Abstract
Previous findings have reported the association between frailty and chronic kidney disease. However, the causality remains ambiguous. This study aimed to determine whether frailty index is causally associated with chronic kidney disease. We obtained the frailty genome-wide association study (GWAS) data and chronic kidney disease GWAS data from the FinnGen R5 (total n = 216,743; case = 3902, control = 212,841) as the exposure and outcome, respectively. A two-sample Mendelian randomization (MR) analysis was primarily conducted using the inverse-variance weighted (IVW), weighted median and MR-Egger regression analyses. Multivariable MR analysis (MVMR) was conducted for additional adjustment. In the two-sample Mendelian randomization analyses, a total of 14 single nucleotide polymorphisms (SNPs) were recognized as effective instrumental variables. The IVW method showed evidence to support a causal association between frailty index and chronic kidney disease (beta = 1.270; 95% CI 0.608 to 1.931; P < 0.001). MR-Egger revealed a causal association between frailty index and chronic kidney disease (beta = 3.612; 95% CI 0.805 to 6.419; P = 0.027). MR-Egger regression revealed that directional pleiotropy was unlikely to be biasing the result (intercept = − 0.053; P = 0.119). The weighted median approach and weighted mode method also demonstrated a causal association between frailty index and chronic kidney disease (beta = 1.148; 95% CI 0.278 to 2.019; P = 0.011; beta = 2.194; 95% CI 0.598 to 3.790; P = 0.018). Cochran’s Q test and the funnel plot indicated no directional pleiotropy. MVMR analysis revealed that the causal association between frailty index and chronic kidney disease remained after adjusting for potential confounders, body-mass index, inflammatory bowel disease, waist-hip ratio, and C-reactive protein. Our study provides evidence of causal association between frailty and chronic kidney disease from genetic perspectives.
Similar content being viewed by others
Introduction
Frailty is commonly characterized as a state resulting from diminished homeostatic reserves in an individual, leading to heightened susceptibility to stressors and a consequent increased likelihood of adverse health outcomes1. Frailty is becoming more prevalent with the aging population trend and is associated with various negative outcomes, including disability, hospitalization, chronic diseases, and increased mortality2,3,4. While a definitive gold standard for identifying frailty remains elusive, there is increasing focus on the frailty index (FI) as a potential key measure5. Comprehending the potential relationship between frailty and age-related diseases, along with the underlying mechanisms, could facilitate tailored management and early intervention strategies for frailty patients.
Chronic kidney disease (CKD), affecting approximately 10% of the worldwide population, posing a substantial challenge to global health systems due to its high mortality rate6,7,8. Frailty is very common among patients with CKD. A meta-analysis revealed a wide variation in frailty prevalence, ranging from 7% among community-dwelling individuals with CKD stages 1–4 as high as 73% in patients undergoing hemodialysis9. However, establishing a definitive causal relationship between frailty and CKD remains challenging, since observational studies may result in reverse causation or leave residual confounding factors unaddressed. The characteristics of the study population, sample size, and the covariates included in the regression models could also introduce potential biases into the research findings.
Mendelian Randomization (MR) is an approach in genetic epidemiology, utilizing summary-level data from genome-wide association studies (GWAS) to infer causal relationships between diverse exposures and their outcomes10. In MR analysis, genetic variants associated with the exposures of interest, while being independent of any confounding factors, serving as instrumental variables (IVs)10. Consequently, MR can alleviate issues related to reverse causation and confounding factors, offering valuable insights into the intrinsic relationship between frailty and CKD. This understanding carries substantial implications for both public health and clinical practice. A recent Genome-Wide Association Study (GWAS) has pinpointed 14 genetic loci that influence the Frailty Index (FI), a widely recognized definition of frailty11,12,13. The FI, based on the accumulation of health deficits, proves particularly effective in assessing frailty at its early stages. Previous study has found that FI is a significant predictor of hospitalization and mortality in nephrology14. However, limitations in sample size have constrained the statistical power of the study. This MR study aims to assessing the causal impacts of genetically predicted FI on the risk of developing CKD using the most comprehensive and extensive GWAS datasets available.
