Abstract
Understanding how large carnivores utilize space is crucial for management planning in human-dominated landscape and enhances the accuracy of population size estimates. However, Eurasian lynx display a large inter-population variation in the size of home ranges across their European range which makes extrapolation to broader areas of a species distribution problematic. This study evaluates variations in home range size for 35 Eurasian lynx in the Western Carpathians during 2011–2022 based on GPS telemetry and explains how intrinsic and environmental factors shape lynx spatial behaviour when facing anthropogenic pressure. The average annual home range size of lynx ranged from 283 (± 42 SE) to 360 (± 60 SE) km2 for males and from 148 (± 50 SE) to 190 (± 70 SE) km2 for females, depending on home range estimator (95% MCP, KDE and AKDE). Females with kittens had smaller annual and summer home ranges compared to non-reproducing females and subadults had smaller home ranges compared to adults. Lynx home range size was explained by availability of roe deer, except for summer, when alternative prey was likely available. We also found clear evidence of human-induced changes in lynx home range size, in particular, forest cover significantly decreased the home range size of male lynx during summer while road density led to an expansion of both annual and summer lynx home ranges. Lynx exhibited consistent fidelity to their home ranges throughout consecutive seasons, showing no seasonal variations. Strong territoriality was observed among competing males maintaining relatively low home range overlaps and considerable distances between centres of activity. The most pronounced tendency for association was observed between males and females, maintaining relatively close proximity year-round. The insights into lynx spatial requirements provided by our study will greatly enhance the accuracy of population size estimates and effectiveness of mitigation measures across the Western Carpathians.
Similar content being viewed by others
Introduction
Animals’ use of space represents an intrinsic aspect of their behaviour which is a result of purposeful actions aimed at safeguarding critical resources in order to fulfil their essential physiological needs, thus shaping the ways in which animals operate and coexist. Understanding the spatial requirements of wildlife, particularly of elusive species such as large carnivores, is essential for strategic planning of conservation initiatives within human-dominated landscapes1. This knowledge plays a key role in the selection of optimal locations and dimensions for protected areas and/or management units2 to encompass home ranges of multiple individuals. Moreover, estimates of home-range sizes are crucial for population size estimates, either through the application of formal distance rules or when extrapolating from local surveys to the population level3,4. Nonetheless, a significant challenge arises in acquiring telemetry data on sufficient number of individuals to estimate home ranges, as the extrapolation from investigated sites to broader areas of a species distribution proves problematic due to considerable variation in home-range sizes between landscapes1,3,5,6.
The Eurasian lynx (Lynx lynx) is a generalist species with a wide distribution, spanning the Palearctic region from Western Europe to the Pacific coast of Siberia and from the northern boreal forests to the Central Asian grasslands and Himalayan mountains7,8. Across its European range, the average home-range size of lynx varies by one order of magnitude1, introducing a significant challenge when transferring data from one study area to another. This variation in home range size is related to intrinsic traits such as sex and age6,9,10 as well as to external factors such as roe deer (Capreolus capreolus) density and environmental productivity1,3,5 and/or conspecific density11,12. The home-range size in human-dominated landscapes is also strongly influenced by anthropogenic activities13. For example, landscape fragmentation can restrict lynx movement, as they are hesitant to leave continuous forested areas and traverse open agricultural lands14. Although lynx can navigate through unfavourable habitats and overcome linear barriers like fenced highways15,16, connectivity between habitat patches is primarily hindered not by the distribution of suitable habitat but rather by elevated road mortality17,18,19.
Here we present findings on the spatial ecology of the Eurasian lynx within the human-dominated landscape of the Western Carpathians (Fig. 1). Our primary objective was to quantify lynx space use and investigate the impact of environmental and anthropogenic factors on lynx home range size, as limited knowledge currently exists from this region. Specifically, our objectives were (1) to evaluate differences in home range size of lynx, considering intrinsic factors and evaluating seasonal variations using three different home range estimators; (2) to investigate environmental and anthropogenic factors that may have shaped lynx home ranges; and (3) to evaluate lynx fidelity to their home ranges and their territoriality towards conspecifics. Based on previous research, we predicted that male lynx would have larger home ranges compared to females. Females with kittens would display notably reduced home ranges compared to non-reproducing females and adult lynx would have larger home ranges compared to subadult lynx5,6,15. We hypothesized that lynx home-range size would be inversely related to roe deer density, its main prey3,11,20. Furthermore, we hypothesized that landscape fragmentation21 and road density22 would significantly influence lynx home range size, although this effect might be season-specific, and, we expect to observe strong fidelity and territoriality in lynx spatial behaviour7. Finally, we discuss further implications arising from our understanding of lynx spatial behaviour for the practical management and conservation efforts in the Western Carpathians.
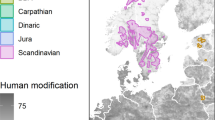
Location of the study and GPS locations of 35 surveyed Eurasian lynx in the Western Carpathians during 2011–2022. The figure was created in ArcMap 10.8 using data from [source: https://www.geoportal.sk/sk/zbgis/na-stiahnutie/] under a CC BY 4.0 license, with permission from the Institute of Geodesy and Cartography Bratislava, original copyright 2022.
Materials and methods
Study area
Our study was conducted within 21, 845 km2 of temperate forests of the Western Carpathians encompassing Slovakia, Czechia and Poland (48°29′42.5″–49°45′57.6″ N; 17°30′27.0″–20°09′04.5″ E; Fig. 1). According to Corine Landcover 201823 forests covered 55% of the study area, of which 14% were deciduous, 24% coniferous, and 17% mixed. Shrubs encompassed 6% of the area and pastures 11%, while agricultural land covered 23% and urban areas 5%. The average road density within the composite home range was 0.63 km/km2 (Fig. 1) including highways (0.01 km/km2), primary roads (0.07 km/km2), secondary roads (0.06 km/km2), tertiary roads (0.18 km/km2) and local roads (0.30 km/km2;23).
