Abstract
The development of synthetic bone substitutes that equal or exceed the efficacy of autologous graft remains challenging. In this study, a rat calvarial defect model was used as a reference to investigate the influence of composition and architecture of 3D-printed cement, with or without bioactives, on tissue regeneration. Printable cement pastes were formulated by combining hyaluronic acid and cement precursors. Cementitious scaffolds were printed with 3 different patterns. After 7 weeks of implantation with or without bone marrow, multiparametric qualitative and quantitative assessments were performed using µCT, SEM, and histology. None of the set-up strategies was as efficient as autologous cancellous bone graft to repair calvarial defects. Nonetheless, the presence of scaffold improved the skull vault closure, particularly when the scaffold was soaked in total bone marrow before implantation. No significant effect of scaffold macro-architecture was observed on tissue mineralization. Magnesium phosphate-based scaffolds (MgP) seemed to induce higher bone formation than their calcium-phosphate-based counterparts. They also displayed a quicker biodegradation and sparse remaining material was found after 7 weeks of implantation. Although further improvements are required to reach clinical settings, this study demonstrated the potential of organo-mineral cements for bone regeneration and highlighted the peculiar properties of MgP-based cements.
Similar content being viewed by others
Introduction
Large bone defects affect millions of individuals every year1, and autologous bone grafts remain the gold standard strategy2. However, this comes with intrinsic limitations and extensive efforts on alternatives based on synthetic materials have been conducted3,4. Among them, due to their relative similarity with physiological hydroxyapatite, calcium phosphate (CaP)-based materials possess one of the longest history of studies5 and the largest clinical use and research development for bone applications6.
CaP bioceramics such as stoichiometric hydroxyapatite, β-tricalcium phosphate, or mixtures (biphasic calcium phosphates) represent the vast majority of synthetic bone fillers—but their limited biodegradation and ability to stimulate bone formation restrain their clinical use. Promising alternatives such as calcium phosphate cements (CPCs), which are more bioactive, have been developed for the past 30 years7,8,9 for minimally invasive bone regeneration. With the rapid development of additive manufacturing, their ability to be injected/extruded also became a notable advantage for the production of personalized scaffolds by soft chemistry (e.g., very limited heating, no aggressive/toxic chemicals, aqueous reaction). This has the significant advantage of allowing the direct incorporation of bioactive substances, whether they stimulate tissue formation (e.g., BMPs, VEGF)10 or prevent infections (e.g., antibiotics, antimicrobial peptides)11.
However, most CPCs and even more macroporous 3D-printed CPCs suffer from their intrinsic fragility and tend to be brittle upon constraint upon mechanical stress9. To address this challenge, two main approaches may be targeted (alone or in combination): (i) inducing fast bone ingrowth (e.g., bioactives) responsible for the mechanical resistance of the construct as soon as possible and (ii) modulating cementitious scaffold architecture and composition to enhance its initial mechanical properties. The latter are also known to be of critical influence on bone formation; hence, a synthetic bioactive-free strategy based on biomaterial-induced bone formation seems to be a promising strategy. This could be reached through the development of innovative cement formulations with improved printability, combining the properties of inorganic cement precursors with organic macromolecules7.
Accordingly, this study aimed to evaluate the biological response induced by 3D-printed macroporous organo-mineral cements. The hereby investigated cements were based on either alpha tricalcium phosphate (Ca3(PO4)2, α-TCP) or anhydrous tri-magnesium phosphate (Mg3(PO4)2, MgP) cement powders, two inorganic precursors abundantly reported in bone tissue engineering and cement applications12,13. Indeed, α-TCP hydrolyses into calcium-deficient hydroxyapatite (CDHA), a compound close to physiological hydroxyapatite14. Yet, depending on the implantation site and local environment, commercial CDHA products tend to undergo tenuous degradation, impeding bone formation dynamics8. Mg-based cements, which are known to have superior intrinsic mechanical properties than CPCs, have been proven to stimulate the formation of vascularized bone tissue and to display faster biodegradation rates that could match the kinetics of bone formation15.
Aiming for clinically relevant formulations, cement precursors were supplemented with hyaluronic acid (HA) in the present study. HA is a natural component of the extracellular matrix (ECM), easier to produce, and easier to handle than other ECM compounds, such as collagen16. Moreover, HA was reported to enhance the osteogenic potential of hydrogels and products for bone tissue engineering17. Apart from the potential biological intrinsic effect induced by HA physico-chemical features (e.g., molecular weight, moieties) and purity (e.g., lipopolysaccharides), combining HA to cement precursor have a significant interest in paste formulation to produce personalized macroporous structures via 3D-printing7. Indeed, the addition of macromolecules to cement precursors can enhance paste stability, injectability and printability, but also modulate the features of the printed structures (e.g., plastic deformability US20230201422A1 patent) and set cements (e.g. mechanical reinforcement)7,9.
In this study, the influence of architecture on bone repair was also questioned, and three scaffold designs were tested. Gyroid architecture was used18 and compared to rectilinear architectures with two angle offsets between layers (60° and 90°), all reported with different mechanical properties and biological outcomes19,20.
Finally, scaffolds were implanted without or with the addition of total bone marrow, a bioactive routinely used in clinical setting which is known to enhance osteogenesis21. This represents a much safer and cheaper strategy until synthetic bioactive doses and release are fully controlled (e.g., risky off-label use of BMP2).
In summary, multiple combinations of material formulations and architectures with or without the addition of autologous bone marrow were investigated to achieve bone repair in a murine calvarial defect model.
Materials and methods
Raw material preparation
Hyaluronic acid with a 2.6 MDa average molecular weight (dispersity 1.75 Mw/Mn) was kindly provided by HTL Biotechnology (France). If no further details, chemicals were purchased from Sigma-Aldrich (Merck, Germany). High-purity apatitic tricalcium phosphate (Ca9(HPO4)(PO4)5OH) was synthesized by aqueous precipitation and extensively characterized (IR, XRD, laser granulometry) by Dr. David Marchat at the Centre for Biomedical and Healthcare Engineering (Mines Saint Etienne, France).
Inorganic cement precursors were obtained as follows. Apatitic tricalcium phosphate and hydrated magnesium phosphate (MgP, Mg3(PO4)2; xH2O) powders were shaped into rods using an isostatic press at 1200 bar for 3 min (Nova Swiss). MgP rods were heat treated at 1050 °C for 5 h in a muffle furnace (heating speed 5 °C/min, Nabertherm, Germany) and then collected once cooled. Apatitic tricalcium phosphate rods were heated at 1364 °C for at least 12 h to reach a complete phase transition to alpha-tricalcium phosphate (α-TCP)22, followed by air quenching (i.e. compressed air flow) to stabilize this metastable phase. MgP and α-TCP rods were finally ground and sieved down to 20–40 µm to obtain the desired inorganic cement precursor powders. This size of particles was defined to ultimately obtain stable pastes printable through 25G cones for hours, reactivity of cements precursors drastically increasing with a decrease in particle diameter.