Materials and methods
Ethics approval
The study used publicly accessible GWAS data, ensuring compliance with ethical considerations as the requisite consents were obtained during the conduct of the original studies.
Instrumental variable selection for frailty index
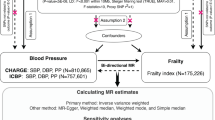
The overall layout of this MR study is illustrated in Fig. 1. Genetic variants that are significantly associated with the FI (P < 5 × 10–8) were sourced from a comprehensive GWAS meta-analysis by Atkins et al.11. This meta-analysis incorporated data from a substantial cohort, including 164,610 participants from the UK Biobank and 10,616 participants from the TwinGene project11. All participants were of European ancestry. The FI was assessed using the “accumulation of deficits” model, a widely recognized approach in frailty research. This model quantifies frailty based on the number and severity of health deficits an individual has. In this case, the FI was calculated using more than 40 self-reported baseline deficit variables, encompassing various aspects of health and functioning11,15. The FI has been extensively validated and is recognized for its efficacy in predicting diverse adverse health outcomes and demonstrating a particular suitability for assessing frailty in younger populations. The meta-analysis of the GWAS has taken into account adjustments for factors such as age, sex, the study center, and the genotyping array used11.
Schematic overview of the study design. (A) The standard MR framework. MR relies on three core assumptions for causal inference: 1. the genetic variants should be related to the exposure (relevance assumption), 2. instrument variables should not be associated with confounders (independence assumption), 3. instrument variables affect the outcome through the exposure of interest only (exclusion restriction assumption, also known as the “no pleiotropy”). (B) Multivariable MR, an extension of univariable MR, utilizes genetic instruments associated with multiple correlated exposures to jointly assess the independent effect of each of the exposures on the outcome. MR Mendelian randomization.
Initially, 14 single nucleotide polymorphisms (SNPs) were selected (Supplementary Table 1). Subsequently, linkage disequilibrium analysis was conducted, resulting in the exclusion of SNPs with r2 > 0.01 and distance > 10,000 kb16. The F-statistic parameters, essential for determining the strength of the identified instrumental variables (IVs), were computed following previously established methodologies16,17. We excluded SNPs with an F-statistic parameter less than 10, as they were considered weak IVs. All 14 SNPs met the selection criteria and were thus incorporated into the MR analysis. In cases where a specific SNP was not available in the CKD datasets, a proxy SNP in linkage disequilibrium (r2 > 0.9) was utilized as a substitute.
CKD dataset
Summary statistics for the associations of the FI-related genetic variants with CKD were obtained from a GWAS dataset called finn-b-N14_CHRONKIDNEYDIS including 3902 cases and 212,841 controls.
Statistical analysis
For each SNP under examination, the Wald ratio we computed. Subsequently, these ratios were aggregated using the multiplicative random-effect inverse-variance weighted (IVW) method. This approach was employed to derive the MR estimates, facilitating a comprehensive assessment of the genetic associations18,19. The IVW method, in its essence, adopts a meta-analysis framework to aggregate Wald ratios from each genetic variant, thereby deriving combined estimates of the effect of exposure on outcomes. This method is particularly effective in delivering consistent causal estimates, assuming that all the genetic variants employed serve as valid IVs19. In addition to the IVW method, our analysis incorporated several supplementary approaches including MR-Egger, weighted mode, and weighted median18. The weighted mode method yields robust causal estimates under the condition that the weights linked to valid IVs are the most significant within any homogenous subset of instruments20. The weighted median method relies on identifying the estimate positioned at the 50th percentile of the empirical distribution, essentially representing the median of the weighted causal estimates21. To evaluate heterogeneity, Cochran’s Q test was conducted, and utilized the MR-Egger regression intercept and MRPRESSO to examine potential pleiotropy22,23. A leave-one-out analysis was carried out to determine whether any single SNP disproportionately influenced the estimated effect. Results were considered statistically significant at P < 0.05. The entirety of the MR analysis was executed using R software version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria) with the TwoSampleMR18 and MRPRESSO packages, along with Zstats v1.0 (www.zstats.net).
