 were docked in the predicted binding sites, such as ASN-2/THR-382, ARG-384/GLN128, LEU-129/ASP-130, LEU-312/SER-1, and THR-353/PRO-2 (Fig. 3F). These results both indicated that the anticancer effect of ANGPTL4 was primarily dependent on LGALS7 expression and interaction to drive the hedgehog pathway, which inhibited GC carcinogenesis, such as proliferation, angiogenesis, migration, invasion, and lymph node metastasis (Fig. 3G).
LGALS7 plays a key role in hedgehog pathway activation by ANGPTL4. (A) The mRNA levels of ANGPTL4, LGALS7, SHH, Smo and Gli-1 were confirmed in the NC, vector, ANGPTL4 OE, and ANGPTL4 OE plus LGALS7 OE groups of MKN7 cells and in the NC, vector, ANGPTL4 OE, and ANGPTL4 OE plus LGALS7 KD groups of AGS cells by PCR. (B) The protein levels of ANGPTL4, LGALS7, SHH, Smo and Gli-1 were confirmed in the NC, vector, ANGPTL4 OE, and ANGPTL4 OE plus LGALS7 OE groups of MKN7 cells and in the NC, vector, ANGPTL4 OE, and ANGPTL4 OE plus LGALS7 KD groups of AGS cells by western blotting. (C) Correlation of LGALS7 with SHH or SMO in GC patients. (D) IF staining analysis was used to test the colocalization of ANGPTL4 and LGALS7 in AGS cells. (E) The interaction between ANGPTL4 and LGALS7 was confirmed by co-IP in AGS cells. (F) Molecular docking analysis showing the molecular binding sites between ANGPTL4 and LGALS7. (G) Schematic diagram showing the effects of the ANGPTL4/LGALS7/Hedgehog pathway on GC cells. *p<0.05, **p<0.01, ***p<0.001.
LGALS7 acts as an important converter for ANGPTL4 in GC cell progression in vitro
Furthermore, we used EdU to confirm the role of LGALS7 in ANGPTL4-induced GC proliferation. LGALS7 OE inhibited the proliferation of MKN7 cells mediated by ANGPTL4 OE, and LGALS7 KD enhanced the proliferation of AGS cells treated with ANGPTL4 OE (Fig. 4A). An FCM assay was utilized to determine whether GC apoptosis is regulated by ANGPTL4. ANGPTL4 OE markedly decreased the apoptosis rate of MKN7 cells, while LGALS7 OE obviously increased the density of apoptosis in MKN7 cells. In AGS cells, ANGPTL4 OE accelerated cell apoptosis, which was reversed by LGALS7 KD (Fig. 4B). Wound healing and Transwell invasion assays indicated that LGALS7 OE inhibited the molecular effects of ANGPTL4 OE on promoting MKN7 cell migration and invasion, and LGALS7 KD reversed the inhibitory effects of ANGPTL4 OE on migration and invasion in AGS cells (Fig. 5A,B).
LGALS7 plays a key role in GC cell proliferation and apoptosis mediated by ANGPTL4. (A) The proliferation ability of MKN7 cells transfected with NC, vector, ANGPTL4 OE, or ANGPTL4 OE plus LGALS7 OE and the proliferation ability of AGS cells transfected with NC, vector, ANGPTL4 OE, or ANGPTL4 OE plus LGALS7 KD determined by EdU analysis (a representative image is displayed at ×200 magnification). (B) FCM analysis was used to confirm the apoptosis ability of MKN7, Vector-MKN7, ANGPTL4 OE-MKN7, ANGPTL4 OE plus Vector-MKN7, ANGPTL4 OE plus LGALS7 OE-MKN7, AGS, Vector-AGS, ANGPTL4 OE-AGS, ANGPTL4 OE plus Vector-AGS, and ANGPTL4 OE plus LGALS7 KD-AGS cells. *p<0.05, **p<0.01, ***p<0.001.
