Abstract
This study aimed to evaluate chitosan (CS)-based formulations loaded with 5% sodium fluoride (NaF) and/or 10% nanohydroxyapatite (nHA) to remineralize the demineralized primary tooth enamel surface. Ninety enamel blocks were demineralized and were divided into six groups (n = 15): (1) CS-based hydrogel, (2) CS-based hydrogel loaded with NaF, (3) CS-based hydrogel loaded with nHA, (4) CS-based hydrogel loaded with NaF and nHA, (5) 5% NaF varnish, and (6) negative control with no intervention. After intervention, the specimens were pH cycled by 2 h immersion in demineralizing solution and 22 h immersion in remineralizing solution for 8 days. The remineralization effects were evaluated by Vickers microhardness measurements and field emission scanning electron microscopy coupled with energy-dispersive X-ray spectrometry (FESEM-EDS). The best mean ± SD percentage microhardness recovery in remineralized enamel (%REMH) was found in group 4 (56.90 ± 5.49). The %REMH of groups 2 (30.74 ± 3.51) and 5 (29.23 ± 5.65) were statistically the same (p = 0.943). FESEM images confirmed partial coverage of the porous demineralized enamel with a newly formed mineralized layer. Based on EDS findings, the Ca/P ratio values of the treated enamel surfaces with CS-based hydrogels ranged between 1.71 and 1.87, and the highest F content was noticed in group 2 (1.02 ± 0.03). Although, all tested CS-based hydrogels demonstrated the potential to repair demineralized enamel, nHA- and NaF-containing CS-based hydrogel showed the highest remineralization effect. We infer that this new hybrid hydrogel is a potentially useful dental material for tooth biomineralization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Dental caries is a biofilm-mediated disease characterized by episodes of demineralization and remineralization that deteriorate tooth structure. The recent caries preventive approaches primarily attempt to inhibit the development of lesions and improve remineralization1. The remineralization systems, categorized into fluoride-based and non-fluoride-based ones, have been widely used for preventive purposes2. The non-fluoride-containing remineralizing materials, mostly based on calcium phosphate, polyphosphate, and natural products, can provide and deposit calcium and phosphate ions over the subsurface and surface lesions2. Sodium fluoride (NaF) is commercially available in the forms of gel, foam, and varnish for professional use3. Extended contact time with the varnish slows down the rapid drop of fluoride level in the oral cavity, increases its uptake by the outermost enamel layer, and enhances the formation of calcium fluoride (CaF2) on the tooth surface4,5. During the pH drop episodes, CaF2 can serve as a fluoride reservoir by releasing its calcium and fluoride ions to reverse the demineralization process5. Nevertheless, topical fluoride application reduces apatite dissolution rather than recovery of the lost minerals6,7. As the main mineral component of bone, enamel, and dentin, hydroxyapatite (HA) has been the subject of dental biomaterial studies due to its bioactive and biocompatible characteristics. HA remineralizes enamel defects through the formation of a new enamel-like layer instead of reinforcing the existing one6,7.
The biomineralization approach for enamel repair mimics the natural mineralization processes and offers an innovative way to treat early-stage enamel defects6,8. An essential step to enamel repair is to identify suitable organic matrices that facilitate the formation and expansion of HA crystals, resulting in the development of enamel-like structures6. Hence, the development of an enamel-like layer that possesses good adhesion and dense interface to the natural enamel has attracted considerable attention9. Amelogenin10, peptide amphiphiles11, glumatic acid combined with nanohydroxyapatite (nHA) particles12, and carboxyl-terminated poly (amidoamine) dendrimers13 have been studied for biomimetic mineralization of enamel defects. An attractive organic candidate for biomineralization is chitosan, a positively charged polymer derived from the deacetylation of chitin6. Chitosan (CS) is cheap, biocompatible, biodegradable, non-toxic, antibacterial, stable, and flexible in hydrated environments, all of which qualify it as an appropriate choice for in situ-forming hydrogels6,14,15,16. The in situ-forming thermoresponsive hydrogel systems transform from a sol state to a gel state as the temperature at the application site (in situ site) changes17. The active regions available in the structure of CS allow for the binding of different bioactive ions necessary for remineralization2. Previous investigations have combined CS with various ingredients like amorphous calcium phosphate (ACP)18,19, calcium phosphates microgel20, HA9,21,22, and fluoride4,23 for biomineralization. Their results have demonstrated a promising method in guiding enamel or dentin repair.