Multivariable MR and covariates
Previous study has found that the genetic association of body-mass index (BMI), inflammatory bowel disease (IBD), waist-hip ratio (WHR), and C-reactive protein (CRP) with the risk of frailty11. Therefore, these four variables were included in the multivariable MR analysis. The multivariable MR analysis (MVMR) incorporated genetic variants linked to at least one exposure, and the causal relationship was estimated using the multivariable IVW method20. BMI and WHR (adjusted for BMI) associated genetic variants were sourced from GIANT consortium24,25. IBD genetic variants were sourced from IIBDGC consortium26. Genetic variants associated with CRP were sourced from the study conducted by Said et al.27.
Results
Results of MR
Genetically predicted frailty index and risk of CKD
A total of 14 SNPs significantly associated with FI were included. IVW method indicated genetically predicted FI is associated with the increased risk of CKD (beta = 1.270; 95% CI 0.608 to 1.931; P < 0.001). Besides, MR-Egger analysis (beta = 3.612; 95% CI 0.805 to 6.419; P = 0.027), weighted median analysis (beta = 1.148, 95% CI 0.278 to 2.019; P = 0.097), and weighted mode analysis (beta = 2.193, 95% CI 0.598 to 3.790; P = 0.018) also indicated comparable results (Table 1, Figs. 2, 3).
Scatter plots of genetic associations with frailty index against the genetic associations with chronic kidney disease. The slopes of each line represent the causal association for each method. The blue line represents the IVW estimate, the green line represents the weighted median estimate, the dark blue line represents the MR‐Egger estimate, the dark green line represents weighted mode. IVW inverse-variance weighted, MR Mendelian randomization.
Sensitivity analysis indicated no heterogeneities (all P > 0.050) in the estimated effects of FI on CKD. MR-Egger regression analysis discovered that directional pleiotropy did not introduce significant bias to the results, with an intercept of − 0.053 (P = 0.119). Furthermore, MR-PRESSO results suggested that there was no evidence of horizontal pleiotropy in this MR study (P = 0.240). Additionally, the result of a leave-one-out analysis affirmed the reliability of estimated effects across varying SNP exclusions (Fig. 4). Altogether, the present MR analysis suggested the association between genetically predicted FI and the risk of any CKD at a significance level of one standard.
“Leave-one-out” analysis examining the causal between frailty index and chronic kidney disease. Each black dot and bar represent the causal estimate and its 95% confidence interval (CI) when an individual SNP is sequentially excluded. The red dot and bar indicate the overall estimate and 95% CI; CI confidence interval, SNP single nucleotide polymorphism, IVW inverse-variance weighted.
Multivariable Mendelian randomisation analysis
We conducted a MVMR analysis that included BMI, CRP, WHR (adjusted for BMI), and IBD as covariates to further assess the causal relationships between genetic predictions of the FI and the risks of CKD. MVMR results demonstrated that the direct causal effect of FI on CKD was still significant after adjusting for BMI (OR 2.408, 95% CI 1.135 to 5.107, P = 0.022), WHR (adjusted for BMI) (OR 2.686, 95% CI 1.090–6.617, P = 0.032), IBD (OR 4.531, 95% CI 1.897 to 10.823, P < 0.001), and CRP (OR 1.855, 95% CI 1.011 to 3.404, P = 0.046) (Fig. 5).
Discussion
To our knowledge, this is the first study to investigate the association between FI and CKD. The FI is widely recognized as a prognostic indicator of adverse outcomes in CKD. However, the causal relationship between FI and CKD remains unanswered. In the present study, we employed four distinct methodologies for MR analysis: the IVW approach, the weighted median method, the MR-egger regression test, and the weighted mode technique. Each method independently pointed towards a positive causal relationship between FI and CKD. After adjusting for BMI, CRP, IBD, and WHR (adjusted for BMI), the effect of genetically predicted FI on CKD remained significant. Consequently, our findings substantiate the hypothesis that FI is causally linked to the progression of CKD.