The Western Carpathians have likely been inhabited by lynx since significant global climate cooling (47.4 kya, 48–54 kya,24,25). At present, the lynx is strictly protected under Annex II (habitat protection) and Annex IV (strictly protected species) of the European Directive 92/43/EEC on the conservation of natural habitats and of wild fauna and flora (the Habitats Directive). Currently, the lynx population in Slovakia, which covers the majority of our study area, is estimated to range between 197 and 337 reproducing adults26 with a stable trend over the past 20 years27. Although lynx occur along the entire Polish part of the Carpathians28 their numbers do not exceed 100 individuals29. The size of the population in the Czech Carpathians is estimated to range between 10 to 12 individuals26.
Lynx captures and collaring
In total, we captured 35 lynx between 2011 and 2022, consisting of 15 adult and 5 subadult males, as well as 9 adult and 6 subadult females (Table S1). Among the females, 5 were reproducing with kittens, while 10 were not reproducing (Table S1). We employed walk-through, double-door wooden or metal box traps (dimensions: 2 × 1 × 1 m), strategically placed at locations identified through prior systematic monitoring as frequently used by lynx26,30. The majority of lynx captures occurred during winter and early spring, aligning with their higher spatial activity during this period, especially mating season (Table S1, Fig. S1). To attract lynx, we applied urine or scats inside some boxes collected from individuals kept in captivity (National ZOO Bojnice and Ostrava Zoological Garden and Botanical Park) or collected by snow tracking of wild individuals. When activated, box traps were under constant surveillance through a GSM alarm system (Kieferle, Gottmadingen—Randegg, Germany; UOV LTA Alarm, Shenzhen, China; Mink Police, Alert House Aps, Denmark; TT3 Trap Transmitter, Vectronic Aerospace, Germany) and GPRS cameras (SPROMISE Camera, Australia; UOvision, China) which promptly notified the responsible person in the event of trap door closure.
All lynx were tranquilized by veterinarian using a mixture of medetomidine (Domitor®) and ketamine (Ketasol® and Narketan®) and reversed with atipamezole (Antisedan®)31,32. The study is reported in accordance with ARRIVE guidelines33 and all procedures were approved by the Ministry of Environment of the Slovak Republic (permits no. 7402/2013-2.2366/2016-2.3; 6933/2019-9.2 [7/19-rozkl.]), by the Ethics Committee of the Czech Academy of Sciences, the Nature Conservation Agency of the Czech Republic and the Ministry of Environment of the Czech Republic (permit no. 6535/BE/2008, 130/2010; SR0031/BE/2019–16; MZP/2020/630/167; MZP/2022/630/735), and by the Regional Directorate for Environmental Protection in Katowice, Poland (permit no. WPN.6401.397.2020.MS) and 1st Local Ethical Committee for Experiments on Animals in Warsaw (permit No. 977/2020). No mortalities occurred during or after captures and no complications were observed due to collaring. All captured animals were medically examined and equipped with GPS (Global Positioning System) collars (18 by LoTek Wireless Inc., Canada; 15 by Vectronic Aerospace GmbH, Germany; and 2 by Followit AB, Sweden). We documented the sex and weight of each captured individual and estimated its age based on tooth wear (kittens 8–11 months, subadults 11 months to 2 years, adults 3 ≥ years;34,35) or by information from systematic camera-trapping. All animals were immediately released at the trapping location. The only exception was the female Lea (ID 83803; Table S1), which was found as an orphan during the winter 2019 and released at the same location after 6 months of rehabilitation. Collars were configured to collect a range of 3–24 fixes daily, maintaining a baseline frequency of three locations per day, interspersed with intensive periods of up to hourly fixes when predation studies were being conducted.
Due to box-trap malfunctions (trap door failures), we missed at least six potential lynx captures. Incidental by-catches included brown bear (Ursus arctos), European badger (Meles meles), red fox (Vulpes vulpes), European wildcat (Felis silvestris), domestic cat (Felis catus), beech marten (Martes foina), pine marten (Martes martes), hazel grouse (Tetrastes bonasia), and tawny owl (Strix aluco), all of which were promptly released.
Home-range estimations
We calculated annual and seasonal lynx home ranges using 95 and 50% (core areas) Minimum Convex Polygons (MCP;36), Kernel home ranges (KDE37), and Autocorrelated Kernel home ranges (AKDE;38) to allow for comparisons with previous studies using different methods. We defined two seasons based on the lynx movement rate throughout the year (Fig. S1). First, we calculated the daily movement rates of all lynx and pooled them across individuals and years. Next, we estimated the effect of the month of survey on the lynx movement rate using Generalized Additive Models (GAMs) and predicted monthly rates at the population level (Fig. S1). Finally, we used mean annual daily movement rate (x̄ = 6.67 km/day) to divide estimates into two seasons, i.e. months with low movement rate typical for spring and summer (1st April–30th September,hereafter summer); and months with high movement rate typical for autumn and winter (1st October–31st March; hereafter winter).
A minimum of 6 months was required to calculate the annual home range3,17,39 and a minimum of 20 locations was required to calculate the seasonal home range2,39. We considered lynx home range as established and permanent, when exhibiting a polygonal movement, and excluded locations representing excursions of adult lynx or dispersion of subadult individuals. Home ranges were calculated using the amt and ctmm package40,41 in R statistical environment42.
