Abstract
The gut microbiota metabolite trimethylamine-N-oxide (TMAO)—derived from dietary phosphatidylcholine—is mechanistically linked to cardiovascular disease (CVD) and increased cardiovascular risk. This study examined the relationship between fasting plasma TMAO levels and 5-year all-cause mortality in a cohort of patients at high risk of cardiovascular events (CORE-Thailand Registry). Of the 134 patients, 123 (92%) had established cardiovascular disease, and 11 (8%) had multiple risk factors. Fasting plasma TMAO levels were measured using nuclear magnetic resonance spectroscopy. Within this prospective cohort study, the median TMAO was 3.81 μM [interquartile range (IQR) 2.89–5.50 μM], with a mean age of 65 ± 11 years; 61% were men, and 39.6% had type II diabetes. Among 134 patients, 65 (49%) were identified as the high-TMAO group (≥ 3.8 μM), and 69 (51%) were identified as the low-TMAO group (< 3.8 μM). After a median follow-up of 58.8 months, the high-TMAO group was associated with a 2.88-fold increased mortality risk. Following adjustment for traditional risk factors, high-sensitivity cardiac troponin-T, estimated glomerular filtration rate, angiotensin-converting enzyme (ACEI), or angiotensin-receptor blocker (ARB) use, the high-TMAO group remained predictive of 5-year all-cause mortality risk (the high-TMAO vs. the low-TMAO group, adjusted hazard ratio 2.73, 95% CI 1.13–6.54; P = 0.025). Among Thai patients at high risk of cardiovascular events, increased plasma TMAO levels portended greater long-term mortality risk.
Similar content being viewed by others
Introduction
The incidence of Cardiovascular Disease (CVD) continues to increase, and it remains the leading cause of death worldwide1. Despite advances in medical treatment and aggressive risk factor modification, a decline in CVD-related mortality has been reported; however, the significant residual risk remains unacceptably high1. It is thus important to identify new proatherogenic biomarkers for predicting mortality, identifying poor prognosis in patients at high risk of cardiovascular (CV) events and potentially serving as targets for CVD prevention.
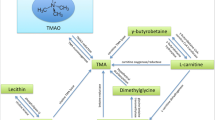
Increasing evidence supports the role of trimethylamine-N-oxide (TMAO)—a gut microbiota metabolite derived from dietary phosphatidylcholine (PC)—in the pathogenesis of CVD2,3,4. PC is a major dietary source of choline and carnitine, often found in red meat, egg yolks, and the liver5. The amount of dietary PC that lead to elevated plasma TMAO levels exhibits interindividual variability, based on factors such as gut microbiota composition, liver function, and overall diet. Generally, habitual consumption of foods high in PC, especially in combination with other factors that promote TMAO production (like low fiber intake or specific gut microbiota profiles), can contribute to higher TMAO levels6. The production of TMAO from dietary PC involves multiple steps. Gut bacteria play a crucial role by converting dietary PC components, such as choline, betaine, and carnitine, into trimethylamine (TMA). The TMA produced in the gut is absorbed into the bloodstream and transported to the liver, where it is oxidized by flavin-containing monooxygenase 3 (FMO3) enzymes to form TMAO. The role of gut microbiota in this process was confirmed in a study showing that plasma TMAO production during a dietary PC challenge was significantly reduced following suppression of the gut microbiota with broad-spectrum antibiotics5,7.
Elevated plasma TMAO levels have been associated with an increased risk of CV events, an independent predictor of subclinical myocardial damage, and a predictor of adverse CVD risk in several studies6,8,9,10,11,12,13.
However, the role of TMAO has limited data in Thai population and some conflicting data about prognification of TMAO in Asian heart failure patients14. The current study examined the relationship between fasting plasma TMAO levels and long-term mortality in Thai participants at high risk of CV events.
Subjects and methods
This was a multicenter, prospective, longitudinal cohort study of Thai patients with high atherosclerotic risk. Data were sourced from a cohort of patients at high risk of CV events recorded in the CORE-Thailand registry. The CORE-Thailand registry includes patients aged ≥ 45 years with established CVD (eCVD) as well as those with multiple risk factors (MRFs), as detailed in the previous publications15. Patients with eCVD included established coronary artery disease (CAD), stroke /transient ischemic attack (TIA), or peripheral artery disease (PAD) while patients with MRFs were defined as the presence of at least three atherosclerosis risk factors, including male > 55 years, female > 65 years, Type II diabetes mellitus (DM) or impaired fasting glucose, hypertension, dyslipidemia, chronic kidney disease (CKD) defined as presence of proteinuria + 1 or estimated glomerular filtration rate (eGFR) < 60 ml/min) and family history of premature CVD.
