Abstract
Antiphospholipid syndrome (APS) is an autoimmune disorder characterized by vascular thrombosis and obstetric morbidity, with accurate laboratory examination of antiphospholipid antibodies (aPLs) being crucial for diagnosis. This study focused on anti-β2 glycoprotein I (aβ2GPI) antibodies and aimed to establish the first population-based cutoff values for aβ2GPI IgA/IgM/IgG antibodies in non-pregnant women of reproductive age in Southwest China. The study cohort comprised 181 healthy women of reproductive age for study. Blood samples were collected on an early morning fast. Anti-β2GPI antibodies including IgA, IgM and IgG were measured in serum using the HOB® BioCLIA kit. According to the Clinical and Laboratory Standards Institute (CLSI) guidelines, the study used non-parametric percentile methods to calculate the 95th, 97.5th, and 99th percentiles cutoff values for aβ2GPI IgA/IgM/IgG antibodies, along with corresponding 90% confidence intervals (CI), while excluding outliers. A total of 168 independent samples were collected for verification, including 85 samples from healthy subjects and 83 samples from APS patients, in order to evaluate the analytical performance of the obtained cutoff values. The 99th percentile cutoff values were 3.36 RU/mL for aβ2GPI IgA, 27.54 RU/mL for aβ2GPI IgM and 1.81 RU/mL for aβ2GPI IgG, which indicated that the levels of aβ2GPI IgM antibodies were generally higher compared to those of IgA and IgG antibodies. Our established reference range was confirmed to be successful in validating the detected values of aβ2GPI antibodies in all healthy controls. With the 99th percentile cutoff value, the sensitivity was 14.46% for aβ2GPI IgA, 22.89% for aβ2GPI IgG, and 9.64% for aβ2GPI IgM in APS patients. This study established population-based cutoff values that are applicable to the local population for the accurate laboratory examination of aβ2GPI antibodies in non-pregnant women of reproductive age. The study also recommends paying more attention to IgM positivity in women of reproductive age.
Similar content being viewed by others
Introduction
Antiphospholipid syndrome (APS) is an autoimmune disorder that is characterized by vascular thrombosis and/or obstetric morbidity in the presence of increased levels of antiphospholipid antibodies (aPLs). According to the the Sydney revised Sapporo criteria1, the diagnosis of APS requires the presence of at least one clinical criterion and one laboratory criterion, with at least one of the three aPLs positives separated by a 12 week interval. As per the updated 2023 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) APS classification criteria, it is recommended that confirmation of aPLs positivity should be carried out within a timeframe of around three years from the documented clinical criterion, as indicated by medical records2. The three antibodies include lupus anticoagulant (LAC), anticardiolipin (aCL) IgG/IgM antibodies, and anti-β2 glycoprotein I (aβ2GPI) IgG/IgM antibodies3. In addition to thrombosis and obstetric morbidity, the clinical symptoms of APS may include livedo reticularis, thrombocytopenia, kidney disease, heart valve disease, and neurological manifestations such as stroke4,5. However, due to the non-specific nature of these clinical manifestations, laboratory testings for aPLs are particularly important for the accurate diagnosis of APS.
Accurate laboratory examination of aPLs is crucial, but the current level of standardization is poor, and there are several issues such as calibration and quantification methods, cutoff values, expression of results and interpretation of reports that need to be addressed3,6,7,8. The appropriate threshold for clinically relevant aPLs levels for the diagnosis of APS is still controversial, and it is recommended that each laboratory establishes an in-house cutoff value based on its own local reagents/instruments. The diagnosis of APS primarily relies on laboratory results, which classify individuals as either positive or negative based on the cut-off values obtained from testing methods for aPLs. According to the classification criteria for definitive APS, the positivity level for aPLs is determined by a cut-off value exceeding the 99th percentile for both aCL and aβ2GPI. The most recent classification criteria indicates that any solid phase aPLs platform can be utilized for routine aPLs testing in hospitals and clinics. Over the past decade, there has been a notable shift towards utilizing solid phase assays on automated platforms, such as fluorescence enzyme immunoassay and chemiluminescence immunoassay (CLIA). These automated assays have gained preference among laboratories over manual enzyme-linked immunoassay (ELISA) due to their enhanced precision and high level of automation9. Nonetheless, it is crucial to note that the diagnostic performance of automated platforms heavily depends on the selection of appropriate cut-off values.