Prey density and anthropogenic impact
We calculated the relative roe deer density based on the average number of harvested roe deer in each hunting ground situated within a lynx home range over a 5-year period (2016–2020), for which data were available in all three countries (Table S2). Harvest data were provided by the local administration bodies responsible for hunting management in the Czech Republic, the Polish National Forest Holding "State Forests," and the National Forest Centre of the Slovak Republic. The management system of roe deer in all three countries is similar. In Czechia and Slovakia, the hunting season for roe deer spans from May 16 to December 31. In Poland, the hunting season for roe deer spans from May 11 to January 15. Annual harvest quotas are calculated from estimated population sizes ("spring counts") and validated by municipal game boards. To derive the relative density of roe deer per 1 km2 across our study area, we calculated the average number of harvested roe deer in each hunting ground, divided it by the area of a hunting ground, and subsequently scaled within the 0–1 interval. This type of index has been previously used for roe deer and other ungulates (e.g.3,20,43,44) and we assumed that the harvest density per hunting ground accurately mirrored roe deer density at large scale because the hunting systems of Slovakia, Czechia, and Poland employ relatively small hunting grounds (averaging 12 km2 in Czechia, 55 km2 in Poland, and 25 km2 in Slovakia). Finally, we calculated the average roe deer density index weighted by the area of hunting ground within a home range of each individual lynx.
The anthropogenic impact was represented by the forest cover and road density within a lynx home range. Forest cover within the home range of each lynx was calculated using Corine Landcover 201823. We extracted coniferous, deciduous and mixed forests and shrubland from the raster map (100 × 100 m) and calculated the percentage of forest cover (%) within a home range of each lynx by dividing the area of forests within each lynx home range by its area using ArcMap 10.545. Similarly, we used a road map of highways, primary, secondary, tertiary roads and local roads from the Global Roads Inventory Project 202346 to calculate road density (km/km2) per each lynx home range by dividing the length of all roads within each lynx home range by its area.
Fidelity and territoriality
In assessing the fidelity of lynx to their home range, we quantified the extent of overlap and computed Euclidean distances between centroids for consecutive home ranges of individual lynx across successive years. To explore social dynamics, we examined the percentage of overlap and Euclidean distances between centroids of 95% home ranges for male-to-male and male-to-female lynx coexisting in the same area concurrently. The investigation of female territoriality was hampered by a lack of available data pertaining to neighbouring individuals. Overlap between home ranges was calculated as a standardized measure, accounting for the geometric mean of the total areas of the two home ranges following Breitenmoser-Würsten et al.5:
where xij represents the overlapping area between areas of home ranges xi and xj.
Statistical analyses
In order to compare annual and seasonal home range sizes between different groups of lynx (i.e. males vs. females, adult vs. subadult, reproducing vs. non-reproducing female), and to assess differences in centroid distances between home ranges, we employed the non-parametric Mann–Whitney U test47. To assess differences between home range estimators, we used a paired t-test. To investigate the influence of environmental variables on lynx home range sizes (annual and seasonal), we pooled data across all home range types (MCP, KDE, AKDE) and used a mixed-effects linear model (LME) implemented within the R package lme448 in R statistical environment. We used log-transformed lynx home range size as a response variable (y), with fixed variables (xi) encompassing an individual's sex (females 0, males 1), prey density, forest cover, and density of roads (including interactions of sex with environmental variables) and the home range estimator was used as a random intercept. Collinearity of the predictor variables was tested by the Pearson’s correlation (threshold ≥ 0.6) and the Akaike’s Information Criterion (AICc) for small sample sizes was used to select the most parsimonious models49. Predictions were visualized using the visreg R package50. To analyse the percentages of overlaps among lynx home ranges, a permutation test51 with 10,000 permutations was conducted for robust statistical assessment of small datasets. For clarity, we report only significant results.
Results
In total, we collected 57,492 locations from 35 lynx collared from 2011 to 2022 (Table S1). The average survey time per animal was 300.6 ± 198.3 days (mean ± SD). From these, we used 47,937 fixes for creating 24 annual home ranges of 14 males and 8 females, including three males with two consecutive annual home ranges (Table S1). Further, we used 25,669 fixes to create 36 summer home ranges of 24 males and 12 females, including 5 males and 1 female with two consecutive summer home ranges, and 14,771 fixes to create 24 winter home ranges of 11 males and 9 females, including 2 males with two consecutive home ranges and 1 male with three consecutive home ranges (Table S1). We also identified three dispersing subadult lynx (2 males and 1 female) in our dataset which were excluded from home-range analyses.
Lynx home range size
Average annual home range (MPC 95%) was 282.9 ± 42.4 km2 (mean ± SE) for males and 147.6 ± 50.3 km2 for females. In summer, male home range size was 203.7 ± 22.0 and female home range size 86.9 ± 21.2, while in winter, 239.8 ± 42.6 and 128.9 ± 36.0 (MPC 95%) respectively. These seasonal difference in home range size of both sexes were, however, nonsignificant. All home range sizes were larger when the other estimators were adopted (Table S3) since MCP consistently produced smaller home ranges compared to KDE (annual: tpaired = − 2.22, P = 0.037; summer: tpaired = − 5.12, P = 0.014; winter: tpaired = − 2.74, P = 0.012) and AKDE (annual: tpaired = − 2.85, P = 0.009; summer: tpaired = − 4.62, P ≤ 0.001; winter: tpaired = − 3.44, P = 0.002). KDE estimates were comparable to AKDE for annual and winter home ranges but were significantly lower than AKDE in summer (tpaired = − 2.19; P = 0.035).