Our Registry had 9,390 patients. However, only 134 patients had TMAO data because only one center can do TMAO measurements. All-cause mortality at five years was tracked by electronic chart review and confirmed by telephone interviews, official hospital records, or death certificates. The median follow-up period was 58.8 months (IQR 46.7–59.9).
The Khon Kaen University Ethics Committee for Human Research approved the study protocol HE611011 and HE671143 per the Declaration of Helsinki.
Laboratory testing
After informed consent was obtained from all the participants, fasting blood samples were collected in EDTA tubes during the hospital visit. Samples were maintained at 4 °C, immediately processed, and frozen at -80 °C until analyzed. Routine laboratory tests were performed on samples using the Abbott Architec platform (Abbott Laboratories), and high-sensitivity cardiac troponin-T (hs-cTnT) was measured using a high-sensitivity (5th generation) assay on a Roche Cobas e411 platform (Roche Diagnostics, Basel, Switzerland). The eGFR was calculated using the diet modification in renal disease (MDRD) equation.
The plasma TMAO levels were determined using a nuclear magnetic resonance spectroscopy (NMR) spectrometer at 400 MHz (Bruker, USA). The Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence was employed to obtain spectra (recycle delay-90°-t1-90°-tm-90°-acquisition) over 64 scans with four dummy scans. Quantification was achieved using the concentration of a known reference signal (TSP) to determine the TMAO concentration. The NMR method to measure TMAO level was highly significant correlated with Mass spectrometry (MS) with R2 = 0.9816.
Statistical analysis
All statistical analyses were performed using SPSS version 28 (SPSS Inc., Chicago, IL, USA). Continuous data are presented as means (standard deviation) or medians (interquartile range) and compared using the Student’s t-test or non-parametric test (Mann–Whitney U Test), as appropriate. Categorical variables are presented as numbers (%) and were compared between the groups using the chi-square test. Based on previous studies8, using the median level of plasma TMAO in the studied population, a plasma TMAO ≥ 3.8 μM was defined as the high-TMAO group, and < 3.8 μM was defined as the low-TMAO group. Kaplan–Meier analysis with Cox proportional hazards regression was used for the time-to-event analysis to determine hazard ratios (HRs) and 95% CIs for 5-year all-cause mortality between the TMAO groups. Adjustments were made for individual traditional cardiovascular risk factors (age, male sex, body mass index, diabetes mellitus, dyslipidemia, hypertension, and smoking status), eCVD, eGFR, hs-cTnT and angiotensin-converting enzyme (ACEI) or angiotensin receptor blocker (ARB) use to predict all-cause mortality. Statistical significance was set at p < 0.05.
Results
Patient characteristics
The baseline characteristics of our study cohort were stratified according to the all-cause death status (Table 1). Overall, the mean age of the patients was 64 years; 61% were men. The median TMAO was 3.81 μM [interquartile range (IQR) 2.89–5.50]. Patients who died were older, received less beta blocker and had higher hs-cTnT level.
Baseline fasting plasma TMAO levels and 5-year all-cause mortality
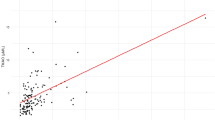
During the median follow-up duration of 58.8 months (IQR 46.7–59.9), 33 (24.6%) deaths occurred in our study cohort. Interestingly, the TMAO levels were significantly higher in patients who died than who survived [4.72 μM (3.39–8.31) vs. 3.58 μM (2.72–4.71), P = 0.004] (Fig. 1).
Figure 2 represents the Kaplan–Meier analysis of TMAO stratified by low (< 3.8 μM) or high-TMAO (≥ 3.8 μM) group, which illustrates a significantly increased risk for all-cause mortality in the high-TMAO group (log-rank, p = 0.007). Importantly, the high-TMAO group was associated with a significant 2.88-fold increased risk of all-cause mortality compared with the low-TMAO group (unadjusted HR 2.88, 95% CI 1.34–6.25, P = 0.007). The significant prognostic value in the high-TMAO group was preserved when adjusted for traditional risk factors (age, male sex, body mass index, diabetes mellitus, dyslipidemia, hypertension, and smoking status) and eCVD, eGFR, hs-cTnT , and ACEI or ARB use (adjusted HR 2.73, 95% CI 1.13–6.54, P = 0.025) (Table 2 and Table S1). In addition, after beta blocker use was added as a covariate, high-TMAO level remained an independent predictor of adverse cardiovascular events (adjusted HR 2.595, 95% CI 1.09–5.13, p = 0.03) (Table S2).