Among the three autoantibodies in the APS classification criteria, aβ2GPI antibodies are associated with the dysfunction of endothelial cells and monocytes and is involved in the pathogenesis of APS10. In vivo experimental animal models have provided substantial evidence supporting the pathogenic role of aβ2GPI antibodies in thrombosis and fetal loss11. Clinical associations have also revealed that aβ2GPI may correlate strongly with thrombotic risk and pregnancy morbidity in patients with APS12. Hence, our primary focus in this study revolves around aβ2GPI.
Establishing dependable cut-off values for aPLs in the diagnosis of APS is of utmost importance. The accurate determination of these values is crucial, as both over-diagnosis and under-diagnosis can lead to significant consequences for patients who are provisionally diagnosed with APS. Since there are limited reports on reference intervals for aβ2GPI measured by CLIA, we conducted a statistical analysis on the cutoff values of aβ2GPI. Due to the potential for severe adverse pregnancy outcomes associated with APS, including preembryonic or embryonic loss, fetal death, preeclampsia and placental insufficiency with severe features, our study primarily focuses on non-pregnant women of reproductive age in Southwest China2. The determination of aβ2GPI IgA, despite not incorporated in the current laboratory criteria for APS, is clinically significant in patients with suspected APS and negative results from other aPLs13. Therefore, the aim of this study is to establish the first population-based cutoff values for aβ2GPI IgA, IgM and IgG antibodies in this region.
Materials and methods
Study cohort
This study cohort consists of a study group and a verification group. The selected population specifically consisted of women of reproductive age, ranging from 15 to 49 years old, which is identified by World Health Organization14. The study group includes 181 healthy women of reproductive age (age range: 15–42 years) between August 2022 and March 2023, at West China Second University Hospital, Sichuan University. None of these women had a clinical history of infectious, neoplastic, hematologic, or rheumatic diseases, including clinical/immunological manifestations of APS. Regarding the selection of healthy volunteers, the exclusion criteria employed during the recruitment process adhered to the Asian project15. All blood samples were collected after informed consent. In addition, 168 independent samples (age range: 21–43 years) were collected for verification between August 2022 and September 2023, including 85 samples from healthy female controls and 83 samples from APS female patients, in order to evaluate the analytical performance of the obtained cutoff values. Samples from APS patients with symptoms such as thrombosis or pregnancy morbidity were included for aPLs testing to calculate the prevalence of each antibody. The diagnosis of APS was made according to the classification criteria for APS1. The study follows the tenets of the declaration of Helsinki. This study was subject to approval by the Ethics Committee of West China Second University Hospital.
Blood sampling
The blood samples for aβ2GPI antibodies were processed and analyzed in accordance with the recommendations outlined in the International Society on Thrombosis and Hemostasis (ISTH) guideline13. Blood samples were collected in 5 mL vacutainer tubes (BD Biosciences, San Diego, CA, USA) without anticoagulants on an early morning fast. Each participant was instructed to fast for a minimum of 8 h before the sample collection. Serum was separated from 5 mL of peripheral blood by centrifugation at room temperature within 30 min of collection. Serum was stored frozen at − 20 ℃ until analysis.
Laboratory analysis
Anti-β2GPI antibodies including IgA, IgM and IgG were measured in serum using the HOB® BioCLIA kit on the SMART 6500 automatic chemiluminescent immunoassay analyzer. Since there is no international recognized standard for aβ2GPI, the results are reported in relative units (RU/mL). The cutoff value was defined as 20 RU/mL as recommended by the manufacturer. Prior to conducting the test, the performance of the kit and the instrument has been verified. This verification process included evaluating parameters such as the detection limit, reference limits, precision, repeatability, linearity, coefficient of variation, as well as internal and external quality control measures, in accordance with the quality requirements outlined by the International Organization for Standardization (ISO) 15189 for medical laboratories16. Negative and positive internal quality controls were included in each run to ensure accuracy and reliability of the results.
Statistical analysis
To determine the cutoff value, it is recommended by the ISTH guideline to use the 99th percentile value based on a healthy volunteer population, with a minimum sample size of 12017,18. As a result, the cutoff value may vary among different laboratories from the one recommended by the manufacturer19. According to the Clinical and Laboratory Standards Institute (CLSI) EP28-A3 guidelines, non-parametric percentile methods should be used to calculate the 95th, 97.5th, and 99th percentiles17.