Frailty is characterized by an increased vulnerability to health issues and affects approximately 1 in 6 older individuals residing in communities28. Frailty heightens the probability of negative consequences such as falls, hospital admissions, disability, and mortality29,30. The conceptual framework of FI posits frailty as a process of accumulating deficits, starting at the cellular level and resulting in diminished organ system redundancy31. The FI, a widely adopted tool in clinical settings for assessing frailty, has been thoroughly validated in numerous previous studies30,32.
In patients before dialysis, the prevalence of frailty varied between 7 and 42.6%33,34. The prevalence of frailty in patients undergoing hemodialysis could be even higher, potentially reaching as high as 73%35. Patients suffering from CKD and exhibiting frailty faced an increased risk of both mortality and hospitalization. The risk of mortality was notably high among patients with CKD, affecting those both on dialysis and in the pre-dialysis stage35,36,37,38. In comparison to non-frail CKD patients, frailty patients almost double the risk of medically urgent falls or fractures39. Additionally, previous study reported that frail CKD undergoing dialysis faced a 2.6 times higher risk of mortality40. In a recent meta-analysis, patients with frailty exhibited an increased risk of all-cause mortality compared to their non-frail counterparts41. Moreover, frail individuals on hemodialysis exhibit an elevated risk of hospital admissions and morality42. Hence, it is essential to offer prompt psychological support and guidance to frail patients.
Several mechanisms have been suggested to explain the relationship between frailty and CKD. A study involving the National Health and Nutrition Examination Survey found that frailty is associated with a significantly higher risk of CKD across all stages, even in individuals with preserved kidney function (eGFR > 60 mL/min/1.73 m2)43. This suggests that frailty may be a risk factor for the development of CKD, rather than just a consequence of existing kidney disease. Frailty, characterized by multi-system impairment including inflammation, immune dysfunction, and metabolic dysregulation, shares common pathways with the development of CKD44. These biological mechanisms contribute to the increased susceptibility of frail individuals to kidney damage, supporting the idea that frailty can lead to the inception of CKD. Several studies have uncovered the relationships between measures of kidney function and frailty45,46,47. A study by the Atherosclerosis Risk in Communities group revealed an association between frailty and decreases in cystatin C-based estimated glomerular filtration rate45. Dalrymple et al. found that lower estimated glomerular filtration rate calculated using serum cystatin C (eGFRcys) correlated with an increased risk of both existing and developing frailty46. In a recent cohort study, Li et al. found that Cystatin C and eGFRcys were associated with trajectories of frailty in the long run47. Besides, chronic inflammation may also play an important role in frailty development. Extant research indicates that inflammation is related to frailty across various chronic diseases, suggesting a ‘shared pathophysiology’ underlying frailty31. Elevated levels of inflammatory markers including C-reactive protein, interleukin-6, and tumor necrosis factor are associated with frailty48,49. Besides, CKD patients exhibit increased levels of proinflammatory cytokines50. Therefore, chronic inflammation is a well-recognized common pathway contributing to both frailty and CKD. However, frailty may independently contribute to the worsening of CKD through several mechanisms. Firstly, frailty is associated with a catabolic state and muscle wasting51, leading to decreased renal function and increased risk of CKD progression. Secondly, frailty often coincides with malnutrition and reduced protein intake, which can adversely affect renal function. Thirdly, frailty is linked to cardiovascular dysfunction52, which can lead to decreased renal perfusion and contribute to CKD. Fourthly, chronic low-grade inflammation is common in frailty and can exacerbate CKD through mechanisms like endothelial dysfunction and fibrosis.