Discussion
Our study demonstrated for the first time, that elevated fasting plasma TMAO levels at baseline provided strong prognostic information about 5-year all-cause mortality in Thai participants at high risk of cardiovascular events from the CORE-Thailand registry. The strong prognostic value was independent of traditional risk factors (age, male sex, body mass index, diabetes mellitus, dyslipidemia, hypertension, and smoking status), cardioprotective medication history (ACEI/ARB), marker of myocardial injury (hs-cTnT), and CVD status. The death rate was significantly higher in patients with higher baseline TMAO levels. The present findings support the incremental prognostic value of TMAO in stable patients at high risk of CV events (92% with eEAD and 8% with MRFs), which provides the opportunity to develop novel therapeutic strategies that target specific pathways of TMAO production and long-term prognosis.
The role of TMAO, a gut microbiota-generated metabolite, plays a vital role in human host metabolism and contributes to associated increased risk of diabetes, obesity, CKD, atherosclerosis, and long-term CVD morbidity and mortality3,6,12,13,17,18,19. These prognosis effects of elevated plasma TMAO were consistent with recent data from several clinical trials in subsets of patients with CAD, PAD, type II DM, CKD, history of heart failure, patients with suspected acute coronary syndrome initially negative for cTnT, and obesity 3,6,7,11,12,20,21. A study of 1,216 patients with type II DM who underwent elective coronary artery catheterization significantly increased the risk of 5-year mortality by up to 2.07-fold after adjustments for traditional risk factors and glycemic control17. Furthermore, elevated plasma TMAO levels can predict the presence of a high coronary atherosclerosis burden and a significant predictor of subclinical myocardial damage12,13,19. These findings are independent of traditional risk factors and other potentially confounding factors.
In the present study cohort, we defined TMAO ≥ 3.8 μM as the high-TMAO group because previous findings from independent study cohorts and meta-analyses showed that dose–response association between TMAO plasma levels and all-cause mortality begin at 3.8 μM8,10. Interestingly, elevated TMAO levels in the high-TMAO group vs. the low-TMAO group are a robust long-term prognostic predictor among patients at a high risk of CV events. Moreover, despite TMAO being cleared by the kidney, dose-dependent elevation in TMAO is noted in patients with CKD22. However, patients in the high-TMAO group remained an independent predictor of poor prognosis, even after adjusting for eGFR. Therefore, it is conceivable that significant residual CV risk may occur in patients with elevated plasma TMAO levels.
The mechanism of the relationship between TMAO and the increased risk of atherosclerosis and prediction of prognosis outcomes has been extensively investigated in real-world patients. Dietary nutrients that are rich in PC, the major dietary source of choline and carnitine (such as red meat), serve as precursors for TMA, which is converted into TMAO by FMO323. Several recent studies have demonstrated that FMO3 is an essential regulator of sterol and tissue cholesterol metabolism, prevents reverse cholesterol transport, and correlates with atherosclerotic plaque size in the arterial wall, which is linked to the development of atherosclerosis 5,23. In contrast, FMO3 knockdown mice showed decreased circulating TMAO levels and attenuated atherosclerotic plaque formation24. Interestingly, recent studies have found that vegans and vegetarians have lower plasma TMAO levels and fecal TMA/TMAO capacities than omnivores5. Significantly, diets rich in red meat or long-term exposure to oral L-carnitine are associated with higher plasma TMAO and TMA/TMAO-generating capacities in humans25,26. Moreover, TMAO has been shown to have direct biological activity in enhancing platelet hyperactivity, which increases the risk of thrombus formation and poor prognosis 27,28.
Thai cuisine typically features a combination of fresh herbs, spices, and aromatic ingredients such as lemongrass. In contrast, Western diets often include a wider use of dairy, wheat-based products, and a focus on individual flavors rather than complex combinations. However, in the urbanization era the diet pattern has been changed to westernized pattern29. The TMAO level in our study was in the same range with the western30.Our findings suggest that measurement of plasma TMAO levels among these high-risk patients should help identify those with residual CV risk, improve our understanding of the link between gut microbiota, TMAO, and long-term prognosis, and help select patients at a high risk of CV events who need more aggressive and specific interventions. Further studies are required to determine whether decreased TMAO with intervention, such as medication or probiotics, can improve the prognosis in patients at a high risk of CV events. Additionally, tissue concentrations of TMA and TMAO may differ from plasma levels, indicating the variable significance of the TMAO pathway across different organs and diseases31. Further studies are warranted.