Cement paste formulations
Cement pastes were obtained by mixing a viscous aqueous phase with a cement precursor. The viscous phase was prepared by dissolving 50% w/v D-glucose in deionized water (role of set retarder). Then, hyaluronic acid was added (5% w/v of deionized water) to reach a homogeneous and viscous aqueous phase. To prevent the development of bacteria and fungi while stored at 4 °C, the viscous solution was supplemented with 1% v/v penicillin/streptomycin (P/S, Thermo Fisher Scientific, Illkirch, France). This last step is highly optional, and P/S is likely degraded during further scaffold autoclaving as they are heat sensitive (acknowledged way to dispose of P/S waste according to NIH23).
CaP-based paste was composed of 55% w/w α-TCP and 45% w/w prepared viscous phase. MgP-based paste was composed of 49.02% w/w MgP precursor, 49.02% prepared viscous phase, and 1.96% w/w hydrochloric acid (HCl, 37%).
3D printing and sterilization
Scaffolds were designed using RegenHu proprietary software Shaper. Cylinders 5 mm in diameter and 1 mm in height were imported into Shaper as the global shape of the desired scaffolds. Three different filling architectures were considered as arrangements of 250 µm cylindrical struts (Fig. 1):
-
(i)
A “triangular” arrangement (T), where the orientation of linear struts was shifted of 60° between each layer
-
(ii)
A “rectilinear” arrangement (R), where the orientation of linear struts was shifted of 90° between each layer
-
(iii)
A “gyroid-like” arrangement (G), where a wavy strut pattern evolved in shape and orientation at each layer.
Scaffold architectures designed for 3D printing by robocasting. Linear structures consisted in rectilinear patterns successively oriented along 0°/60°/120° (T, 60°) or 0°/90° (R, 90°). Gyroid structure consisted in ‘’wave-like’’ pattern whose orientation and shape evolve layer-by-layer to form a triply periodic minimal structure. Each architecture is represented by a symbol to ease Figs. 6, 7, and 8 reading experience.
Scaffolds were printed on glass slides in a clean but nonsterile environment via a pneumatic stand dispenser (PSD) equipped with a 25G nozzle (R-Gen 200, RegenHU, Switzerland). The printing speed was fixed at 1.5 mm s−1 to ensure maximal deposition accuracy and the printing pressure was modulated depending on the paste formulation (150–170 and 200–220 kPa for CaP- and MgP-based formulations, respectively). Paste formulations, due to the presence of HA and D-glucose, could enable long printing sessions of at least a day; airtight storage of the cartridges at 4 °C may prolong even more their use.
After drying, scaffolds were bagged and then autoclaved (121 °C, 15 min, 220 kPa) to both sterilize them and speed up the cement setting reaction as extensively described by the work of M.P. Ginebra’s team24,25.
Physicochemical characterization
Phase characterization
Raw inorganic materials, cement precursors, and scaffolds before and after autoclaving were characterized by X-ray diffraction (XRD) and infrared spectroscopy (IR). Prior to analysis, scaffolds were finely ground with an agate mortar and pestle.
XRD analyses were performed with a D8 Advance Serie II diffractometer (Cu Kα, LinxEye 1D Si detector, Ge [1 1 1] monochromator) with an 8–90 2θ angular range (step size 0.01°, counting time 0.2 s per step). Crystalline phases were identified with HighScore software (Malvern Panalytical) using the Crystallography Open Database (COD). Fourier transform infrared (FTIR) spectroscopy was carried out using a Magna IR-550 spectrometer equipped with a diamond ATR module (single reflection). Spectra were obtained as an average of 64 scans from 4000 to 400 cm−1 with a 2 cm−1 resolution.
Morphological analyses of scaffolds
Information about scaffold macro- to meso-architecture was obtained by microcomputed tomography (µCT, SkyScan 1272, Bruker, USA). Acquisitions were performed with a voxel resolution of 10.7 µm, a rotation step of 0.35° (over 360°), a 70 kV tension and 142 µA intensity (0.5 Al filter), and frame averaging of 3. NRecon software (SkyScan, Bruker) allowed for the 3D reconstructions, with optimized misalignment compensation, ring artifact reduction, and beam hardening correction for each sample.
Micro- and nanoarchitectures were observed with scanning electron microscopy (SEM, GeminiSEM 460, Zeiss) after printing and autoclaving followed by 1, 3, or 7 days of immersion in phosphate buffer saline (PBS 1 ×) solution at 37 °C. Before analyses, scaffolds were sputtered with approximately 10 nm of gold (Quorum Q150R ES, UK). Imaging was performed with an acceleration voltage of 5 kV at a working distance of 10.6 mm using either a secondary electron detector SE2 or an InLens SE detector.
Mechanical testing
Compressive strength and deformation were measured on non-macroporous autoclaved cement cylinders (⌀ = 3 mm, h = 5 mm) with a universal testing machine TA HDplus (Stable Microsystem) equipped with a 500 kgf cell. The compression speed was fixed at 0.5 mm/s, and the load was recorded at a 60 Hz frequency. Young moduli were approximated from these assays, as the testing machine did not display an extensometer. Comparison with purely inorganic cements with similar powder/liquid ratio was considered, but not possible due to their pulverulence and poor handling properties.
Dissolution assay
Static dissolution assays in PBS 1 × (50 mL per sample) at 37 °C were performed on autoclaved cement cylinders (⌀ = 3 mm, h = 5 mm). PBS 1 × was completely renewed once a week. Cylinders were weighed before immersion and from 3 times a week (early stages) to once a week (later timepoints) throughout the entire experiment (5 months).
At increasing intervals throughout the 5 months of the experiment: from initially 3 times to 1 a week after 2 months.