Normality was assessed using the Shapiro–Wilk test. Data having a skewed distribution were reported as the median and the interquartile range (25th–75th percentiles). To remove potential outliers, we followed the CLSI guideline and used the block procedure. All statistical analyses and graphs were performed using Microsoft Excel and GraphPad Prism9. The study cohort participants were divided into four age groups: Age1 (15–20 years), Age2 (21–30 years), and Age3 (31–42 years). The non-parametric Kruskal–Wallis test was utilized to compare the levels of aβ2GPI antibodies among different age groups. The receiver operator characteristic (ROC) curve analysis was generated to evaluate areas under the ROC curve (AUC) with a 95% confidence interval (CI). A P-value less than 0.05 was considered statistically significant.
Results
The study cohort was divided into a study group and a verification group. The study group consisted of 181 healthy women of reproductive age (median age 29, range 15–42 years) used for establishing the cutoff values. The verification group comprised 85 healthy female controls (median age 31, range 21–41 years) and 83 APS female patients (median age 30, range 23–43 years) used for validating the cutoff values.
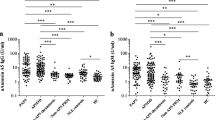
The study group participants were divided into four age groups: Age1 (15–20 years, n = 16), Age2 (21–30 years, n = 84), and Age3 (31–42 years, n = 81). Table 1 displayed the median and quantiles for aβ2GPI antibodies across different age subgroups. There were no statistically significant differences in the levels of aβ2GPI IgA/IgM/IgG antibodies between different age groups (P > 0.05) as shown in Fig. S1. Consequently, data from all age groups were combined to establish the cutoff value for the antibodies.
According to the results of normality analysis, it was observed that the distribution of aβ2GPI IgA/IgM/IgG antibodies in the study group was non-Gaussian. Due to clustering of low values at the bottom of the distribution, not all datasets could be normalized. The datasets for aβ2GPI IgG and IgM still exhibited a skewed distribution after a logarithmic transformation. Therefore, potential outliers were identified by the ‘block procedure’ as suggested in the CLSI guideline.
The block procedure method was utilized to remove outliers, resulting in the exclusion of only one outlier (55.23RU/mL) from the aβ2GPI IgM dataset, despite one or two other values being visually identified as outliers. Therefore, by combining visual inspection with the block procedure, two additional outliers (46.51 and 34.13 RU/mL) were identified and removed. The number of outliers excluded from aβ2GPI IgA and IgG was 1 and 1, respectively.
After excluding outliers, the non-parametric method was used to calculate the 95th, 97.5th, and 99th percentiles of aβ2GPI IgA/IgM/IgG antibodies, along with their corresponding 90% confidence intervals (CI), as shown in Table 2. The 99th percentile cutoff values were 3.36 RU/mL for aβ2GPI IgA, 27.54 RU/mL for aβ2GPI IgM and 1.81 RU/mL for aβ2GPI IgG.
Table 3 demonstrated that the detected values of aβ2GPI antibodies in all healthy controls were within our established reference range, confirming successful verification and achieving a specificity of 100% for APS diagnosis.
Table 4 displayed the prevalence of aPLs testing in the APS samples (n = 83). The data was presented as the number and percentage of positive samples, determined by the 95th, 97th, and 99th cut-off values established in this study, as well as the cut-off values suggested by the manufacturer. With the 99th percentile cutoff value, the sensitivities were 14.46% for aβ2GPI IgA, 22.89% for aβ2GPI IgG, and 9.64% for aβ2GPI IgM in APS patients. With the manufacturer’s cutoff value, the sensitivity was 1.20% for aβ2GPI IgA, 4.82% for aβ2GPI IgG, and 12.05% for aβ2GPI IgM in APS patients. In the verification group, the ROC analysis revealed the following AUC values for distinguishing patients with APS with healthy controls: 0.881 (95% CI 0.831–0.931) for aβ2GPI IgA, 0.967 (95% CI 0.942–0.991) for aβ2GPI IgG, and 0.719 (95% CI 0.643–0.795) for aβ2GPI IgM (all P values < 0.0001).
Discussion
To our knowledge, this is the first study to establish cutoff values for aβ2GPI antibodies in women of reproductive age in Southwest China.