Males exhibited significantly larger annual, and seasonal home ranges than females across all home range estimators (Fig. 2a, b, c; Table S3). Reproducing females had smaller annual (77.7 ± 12.0; MPC 95%) and summer (50.1 ± 8.3; MCP 95%) home ranges compared to non-reproducing females (175.5 ± 67.9 and 108.0 ± 30.8, respectively), although this difference was significant only with KDE in summer (Fig. 2d, e; Table S4). Also, winter home ranges of non-reproducing females (140.8 ± 45.9; MCP 95%) were slightly larger than reproducing females, however, this difference was not significant (Fig. 2f). While adult lynx generally exhibited larger home ranges (239.9 ± 82.6; MPC 95%) than subadults (201.4 ± 23.4; MCP 95%), this difference was statistically significant only in winter (Fig. 2g, h, i; Table S5). Core areas (85.5 ± 15.5 km2 for males and 58.1 ± 43.1 km2 for females; MCP 95%), constituted, on average, 24–29% of annual home ranges, 24–33% of summer home ranges and 22–25% of winter home ranges, depending on the home range estimating method (Table S6). Subadult lynx dispersed for 12–90 km (70.3 ± 11.3 km in males and 21.6 ± 8.9 km in females; mean ± SE) and exhibited an average annual home range size of 521.73 km2 (MCP 95%; Table S5).
Comparisons of annual, summer and winter home ranges between male (blue) and female (red), non-reproducing female (orange) and reproducing female (purple), and adult (yellow) and subadult (green) Eurasian lynx (Lynx lynx) in the Western Carpathians during 2011–2022 using Mann–Whitney U test for three different home range estimation methods (see details in Supplementary material, Tables S1, S3, S3).
Environmental factors driving lynx home range size
Annual lynx home range size decreased with increasing roe deer density within it (Fig. 3a) and home range size expanded with increasing road density (Fig. 3d; Table S8). Although, male lynx home range significantly decreased in summer in response to increasing forest cover, it had no effect on home ranges of females (Fig. 3b). Consistent with the annual period, lynx increased summer home range size with increasing road density (Fig. 3e). In winter, we found prey density to be the only significant factor affecting lynx home range size (Fig. 3c; Table S8). However, only the females' home range size significantly decreased with increasing prey density, while the males' home ranges remained almost constant. According to LME, lynx home ranges did not change significantly over the 12 years of our study.
Fidelity and territoriality
The mean overlap of successive annual home ranges for individual males varied between 76 and 78%, depending on the home range estimator and did not change during seasons (Table S9). The mean distance between centroids of successive annual home ranges for males was 2.4–2.7 km (Table S8). During summer, centroid distances ranged between 3.5 and 3.7 km, and during winter, they decreased to 2.1–2.4 km, however, this difference was not statistically significant. Notably, the observed overlap in the summer home ranges of female Lea (ID 83803) was much smaller, at 38–42%, and the distance between centroids in her home ranges was greater, at 4.4–4.9 km (Table S9).
Neighbouring males shared on average 15–18% of their annual home ranges and the mean distance between centres of their home ranges was 16.9–20.7 km (Table S10). During summer, the mean overlap ranged between 5.6 and 11.6% and increased in winter to 12.8–31.2%, however, this increase was significant only by KDE (KDE: Test statistic = 0.787, P = − 0.045). Nonetheless, the distance between centres of summer home ranges 13.7–16.4 km was similar to those in winter 12.1–18.7 km.
Males and females shared 44–46% of their annual home ranges and the mean distance between centres of their home ranges was 8.5–9.1 km, depending on home range estimator (Table S10). During summer, the mean overlap ranged between 17 and 27%, increasing in the winter to 43–51%. This increase was statistically significant only when measured using the AKDE estimator, which accounts for temporal autocorrelation in tracking data (AKDE: Test statistic = − 0.266, P = 0.034). The distance between summer home range centroids of lynx of different sex was 9.7–11.6 km and slightly decreased to 7.3–7.9 km in winter but this change was not significant.
Discussion
Lynx home ranges in the Western Carpathians were 1.7–10.5 times larger than previously estimated by less precise methods52,53 and were comparable to populations in Bohemian Forest, Czechia and Bavarian Forest, Germany54, Białowieża Primeval Forest, Poland6, Swiss Jura, Switzerland5,55, and Macedonia9. Eurasian lynx display a large between-population variation in the size of home ranges across their European range. The smallest average home ranges (169 km2 for males and 100 km2 for females; MCP 100%) were in northwestern Alps, Switzerland15, while the largest (1857 km2 for males and 916 km2 for females; MCP 95%) were along the Barents Sea coast of northern Norway (1; Table S11). Notably, MCP tended to provide smaller estimates compared to KDE and AKDE, a pattern observed in other studies (e.g.6,9). Estimators such as KDE and AKDE incorporate the spatial and temporal autocorrelation of locations. These components can result in larger estimated home ranges compared to MCP, which treats all location points equally without considering their spatial distribution and temporal sequence.
We observed substantial within-population variation in the size of lynx home ranges influenced by intrinsic factors. As predicted, male lynx displayed significantly larger home ranges compared to females (Fig. 2a, b, c), a trend consistent with observations in other populations2,54,56. This pattern is driven by the inherent necessity of males to maximise their reproductive success by accessing multiple receptive females57,58. Females with kittens had reduced annual and summer home ranges compared to non-reproducing females, comparable to observations from Białowieża NP, Poland6, Central Norway10 and populations in Switzerland7. This is likely linked to a maternal behaviour59,60 when reproducing females restrict movement due to limited mobility of kittens11,61. As kittens become larger and more mobile, females expand their winter home ranges to access to increased demand for prey62. Subadult lynx had smaller home ranges compared to adults, consistent with findings from populations in Poland6, Norway10, and Switzerland7. Subadults have smaller home ranges than adults due to factors such as developing hunting skills5, competition for prime territories from adults, and avoiding potential conflicts before reaching reproductive maturity55,56,58. Furthermore, resident lynx drove subadults to disperse and search for new territories within a ~ 50 km radius, similar to previous studies6,14.
Lynx maintained a relatively consistent home range size across seasons which is in conformity with studies from Poland6, Switzerland7, Sweden63 and Norway61. Despite having migratory prey sources, the migratory distances of roe deer in Europe are relatively short, with recorded distances of 5.5 up to 12 km, and only a fraction of the roe deer population (~ 32–40%) migrates, while resident individuals remain distributed throughout the area year-round64,65. Consequently, the availability and distribution of prey remain relatively stable across extensive lynx home ranges, reducing the necessity for significant alterations in its seasonal size.