There is still a crucial demand to develop a simple strategy to simulate biomineralization. From this perspective, the present study aimed to evaluate the prepared CS-based in situ-gel forming formulation containing NaF and/or nHA to repair initially demineralized primary tooth enamel surface in comparison with commercially available NaF varnish. The changes in the enamel microhardness and surface morphology of the newly remineralized enamel surface were investigated by Vickers microhardness measurements and field emission scanning electron microscopy coupled with energy-dispersive X-ray spectrometry (FESEM-EDS).
Methods and materials
Study design
In total, 102 sound primary canine teeth, extracted for orthodontic reasons, were obtained in accordance with the established Ethics Review Committee of Shiraz University of Medical Sciences protocol (IR.SUMS.DENTAL.REC.1401.120). Written informed consents were collected from the parents/guardians of the individuals aged 8–10 years for the use of the teeth for the present study. After a thorough cleaning and debridement with a prophylaxis brush, the teeth were disinfected by immersion in 0.1% chloramine T solution for 1 month. The samples were kept in weekly-replaced deionized water at room temperature until use. Defects, fracture lines on the enamel surface under a stereomicroscope (Motic K, Wetzlar, Germany) at ×20 magnification, and surface hardness values below 250 VHN at the baseline stage were considered as the exclusion criteria. Besides, samples with significant differences in VHN values at the baseline and after demineralization stage were excluded. These criteria would have ensured no statistically significant differences between groups before the intervention and the pH cycling procedure. Ninety teeth fulfilled the inclusion criteria. The surface microhardness and enamel microstructure were evaluated at three stages: sound (baseline), demineralized, and remineralized enamel.
Synthesis of various in situ gel-forming chitosan-based formulations
The NaF varnish used was produced by FluoroDose®, Centrix Inc., Shelton, USA. The chitosan hydrogel formulation was prepared as described in our previous study utilizing intermediate molecular weight CS ((C6H11NO4)n), tripolyphosphate (TPP) (Na5P3O10), poloxamer 407 (Pluronic F- 127), glacial acetic acid (CH3COOH), glycerophosphate disodium salt hydrate (C3H9Na2O7P), nHA (Ca5(PO4)3(OH)), and sodium fluoride (NaF), all produced by Merck, Darmstadt, Germany17. Briefly, 1% w/v medium molecular weight CS (MMWC; 75–85% deacetylated) was mixed with 0.1 N acetic acid solution under magnetic stirring at room temperature until total dispersion. The CS-based hydrogel was prepared by adding 22% w/v of poloxamer 407 to the CS solution. For loading the formulation with NaF and/or nHA, 10% w/w nHA and/or 5% w/w NaF were dissolved in the dispersion gradually while stirring at 125 rpm. Subsequently, the hydrogels were placed in the ice bath and allowed to cool for approximately 30 min. Then, the dispersions were mixed dropwise with 0.5% w/v TPP and 14.5% w/v glycerophosphate disodium salt hydrate on a stirrer for 1 h. The change from sol state to gel form was verified when the mixtures were transferred from the ice bath to the water bath at 37 °C after 30 s17.
Sample preparation
We removed the roots about 2 mm below the cemento-enamel junction and embedded the crowns in cold-curing acrylic resin with the labial surface aligned parallel to the mold to prepare the enamel blocks. After polishing the tooth surfaces with 600-, 800-, 1200-, 2400-, and 4000-grit waterproof silicon carbide papers (Buehler, Lake Bluff, IL, USA) and 1 μm aluminum oxide, the samples were then subjected to a 5 min cleaning process in an ultrasonic bath. Afterward, the samples underwent a 20 s washing process with distilled water. Once the teeth were dried, they were covered with two coats of nail polish, leaving a 2 × 4 mm exposed window on the flattest surface of the enamel24.
Induction of initial caries lesion
For initial caries lesion induction, 90 blocks were subjected to demineralizing solution at 37 °C for 96 h (15 mL/sample) consisting of 0.1 mM lactic acid solution, 3 mM CaCl2, 3 mM KH2PO4, and 0.1% carboxymethyl cellulose at the pH of 4.525. We refreshed the solution after 48 h and rinsed each sample with deionized water after 96 h24.