Evaluation of ionic release
Ionic release was performed in compliance with ISO10993 guidelines regarding the biological evaluation of medical devices. Extracts preparation was performed in a comparable manner to cytocompatibility assay (see “Cytocompatibility”). Briefly, ⌀5 × 1 mm cylindric materials were placed in a 200 µL PBS 1X extraction bath. After 2h (day 0) and 1, 2, 3 and 7 days (N = 3 per timepoint), medium was collected and entirely replaced by PBS 1 × . All extracts were processed by ICP-OES (Inductively coupled plasma optical emission spectrometry-iCAP 6300 Thermo Scientific). Sample preparation was designed so that all ionic concentrations are included in the analytic range of the device. Volumes are indicated for clarity purpose; however, every analyte was weighted on a 0.1 mg precision balance. First, Ca, Mg and P concentrations were grossly evaluated on 6 samples (50 µL) randomly picked and diluted 60 times in 1% v/w nitric acid using a 1 ppm in Ca, P and Mg standard solution as a reference. For accurate ionic analyses, 7 Ca, Mg and P standard were prepared by dilution of 1000 ppm standard in 1% w/v nitric acid to reach concentration ranging from 0.2 to 15, 15 to 0.2 and 0.1 to 4 ppm, respectively. Analytes were prepared by diluting 100 µL of the extract in 5.9 mL of 1% w/v nitric acid, PBS 1 × (diluted 60 ×) and 1% w/v nitric acid serving as reference and blank, respectively. Each analyte was titrated 5 times, standard solution, blank and PBS 1 × reference being analyzed at regular intervals to assess a possible deviation of the ICP-OES device. Determination of Ca (393.366, 396.847 & 422.673), Mg (279.553, 280.270 & 285.213) and P (177.495, 178.284, 213.618) concentrations was carried out using 3 different rays to prevent possible bias and assess the robustness of the analytic method.
Biological assays
Cell source
L929 (ECACC, London, UK) cells were amplified in Dulbecco’s Minimal Essential Medium (DMEM) supplemented with 10% FBS (Eurobio, Collebeato, Italy) and 1% Penicillin/Streptomycin (ThermoFisher Scientific, Illkirch, France) at 37 °C, 5%CO2, and 95% air in a humidity-saturated atmosphere. Cells retrieving for passaging or experimental seeding was performed under trypsin/EDTA (ThermoFisher Scientific, Illkirch, France) action.
Cytocompatibility
Cytotoxicity assays were performed in compliance with ISO10993-5 guidelines regarding in vitro cytotoxicity assays in the biological evaluation of medical devices26. Briefly, ⌀5 × 1 mm cylindric materials were placed in a 200 µL culture medium extraction bath for 72 h at 37 °C in 37 °C, 5%CO2, 95% air and saturated humidity atmosphere. L929 were seeded in plastic well plate at a density of 15.103 cells/cm2 and cultured in standard culture medium for 24h. Culture medium was then replaced by extracted solutions, undiluted (0:1), and diluted 2 (1:1) or 10 (1:9) times. Dimethyl sulfoxide (DMSO, Sigma‒Aldrich, Darmstadt, Germany) was used as negative control (cell death inducer). Metabolic activity (CCK8, Tebubio, Le Perray en Yvelines, France) and DNA quantification (Picogreen, Life Technologies, Carlsbad, USA) assays were performed 24 h after cell exposure to material extracts according to the manufacturer’s protocol (N = 3).
Animal experiment
All experiments were performed following the European 2010/63/UE Directive on the protection of animals and ARRIVE guidelines used for scientific purposes, and conducted to comply with SYRCLE risk of bias tool guidelines27,28. The study was registered under European, French, and local ethical committee approval (APAFIS #24,288). A total of 49 Lewis 1A-haplotype RT1a male, 10-week-old rats (n = 7 per condition) from a certified breeder (Janvier Labs, Le Genest-Saint-Isle, France) were initially used. Animals were randomly selected, housed, and allocated to the different experimental conditions. Explants were systematically removed from data analysis when observations met the following exclusion criteria: scaffold breaking or displacement greater than 50% of the defect volume or damage to the calvarial area of interest during the retrieval operation. Explant characterization was performed blindy.
Surgery
Surgeries were performed based on a previously described procedure29 (Fig. 2). Briefly, all rats were anesthetized by isoflurane inflow (2%) and received local subcutaneous lidocaine injection (0.1%). The calvaria were exposed by incision of the skin from the forehead to the neck. Two subcritical size defects were created per animal with a ⌀5 mm circular trephine (Komet Medical, Lemgo, Germany) with saline solution irrigation on opposite sides of the sagittal suture. Each animal received two randomly assigned scaffolds and was sutured (Ethylon 5.0, Ethicon, Raritan (NJ), USA). Buprenorphine hydrochloride (Buprecare; Animalcare, Dunnington, U.K.) was administered 30 min before surgery and every 8 h for the two following days. Animals were euthanized after 7 weeks by CO2 inhalation.
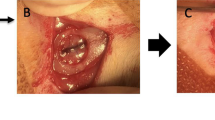
Scaffold implantation in Lewis rats. All animals were anesthetized and maintained under isoflurane inflow during the surgical procedure (A). An incision through the skin revealed the skull (B), ⌀5 mm bilateral calvarial defect was created symmetrically with regard of the sagittal suture (C), scaffolds were implanted (D), and incision was finally closed with 4.0 prolene sutures (E).
Explant characterization
After 7 weeks of implantation, the calvarias were recovered and immediately fixed in 4% paraformaldehyde for 48 h. After rinsing with PBS 1X, calvarias were scanned by µCT before further dehydration and embedding for scanning electron microscopy (SEM) and histological analyses.
Microcomputed tomography
Calvarias were imaged using a Skyscan-1272 high-resolution 3D X-ray microcomputed tomography (µCT) system (Bruker, Kontich, Belgium) with parameters described in Sect. "Morphological analyses of scaffolds". Data segmentation was performed with a deep-learning routine, as described in Sect. "Deep learning image segmentation". Three volumes of interest (VOIs) were defined, one comprising the entire defect (VOI1, ⌀ 5 mm, h = 1 mm), another including exclusively the center of the defect (VOI2, ⌀ 3.5 mm, h = 1 mm), and the last one representing the peripheral area of the defect (VOI3 = VOI1-VOI2).
A bridging score was defined by 3 independent researchers to qualitatively assess the closure of the calvarial defect, as recommended by Spicer et al.30 due to its relevance for this in vivo model: from 0, indicating only peripheral bone formation, to 5, meaning complete healing of the defect.
Embedding
Calvarias were dehydrated by soaking in a graded series of ethanol baths (70, 80, 90, and 100%). Embedding was performed with Technovit® 9100 resin (Kulzer, Germany) according to the manufacturer’s instructions. In short, dehydrated samples were washed with xylene and then preinfiltrated with a 1:1 mixture of xylene and stable Technovit followed by a mixture of destabilized Technovit and hardener. They were finally oriented in their respective histology cassettes, immersed in Technovit polymerization solution and then stored at − 20 °C for 5 days to induce the crosslinking reaction. Embedded samples were processed with a Polycut Leica microtome with a tungsten carbide blade to obtain 5 µm thick slides for histological staining and SEM observations.
Scanning electron microscopy.