Our study confirms the hypothesis that lynx home range size is significantly influenced by prey availability. Specifically, we observed that lynx, especially females, reduced annual and winter home range size with increasing roe deer density (Fig. 3a, c), consistent with related studies at regional and continental scales1,3,62,66. This reduction in home range size can be attributed to the higher availability of roe deer, the primary prey of lynx, within a smaller area. By optimizing their home range sizes in response to prey abundance, lynx, and particularly females, effectively balance resource utilization and energy expenditure, leading to a negative correlation between home range size and roe deer density3,11,20 as also observed in Canada lynx (Lynx canadensis,67,68,69). However, roe deer density, had no effect on lynx home ranges during summer, nor on male home ranges during winter. This can be explained by the availability of alternative prey species during summer (i.e., red deer offspring, rodents and birds70,71,72), potentially mitigating the impact of roe deer density on their summer home range size. When these alternative prey sources are abundant and easily obtainable, lynx may not need to adjust their home ranges in response to fluctuations in roe deer density7,20 (Schmidt et al. 200873). Conversely, during winter, male lynx may prioritize the territory maintenance and mate-seeking behaviours, which could most likely outweigh the effect of prey density on their home range3,6,11,55. Alternatively, our results might have been affected by using only annual harvest data instead of seasonal population sizes of roe deer, however, such data are unavailable.
Our analysis uncovered compelling evidence of human-induced habitat changes on lynx home range size, both annually and during the summer months. We found that the extent of forest cover significantly decreased the home range size of males during summer, whereas no such relationship was observed for females (Fig. 3b). While reproducing females may prioritize maternal duties, such as denning and caring for dependent offspring, and non-reproducing females exploring larger areas for prey and suitable habitats within their summer home ranges regardless of forest cover, the abundance of prey in forested habitats and less territorial behaviour could enable males to maintain smaller home ranges7,11,21. Nevertheless, increasing habitat fragmentation forces lynx to traverse open areas to access other forest patches, elevating the risk of human-induced mortality74 (Heurich et al. 201875). Our findings indicate that road density significantly influenced lynx spatial behaviour, leading to an expansion of both annual and summer home ranges with increasing road density (Fig. 3d, e). Notably, heavily utilized roads, especially fenced highways, exacerbate habitat fragmentation, compelling lynx to cover larger distances to circumvent them or risk collision with vehicles14,17. Although conspecific density is also known to influence lynx home range size in Europe (e.g.2,5,11), we were not able to evaluate its impact due to unavailable data for all surveyed lynx.
Male lynx exhibited relatively strong fidelity to their consecutive home ranges (˃ 70%) without seasonal variations, consistent with findings from Switzerland5,15,16 and Scandinavia1. Territorial behaviour, particularly among males, often involves marking and defending territories, supporting the hypothesis that loss of territory and associated landscape familiarity can significantly decrease lynx fitness61,76. This strong territoriality among males is further evidenced by relatively low home range overlaps (< 20%) and considerable distances between competing males, similar to observations in the northwestern Alps15, northeastern Switzerland16 and Swiss Jura Mountains5. Female summer home range overlap was significantly lower (~ 40%) compared to prior studies, which reported up to 75%. However, more research is required to draw meaningful conclusions about female home range fidelity. The most pronounced tendency for association was observed between males and females reaching its peak in winter (during mating season with an overlap of ~ 50%) and decreasing to a minimum in summer (≤ 20%). Lynx are strongly territorial and tend to avoid direct contacts with conspecifics to minimize conflicts and competition for prey7,67,69,77. Social interactions peak in winter due to mating, with males competing with each other to enhance access to females, while reproducing females are equally actively searching for mates10,11,55,56.
The insights into lynx movement provided by our study have clear implications because they will greatly enhance the accuracy of population size estimates across the Western Carpathians4. Our findings align with robust monitoring indicating considerable anthropogenic influence on the Western Carpathian lynx population26,30,78. Considering the substantial impact of human-induced mortality on the lynx population, such as vehicle collisions (32 lynx killed in vehicle collisions in Slovakia during our study) but also illegal killings (4 out of 35 surveyed lynx, i.e., 11.4%, were killed illegally, with another 3 lynx, 8.6%, suspected), we suggest to implement effective mitigation measures. One crucial strategy involves enhancing habitat connectivity, especially in cross-border areas and regions where key habitats are fragmented by fenced highways. By establishing wildlife crossings within natural wildlife corridors, we can facilitate safe passage for lynx and other wildlife, reducing the risk of collisions and promoting genetic exchange among fragmented populations76. Another strategy involves establishing a programme to detect, reduce, and prevent lynx illegal killing as suggested by Arlettaz et al.73. Engaging hunters, foresters and local communities is crucial for promoting coexistence and mitigating conflicts in large carnivore conservation efforts27. Range-wide collaborative approaches involving key stakeholders in lynx monitoring, conservation, and education programs can enhance sustainable management practices and efforts in the Carpathians and Europe79.
Data availability
The datasets generated during and/or analysed during the current study are available in the [EUROMAMMALS] repository, [https://euromammals.org/eurolynx/], and / or from the corresponding author on reasonable request.
References
Linnell, J. D. C., Mattisson, J. & Odden, J. Extreme home range sizes among Eurasian lynx at the northern edge of their biogeographic range. Nat. Ecol. Evol. 11, 10. https://doi.org/10.1002/ece3.74365001-5009 (2021).
Linnell, J. D. C. et al. Home range size and choice of management strategy for lynx in Scandinavia. J. Environ. Manag. 27, 869–879 (2001).