LGALS7 plays a key role in GC cell migration, invasion and angiogenesis mediated by ANGPTL4. (A) The migration ability of MKN7 cells transfected with NC, vector, ANGPTL4 OE, or ANGPTL4 OE plus LGALS7 OE and the migration ability of AGS cells transfected with NC, vector, ANGPTL4 OE, or ANGPTL4 OE plus LGALS7 KD determined by wound healing analysis (a representative image is displayed at 40× magnification). (B) Transwell invasion analysis was used to confirm the invasion ability of MKN7, Vector-MKN7, ANGPTL4 OE-MKN7, ANGPTL4 OE plus Vector-MKN7, ANGPTL4 OE plus LGALS7 OE-MKN7, AGS, Vector-AGS, ANGPTL4 OE-AGS, ANGPTL4 OE plus Vector-AGS, and ANGPTL4 OE plus LGALS7 KD-AGS cells (a representative image is shown at ×200 magnification). (C) Tube formation analysis was used to confirm angiogenesis in MKN7, Vector-MKN7, ANGPTL4 OE-MKN7, ANGPTL4 OE plus Vector-MKN7, ANGPTL4 OE plus LGALS7 OE-MKN7, AGS, Vector-AGS, ANGPTL4 OE-AGS, ANGPTL4 OE plus Vector-AGS, and ANGPTL4 OE plus LGALS7 KD-AGS cells (a representative image is shown at ×100 magnification). The proliferation and migration ability of HUVECs cocultured in conditioned medium from MKN7 cells transfected with NC, vector, ANGPTL4 OE, or ANGPTL4 OE plus LGALS7 OE and the proliferation and migration ability of AGS cells transfected with NC, vector, ANGPTL4 OE, or ANGPTL4 OE plus LGALS7 KD determined by (D) EdU analysis (a representative image is displayed at ×200 magnification) and (E) wound healing assay (a representative image is displayed at ×40 magnification). ***p < 0.001.
Angiogenesis analysis also revealed that LGALS7 OE inhibited the ratio of tube length mediated by ANGPTL4 OE in MKN7 cells, and LGALS7 KD enhanced the ratio of tube length mediated by ANGPTL4 OE in AGS cells (Fig. 5C). EdU analysis revealed that ANGPTL4 OE markedly enhanced the proliferation ability of HUVECs cultured in conditioned medium from MKN7 cells, while LGALS7 OE markedly reduced the proliferation ability of HUVECs cultured in conditioned medium from MKN7 cells. In HUVECs cultured in AGS conditioned medium, compared to the NC and vector groups, the ANGPTL4 OE group exhibited inhibited cell proliferation, which were rescued by LGALS7 KD (Fig. 5D). A wound healing assay showed that LGALS7 OE rescued the molecular effects of ANGPTL4 OE on the migration of HUVECs cultured in conditioned medium from MKN7 cells, and LGALS7 KD rescued the migration of HUVECs cultured in conditioned medium from AGS cells (Fig. 5E).
The role of ANGPTL4 in GC progression in vivo
We further confirmed the molecular effects of ANGPTL4 using MKN7 and AGS xenograft tumour mouse models. The tumour weight and volume of MKN7 xenografts transfected with ANGPTL4 OE were significantly greater than those of MKN7 xenografts transfected with empty vector, but the weights and volumes of AGS xenografts transfected with ANGPTL4 OE were significantly less than those of AGS xenografts transfected with empty vector (Fig. 6A). Next, we used IHC staining to detect AGS and MKN7 xenografts. LGALS7 expression did not significantly change in ANGPTL4 OE xenografts; Bcl-2, CCNB1, Cdc25A, Ki-67, PCNA, Vimentin, Shh, and Gli1 expression significantly increased in ANGPTL4 OE xenografts; and Bax and E-cadherin expression significantly decreased in ANGPTL4 OE in MKN7 xenografts. The expression of LGALS7, Bax, and E-cadherin was significantly increased, but the expression of Bcl-2, CCNB1, Cdc25A, Ki-67, PCNA, Vimentin, Shh, and Gli1 was obviously decreased in AGS xenografts transfected with ANGPTL4 OE (Fig. 6B).