In our study, we observed that the levels of aβ2GPI IgM antibodies were generally higher than those of IgA and IgG antibodies, which is pertinent to our study population. This finding is consistent with the known fact that the IgM isotype is important in obstetric APS: it was reported that isolated aPLs IgM positivity is rare in thrombotic APS, but more common in obstetric APS20; study has found that aβ2GPI antibodies, especially of IgM isotype, are frequent in pure obstetrical APS21. Even though IgG antiphospholipid antibodies have a higher weighted score (5–7) compared to IgM (1) for the diagnosis of APS based on the new criteria2, this disparity could be attributed to population differentiation. Thus, it is recommended to pay more attention to IgM positivity in women of reproductive age in the future.
As recommended by Sydney revised Sapporo criteria1 and the ISTH guidelines13, the 99th percentile of antibody levels is recommended as the cutoff value for the diagnosis of APS. The population-based 99th percentile cutoff values for aβ2GPI IgA and IgG were much lower than the manufacturer’s recommended cutoff values (20 RU/mL), while for aβ2GPI IgM, values exceeded the manufacturer's recommended cutoff values. Numerous studies have examined the calculation of aPLs cutoff values, utilizing different methodologies and manufacturers, making the corresponding aPLs units non-comparable. Most of these studies have consistently demonstrated that in-house cutoff values tend to be higher than the manufacturer's recommended cutoff values, as observed in our analysis of aβ2GPI IgM22,23. Notably, two studies conducted in Germany and Italy have reported that the in-house 99th percentile cutoff values are lower than the manufacturer’s recommended cutoff values9,24. These variations can be attributed to factors such as the number of healthy controls selected for cutoff value calculation and the racial composition of the study population.
Vanoverschelde et al.23 sent the questionnaire to the scientific and standardization committee on lupus anticoagulant/antiphospholipid antibodies (SSC-aPL) members and participants of the “lupus program”, and they reported that 41.1% of the laboratories calculated in-house cutoff values. The majority of laboratories did not establish their in-house cutoff values due to considerations of the cost and availability of recruiting healthy volunteers. According to the CLSI guidelines, it is recommended to recruit a minimum of 120 local healthy volunteers for the establishment of the cutoff values and 20 healthy volunteers for the verification of the cutoff values17. In the present study, the population-based cutoff values were established in 181 healthy controls, enabling the calculation of corresponding 90% confidence intervals for antibodies according to the recommendations in the CLSI guidelines.
In addition, we evaluated the sensitivity and specificity of aβ2GPI IgA/IgM/IgG in 85 healthy controls and 83 APS patients using both the manufacturer’s and locally calculated 99th percentiles as cutoff values. As expected, using a higher cutoff value, particularly for aβ2GPI IgM, decreased the positivity rate of antibodies in the APS patient cohort. However, our study may have been subject to bias in terms of the number and types of populations that were selected. The cutoff values for aβ2GPI IgA and IgG were considerably lower than those recommended by the manufacturer. This led to a statistically inevitable increase in sensitivity, but it is important to note that the results should be interpreted with caution.
Our previous findings showed that the isolated positive rate of aβ2GPI IgM was significantly higher than that of IgG and IgA (unpublished data). Based on our analysis, it’s possible that the cutoff values provided by the manufacturer may not be appropriate for our hospital’s population, as they were developed for all genders and age groups, whereas women of reproductive age constitute a unique demographic, such as the higher estrogen levels and the heavier social involvement. Therefore, we suspect that in previous tests, the use of manufacturer’s higher cutoff values for aβ2GPI IgA and IgG may have resulted in some varying degrees of false negatives and missed diagnoses. Nevertheless, given the relatively small size of our study population, more data is needed to validate this hypothesis. In summary, according to the Sydney revised Sapporo criteria1 for APS, it is recommended that each laboratory establish its own in-house cutoff values by testing a cohort of normal volunteers that represents the local population and applying the 99th percentile.
The study adhered strictly to CLSI guidelines regarding sample size and handling of outliers. Our study findings highlight the importance of excluding outliers prior to calculating cutoff values, as there is a huge difference in the results of the 99th percentile calculation before and after outlier removal. As suggested by CLSI EP28-A3 guideline, there are several available methods to detect outliers, such as Reed method, and Tukey method25,26. However, these methods have their limitations to some extent when applied to our data distribution. If there are two or three outliers located in the same tail of the distribution, such as in the aβ2GPI IgM dataset, the Reed method (one-third rule) may not accurately identify the most extreme outlier as statistically significant25. The Tukey method requires the data belonging to the Gaussian distribution27. Despite applying the Box-Cox transformation, we were unable to achieve a normal distribution for our results. Therefore, we used the block procedure combined the visual inspection to identify outliers in our population.