Herfindal, I., Linnell, J. D. C., Odden, J., Nilsen, E. B. & Andersen, R. Prey density, environmental productivity, and home range size in the Eurasian lynx (Lynx lynx). J. Zool. 265, 63–71 (2005).
Palmero, S., Premier, J., Kramer-Schadt, S., Monterroso, P. & Heurich, M. Sampling variables and their thresholds for the precise estimation of wild felid population density with camera traps and spatial capture–recapture methods. Mam. Rev. 53, 223–237 (2023).
Breitenmoser-Würsten, C. et al. Spatial and social stability of a Eurasian lynx Lynx lynx population: An assessment of 10 years of observation in the Jura Mountains. Wildl. Biol. 13, 365–380 (2007).
Schmidt, K., Jędrzejewski, W. & Okarma, H. Spatial organization and social relations in the Eurasian lynx population in Bialowieza Primeval Forest, Poland. Acta Theriol. 42, 289–312 (1997).
Breitenmoser, U. & Breitenmoser-Würsten, C. D. Luchs—ein Grossraubtier in der Kulturlandschaft (Salm Verlag, 2008).
von Arx, M. The IUCN red list of threatened species: Lynx lynx (Europe assessment) (amended version of 2018 assessment). https://doi.org/10.2305/IUCN.UK.2020-3.RLTS.T12519A177350310.en (2020).
Melovski, D. et al. First insight into the spatial and foraging ecology of the critically endangered Balkan lynx (Lynx lynx balcanicus, Buresh 1941). Hystrix 31, 26–34 (2020).
Sunde, P., Kvam, T., Moa, P., Negard, A. & Overskaug, K. Space use by Eurasian lynxes Lynx lynx in central Norway. Acta Theriol. 45, 507–524 (2000).
Aronsson, M. et al. Intensity of space use reveals conditional sex-specific effects of prey and conspecific density on home range size. Nat. Ecol. Evol. 6, 2957–2967 (2016).
Pesenti, E. & Zimmermann, F. Density estimations of the Eurasian lynx (Lynx lynx) in the Swiss Alps. J. Mammal. 94, 73–81 (2013).
Ripari, L. et al. Human disturbance is the most limiting factor driving habitat selection of a large carnivore throughout Continental Europe. Biol. Conserv. 266, 109446 (2022).
Zimmermann, F., Breitenmoser-Würsten, C. & Breitenmoser, U. Importance of dispersal for the expansion of a Eurasian lynx Lynx lynx population in a fragmented landscape. Oryx 41, 358–368 (2007).
Breitenmoser-Würsten, C. et al. Untersuchungen zur Luchspopulation in den Nordwestalpen der Schweiz 1997–2000. KORA Beicht 9, 1–88 (2001).
Ryser, A. et al. Luchsumsiedlung Nordostschweiz 2001–2003, Schlussbericht Modul Luchs des Projektes LUNO. KORA Bericht 22, 1–60 (2004).
Basille, M. et al. Selecting habitat to survive: The impact of road density on survival in a large carnivore. PloS One 8, e65493. https://doi.org/10.1371/journal.pone.0065493 (2013).
Gehr, B. et al. landscape of coexistence for a large predator in a human dominated landscape. Oikos 126, 1389–1399 (2017).
Kramer-Schadt, S., Revilla, E., Wiegand, T. & Breitenmoser, U. Fragmented landscapes, road mortality and patch connectivity: Modelling influences on the dispersal of Eurasian lynx. J. Appl. Ecol 41, 711–723 (2004).
Andrén, H. & Liberg, O. Numerical response of predator to prey: dynamic interactions and population cycles in Eurasian Lynx and roe deer. Ecol. Monogr. 94, e1594. https://doi.org/10.1002/ecm.1594 (2024).
Filla, M. et al. Habitat selection by Eurasian lynx (Lynx lynx) is primarily driven by avoidance of human activity during day and prey availability during night. Ecol. Evol. 7, 6367–6381 (2017).
Basille, M. et al. What shapes Eurasian lynx distribution in human dominated landscapes: Selecting prey or avoiding people?. Ecography 32, 683–691 (2009).
European Copernicus Programme. CORINE Land Cover 2018. https://land.copernicus.eu/en/products/corine-land-cover/clc2018 (2023).
Bazzicalupo, E. et al. History, demography and genetic status of Balkan and Caucasian Lynx lynx (Linnaeus, 1758) populations revealed by genome-wide variation. Divers. Distrib. 28, 65–82 (2022).
Lucena-Perez, M. et al. Ancient genome provides insights into the history of Eurasian lynx in Iberia and Western Europe. Quat. Sci. Rev. 285, 107518. https://doi.org/10.1016/j.quascirev.2022.107518 (2022).
Duľa, M. et al. Multi-seasonal systematic camera-trapping reveals fluctuating densities and high turnover rates of Carpathian lynx on the western edge of its native range. Sci. Rep. 11, 9236. https://doi.org/10.1038/s41598-021-88348-8 (2021).
Kubala, J. et al. Conservation needs of the Carpathian lynx population. CATnews Spec. Iss. 14, 12–15 (2021).
Niedziałkowska, M. et al. Environmental correlates of Eurasian lynx occurrence in Poland—Large scale census and GIS mapping. Biol. Conserv. 133, 63–69 (2006).
Jędrzejewski, W., Nowak, S., Schmidt, K. & Jędrzejewska, B. The wolf and the lynx in Poland—results of a census conducted in 2001. Kosmos 51, 491–499 (2002).
Kubala, J. et al. Robust monitoring of the Eurasian lynx Lynx lynx in the Slovak Carpathians reveals lower numbers than officially reported. Oryx 53, 548–556 (2019).
LifeLynx. Protocol for Eurasian lynx (Lynx lynx) capture, narcosis, transport, and quarantine in the Slovak Carpathians. https://www.lifelynx.eu/wp-content/uploads/2018/06/C1_Protocol-for-lynx-capture-narcosis-transport-and-quarantine-in-the-Slovak-Carpathians.pdf (2018).