Resin-embedded samples were mirror polished and then sputtered with 10 nm of gold–palladium (Desk V, Denton Vacuum, USA) for SEM analyses (GeminiSEM 300, Zeiss, Germany). Imaging was performed in backscattering electron (BSE) with a 15 kV voltage (aperture 60 µm) at a magnification of × 80 (scanning speed 7). SmartStich software was used to merge local images into a single high-resolution image of the whole sample. Image segmentation was performed with a deep-learning routine, as described in Sect. "Deep learning image segmentation".
Histology
Histological sections were stained with hematoxylin/eosin/saffron (HES) and Von Kossa/toluidine blue (VK/BT). Sections were scanned with an automated slide reader (NanoZoomer®, Hamamatsu Photonics, Japan) at × 40 magnification for qualitative assessment of ongoing bone regeneration within the defect.
Deep learning image segmentation
As the respective densities of native healthy bone, neo-formed bone, and calcium and magnesium phosphate-based cements are comparable, their gray values in µCT are often superimposed. Image segmentation by thresholding to obtain reliable quantitative information is consequently difficult and time-consuming. Therefore, µCT data were processed by neural networks with the segmentation wizard of Dragonfly software (ORS, Canada)7,31. The following workflow was set-up:
-
(i)
Pre-architectured neural networks were generated with a depth level of 5 and a patch size of 32. In particular, Dragonfly U-Net 3D was used to improve the robustness of the results.
-
(ii)
Manual segmentation of a representative region of interest (ROI) was performed as initial training data.
-
(iii)
The Adadelta optimization algorithm and a stride ratio of 0.5 were selected to train the neural network. First, a 10 × data augmentation was selected to artificially generate additional data using a combination of horizontal and vertical flips, rotations, scaling operations (90–110%), and shear (2° max).
-
(iv)
As the training of the neural network advanced, other representative ROIs were defined, automatically segmented (prediction), and manually corrected to feed the model. Data augmentation was reduced as real input data were generated.
-
(v)
Training of the neural network was carried out until the segmentation of the images was validated by 3 independent researchers.
-
(vi)
The neural network was updated for each new sample, making the training far less demanding.
This workflow was adapted for the treatment of SEM data with particularly the use of a pre-architectured U-Net neural network (depth level of 4 and patch size of 32).
Statistical analysis
All tests were performed using GraphPad Prism version 8.0.0 (GraphPad Software, San Diego, California, USA). Nonparametric tests were selected when a Gaussian distribution of values could not be found by a normality test or when the sample size did not satisfy parametric test requirements. The H0 hypothesis was rejected when p-value < 0.05. Outlier values determined by the ROUT method32 were removed from the analysis.
Results
Printability of cements and scaffold production
The ratio of cement precursor powder and viscous aqueous phase was optimized to reach optimal printability. Once loaded in a syringe and protected from the ambient atmosphere, both formulations could be printed for at least 3 h with possible but very limited clogging of 25G nozzles. Auto-supported macroporous structures could be easily printed with both formulations (Supplementary Fig. 1), with a printing speed of 1.5 mm/s and pressures varying from 150 to 200 kPa (Fig. 3). Once dried, the thread sizes were approximately 230 and 220 µm for CaP- and MgP-based scaffolds, respectively. Meso-sized bubbles (10–30 µm), trapped within cement thread, could be frequently observed (Fig. 3). Scaffolds were sterilized by autoclaving prior to the animal experiment, which also induced cement setting and consequently the hardening of the 3D-printed structures.
(A) µCT imaging of the different 3D-printed architectures, i.e., a triangular (T, 60°), a rectilinear (R, 90°), and a gyroid (G) arrangement from left to right. Mesoporosity within the printed threads can be observed for each architecture on their respective cross-sections. (B) ATR-FTIR analyses of the raw powders, cement precursors, and non-autoclaved and autoclaved CaP- (left) and MgP- (right) based scaffolds. (C) XRD analyses of the raw powders, cement precursors, and non-autoclaved and autoclaved CaP- (left) and MgP- (right) based scaffolds. SEM imaging of the evolution of cement micro- to nano- features (cement setting) induced by the sterilization process for CaP (D, before autoclaving) and (E, after autoclaving) and MgP-based scaffolds (F then G), respectively.
As demonstrated by the XRD and FTIR analyses and supplemented by SEM images (Fig. 3), the mechanical strength of the structure after printing was ensured by the drying of the viscous organic phase, which wrapped around the cement precursor particles, rather than a cementitious setting reaction. In contrast, the setting reaction was induced by autoclaving, and partial degradation of hyaluronic acid occurred. This phase transition (α-TCP to CDHA and β-TCP for CaP-based scaffolds and Mg3(PO4)2 to Mg3(PO4)2; xH2O for MgP-based scaffolds) is well illustrated by the micro- to nanomorphological evolution of the structure (Fig. 4A). Indeed, microparticles of cement precursors were converted into entangled nanoobjects in the shape of needles and petals for CaP- and MgP-based scaffolds, respectively. HA does not seem to be fully degraded after autoclaving, as illustrated in Figs. 2E and 4A where a polymeric membrane covers the mineral precipitates (Supplementary Fig. 2 displays an enlargement of these SEM pictures).
(A) Scanning electron microscopy imaging of CaP and MgP-based formulations. The organic phase (upper left corner of the upper left picture) and structural changes can be observed after samples were soaked for 7 days in PBS, as the needle-like shape of the CaP-based material turned into a scale-like shape close to what the MgP-based formulation displayed. Scale bars = 1 µm. (B) Ionic release of the materials. After early immersion in PBS, ionic release of Ca and Mg tends to stabilize throughout time. (C) Dissolution assay of CaP and MgP-based formulations. All samples were soaked in PBS at 37 °C for 5 months, regularly weighed and compared to their initial mass. MgP-based materials were found to progressively lose their mass, exhibiting higher dissolution than their CaP-based counterparts. N = 3, Multiple t-tests with Bonferroni–Dunn method *: adjusted p-value < 0.05.
Physico-chemical characterization of bulk cements
The intrinsic mechanical properties of bulk cements were assessed by compression assays on non-macroporous cylinders. While their fracture strength was comparable (i.e., 4.92 ± 0.94 MPa and 3.92 ± 0.99 MPa for CaP- and MgP-based cements, respectively), CaP-based cement displayed a fracture deformation of 6.09 ± 1.38%, which is approximately three times that of MgP-based cements (1.88 ± 0.65%). For both cements, plastic deformation was very limited as a sign of their brittle behavior.