Study limitations
This study recruited patients at high risk of CV events, mainly from tertiary care centers, and recruited only patients who had TMAO data; therefore, there was a higher proportion of patients with eCVD, and we cannot exclude the selection bias. As plasma TMAO was only analyzed once, we were unable to assess the prognostic significance of fluctuations in TMAO levels over time. The diet pattern such as amount of red meat which may associate with plasma TMAO level was not recorded. Despite these limitations, our findings provide a mechanistic link and strong prognostic information about 5-year all-cause mortality.
Conclusions
Elevated fasting plasma TMAO is an independent predictor of increased long-term mortality risk among patients at high risk of CV events.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- CAD:
-
Coronary artery disease
- CVD:
-
Cardiovascular disease
- TMAO:
-
Trimethylamine-N-oxide
References
Tsao, C. W. et al. Heart Disease and Stroke Statistics-2023 update: A report from the American Heart Association. Circulation 147, e93–e621. https://doi.org/10.1161/cir.0000000000001123 (2023).
Jonsson, A. L. & Bäckhed, F. Role of gut microbiota in atherosclerosis. Nat. Rev. Cardiol. 14, 79–87. https://doi.org/10.1038/nrcardio.2016.183 (2017).
Kanitsoraphan, C., Rattanawong, P., Charoensri, S. & Senthong, V. Trimethylamine N-oxide and risk of cardiovascular disease and mortality. Curr. Nutr. Rep. 7, 207–213. https://doi.org/10.1007/s13668-018-0252-z (2018).
Wang, M. et al. Trimethylamine N-oxide is associated with long-term mortality risk: The multi-ethnic study of atherosclerosis. Eur. Heart J. 44, 1608–1618. https://doi.org/10.1093/eurheartj/ehad089 (2023).
Koeth, R. A. et al. Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 19, 576–585. https://doi.org/10.1038/nm.3145 (2013).
Tang, W. H. W., Bäckhed, F., Landmesser, U. & Hazen, S. L. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J. Am. Coll. Cardiol. 73, 2089–2105. https://doi.org/10.1016/j.jacc.2019.03.024 (2019).
Tang, W. H. et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Eng. J. Med. 368, 1575–1584. https://doi.org/10.1056/NEJMoa1109400 (2013).
Heianza, Y., Ma, W., Manson, J. E., Rexrode, K. M. & Qi, L. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: A systematic review and meta-analysis of prospective studies. J. Am. Heart Assoc. https://doi.org/10.1161/jaha.116.004947 (2017).
Qi, J. et al. Circulating trimethylamine N-oxide and the risk of cardiovascular diseases: A systematic review and meta-analysis of 11 prospective cohort studies. J. Cell Mol. Med. 22, 185–194. https://doi.org/10.1111/jcmm.13307 (2018).
Schiattarella, G. G. et al. Gut microbe-generated metabolite trimethylamine-N-oxide as cardiovascular risk biomarker: A systematic review and dose-response meta-analysis. Eur. Heart J. 38, 2948–2956. https://doi.org/10.1093/eurheartj/ehx342 (2017).
Senthong, V. et al. Trimethylamine N-oxide and mortality risk in patients with peripheral artery disease. J. Am. Heart Assoc. https://doi.org/10.1161/jaha.116.004237 (2016).
Senthong, V. et al. Intestinal microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: The contributory role of intestinal microbiota in a COURAGE-like patient cohort. J. Am. Heart Assoc. https://doi.org/10.1161/jaha.115.002816 (2016).
Senthong, V. et al. Gut microbiota-generated metabolite, trimethylamine-N-oxide, and subclinical myocardial damage: A multicenter study from Thailand. Sci. Rep. 11, 14963. https://doi.org/10.1038/s41598-021-93803-7 (2021).
Yazaki, Y. et al. Ethnic differences in association of outcomes with trimethylamine N-oxide in acute heart failure patients. ESC Heart Fail. 7, 2373–2378. https://doi.org/10.1002/ehf2.12777 (2020).