ANGPTL4 promotes growth, lymphangiogenesis, angiogenesis and metastasis in lymph nodes in a manner dependent on LGALS7 in vivo. (A) The weight and volume of xenografts of vector-MKN7, ANGPTL4 OE-MKN7, vector-AGS, and ANGPTL4 OE-AGS cells. (B) IHC staining for the expression of ANGPTL4, LGALS7, Bax, Bcl-2, CCNB1, Cdc25A, Ki-67, PCNA, Vimentin, E-cad, Shh and Gli1 in the xenografts of Vector-MKN7, ANGPTL4 OE-MKN7, Vector-AGS, and ANGPTL4 OE-AGS (a representative image is displayed at 400× magnification). (C) The volume of lymph nodes in vector-MKN7, ANGPTL4 OE-MKN7, vector-AGS, and ANGPTL4 OE-AGS cells. (D) Lyve1 and CD34 protein expression in the xenografts of vector-MKN7, ANGPTL4 OE-MKN7, vector-AGS, and ANGPTL4 OE-AGS cells in different groups. (E) ANGPTL4 expression in the lymph nodes from the xenografts of Vector-MKN7, ANGPTL4 OE-MKN7, Vector-AGS, and ANGPTL4 OE-AGS cells detected by IHC (a representative image is displayed at 400× magnification), *p<0.05, ***p<0.001 .
Then, we established a popliteal lymph node metastasis model using MKN7/AGS-ANGPTL4 cells to confirm the effect of ANGPTL4 on GC angiogenesis and lymphatic metastasis. The blood vessel length and density in vivo were significantly increased in MKN7 cells transfected with ANGPTL4 OE but decreased in AGS cells transfected with ANGPTL4 OE (Fig. 6C). We further found that popliteal lymph nodes derived from MKN7 cells transfected with ANGPTL4 OE were obviously larger than those from the vector group. On the other hand, popliteal lymph nodes derived from AGS cells transfected with ANGPTL4 OE cells were smaller in volume (Fig. 6C).
Finally, we tested the expression of Lyve-1 and CD34 to confirm the effects on lymphangiogenesis and angiogenesis in xenografts and showed that ANGPTL4 increased the level of lymphangiogenesis and angiogenesis in MKN7 cells but reduced the level of angiogenesis in AGS cells (Fig. 6D). Lymphangiogenesis is significantly correlated with lymphatic metastasis23, and angiogenesis is responsible for cancer cell progression from the lymph node to distant metastasis. Hence, we confirmed the association between ANGPTL4 levels and GC lymphatic metastasis. Compared with those in the vector group, high ANGPTL4 expression was detected in GC cells in the lymph nodes of the MKN7-ANGPTL4 OE group, but high ANGPTL4 expression were detected in the AGS-ANGPTL4 OE group (Fig. 6E). Furthermore, lymph node metastasis was greater in the MKN7-ANGPTL4 OE group than in the MKN7-vector group but was lower in the AGS-ANGPTL4 OE group than in the AGS-vector group. These results indicated that LGALS7 inhibited lymphangiogenesis and angiogenesis in GC, resulting in lymphatic and distant metastasis.
Discussion
Multiple studies have demonstrated that ANGPTL4 is involved in the progression of malignant tumours, including ovarian cancer10, GC9, colon cancer24, lung cancer8 and breast cancer6. Xiao et al. suggested that ANGPTL4 is an oncogene that drives lung cancer proliferation by regulating glutamine metabolism and fatty acid oxidation25. Shen et al. indicated that the ability of oleic acid to accelerate colorectal cancer metastasis is dependent on ANGPTL426. Yang and his colleagues found that ANGPTL4 increased proliferation by activating the Akt pathway in thyroid cancer27. Avalle et al. reported that ANGPTL4 accelerated breast cancer development6. Zhang and his colleagues indicated that ANGPTL4 induced radioresistance by repressing ferroptosis in a hypoxic tumour microenvironment in lung cancer cells8. Nakayama et al. reported that ANGPTL4 accelerated metastasis and blood vessel invasion in colon cancer patients28. Lin et al. found that ANGPTL4 impeded osteosarcoma progression by remodelling branched chain amino acids29. These findings both indicated the significance of ANGPTL4 in the development and progression of cancer, indicating that ANGPTL4 expression might be involved in the progression of GC.