The strength of this study lies in the establishment of cutoff values for aβ2GPI antibodies in women of reproductive age in Southwest China for the first time. Due to limitations in the selection of populations, the results may differ significantly from those of the manufacturer. However, given that this hospital specializes women’s and children's healthcare, the vast majority of those who undergo aPLs testing are women of reproductive age. Therefore, we consider the establishment of a reference range applicable to our testing population in our own laboratory to be crucial. The age range of women of reproductive age in this study spans from 15 to 49 years, with the majority concentrated in the 20 s and 30 s. Individuals below 20 years and above 40 years are less represented within this cohort. Therefore, there may be a bias in the age grouping, which constitutes a limitation of this study.
As indicated by previous findings, a multicenter approach has been proposed as a more effective alternative for establishing cutoff values, which can enhance the accuracy of determining cutoff values by involving a larger number of healthy blood bank donors28. Achieving uniformity in the calculation of cutoff values is essential, as it holds the potential to improve and standardize the interpretation of test results, ultimately facilitating the complex diagnosis of APS. Consequently, future research efforts should be directed towards exploring these aspects further.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
References
Miyakis, S. et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J. Thromb. Haemost. 4(2), 295–306. https://doi.org/10.1111/j.1538-7836.2006.01753.x (2006).
Barbhaiya, M. et al. 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Ann. Rheum. Dis. 82(10), 1258–1270. https://doi.org/10.1136/ard-2023-224609 (2023).
Devreese, K. M. J., Ortel, T. L., Pengo, V. & de Laat, B. Laboratory criteria for antiphospholipid syndrome: Communication from the SSC of the ISTH. J. Thromb. Haemost. 16(4), 809–813. https://doi.org/10.1111/jth.13976 (2018).
Abreu, M. M. et al. The relevance of “non-criteria” clinical manifestations of antiphospholipid syndrome: 14th International congress on antiphospholipid antibodies technical task force report on antiphospholipid syndrome clinical features. Autoimmun. Rev. 14(5), 401–414. https://doi.org/10.1016/j.autrev.2015.01.002 (2015).
Bernardoff, I. et al. Antiphospholipid antibodies and the risk of autoimmune hemolytic anemia in patients with systemic lupus erythematosus: A systematic review and meta-analysis. Autoimmun. Rev. 21(1), 102913. https://doi.org/10.1016/j.autrev.2021.102913 (2022).
Devreese, K. M. Antiphospholipid antibody testing and standardization. Int. J. Lab. Hematol. 36(3), 352–363. https://doi.org/10.1111/ijlh.12234 (2014).
Favaloro, E. J. & Wong, R. C. Antiphospholipid antibody testing for the antiphospholipid syndrome: A comprehensive practical review including a synopsis of challenges and recent guidelines. Pathology 46(6), 481–495. https://doi.org/10.1097/pat.0000000000000142 (2014).
Favaloro, E. J. & Wong, R. C. Laboratory testing for the antiphospholipid syndrome: Making sense of antiphospholipid antibody assays. Clin. Chem. Lab. Med. 49(3), 447–461. https://doi.org/10.1515/cclm.2011.064 (2011).
Bor, M. V., Jacobsen, I. S., Gram, J. B. & Sidelmann, J. J. Revisiting the Phadia/EliA cut-off values for anticardiolipin and anti-β2-glycoprotein I antibodies: A systematic evaluation according to the guidelines. Lupus 27(9), 1446–1454. https://doi.org/10.1177/0961203318776105 (2018).
Bai, A. β2-glycoprotein I and its antibodies involve in the pathogenesis of the antiphospholipid syndrome. Immunol. Lett. 186, 15–19. https://doi.org/10.1016/j.imlet.2017.03.013 (2017).
Meroni, P. L. et al. Role of anti-beta2 glycoprotein I antibodies in antiphospholipid syndrome: In vitro and in vivo studies. Clin. Rev. Allergy Immunol. 32(1), 67–74. https://doi.org/10.1007/bf02686083 (2007).
Banzato, A. et al. Antibodies to domain I of β(2)glycoprotein I are in close relation to patients risk categories in antiphospholipid syndrome (APS). Thromb. Res. 128(6), 583–586. https://doi.org/10.1016/j.thromres.2011.04.021 (2011).