Phrommintikul, A. et al. Management of atherosclerosis risk factors for patients at high cardiovascular risk in real-world practice: A multicentre study. Singap. Med. J. 58, 535–542. https://doi.org/10.11622/smedj.2017044 (2017).
Garcia, E. et al. NMR quantification of trimethylamine-N-oxide in human serum and plasma in the clinical laboratory setting. Clin. Biochem. 50, 947–955. https://doi.org/10.1016/j.clinbiochem.2017.06.003 (2017).
Tang, W. H. et al. Increased trimethylamine N-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin. Chem. 63, 297–306. https://doi.org/10.1373/clinchem.2016.263640 (2017).
Gruppen, E. G. et al. TMAO is associated with mortality: impact of modestly impaired renal function. Sci. Rep. 7, 13781. https://doi.org/10.1038/s41598-017-13739-9 (2017).
Senthong, V. et al. Plasma trimethylamine N-oxide, a gut microbe-generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J. Am. Coll. Cardiol. 67, 2620–2628. https://doi.org/10.1016/j.jacc.2016.03.546 (2016).
Wang, Z. et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63. https://doi.org/10.1038/nature09922 (2011).
Trøseid, M. et al. Microbiota-dependent metabolite trimethylamine-N-oxide is associated with disease severity and survival of patients with chronic heart failure. J. Intern. Med. 277, 717–726. https://doi.org/10.1111/joim.12328 (2015).
Tang, W. H. et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Cir. Res. 116, 448–455. https://doi.org/10.1161/circresaha.116.305360 (2015).
Warrier, M. et al. The TMAO-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 10, 326–338. https://doi.org/10.1016/j.celrep.2014.12.036 (2015).
Shih, D. M. et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J. Lipid Res. 56, 22–37. https://doi.org/10.1194/jlr.M051680 (2015).
Fukami, K. et al. Oral l-carnitine supplementation increases trimethylamine-N-oxide but reduces markers of vascular injury in hemodialysis patients. J. Cardio Pharmacol. 65, 289–295. https://doi.org/10.1097/fjc.0000000000000197 (2015).
Miller, M. J. et al. Chronic oral l-carnitine supplementation drives marked plasma TMAO elevations in patients with organic acidemias despite dietary meat restrictions. JIMD Rep. 30, 39–44. https://doi.org/10.1007/8904_2016_539 (2016).
Li, X. S. et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 38, 814–824. https://doi.org/10.1093/eurheartj/ehw582 (2017).
Zhu, W. et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 165, 111–124. https://doi.org/10.1016/j.cell.2016.02.011 (2016).
Papier, K. et al. Social demography of transitional dietary patterns in Thailand: Prospective evidence from the Thai Cohort Study. Nutrients https://doi.org/10.3390/nu9111173 (2017).
Heianza, Y. et al. Long-term changes in gut microbial metabolite trimethylamine N-oxide and coronary heart disease risk. J. Am. Coll. Cardiol. 75, 763–772. https://doi.org/10.1016/j.jacc.2019.11.060 (2020).
Maksymiuk, K. M. et al. Mice, rats, and guinea pigs differ in FMOs expression and tissue concentration of TMAO, a gut bacteria-derived biomarker of cardiovascular and metabolic diseases. PloS One 19, e0297474. https://doi.org/10.1371/journal.pone.0297474 (2024).
Acknowledgements
We thank Mr. Bryan Roderick Hamman—under aegis of the Publication Clinic KKU, Thailand—for assistance with the English-language presentation of the manuscript.
Funding
This research was supported by the Heart Association of Thailand under the Royal Patronage of H.M. the King, National Research Council of Thailand and the Fundamental Fund of Khon Kaen University which has received funding support from the National Science, Research and Innovation Fund, Thailand (project number 67A103000113).
Author information
Authors and Affiliations
Consortia
Contributions
V.S. study conception and design, data collection, helped to run TMAO levels, analysis and interpretation of result, wrote the main manuscript and manuscript submitted. A.P. study conception and design, interpretation of result, revise manuscript, critical reading and final approval S.K., C.W. and P.S. critical reading J.P. critical reading and run TMAO levels.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Senthong, V., Kiatchoosakun, S., Wongvipaporn, C. et al. Trimethylamine-N-oxide and 5-year mortality: the role of gut microbiota-generated metabolite from the CORE-Thailand cohort. Sci Rep 14, 21264 (2024). https://doi.org/10.1038/s41598-024-71479-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71479-z
- Springer Nature Limited