Importantly, ANGPTL4 appears to have a dual role in the development of GC, and previous studies have shown contradictory findings. Qin et al. reported that ANGPTL4 might be a tumour suppressor gene that represses tumorigenesis in GC cells, especially in AGS cells30. Okochi-Takada and his colleagues suggested that ANGPTL4, a secreted protein, inhibited angiogenesis to impede GC growth5. Kubo and his colleagues suggested that the ANGPTL4 protein level was strongly associated with longer survival time in GC patients31. However, Nakayama and his colleagues reported that ANGPTL4 was significantly and positively correlated with venous invasion to promote metastasis in GC patients21. Baba et al. suggested that ANGPTL4 promoted cancer growth and anoikis resistance via the FAK/Src/PI3K-Akt/ERK pathway in GC9. Chen et al. reported that ANGPTL4 is an oncogene that promotes proliferation and invasion in SNU1 and BGC823 cells32. In our study, we found that ANGPTL4 plays a paradoxical role in different GC cell lines, including AGS, MKN7 and SNU5. ANGPTL4 promoted proliferation, migration, invasion, apoptosis escape and angiogenesis in MKN7 and SNU5 cells but inhibited these cell functions in AGS cells. Therefore, our results also support previous studies showing that ANGPTL4 may have different molecular biological effects in different GC cell lines. Moreover, this contradictory effect of ANGPTL4 may be due to the heterogeneity of tumour cells. According to the ATCC® Company tumour cell description, primary tumour-derived AGS cells are frequently associated with mutations in four genes, namely, CDH1, CTNNB1, KRAS and PI3KCA. CDH1, CDKN2A and TP53 mutations are frequently found in SNU5 cells33. MKN7 is a well-differentiated tubular gastric adenocarcinoma cell line derived from lymph node metastasis34. Therefore, gastric cancer cells with highly metastatic characteristics may be further enhanced by ANGPTL4, but the exact molecular mechanism involved is still unknown.
To further analyse the molecular mechanisms responsible for these contradictory effects in GC, we used RNA-seq to analyse AGS-ANGPTL4 OE and AGS-Vector cells. We found that six genes, namely, TSTD3, COMMD3-BMI1, H4C5, LGALS7B, RARRES2 and LGALS7, were differentially expressed after ANGPTL4 was overexpressed, and the functions of LGALS7 and ANGPTL4 were highly consistent according to GSVA enrichment analysis. A previous study revealed that LGALS7 expression is reduced in GC patients and is epigenetically decreased by DNA promoter hypermethylation14. LGALS7, an important binding protein, interacts with glycoproteins to regulate their activation and function11. We found that LGALS7 interacted and bound with ANGPTL4 to inhibit proliferation, migration, invasion, apoptosis escape and angiogenesis in AGS and MKN7 cells, which indicated that LGALS7 might be an important tumour suppressor gene downstream of ANGPTL4.
Moreover, ANGPTL4 upregulated LGALS7 mRNA in AGS cells. The underlying mechanism may be that ANGPTL4 has a powerful tumour metabolic reprogramming effect35. However, tumour metabolic reprogramming is often closely related to epigenetic modifications, especially DNA methylation36. It has been reported that LGALS7 is inhibited by DNA hypermethylation in AGS cells14. Taken together with our results, these findings indicate that ANGPTL4 most likely promotes the transcription of LGALS7 by reducing the level of the CpG methylation of LGALS7 through metabolic reprogramming in AGS cells. In MKN7 cells, a lack of LGALS7 led to the function of the proto-oncogene ANGPTL4. However, AGS cells had increased LGALS7 levels, and ANGPTL4 promoted the expression of LGALS7 in AGS cells. This results in ANGPTL4 having a tumour suppressor effect on AGS cells. Therefore, the presence of LGALS7 is one of the important factors leading to the contradictory roles of ANGPTL4 in GC.
The hedgehog signalling pathway, a significant molecular mechanism, is ectopically activated to drive poor prognosis in GC patients37. According to our above results (Fig. 3), it is crucial to confirm the potential functions of the ANGPTL4-LGALS7 axis in activating the hedgehog pathway in GC. Sun et al. reported that PPARγ enhanced the expression of multiple genes, including ANGPTL4, PLIN2, SQSTM1 and DDIT3, in human meibomian gland epithelial cells and is enriched in many pathways, such as the Hedgehog pathway38. We found that LGALS7 strongly repressed hedgehog pathway activation in AGS and MKN7 cells. These results indicated that LGALS7 is a significant regulator downstream of ANGPTL4 for the activation of hedgehog.
In conclusion, this study revealed the role of the ANGPTL4-LGALS7 axis in hedgehog pathway activation for GC progression. Compared to previous studies21,30,31,32, our study partly elucidated the contradictory effect of ANGPTL4 on GC progression. The absence of LGALS7 is an important factor that determines the role of ANGPTL4 in the occurrence and development of GC. Thus, our results may be useful both clinically and practically.
Conclusion
LGALS7 is a key factor involved in the contradictory effects of ANGPTL4 on activating the hedgehog pathway to drive GC progression, including proliferation, migration, invasion, apoptosis escape, angiogenesis and lymphangiogenesis.