To assess the in vivo implantation passive influence over material aspect and dissolution, autoclaved cement cylinders were immersed in PBS (Fig. 4A). After 7 days and beyond, no trace of HA residues could be observed. Surface topography of CaP-based and MgP-based cement evolved with a modification of both shape and size of initial nanoscale precipitates, which indicated a total conversion of the cementitious precursors and a maturation of the cements toward thermodynamically stable inorganic phases.
These topologic changes are associated with cement setting and maturation, which are coherent with the ionic release measured by ICP-OES within the 1st week (Fig. 4B): Ca2+ and Mg2+ release in PBS 1X is relatively high in the first hours of immersion (123 and 395 ppm at 2h, respectively) for CaP and MgP based cements, then tend to stabilize at lower amounts (47 and 88 ppm at day 7, respectively) throughout time.
In the first three weeks, the weights of the samples (Fig. 4C) slightly increased in both conditions and stabilized at approximately 105% and 103%, respectively for the CaP- and MgP-based cements. While CaP-based cements remained stable throughout time, MgP cements started to dissolve after a month. A significant difference between the dissolution of the two cements was found after three months in PBS at 37 °C and increased markedly until the end of the assay at 5 months.
Biocompatibility
To evaluate the impact of material biodegradation regarding in vitro cytotoxicity in compliance with ISO 10993-5 norm guidelines, a metabolic activity assay was performed on L929 cells (Fig. 5). MgP-based materials' cytocompatibility was found with a tenfold dilution of extracts in DMEM, while CaP-based materials were compatible with a twofold dilution (Fig. 5A). No significant difference was found between cells in standard medium and any of the CaP-based material extracts. These results were supported by the DNA quantification assay reported for metabolic activity (Fig. 5B), where no difference was found between all conditions tested, showing the consistency of L929 metabolic activity regardless of the presence of extracts. To summarize, CaP-based materials were found to match the cytocompatibility threshold with a 1:1 dilution and MgP-based materials with a 1:9 dilution in DMEM. These difference in cytocompatibility at early stages between the 2 biomaterials could be explained by their respective ion release, where too high amount of calcium, magnesium or phosphate are known to be detrimental for cells33,34,35 , and particularly fibroblasts36,37.
Metabolic activity of L929 cells exposed to different material extracts compared to standard culture medium DMEM. A total of 15.103 cells mm−2 were seeded on day 0, and measurements were performed after 24 h of exposure. The normalized formazan-measured absorbance (A) shows the cell viability via NADH dehydrogenase activity over time, and the red line represents the ISO10993-5 threshold under which cytotoxicity is found. The metabolic activity of cells is constant with DNA concentration (B). N = 3, Kruskal–Wallis test #: p < 0.05 compared to L929 w/DMEM.
Material-induced bone regeneration
Observation of µCT data revealed a complete or nearly complete closure of the calvarial defect when treated with cancellous bone. When left empty, bone formation was observed in the vast majority of the peripheral area of the defect. For defects treated with scaffolds, bone formation in the peripheral area often secured the structure, and defect bridging was uneven within the same experimental group, from a few spicules in the center of the scaffold macroporous architecture to complete closure of the defect. The addition of total bone marrow to the scaffold prior to implantation tended to improve bone formation and defect closure (Fig. 6).
Bridging score† (A) of bone defects 7 weeks post-surgery appreciated via µCT. Autologous grafts were associated with the highest score. Overall, the addition of TBM enhanced bone bridging. Red bar = median; Kruskal‒Wallis test p-value = 0.039, but no Dunn’s multiple comparisons displayed any significance between groups. SEM illustration (B) of controls and material conditions. Scale bar = 1 mm. † Adapted from Spicer et al. and Patel et al.30,38. 0: No bone formation other than peripheral. 1: A few bony spicules dispersed at the center of the defect. 2: Bony from defect border to center. 3: Bony bridging through 1 axis. 4: Bony bridging through 2 axes. 5: almost to fully regenerated defect.
At first glance, the only difference between CaP- and MgP-based scaffolds was the nearly complete biodegradation of the latter, which was not observable with µCT. Several CaP-based scaffolds crumbled throughout the implantation time and spread around the defect. These events seemed to be less likely with the linear 90° architecture, a phenomenon that was confirmed by further analysis of segmented µCT images (Fig. 7).
(A) Influence of architecture in scaffold displacement off the defect area for CaP-based formulations. The R scaffolds were found to be less subject to displacement compared to the two other different architectures assessed. Kruskal‒Wallis test, *: p-value < 0.05). (B) Micro-CT illustration of an off-side displacement of broken material (yellow) over the bone (white) defect zone (illustrated as the red ring, ⌀5 mm) 7 weeks post implantation.
While gray values of voxels relative to bone and cement were superimposed, precise segmentation of the µCT data was carried out thanks to deep-learning routines. The quantitative results are recapitulated in Table 1 and illustrated in Fig. 8.
Assessments of mineralized tissue formation 7 weeks after surgery. Mineralized tissue fractions (MTF) in total Volume of interest (VOI) (panel A) were measured via deep learning analysis of micro-CT acquisitions. No differences were found between all conditions, except for CaP lin60 TBM-, CaP lin90 TBM-, and MgP gyr TBM-, all compared to autologous graft. Looking at grouped variables (formulation, presence/absence of total bone marrow), CaP-based formulations and absence of TBM were found to be of higher significant difference compared to autologous grafts. Within defects, mineralization (panel B) was found to occur mostly at the edges, with CaP-based formulations correlated with a central/peripheral balance closer to autologous graft. CaP residual material was detected with micro-CT as Residual Material Fraction (RMF) and is displayed cumulated with MTF (panel C). With RMF, the fraction in VOI occupied by mineral content overcome autologous graft associated MTF. Kruskal–Wallis test, # or *: p < 0.05 compared to AG.
Total mineralized tissue fractions (MTF) were determined (Fig. 8, panel A, upper side) in a volume of interest (VOI) matching the size of the generated defects and implanted scaffolds (i.e., ⌀ 5 × h 0.9 mm cylinder). The MTF of defects treated with autografts was significantly higher (42.7 ± 11%) than that for all the other conditions. Loading scaffolds with total bone marrow seemed to be beneficial for MTF, but the results remained nonsignificant (12.7 ± 6.8% vs 18.8 ± 11%).
The distribution of mineralized tissue (Fig. 8, panel B) was quite even for AG, with a peripheral mineralization of 56.2% (VOI3) and a central mineralization of 43.8% (VOI2). In contrast, for empty defects, the regeneration dynamic was centripetal, with 78.3% of new mineralized tissue in VOI3. The formation of mineral tissues within defects treated with scaffolds was in between these extremes, with a more central repartition of CaP- vs MgP-based materials (29.1% vs 23.8%). Overall, a tendency to shift toward AG values when bone marrow was added was also noted.