Several limitations should be mentioned in this study. First, the issue of population stratification stands as a potential biasing factor in the MR analysis, given the observed tendency for older frailty patients and predominantly female53. Secondly, the presence of pleiotropy, especially horizontal pleiotropy, is an almost inescapable aspect of MR analysis. Although the MR-Egger intercept test was conducted to mitigate horizontal pleiotropy, complete mitigation remains challenging. Thirdly, the relatively low adoption rate poses the limitation, particularly in countries with low or middle income, where FI utilization is not widespread. Moreover, prior studies have indicated a higher probability of frailty among individuals with lower socioeconomic status. However, the reliance on GWAS data for FI predominantly derived from the UK Biobank may introduce selection bias. This is because individuals with lower socioeconomic status, who are more likely to exhibit frailty, may be underrepresented in the UK Biobank. Additionally, participants in the UK Biobank tend to be healthier than the general population, which could skew our findings towards a healthier demographic. Fourthly, our study relies on cross-sectional data, which limits the ability to assess the temporal relationship between FI and CKD. Future research should involve longitudinal data to better capture the temporal dynamics between FI and CKD. Besides, the inclusion of only individuals of European populations limits the generalizability of the findings to other ethnic groups. We will extend our research to include diverse ethnic populations in future studies to enhance the applicability of our findings globally. Fifth, while the study establishes a causal relationship between FI and CKD, it does not provide insights into the underlying biological mechanisms. We will integrate additional omics data (e.g., transcriptomics, proteomics) in future studies to help elucidate the molecular pathways involved. We are also considering collaborations with experts in the field of systems biology and bioinformatics to ensure a multidisciplinary approach to dissecting these complex mechanisms. Last but not least, we did not replicate our results in an independent cohort due to resource constraint. We plan to collaborate with other research groups to access independent cohorts for replication studies.
In conclusion, this MR analysis supported that genetically predicted FI is probably associated with an increased risk of CKD. Our results suggest that FI may play an important role in the development of CKD. The findings might offer a chance to explore the mechanisms of FI on the development of CKD.
Data availability
The datasets analyzed in the current study are publicly available from the public datasets (https://gwas.mrcieu.ac.uk/datasets/ebi-a-GCST90020053/ for ebi-a-GCST90020053 and https://gwas.mrcieu.ac.uk/datasets/finn-b-N14_CHRONKIDNEYDIS/ for finn-b-N14_CHRONKIDNEYDIS).
References
Buta, B. J. et al. Frailty assessment instruments: Systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res. Rev. 26, 53–61 (2016).
Veronese, N. et al. Prevalence of multidimensional frailty and pre-frailty in older people in different settings: A systematic review and meta-analysis. Ageing Res. Rev. 72, 101498 (2021).
Evans, N. R. et al. Frailty and cerebrovascular disease: Concepts and clinical implications for stroke medicine. Int. J. Stroke 17(3), 251–259 (2022).
Tan, L. F., Lim, Z. Y., Choe, R., Seetharaman, S. & Merchant, R. Screening for frailty and sarcopenia among older persons in medical outpatient clinics and its associations with healthcare burden. J. Am. Med. Dir. Assoc. 18(7), 583–587 (2017).
Rockwood, K. et al. A global clinical measure of fitness and frailty in elderly people. Can. Med. Assoc. J. 173(5), 489–495 (2005).
Vos, T. et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 390(10100), 1211–1259 (2017).
Woo, K. T., Choong, H. L., Wong, K. S., Tan, H. B. & Chan, C. M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 81(10), 1044–1045 (2012).
Ene-Iordache, B. et al. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): A cross-sectional study. Lancet Glob. Health 4(5), e307–e319 (2016).
Li, B. H. et al. The prevalence and influencing factors of frailty in patients with chronic kidney disease: A systematic review and meta-analysis. Int. Urol. Nephrol. 56, 767 (2023).
Lawlor, D. A., Harbord, R. M., Sterne, J. A., Timpson, N. & Davey, S. G. Mendelian randomization: Using genes as instruments for making causal inferences in epidemiology. Stat. Med. 27(8), 1133–1163 (2008).
Atkins, J. L. et al. A genome-wide association study of the frailty index highlights brain pathways in ageing. Aging Cell 20(9), e13459 (2021).
Blodgett, J., Theou, O., Kirkland, S., Andreou, P. & Rockwood, K. Frailty in NHANES: Comparing the frailty index and phenotype. Arch. Gerontol. Geriatr. 60(3), 464–470 (2015).
Liu, W. et al. Genetically predicted frailty index and risk of stroke and Alzheimer’s disease. Eur. J. Neurol. 29(7), 1913–1921 (2022).
Vezza, C. et al. Use of the frailty index in older persons with chronic kidney disease. J. Am. Med. Dir. Assoc. 20(9), 1179–1180 (2019).