Devreese, K. M. J. Testing for antiphospholipid antibodies: Advances and best practices. Int. J. Lab. Hematol. 42, 49–58. https://doi.org/10.1111/ijlh.13195 (2020).
World Health Organization. Women of reproductive age (15–49 years) population (thousands). https://www.who.int/data/gho/indicator-metadata-registry/imr-details/women-of-reproductive-age-(15-49-years)-population-(thousands) (2024).
Ichihara, K. et al. The Asian project for collaborative derivation of reference intervals: (1) strategy and major results of standardized analytes. Clin. Chem. Lab. Med. 51(7), 1429–1442. https://doi.org/10.1515/cclm-2012-0421 (2013).
ISO 15189:2022. Medical laboratories—Requirements for quality and competence. https://www.iso.org/standard/76677.html (2022).
Clinical and Laboratory Standards Institute (2015). Defining, establishing, and verifying reference intervals in the clinical laboratory, 3rd edition. CLSI document EP28-A3c. Wayne PA: Clinical and Laboratory Standards Institute. (2015).
Devreese, K. M. et al. Testing for antiphospholipid antibodies with solid phase assays: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 12(5), 792–795. https://doi.org/10.1111/jth.12537 (2014).
Devreese, K. M. & Van Hoecke, F. Anticardiolipin and anti-β2glycoprotein-I antibody cut-off values in the diagnosis of antiphospholipid syndrome: More than calculating the in-house 99th percentiles, even for new automated assays. Thromb. Res. 128(6), 598–600. https://doi.org/10.1016/j.thromres.2011.06.023 (2011).
Chayoua, W. et al. The (non-)sense of detecting anti-cardiolipin and anti-β2glycoprotein I IgM antibodies in the antiphospholipid syndrome. J. Thromb. Haemost. 18(1), 169–179. https://doi.org/10.1111/jth.14633 (2020).
Tincani, A., Andreoli, L., Casu, C., Cattaneo, R. & Meroni, P. Antiphospholipid antibody profile: Implications for the evaluation and management of patients. Lupus 19(4), 432–435. https://doi.org/10.1177/0961203310361491 (2010).
Grossi, V. et al. Two novel technologies for the detection of anti-cardiolipin and anti β2-glycoprotein antibodies in the real life: Chemiluminescent in comparison to the addressable laser bead immunoassays. Immunol. Investig. 49, 58–68. https://doi.org/10.1080/08820139.2019.1647233 (2020).
Vanoverschelde, L., Kelchtermans, H., Musial, J., de Laat, B. & Devreese, K. M. J. Influence of anticardiolipin and anti-β2 glycoprotein I antibody cutoff values on antiphospholipid syndrome classification. Res. Pract. Thromb. Haemost. 3(3), 515–527. https://doi.org/10.1002/rth2.12207 (2019).
Montaruli, B. et al. Anti-cardiolipin and anti-β2-glycoprotein I antibodies: Normal reference ranges in northwestern Italy. Lupus 21(7), 799–801. https://doi.org/10.1177/0961203312442260 (2012).
Reed, A. H., Henry, R. J. & Mason, W. B. Influence of statistical method used on the resulting estimate of normal range. Clin. Chem. 17(4), 275–284 (1971).
Dixon, W. Processing data for outliers. Biometrics 9(1), 74–89 (1953).
Tukey, J. W. Exploratory Data Analysis 688 (Addison-Wesley, 1977).
Fontana, P., Poncet, A., Lindhoff-Last, E., de Moerloose, P. & Devreese, K. M. Refinement of the cutoff values of the HemosIL AcuStar assay for the detection of anticardiolipin and anti-beta2 glycoprotein-1 antibodies. J. Thromb. Haemost. 12(12), 2034–2037. https://doi.org/10.1111/jth.12732 (2014).
Acknowledgements
This research was supported by the grants from the Grants (22H0215). This work was supported by the Fundamental Research Funds for the Central Universities (SCU2022F4080).
Author information
Authors and Affiliations
Contributions
CL and LP designed the study. LY and MZ interpreted the data. YG, YD and TL performed the tests. YJ and WL reviewed the paper. WL contributed to the revision stage. All authors approved the final manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Liu, C., Yan, L., Zhang, M. et al. Establishment of cutoff values for anti-β2 glycoprotein I antibodies in women of reproductive age in Southwest China. Sci Rep 14, 20529 (2024). https://doi.org/10.1038/s41598-024-71549-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71549-2
- Springer Nature Limited