Residual material fraction was only observable for CaP-based formulation, the remaining MgP biomaterial being very faintly radiopaque to X-rays (Fig. 8, panel C). The total volume occupancy observed within the VOI (MTF + RMF -if applicable-) for CaP-based conditions was found to match and even exceed the volume fraction corresponding to AG.
Histological staining (Fig. 9) provided a qualitative appreciation of the different biomaterial-induced biological responses. Histological slices for the remaining non-displayed conditions are available in Supplementary Fig. 3 as the architecture of CaP- or MgP-based scaffold did not seem to have a significant influence.
Von Kossa and hematoxylin–eosin staining of calvarial cross sections. Edge-to-edge defect representations and close-ups show large bone formation in autologous graft conditions and only the formation of fibrous tissue in empty defect samples (positive and negative controls, A and B, respectively). CaP-based material (C and D, yellow *) was found to be stained black with VK, although its blurry structure was distinct from organized layers of bone tissue (white arrows for bone adjacent to material, and ‡ for edge bone). Overall, much less material could be found in Mg-based samples (E and F) compared to their CaP-based counterparts.
Overall, no structural markers of inflammation or foreign body reaction were found, and all implants—for those remaining after 7 weeks—were well integrated with host tissues. Autologous graft (AG), as a positive control condition, was associated with the highest surfaces of physiological bone-looking mineralized tissue formation. Empty defect conditions failed to show significant mineralized tissue within the area of the defect (comprised between the black arrows), as expected. Residual material could be easily identified for CaP-based formulation due to its black VK and very light pink HES colors, with a nonorganized structure. Neo-formed mineralized tissue was found intricated at the periphery of CaP-based material (white arrows). With MgP-based formulations, while larger areas of neo-formed mineralized tissue were found compared to CaP-based materials, few to merely traces of material could be identified after the 7 weeks. The MgP (T, 60°) + TBM example with a large area of MgP-based material displayed here stands as an illustrative example yet is not representative of most MgP-based scaffolds that are largely biodegraded. Additional images are provided in Supplementary Fig. 3. Overall, for MgP-based formulations, soft tissue was found in spaces occupied by residual material for CaP-based formulations.
Discussion
While many materials can be considered good candidates for bone regeneration39, 3D printing of cement-like formulations is relatively recent40,41,42. Although ceramics have been extensively studied and used for decades, the sintering step at an elevated temperature may limit the material-induced biological response and is less convenient for the supplementation with biological additives. In contrast, the use of cement unveils the possibility of low-temperature hardening adapted to the development of more complex hybrid materials and the incorporation of fragile bioactive substances43. Among the cements used in bone tissue engineering, calcium phosphate cements (CPCs) are usually preferred for their constitutive affinity and long clinical history with bone tissue6,44. Indeed, calcium-phosphate cements are also known for their cytocompatibility and bioactivity regarding several tissue-derived stem cell types and therefore exert strong potential for skeletal regenerative medicine45. The use of substituted calcium-phosphate cements is also raising the question of other substitutes, and among them, magnesium-substituted calcium phosphates are extensively studied46. Indeed, magnesium was reported to have dual osteogenic and angiogenic effects, which are decisive aspects of critical bone defect regeneration47. In addition, magnesium phosphate cements (or Mg substituted CPCs) are known for their more adequate biodegradation rate48, a major drawback of CPCs. Consequently, magnesium phosphate cements are drawing increasing interest in bone regeneration.
In this study, a traditional CPC formulation and its magnesium phosphate-based counterpart were combined with a well-known macromolecule: hyaluronic acid. The benefits of this strategy include a potential increase in bioactivity compared to purely inorganic cements49 and a modulation of the physicochemical and mechanical properties of the set cement7. The latter grants the ability to easily 3D-print cementitious formulations to obtain macroporous structures, a tremendous limitation of traditional cements.
The developed organo-mineral cements (Patent WO2021209616A1) displayed intrinsic mechanical properties within the known range of CaP- or MgP-based purely inorganic cements (e.g., rupture strength 1–80 MPa)13,50, despite a very low powder:liquid ratio (P:L = 1.2:1 and 1:1, respectively). These are known to be relevant for non or low-loading bone reconstruction, as they match the mechanical properties of cancellous bone51.
To generate macroporosity and thereby enhance bone regeneration potential, the use of a 3D printing technique enabled tailoring the internal architecture of the cementitious structures with precision. However, the introduction of macroporosity also posed a challenge, as it has been reported to influence the mechanical properties of the printed cements52. Thus, the breaking or displacement events observed after 7 weeks of in vivo implantation could be related to the scaffold architectural design. Interestingly, among the three investigated architectures, the linear 90° geometry was associated with a significantly lower volume of material outside the defect area. This could be explained by the strut arrangement of these structures, combining both a higher number of contact points and smaller inter-strut spaces, two critical aspects of structural self-supporting ability24,53. Overall, scaffold fragility should be improved in further investigations (e.g., using reticulating macromolecules: patent EP23305052.5, 2023), despite the developed CaP-based formulation already demonstrating improved deformation ability (≈ 6% before fracture, patent WO2021209616A1 2021).
Despite these advancements, no experimental condition was able to match the efficacy of AG for calvarial regeneration. Mineralized tissue formation observed within scaffolds was in the same range as that in most studies (without bioactives)9, which validates the ability of the scaffolds to support bone regeneration. In addition, the set-up of deep learning routines significantly improved data segmentation, and mineralized tissues tend to be over-evaluated in these complex settings when manual segmentation (e.g., thresholding, propagation) is performed, even by seasoned researchers31.
CaP-based scaffolds were subject to strong tissue integration, and tissue mineralization occurred both at the periphery and center of the defect. However, their very limited biodegradation (in correlation with their low in vitro dissolution) may have impaired early bone formation within the defect54. In contrast, MgP-based scaffolds were largely biodegraded after 7 weeks of implantation, suggesting strong cellular resorption activity with regard to their early in vitro stability. The distribution and overall morphology of bone formation seem to indicate that MgP-based scaffold biodegradation occurred too prematurely, thus depriving the local environment of the mechanical support required for bone formation55. Determining a suitable balance between sustained osteoconduction and permissive biodegradability with these organo-mineral scaffolds will be the next challenge to be addressed. To do so, various substituted CaP cement precursors, magnesium phosphate cements, or CaP/MgP mixtures will be targeted56.
As the main objective remains to obtain long-term ad integrum bone repair, biofunctionalization of materials should also be a central consideration for future directions. Despite its cautious use due to undesirable side effects, BMP2 remains an efficient, clinically used treatment to promote ossification57,58. Future investigations should then consider adding a relevant concentration of BMP259 to the material formulation or other growth factors to emphasize vascularization events such as VEGF60. However, this type of biofunctionalization can only be performed with low-temperature hardening to preserve such active substances. Thus, the first step toward these improvements remains the development of a non-brittle 3D printable cement formulation with physiologically relevant temperature hardening and satisfying bone regeneration potential.