Signer, S. et al. Luchsumsiedlungen aus der Schweiz von 2016–2020 in den Pfälzerwald und in die Kalkalpen. KORA Bericht 100, 1–26 (2021).
Percie du Sert, N. et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. https://doi.org/10.1371/journal.pbio.3000410 (2020).
Marti, I. & Ryser-Degiorgis, M. P. A tooth wear scoring scheme for age estimation of the Eurasian lynx (Lynx lynx) under field conditions. Eur. J. Wildl. Res. 64, 1–13 (2018).
Marti, I. & Ryser-Degiorgis, M. P. Morphometric characteristics of free-ranging Eurasian lynx Lynx lynx in Switzerland and their suitability for age estimation. Wildl. Biol. 1, 1–10 (2018).
Mohr, C. O. Table of equivalent populations of North American small mammals. Am. Midl. Nat. 37, 223–249 (1947).
Worton, B. J. Kernel methods for estimating the utilization distribution in home-range studies. Ecology 70, 164–168 (1989).
Fleming, C. H. et al. Rigorous home range estimation with movement data: A new autocorrelated kernel density estimator. Ecology 96, 1182–1188 (2015).
White, S., Briers, R. A., Bouyer, Y., Odden, J. & Linnell, J. D. C. Eurasian lynx natal den site and maternal home-range selection in multi-use landscapes of Norway. J. Zool. 297, 87–98 (2015).
Calabrese, J. M., Fleming, C. H. & Gurarie, E. ctmm: An R package for analysing animal relocation data as a continuous-time stochastic process. MEE 7(9), 1124–1132 (2016).
Signer, J., Fieberg, J. & Avgar, T. Animal movement tools (Amt): R package for managing tracking data and conducting habitat selection analyses. Nat. Ecol. Evol. 9, 880–890 (2019).
R Core Team. R: A language and environment for statistical computing. https://www.R-project.org/ (R Foundation for Statistical Computing, 2021).
Mysterud, A. et al. Monitoring population size of red deer Cervus elaphus: An evaluation of two types of census data from Norway. Wildl. Biol. 13, 285–298 (2007).
Ueno, M., Solberg, E. J., Iijima, H., Rolandsen, C. M. & Gangsei, L. E. Performance of hunting statistics as spatiotemporal density indices of moose (Alces alces) in Norway. Ecosphere 5, 1–20 (2014).
ESRI. ArcGIS Desktop: Release 10.5. Environmental Systems Research Institute (2016)
Meijer, J. R., Huijbregts, M. A. J., Schotten, C. G. J. & Schipper, A. M. Global patterns of current and future road infrastructure. Environ. Res. Lett. 13(6), 064006. https://doi.org/10.1088/1748-9326/aacb93 (2018).
Mann, H. B. & Whitney, D. R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 18, 50–60 (1947).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Anderson, D. R. & Burnham, K. P. Avoiding pitfalls when using information-theoretic methods. J. Wildl. Manag. 66, 912–918 (2002).
Breheny, P. & Burchett, W. Visualization of regression models using visreg. R. J. 9, 1–56 (2017).
Good, P. I. Permutation, Parametric and Bootstrap Tests of Hypotheses 3rd edn, 1–315 (Springer, 2005).
Hell, P. Der Luchs und seine Erhaltung in Europa (Schaffhausen, 1971).
Okarma, H., Śnieżko, S. & Śmietana, W. Home ranges of Eurasian lynx Lynx lynx in the Polish Carpathian Mountains. Wildl. Biol. 13, 481–487 (2007).
Magg, N. et al. Habitat availability is not limiting the distribution of the Bohemian-Bavarian lynx Lynx lynx population. Oryx 50(4), 742–752 (2016).
Breitenmoser, U. et al. Spatial organization and recruitment of lynx (Lynx lynx) in a re-introduced population in the Swiss Jura Mountains. J. Zool. 231, 449–464 (1993).
Jędrzejewski, W., Schmidt, K., Okarma, H. & Kowalczyk, R. Movement pattern and home range use by the Eurasian lynx in Białowieża Primeval Forest (Poland). Ann. Zool. Fenn. 39, 29–41 (1996).
Eisenberg, J. F. Life history strategies of the felidae: Variations on a common theme. In Cats of the World: Biology, Conservation, and Management (eds Miller, S. D. & Everett, D. D.) 293–303 (National Wildlife Federation, 1986).
Sandell, M. The mating tactics and spacing patterns of solitary carnivores. In Carnivore Behavior, Ecology, and Evolution (ed. Gittleman, J. L.) 164–182 (Springer, 1989).
Davies, N. B. Mating system. In Behavioural Ecology-An Evolutionary Approach 263–294 (Wiley, Hoboken, 1991).
Sunquist, M. & Sunquist, F. Wild Cats of the World (University of Chicago Press, 2017).
Walton, Z., Mattisson, J., Linnell, J. D. C., Stien, A. & Odden, J. The cost of migratory prey: seasonal changes in semi-domestic reindeer distribution influences breeding success of Eurasian lynx in northern Norway. Oikos 126, 642–650 (2017).
Molinari-Jobin, A. et al. Variation in diet, prey selectivity and home-range size of Eurasian lynx Lynx lynx in Switzerland. Wildl. Biol. 13, 393–405 (2007).
Danell, K., Bergström, R., Duncan, P. & Pastor, J. Large Herbivore Ecology, Ecosystem Dynamics and Conservation Vol. 11 (Cambridge University Press, 2006).
Cagnacci, F. et al. Partial migration in roe deer: Migratory and resident tactics are end points of a behavioural gradient determined by ecological factors. Oikos 120, 1790–1802 (2011).