Group allocation and enamel remineralization protocol
After demineralization induction, 90 teeth were randomly allocated to six groups (n = 15) as follows:
Group 1: CS-based hydrogel.
Group 2: CS-based hydrogel loaded with NaF.
Group 3: CS-based hydrogel loaded with nHA.
Group 4: CS-based hydrogel loaded with NaF and nHA.
Group 5: NaF varnish (positive control).
Group 6: No intervention (negative control).
A thin layer of respective hydrogel (groups 1 to 4) or varnish (group 5) was applied using a clean microbrush on the demineralized enamel surface and left to dry for 4 min, while group 6 received no intervention. The samples were then immediately transferred to artificial saliva (15 mL per sample) and stored at 37 °C for 6 h to facilitate ionic exchange with the enamel26. The components of the artificial saliva were 0.2 mM glucose, 0.1 mM C8H15NaO, 9.9 mM NaCl, 1.5 mM CaCl2.H2O, 3 mM NH4Cl, 17 mM KCl, 2 mM NaSCN, 2.4 mM K2HPO4, 3.3 mM urea, 2.4 mM NaH2PO4, and 11 µM ascorbic acid (pH 6.8)27. After 24 h, the hydrogel or varnish coating was carefully removed with gauze, and any excess was cleaned with cotton swabs soaked in 50% acetone solution26. Then, the blocks were rinsed for 30 s with deionized water.
pH cycling model
To better imitate the oral environment, an 8-day pH cycling model was applied for each tooth following the methodology described by Mohd Said et al.26. In summary, each block was placed for 2 h in the demineralization solution (0.05 M acetate buffer, pH 5.0 and containing 1.28 mM Ca, 0.74 mM P, and 0.03 µg F/mL) and for 22 h in the remineralization solution (1.5 mM Ca, 0.9 mM P, 150 mM KCl, 0.05 µg F/mL in 0.1 M Tris buffer, pH 7.0) at 37 °C. The solutions were refreshed on the fourth day to avert both depletion and solution oversaturation. The amount of enamel remineralization was assessed at the end of the 8 day cycle.
Surface microhardness assessment
The microhardness values of each group (n = 15) were determined by a Vickers diamond indenter device (ZwickRoell, Fürstenfeld, Austria) with a 100 g force for 15 s. Measurements were taken at three points of 100 µm distance for each sample. The measurements were performed at the baseline (sound enamel), initial caries lesion induction (demineralized enamel), and after pH cycling (remineralized enamel). After calculation of the average Vickers hardness number (VHN), the following equation was used to calculate the percentage microhardness recovery in remineralized enamel (%REMH) for each group:
A single examiner used the same calibrated machine to conduct all measurements at the three stages.
Surface morphology assessment
Upon completion of the intervention, two samples were randomly chosen from each group for topographic evaluation, resulting in a total of 12 samples. The enamel surfaces were analyzed for their morphological microstructure using FESEM-EDS (MIRA3, TSCAN, Brno, Czech Republic) at an accelerating voltage of 15 kV. The magnification levels used for the examination were ×15,000, ×75,000, and ×150,00017. The selected 12 samples were subjected to dehydration using a sequence of ethanol solutions and were gold-coated in a vacuum evaporator before FESEM-EDS characterization for 1 min at 20 mA. The elemental composition of the samples, including carbon (C), oxygen (O), calcium (Ca), phosphorus (P), sodium (Na), and fluorine (F) was quantified in weight (wt.%) using EDS. Additionally, the EDS mapping was conducted to illustrate the spatial location of elements on the treated enamel surfaces.
Statistical analysis
All measurements are represented as mean value ± the standard deviation (± SD) of the mean, and data analysis was conducted using SPSS version 22 software (IBM, NY, USA). The Shapiro–Wilk test was used to assess the normality of the data distribution. One-way ANOVA and Tukey HSD post hoc tests were used to compare the surface microhardness values in the six different groups. Besides, repeated measure ANOVA and Mauchly’s test of sphericity were used for intra-group comparisons at different time points (sound, demineralized, and remineralized enamel microhardness values). The significant level was set to 0.05 for all statistical tests.