Conclusion
Through the combination of hyaluronic acid and cement precursors, the printability of the cementitious formulation was significantly improved, leading to the production of auto-supported macroporous structures. Although the mechanical properties of the autoclaved scaffolds remained low (partly due to their high macroporosity), the presence of HA interestingly improved the rupture deformation of CaP-based structures, which is a step further the development of personalized 3D-printed cementitious implants with relevant mechanical handling. MgP-based scaffold supported a limited bone formation due to their premature biodegradation. Future investigations should focus on formulation and architectural upgrades for improved scaffold robustness upon implantation, refined degradation behavior, and enhanced biological functionalization.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Wildemann, B. et al. Non-union bone fractures. Nat. Rev. Dis. Primers 7, 1–21 (2021).
Bergh, C., Wennergren, D., Möller, M. & Brisby, H. Fracture incidence in adults in relation to age and gender: A study of 27,169 fractures in the Swedish Fracture Register in a well-defined catchment area. PLOS ONE 15, e0244291 (2020).
Fernandez de Grado, G. et al. Bone substitutes: a review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 9, 2041731418776819 (2018).
Valtanen, R. S., Yang, Y. P., Gurtner, G. C., Maloney, W. J. & Lowenberg, D. W. Synthetic and bone tissue engineering graft substitutes: What is the future?. Injury 52(Suppl 2), S72–S77 (2021).
Dorozhkin, S. V. A detailed history of calcium orthophosphates from 1770s till 1950. Mater. Sci. Eng. C Mater. Biol. Appl. 33, 3085–3110 (2013).
Canillas, M., Pena, P., de Aza, A. H. & Rodríguez, M. A. Calcium phosphates for biomedical applications. Boletín de la Sociedad Española de Cerámica y Vidrio 56, 91–112 (2017).
Moussi, H., Weiss, P., Bideau, J. L., Gautier, H. & Charbonnier, B. Injectable macromolecule-based calcium phosphate bone substitutes. Mater. Adv. 3, 6125–6141 (2022).
Schröter, L., Kaiser, F., Stein, S., Gbureck, U. & Ignatius, A. Biological and mechanical performance and degradation characteristics of calcium phosphate cements in large animals and humans. Acta Biomater. 117, 1–20 (2020).
Lodoso-Torrecilla, I., van den Beucken, J. J. J. P. & Jansen, J. A. Calcium phosphate cements: Optimization toward biodegradability. Acta Biomater. 119, 1–12 (2021).
Dimitriou, R., Tsiridis, E. & Giannoudis, P. V. Current concepts of molecular aspects of bone healing. Injury 36, 1392–1404 (2005).
Hao, Z. et al. Antimicrobial peptides for bone tissue engineering: Diversity, effects and applications. Front. Bioeng. Biotechnol. 10, 1030162 (2022).
Carrodeguas, R. G. & De Aza, S. α-Tricalcium phosphate: synthesis, properties and biomedical applications. Acta Biomater. 7, 3536–3546 (2011).
Ostrowski, N., Roy, A. & Kumta, P. N. Magnesium phosphate cement systems for hard tissue applications: A review. ACS Biomater. Sci. Eng. 2, 1067–1083 (2016).
Sadowska, J. M. et al. The effect of biomimetic calcium deficient hydroxyapatite and sintered β-tricalcium phosphate on osteoimmune reaction and osteogenesis. Acta Biomater. 96, 605–618 (2019).
Amukarimi, S. & Mozafari, M. Biodegradable magnesium-based biomaterials: An overview of challenges and opportunities. Med. Comm. 2020(2), 123–144 (2021).
Zheng, Z., Patel, M. & Patel, R. Hyaluronic acid-based materials for bone regeneration: A review. React. Funct. Polym. 171, 105151 (2022).
Hwang, H. S. & Lee, C.-S. Recent progress in hyaluronic-acid-based hydrogels for bone tissue engineering. Gels 9, 588 (2023).
Shen, M. et al. Bioceramic scaffolds with triply periodic minimal surface architectures guide early-stage bone regeneration. Bioact. Mater. 25, 374–386 (2023).
Bartnikowski, M., Klein, T. J., Melchels, F. P. W. & Woodruff, M. A. Effects of scaffold architecture on mechanical characteristics and osteoblast response to static and perfusion bioreactor cultures. Biotechnol. Bioeng. 111, 1440–1451 (2014).
Berner, A. et al. Effects of scaffold architecture on cranial bone healing. Int. J. Oral Maxillofac. Surg. 43, 506–513 (2014).
Guerrero, J. et al. The use of total human bone marrow fraction in a direct three-dimensional expansion approach for bone tissue engineering applications: Focus on angiogenesis and osteogenesis. Tissue Eng. Part A 21, 861–874 (2015).
Yubao, L., Xingdong, Z. & de Groot, K. Hydrolysis and phase transition of alpha-tricalcium phosphate. Biomaterials 18, 737–741 (1997).
Waste Management Services. https://orf.od.nih.gov/EnvironmentalProtection/WasteDisposal/Pages/default.aspx.
Raymond, S. et al. Accelerated hardening of nanotextured 3D-plotted self-setting calcium phosphate inks. Acta Biomater. 75, 451–462 (2018).
Barba, A. et al. Osteoinduction by foamed and 3D-printed calcium phosphate scaffolds: Effect of nanostructure and pore architecture. ACS Appl. Mater. Interfaces 9, 41722–41736 (2017).
International Organization for Standardization. ISO 10993-5:2009(en), Biological evaluation of medical devices— Part 5: Tests for in vitro cytotoxicity. https://www.iso.org/obp/ui/fr/#iso:std:iso:10993:-5:ed-3:v1:en.
Hooijmans, C. R. et al. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 14, 43 (2014).
The Cochrane Collaboration. Cochrane Handbook for Systematic Reviews of Interventions, 2nd Edition|Wiley. Wiley.com https://www.wiley.com/en-us/Cochrane+Handbook+for+Systematic+Reviews+of+Interventions%2C+2nd+Edition-p-9781119536628.
Paré, A. et al. Tailored three-dimensionally printed triply periodic calcium phosphate implants: A preclinical study for craniofacial bone repair. ACS Biomater. Sci. Eng. 6, 553–563 (2020).
Spicer, P. P. et al. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 7, 1918–1929 (2012).