Ramanzin, M., Sturaro, E. & Zanon, D. Seasonal migration and home range of roe deer (Capreolus capreolus) in the Italian eastern Alps. Can. J. Zool. 85(2), 280–289 (2007).
Bouyer, Y. et al. Eurasian lynx habitat selection in human-modified landscape in Norway: Effects of different human habitat modifications and behavioral states. Biol. Conserv. 191, 291–299 (2015).
Burdett, C. L., Moen, R. A., Niemi, G. J. & Mech, L. D. Defining space use and movements of Canada lynx with global positioning system telemetry. J. Mammal. 88(2), 457–467 (2007).
O’Donoghue, M. et al. Cyclical dynamics and behaviour of Canada lynx in northern Canada. In Biology and Conservation of Wild Felids (eds Macdonald, D. W. & Loveridge, A. J.) 521–536 (Oxford, 2010).
Vashon, J. H. et al. Spatial ecology of a Canada lynx population in northern Maine. J. Wildl. Manag. 72(7), 1479–1487 (2008).
Buzan, E. et al. Molecular analysis of scats revealed diet and prey choice of grey wolves and Eurasian lynx in the contact zone between the Dinaric Mountains and the Alps. Front. Zool. 21, 9. https://doi.org/10.1186/s12983-024-00530-6 (2024).
Duľa, M. et al. The first insight into hunting and feeding behaviour of the Eurasian lynx in the Western Carpathians. Mamm. Res. 68, 237–242 (2023).
Mysłajek, R. W., Stachyra, P., Figura, M. & Nowak, S. Food habits of the Eurasian lynx Lynx lynx in southeast Poland. J. Vertebr. Biol. 71, 1–7 (2021).
Schmidt, K. Behavioural and spatial adaptation of the Eurasian lynx to a decline in prey availability. Acta Theriol, 53, 1–16 (2008).
Arlettaz, R. et al. Poaching threatens the establishment of a lynx population, highlighting the need for a centralized judiciary approach. Front. Conserv. Sci. 2, 665000. https://doi.org/10.3389/fcosc.2021.665000 (2021).
Heurich, M. et al. Illegal hunting as a major driver of the source-sink dynamics of a reintroduced lynx population in Central Europe. Biol. Conserv. 224, 355–365 (2018).
Van Moorter, B. et al. Memory keeps you at home: A mechanistic model for home range emergence. Oikos 118(5), 641–652 (2009).
Wőlfl, M. Zur intersexuellen Konkurrenzverminderung beim Eurasischen Luchs (Lynx lynx L.) in der Schweiz (Universität München, 1993).
Kubala, J. et al. The coat pattern in the Carpathian population of Eurasian lynx has changed: A sign of demographic bottleneck and limited connectivity. Eur. J. Wildl. Res. 66, 2. https://doi.org/10.1007/s10344-019-1338-7 (2020).
Bonn Lynx Expert Group. Recommendations for the conservation of the Eurasian lynx in Western and Central Europe. CATnews Spec. Iss. 14, 78–86 (2021).
Acknowledgements
We thank all institutions, fieldworkers and students participating in this study, particularly the Ministry of the Environment of the Slovak Republic, the State Nature Conservancy of the Slovak Republic, the Forests of the Slovak Republic enterprise, the Ministry of the Environment of the Czech Republic, the Nature Conservation Agency of the Czech Republic, the Forests of the Czech Republic, state enterprise, the Zoo Bojnice, the Zoo Ostrava, and the ČSOP Salamandr. For veterinary and field assistance, we would like to thank Lukáš Pavlačík, Pavel Forejtek†, Ivo Šturm, Ladislav Fiala, Vladimír Piaček, Izabela Całus. Jaroslav Brndiar, Eva Gregorová, Nuno Filipe De Campos Peixoto Guimaraes, Petr, Konupka, Peter Kováč, Mirko Krejči and Štefan Zatroch†.
Funding
The Slovak part of this study was funded by the European Commission (EU Structural Funds and the Cohesion Fund) and the Ministry of the Environment of the Slovak Republic (LIFE13 NAT/DE/000755, and LIFE16 NAT/SI/000634), by the Operational Programme Quality of Environment (No. ITMS 310011L489), FORRES No. ITMS 313011T678), and FOMON (No. ITMS 313011V465) by the Operational Programme Integrated Infrastructure, as well as Centre of Excellence (No. ITMS 26220120006), and Completing the Centre of Excellence (No. ITMS 26220120049), supported by the Operational Programme Research and Development (European Regional Development Fund). The Czech part was funded by the Operational Programme Environment via State Environmental Fund of the Czech Republic and by the Nature Conservation Agency of the Czech Republic (project No. 9028766, 2011–2014), and partially supported by the European Regional Development Fund, the Cross-border Cooperation Program Slovak Republic – Czech Republic 2014 – 2020 (INTERREG V-A SK-CZ 304021D016), the Danube Transnational Programme (No. DTP3-314–2.3), the Friends of the Earth Czech Republic, Carnivore Conservation Programme, and by the Operational Programme Environment (No. CZ.05.4.27/0.0/0.0/20_139/0012815). The Polish part was funded by the statutory budget of the Association for Nature “Wolf”, Poland.
Author information
Authors and Affiliations
Contributions
J.K., P.S., J.S., S.F., M.D., J.K.-P., R.M., and S.N. designed research; all authors performed research; J.K., P.S., J.S., S.F., M.D., J.K.-P., R.M., S.N., M.K., M.S.L., P.K., and R.K. wrote the manuscript and prepared figures; all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jakub, K., Johannes, S., Slavomír, F. et al. Factors shaping home ranges of Eurasian lynx (Lynx lynx) in the Western Carpathians. Sci Rep 14, 21600 (2024). https://doi.org/10.1038/s41598-024-71800-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71800-w
- Springer Nature Limited