Data availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
References
Rawla, P. & Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz. Gastroenterol. 14, 26–38 (2019).
Fock, K. M. Review article: The epidemiology and prevention of gastric cancer. Aliment Pharmacol. Ther. 40, 250–260 (2014).
Fernández-Hernando, C. & Suárez, Y. ANGPTL4: A multifunctional protein involved in metabolism and vascular homeostasis. Curr. Opin. Hematol. 27, 206–213 (2020).
Yang, W. H. et al. A TAZ-ANGPTL4-NOX2 axis regulates ferroptotic cell death and chemoresistance in epithelial ovarian cancer. Mol. Cancer Res. 18, 79–90 (2020).
Okochi-Takada, E. et al. ANGPTL4 is a secreted tumor suppressor that inhibits angiogenesis. Oncogene 33, 2273–2278 (2014).
Avalle, L. et al. STAT3 induces breast cancer growth via ANGPTL4, MMP13 and STC1 secretion by cancer associated fibroblasts. Oncogene 41, 1456–1467 (2022).
Zheng, X. et al. ANGPTL4-mediated promotion of glycolysis facilitates the colonization of Fusobacterium nucleatum in colorectal cancer. Cancer Res. 81, 6157–6170 (2021).
Zhang, Y. et al. Exosomal protein angiopoietin-like 4 mediated radioresistance of lung cancer by inhibiting ferroptosis under hypoxic microenvironment. Br. J. Cancer 127, 1760–1772 (2022).
Baba, K. et al. Hypoxia-induced ANGPTL4 sustains tumour growth and anoikis resistance through different mechanisms in scirrhous gastric cancer cell lines. Sci. Rep. 7, 11127 (2017).
Bajwa, P. et al. Cancer-associated mesothelial cell-derived ANGPTL4 and STC1 promote the early steps of ovarian cancer metastasis. JCI Insight 8, e163019 (2023).
Kaur, M., Kaur, T., Kamboj, S. S. & Singh, J. Roles of galectin-7 in cancer. Asian Pac. J. Cancer Prev. 17, 455–461 (2016).
St-Pierre, Y., Campion, C. G. & Grosset, A. A. A distinctive role for galectin-7 in cancer?. Front. Biosci. (Landmark Ed) 17, 438–450 (2012).
Saussez, S. & Kiss, R. Galectin-7. Cell. Mol. Life Sci. 63, 686–697 (2006).
Kim, S. J., Hwang, J. A., Ro, J. Y., Lee, Y. S. & Chun, K. H. Galectin-7 is epigenetically-regulated tumor suppressor in gastric cancer. Oncotarget 4, 1461–1471 (2013).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Kanehisa, M. Toward understanding the origin and evolution of cellular organisms. Protein Sci. 28, 1947–1951 (2019).
Kanehisa, M., Furumichi, M., Sato, Y., Kawashima, M. & Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 51, D587–D592 (2023).
Li, Y. K. et al. Validation of ESM1 related to ovarian cancer and the biological function and prognostic significance. Int. J. Biol. Sci. 19, 258–280 (2023).
Zeng, Y., Ren, M., Li, Y., Liu, Y. & Su, Q. Knockdown of RhoGDI2 represses human gastric cancer cell proliferation, invasion and drug resistance via the Rac1/Pak1/LIMK1 pathway. Cancer Lett. 492, 136–146 (2020).
Zhang, J. et al. Identification of bromodomain-containing proteins prognostic value and expression significance based on a genomic landscape analysis of ovarian serous cystadenocarcinoma. Front. Oncol. 12, 1021558 (2022).
Nakayama, T. et al. Expression of angiopoietin-like 4 in human gastric cancer: ANGPTL4 promotes venous invasion. Oncol. Rep. 24, 599–606 (2010).
Akyala, A. I. & Peppelenbosch, M. P. Gastric cancer and Hedgehog signaling pathway: Emerging new paradigms. Genes Cancer 9, 1–10 (2018).
Tobler, N. E. & Detmar, M. Tumor and lymph node lymphangiogenesis–impact on cancer metastasis. J. Leukoc. Biol. 80, 691–696 (2006).
Xu, S. et al. STAT2-induced linc02231 promotes tumorigenesis and angiogenesis through modulation of hnRNPA1/ANGPTL4 in colorectal cancer. J. Gene Med. 8, e3506 (2023).