Paré, A. et al. Standardized and axially vascularized calcium phosphate-based implants for segmental mandibular defects: A promising proof of concept. Acta Biomater. 154, 626–640 (2022).
Motulsky, H. J. & Brown, R. E. Detecting outliers when fitting data with nonlinear regression - a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 7, 123 (2006).
Przekora, A., Czechowska, J., Pijocha, D., Ślósarczyk, A. & Ginalska, G. Do novel cement-type biomaterials reveal ion reactivity that affects cell viability in vitro?. Open Life Sci. 9, 277–289 (2014).
Feyerabend, F. et al. Evaluation of short-term effects of rare earth and other elements used in magnesium alloys on primary cells and cell lines. Acta Biomater. 6, 1834–1842 (2010).
Wang, J. et al. Recommendation for modifying current cytotoxicity testing standards for biodegradable magnesium-based materials. Acta Biomater. 21, 237–249 (2015).
Zhen, Z., Liu, X., Huang, T., Xi, T. & Zheng, Y. Hemolysis and cytotoxicity mechanisms of biodegradable magnesium and its alloys. Mater. Sci. Eng.: C 46, 202–206 (2015).
Tamai, M., Nakaoka, R. & Tsuchiya, T. Cytotoxicity of various calcium phosphate ceramics. Key Eng. Mater. 309–311, 263–266 (2006).
Patel, Z. S. et al. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 43, 931–940 (2008).
Cheng, L. et al. 3D Printing of Micro- and Nanoscale Bone Substitutes: A Review on Technical and Translational Perspectives. Int J Nanomedicine 16, 4289–4319 (2021).
Trombetta, R., Inzana, J. A., Schwarz, E. M., Kates, S. L. & Awad, H. A. 3D Printing of calcium phosphate ceramics for bone tissue engineering and drug delivery. Ann. Biomed. Eng. 45, 23–44 (2017).
Richter, R. F. et al. Treatment of critical bone defects using calcium phosphate cement and mesoporous bioactive glass providing spatiotemporal drug delivery. Bioact. Mater. 28, 402–419 (2023).
Korn, P. et al. 3D printing of bone grafts for cleft alveolar osteoplasty—in vivo evaluation in a preclinical model. Front. Bioeng. Biotechnol. https://doi.org/10.3389/fbioe.2020.00217 (2020).
Araújo, M. V. F., Mendes, V. C., Chattopadhyay, P. & Davies, J. E. Low-temperature particulate calcium phosphates for bone regeneration. Clin. Oral Implants Res. 21, 632–641 (2010).
Bonjour, J.-P. Calcium and phosphate: A duet of ions playing for bone health. J. Am. Coll. Nutr. 30, 438S-448S (2011).
Wang, P. et al. Stem cells and calcium phosphate cement scaffolds for bone regeneration. J. Dent. Res. 93, 618–625 (2014).
Uppal, G., Thakur, A., Chauhan, A. & Bala, S. Magnesium based implants for functional bone tissue regeneration—a review. J. Magnes. Alloys 10, 356–386 (2022).
Liu, W. et al. Magnesium promotes bone formation and angiogenesis by enhancing MC3T3-E1 secretion of PDGF-BB. Biochem. Biophys. Res. Commun. 528, 664–670 (2020).
Kowalewicz, K. et al. Comparison of degradation behavior and osseointegration of 3D powder-printed calcium magnesium phosphate cement scaffolds with alkaline or acid post-treatment. Front. Bioeng. Biotechnol. https://doi.org/10.3389/fbioe.2022.998254 (2022).
Zhai, P. et al. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 151, 1224–1239 (2020).
Tronco, M. C., Cassel, J. B. & dos Santos, L. A. α-TCP-based calcium phosphate cements: A critical review. Acta Biomater. 151, 70–87 (2022).
Gibson, L. J. The mechanical behaviour of cancellous bone. J. Biomech. 18, 317–328 (1985).
Bignon, A. et al. Effect of micro- and macroporosity of bone substitutes on their mechanical properties and cellular response. J. Mater. Sci. Mater. Med. 14, 1089–1097 (2003).
Habib, M. A. & Khoda, B. Development of clay based novel bio-ink for 3D bio-printing process. Proc. Manuf. 26, 846–856 (2018).
Wei, S., Ma, J.-X., Xu, L., Gu, X.-S. & Ma, X.-L. Biodegradable materials for bone defect repair. Mil. Med. Res. 7, 54 (2020).
Guimarães, C. F., Gasperini, L., Marques, A. P. & Reis, R. L. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater. 5, 351–370 (2020).
Kaiser, F. et al. Accelerated bone regeneration through rational design of magnesium phosphate cements. Acta Biomater. 145, 358–371 (2022).
James, A. W. et al. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng. Part B Rev. 22, 284–297 (2016).
Gillman, C. E. & Jayasuriya, A. C. FDA-approved bone grafts and bone graft substitute devices in bone regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 130, 112466 (2021).
Schmidt-Bleek, K., Willie, B. M., Schwabe, P., Seemann, P. & Duda, G. N. BMPs in bone regeneration: Less is more effective, a paradigm-shift. Cytokine Growth Factor Rev. 27, 141–148 (2016).
Zhang, W. et al. VEGF and BMP-2 promote bone regeneration by facilitating bone marrow stem cell homing and differentiation. Eur. Cell Mater. 27, 1–11 (2014).
Acknowledgements
The authors thank Dr. David Marchat (Mines St Etienne) for the supply and characterization of apatitic tricalcium phosphate. The authors also acknowledge the BIO3 platform from the Inserm/NU/ONIRIS UMR1229 RMeS Laboratory, and the SC3M platform from the Inserm/NU/ONIRIS UMR1229 RMeS Laboratory and SFR François Bonamy-UMS 016. The authors thank Carole La (LPG-UMR 6112, Nantes Université) for her help regarding Ca, Mg, P content titrations using ICP-OES.
Funding
This work was funded by the ANR-GIJaw project 20CE17-0018 grant.
Author information
Authors and Affiliations
Contributions
T.N., P.A., W.P., C.P., and C.B. contributed to the conception and/or design of the work. T.N., R.S., R.T., D.M., V.J., B.L., and C.B. performed acquisitions. T.N., R.S., and C.B. interpreted the data. T.N. and C.B. drafted the manuscript. All authors have approved the submitted version. All authors have agreed both to be personally accountable for the author's own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even those in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The animal study was registered under ethical committee approval number APAFIS 24288.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Nicolas, T., Ségolène, R., Thierry, R. et al. Multiparametric influence of 3D-printed organo-mineral scaffolds on bone regeneration. Sci Rep 14, 20848 (2024). https://doi.org/10.1038/s41598-024-71698-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71698-4
- Springer Nature Limited