Mitnitski, A. B., Mogilner, A. J. & Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 1, 323–336 (2001).
Burgess, S. & Thompson, S. G. Avoiding bias from weak instruments in Mendelian randomization studies. Int. J. Epidemiol. 40(3), 755–764 (2011).
Burgess, S., Small, D. S. & Thompson, S. G. A review of instrumental variable estimators for Mendelian randomization. Stat. Methods Med. Res. 26(5), 2333–2355 (2017).
Hemani, G. et al. The MR-Base platform supports systematic causal inference across the human phenome. eLife 7, 8 (2018).
Burgess, S., Butterworth, A. & Thompson, S. G. Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37(7), 658–665 (2013).
Burgess, S. & Thompson, S. G. Multivariable Mendelian randomization: The use of pleiotropic genetic variants to estimate causal effects. Am. J. Epidemiol. 181(4), 251–260 (2015).
Burgess, S., Bowden, J., Fall, T., Ingelsson, E. & Thompson, S. G. Sensitivity analyses for robust causal inference from Mendelian randomization analyses with multiple genetic variants. Epidemiology 28(1), 30–42 (2017).
Bowden, J. et al. Assessing the suitability of summary data for two-sample Mendelian randomization analyses using MR-Egger regression: The role of the I2 statistic. Int. J. Epidemiol. 45(6), 1961–1974 (2016).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21(11), 1539–1558 (2002).
Locke, A. E. et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 518(7538), 197–206 (2015).
Shungin, D. et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature 518(7538), 187–196 (2015).
Liu, J. Z. et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 47(9), 979–986 (2015).
Said, S. et al. Genetic analysis of over half a million people characterises C-reactive protein loci. Nat. Commun. 13(1), 2198 (2022).
Ofori-Asenso, R. et al. Global incidence of frailty and prefrailty among community-dwelling older adults: A systematic review and meta-analysis. JAMA Netw. Open 2(8), e198398 (2019).
Walston, J., Buta, B. & Xue, Q. L. Frailty screening and interventions: Considerations for clinical practice. Clin. Geriatr. Med. 34(1), 25–38 (2018).
Hoogendijk, E. O. et al. Frailty: Implications for clinical practice and public health. Lancet 394(10206), 1365–1375 (2019).
Jeffery, C. A., Shum, D. W. & Hubbard, R. E. Emerging drug therapies for frailty. Maturitas 74(1), 21–25 (2013).
Dent, E., Kowal, P. & Hoogendijk, E. O. Frailty measurement in research and clinical practice: A review. Eur. J. Intern. Med. 31, 3–10 (2016).
Reese, P. P. et al. Physical performance and frailty in chronic kidney disease. Am. J. Nephrol. 38(4), 307–315 (2013).
Mansur, H. N., Colugnati, F. A., Grincenkov, F. R. & Bastos, M. G. Frailty and quality of life: A cross-sectional study of Brazilian patients with pre-dialysis chronic kidney disease. Health Qual. Life Outcomes 12, 27 (2014).
Bao, Y., Dalrymple, L., Chertow, G. M., Kaysen, G. A. & Johansen, K. L. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch. Intern. Med. 172(14), 1071–1077 (2012).
Pugh, J. et al. Frailty and comorbidity are independent predictors of outcome in patients referred for pre-dialysis education. Clin. Kidney J. 9(2), 324–329 (2016).
King, S. J. et al. A prospective, observational study of frailty, quality of life and dialysis in older people with advanced chronic kidney disease. BMC Geriatr. 23(1), 664 (2023).
Alfaadhel, T. A. et al. Frailty and mortality in dialysis: Evaluation of a clinical frailty scale. Clin. J. Am. Soc. Nephrol. 10(5), 832–840 (2015).
Delgado, C. et al. Association of self-reported frailty with falls and fractures among patients new to dialysis. Am. J. Nephrol. 42(2), 134–140 (2015).
McAdams-DeMarco, M. A. et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J. Am. Geriatr. Soc. 61(6), 896–901 (2013).