Results
The mean ± SD for enamel microhardness (EMH) values for each group at different time points are presented in Table 1. The sound enamel microhardness values ranged from 323.38 to 337.40 VHN, with an average of 329.57 ± 2.81 VHN, with no significant differences between the groups (p = 0.860). The initial caries induction led to a substantial decrease in EMH values in all groups (all, p < 0.001). There was no statistically significant difference in EMH values following demineralization (p = 0.749). The hydrogel or varnish application and 8 day pH cycling process led to a dramatic increase in EMH values of all intervention groups as compared to the demineralized status (all, p < 0.001). The negative control group resulted in a slight increase in EMH values after pH cycling with no statistically significance difference (p = 0.095). EMH values in groups 2 and 5 were statistically the same after remineralization and pH cycling (p = 0.911).
Although all intervention groups showed degrees of recovery of EMH values (%REMH) after varnish and hydrogel application (all, p < 0.001), the best recovery in EMH value was found in group 4 (56.90 ± 5.49). The %REMH of groups 2 and 5 were not statistically different (p = 0.943). Group 1 demonstrated degrees of recovery in EMH values (10.02 ± 3.41).
The effects of various CS-based hydrogels and NaF varnish application after 8 days of pH cycling are presented in FESEM images (Fig. 1a–f). As shown in FESEM images, the porous demineralized enamel was partially covered with a mineralized layer (Fig. 1a–e). Mineral precipitates could occupy the porous areas and defects of the demineralized enamel to some extent. The findings confirm the formation of a new mineral layer on enamel surfaces treated with the CS hydrogel. Most areas of the enamel surface in the negative control group demonstrated loss of the normal enamel structure due to loss of organic and inorganic substances (Fig. 1f).
The FESEM images of treated enamel surfaces with various CS-based hydrogels and NaF varnish application after 8 days of pH cycling. (a) CS-based hydrogel, (b) CS-based hydrogel loaded with NaF, (c) CS-based hydrogel loaded with nHA, (d) CS-based hydrogel loaded with NaF and nHA, (e) NaF varnish (positive control), and (f) negative control. The white arrows show the loss of the normal enamel structure due to dissolved minerals and organic materials.
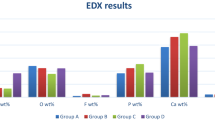
The values of F, Ca, and P elemental composition in weight (wt.%) and the Ca/P ratio of the enamel surface are presented in Table 2. The value of the Ca/P ratio of the treated enamel surfaces with hydrogel and varnish varied between 1.71 and 1.87. The values for the hydrogel and varnish groups demonstrated a higher Ca/P ratio than the theoretical value of the carbonated hydroxyapatite of natural enamel (1.67). The highest F content was noticed in group 2, followed by groups 4, 5, 3, 1, and 6, respectively. The EDS elemental mappings of the nHA/NaF/CS-based in situ forming gel and negative control group for F, Ca, and P ions are presented in Fig. 2.
Discussion
This study aimed to evaluate the remineralization potential of various CS-based in situ forming gels on demineralized primary tooth enamel, with an emphasis on the clinical application of biomineralizing materials.