Xiao, S. et al. ANGPTL4 regulate glutamine metabolism and fatty acid oxidation in nonsmall cell lung cancer cells. J. Cell. Mol. Med. 26, 1876–1885 (2022).
Shen, C. J. et al. Oleic acid-induced NOX4 is dependent on ANGPTL4 expression to promote human colorectal cancer metastasis. Theranostics 10, 7083–7099 (2020).
Yang, L. et al. ANGPTL4 promotes the proliferation of papillary thyroid cancer via AKT pathway. Onco Targets Ther. 13, 2299–2309 (2020).
Nakayama, T. et al. Expression of angiopoietin-like 4 (ANGPTL4) in human colorectal cancer: ANGPTL4 promotes venous invasion and distant metastasis. Oncol. Rep. 25, 929–935 (2011).
Lin, S. et al. ANGPTL4 negatively regulates the progression of osteosarcoma by remodeling branched-chain amino acid metabolism. Cell Death Discov. 8, 225 (2022).
Qian, P. et al. LMX1A inhibits C-Myc expression through ANGPTL4 to exert tumor suppressive role in gastric cancer. PLoS One 14, e0221640 (2019).
Kubo, H. et al. Regulation and clinical significance of the hypoxia-induced expression of ANGPTL4 in gastric cancer. Oncol. Lett. 11, 1026–1034 (2016).
Chen, J. W., Luo, Y. J., Yang, Z. F., Wen, L. Q. & Huang, L. Knockdown of angiopoietin-like 4 inhibits the development of human gastric cancer. Oncol. Rep. 39, 1739–1746 (2018).
Park, J. G. et al. Characteristics of cell lines established from human gastric carcinoma. Cancer Res. 50, 2773–2780 (1990).
Motoyama, T., Hojo, H. & Watanabe, H. Comparison of seven cell lines derived from human gastric carcinomas. Acta Pathol. Japonica 36, 65–83 (1986).
Li, Y. K. et al. ANGPTL4 accelerates ovarian serous cystadenocarcinoma carcinogenesis and angiogenesis in the tumor microenvironment by activating the JAK2/STAT3 pathway and interacting with ESM1. J. Transl. Med. 22, 46 (2024).
Xu, X. et al. Metabolic reprogramming and epigenetic modifications in cancer: From the impacts and mechanisms to the treatment potential. Exp. Mol. Med. 55, 1357–1370 (2023).
Katoh, Y. & Katoh, M. Hedgehog signaling pathway and gastric cancer. Cancer Biol. Ther. 4, 1050–1054 (2005).
Kim, S. W., Brown, D. J. & Jester, J. V. Transcriptome analysis after PPARγ activation in human meibomian gland epithelial cells (hMGEC). Ocul. Surf. 17, 809–816 (2019).
Funding
This work is supported by the Key project of Hunan Provincial Department of Education (22A0317) and the Undergraduate research learning and innovative experimental 2019 project (Grant No. X2019155).
Author information
Authors and Affiliations
Contributions
J Xie, J Zou, YK Li, and XY Lei conceived, designed, and supervised the study. TY Fan, GQ Shi, HG Shan and WC Zhou collected and analyzed clinical human samples. J Xie, T Zeng and WC Zhou performed in vitro and in vivo experiments and analyzed the data. J Xie, J Zou and YK Li wrote the paper. All authors drafted the article and revised it critically for important intellectual content, and with final approval of the version submitted to the journal.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics statement
The Second Affiliated Hospital of the University of South China’s research ethics committee approved the study after receiving written informed consent from each patient. Human tissues were collected with Institutional Review Board (IRB) approval (#SAHUSC2023098) from the Second Affiliated Hospital of the University of South China. Experiments followed the guidelines and regulations of the IRB and all patients provided informed consent. All methods were carried out in accordance with relevant guidelines and regulations. All methods are reported in accordance with ARRIVE guidelines (https://arriveguidelines.org).
Patient consent for publication
After detailed patient education, all patients consented to the off-label use of the substance and publication of the results.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xie, J., Li, Y., Zeng, T. et al. ANGPTL4 plays a paradoxical role in gastric cancer through the LGALS7 and Hedgehog pathways. Sci Rep 14, 23173 (2024). https://doi.org/10.1038/s41598-024-71415-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71415-1
- Springer Nature Limited