Lee HJ, Son YJ. Prevalence and associated factors of frailty and mortality in patients with end-stage renal disease undergoing hemodialysis: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021;18(7), 3471.
Soldati, A., Poggi, M. M., Azzolino, D., Vettoretti, S. & Cesari, M. Frailty index and adverse outcomes in older patients in haemodialysis. Arch. Gerontol. Geriatr. 101, 104673 (2022).
Wilhelm-Leen, E. R., Hall, Y. N., Tamura, M. K. & Chertow, G. M. Frailty and chronic kidney disease: The Third National Health and Nutrition Evaluation Survey. Am. J. Med. 122(7), 664–671 (2009).
Lorenz, E. C. et al. Frailty in CKD and transplantation. Kidney Int. Rep. 6(9), 2270–2280 (2021).
Ballew, S. H. et al. Frailty, kidney function, and polypharmacy: The atherosclerosis risk in communities (ARIC) study. Am. J. Kidney Dis. 69(2), 228–236 (2017).
Dalrymple, L. S. et al. Kidney function and prevalent and incident frailty. Clin. J. Am. Soc. Nephrol. 8(12), 2091–2099 (2013).
Li, C. et al. Association of cystatin C kidney function measures with long-term deficit-accumulation frailty trajectories and physical function decline. JAMA Netw. Open 5(9), e2234208 (2022).
Soysal, P. et al. Inflammation and frailty in the elderly: A systematic review and meta-analysis. Ageing Res. Rev. 31, 1–8 (2016).
Nascimento, C. M. C. et al. Higher levels of tumor necrosis factor β are associated with frailty in socially vulnerable community-dwelling older adults. BMC Geriatr. 18(1), 268 (2018).
Chowdhury, R., Peel, N. M., Krosch, M. & Hubbard, R. E. Frailty and chronic kidney disease: A systematic review. Arch. Gerontol. Geriatr. 68, 135–142 (2017).
Kennard, A. L., Glasgow, N. J., Rainsford, S. E. & Talaulikar, G. S. Narrative review: Clinical implications and assessment of frailty in patients with advanced CKD. Kidney Int. Rep. 9(4), 791–806 (2024).
He, D. et al. Changes in frailty and incident cardiovascular disease in three prospective cohorts. Eur. Heart J. 45(12), 1058–1068 (2024).
Damluji, A. A. et al. Frailty and cardiovascular outcomes in the National Health and Aging Trends Study. Eur. Heart J. 42(37), 3856–3865 (2021).
Acknowledgements
The authors thank all the investigators of corresponding genetic consortia and GWAS for providing the data publicly.
Funding
This work was supported by the Hainan Province 'Nanhai Rising Star' Healthcare Talent Platform Project (NHXX-WJW-2023003), the Key Science and Technology Project of Hainan Province (ZDYF2024SHFZ058), the National Nature Science Foundation of China [Grant Numbers 82271977; 81971602; 82160327; 82160327; 81801684], Hainan Medical University Undergraduate Innovation and Entrepreneurship Training Program (X202311810019), and the Program of Hainan Association for Science and Technology Plans to Youth R & D Innovation (QCXM201919). This project was supported by the Key Science and Technology Project of Hainan Province (ZDYF2021SHFZ239, ZDYF2021SHFZ246), the Hainan Academician Innovation Platform Fund, and the Hainan Province Clinical Medical Center.
Author information
Authors and Affiliations
Contributions
Hui Juan Chen and Jie Qiu led the analysis and writing of this paper. All authors read and contributed to the drafting of the paper. Yihao Guo did the interpretation of the data. Feng Chen conceived and designed the experiment. All authors reviewed the manuscript and approved the publication of our manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The included genome-wide association studies (GWASs) had obtained the necessary ethical approvals from the relevant committees and written informed consent was obtained from all individuals involved in these studies. This study based on the summary-level data requires no ethical permit.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, H.J., Qiu, J., Guo, Y. et al. Genetically predicted frailty index and risk of chronic kidney disease. Sci Rep 14, 21862 (2024). https://doi.org/10.1038/s41598-024-71881-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71881-7
- Springer Nature Limited