Microhardness evaluation using a Vickers diamond indenter device is a common non-destructive technique for the determination of EMH values. The baseline EMH values of all samples ranged from 323.38 to 337.40 VHN, which was consistent with previously reported surface hardness values of sound enamel24,28. EMH values of all samples reduced significantly after initial caries lesions formation as a result of enamel mineral loss. Based on our results, CS-based hydrogel loaded with NaF and nHA was the most effective compound in recovering the EMH scores (%REMH = 56.90 ± 5.49%). This finding might be attributed to the synergistic effects of nHA and NaF, as stated by previous studies29,30. Besides, it is possible that fluoride ions were incorporated into the nHA structure, resulting in the formation of fluorohydroxyapatite. The CS-based hydrogel loaded with nHA demonstrated superior efficacy in EMH recovery than NaF-containing hydrogel and/or varnish. The smaller particle size of nHA compared to NaF potentially facilitates its penetration through the interprismatic enamel spaces, leading to a constructive interdigitation within the enamel structure31. Hybrid hydrogels loaded with 10% nHA enhanced biomineralization by stimulating the formation of a new dentin-like layer rich in HA32. As stated earlier, fluoride compounds are capable of only replacing the lost minerals6,7. We found no significant difference between NaF-containing CS-based hydrogel and NaF varnish in terms of EMH. Both the fluoride varnish and the formulated CS-based hydrogel have high concentrations of fluoride (5% NaF and 22,600 ppm F ion)4,17. Even 0.1 ppm of fluoride in saliva can provide caries protection33. CS-based hydrogel free of nHA and/or NaF could also recover the EMH values to some extent (10.02 ± 3.41). This finding can be attributed to the CS structure and the demineralized enamel surface. CS is a positively charged polymer that can gradually release bioactive substances. Its structure modification is due to the existence of repeated units of the primary amine group at the C-2 position34. The cationic nature of CS enables its adhesion to negatively charged surfaces, including demineralized enamel6,8,35. The demineralized enamel surface has a negative charge as a result of the loss of calcium ions2. Due to the amine group chelating ability and tight binding to the enamel surface, CS serves as an excellent framework for the regeneration of the new mineralized tissue by facilitating the attachment of Ca and P ions6,8,35. Arnaud et al. proposed that chitosan disrupts the enamel demineralization process by preventing phosphorus release and acid penetration prevention36. Besides, the positive charge of CS allows for antibacterial and anticaries effects by firmly adhesion to negative charge of bacterial cell membrane and causing cell membrane disruption15,16.
FESEM images showed substantial deposits of minerals in samples treated with hydrogels and varnish. As previously mentioned, CS can bind to negatively charged surfaces6,8,35. Hence, it is reasonable to propose that the slight remineralization effect in CS-based hydrogel (group 1) might have been induced by CS attraction of apatite formation on the demineralized enamel surface (Fig. 1a). Following the application of various CS hydrogels, there was evidence of enamel repair by the formation of a new mineralized layer. The newly grown layer of CS groups (groups 1 to 4) shows the spheroid HA particles, which is in line with Muşat et al. findings8. We propose that nHA and NaF-containing CS hydrogel served as a nucleating scaffold for the accumulation and rearrangement of high concentrations of Ca, P, and F ions, which eventually resulted in the formation of a new enamel-like layer. The mineral deposits on the demineralized surfaces filled the voids, leading to a relatively intact subsurface, as shown in Fig. 1d. The FESEM observations of the negative control group (demineralized-pH-cycled enamel surface) showed a loss of normal enamel integrity. However, scarce areas of decreased surface irregularities were evident, which might be attributed to the pH cycling process in the negative control group.
Ca/P ratio of the control and remineralized enamel in different groups were calculated from Ca and P Wt.% of EDS results. The Ca/P ratio of enamel surfaces treated with CS hydrogels or varnish confirmed their remineralization potential. A Ca/P ratio of 1.6 can result in an ideal enamel remineralization and mineral supersaturation37. Therefore, it is logical to propose that providing an additional source for ions can improve enamel remineralization. For the control group, the Ca/P ratio reduced to 1.49 ± 0.0039 as a result of mineral diminution on demineralized enamel surfaces. Following intervention and pH cycling, all intervention groups showed recovery in the Ca/P ratio. The findings indicated that the remineralizing compounds used could partially compensate for mineral loss. Based on our results, the Ca/P ratio was 1.71 ± 0.0002 to 1.87 ± 0.0039 for four CS-based hydrogels and 1.84 ± 0.0038 for the NaF varnish group. The higher Ca/P ratio in nHA and/or NaF-containing CS-based in situ-forming gel points out possibly better resistance to further chemical attack. In addition to the Ca, P, and F content of pH cycling solutions, TPP, glycerophosphate disodium salt hydrate, and nHA contain P element in their chemical structure. Besides, nHA and NaF contain Ca and F elements, respectively17. The higher amount of P element in the NaF-containing hydrogel that the NaF varnish might explain the negligible difference in the Ca/P ratio of the NaF-containing hydrogel. Interestingly, electrostatic forces cause CS to adhere to the phosphate groups in nHA and TPP, as well as the F ion in NaF38. Besides, the hydroxyl and amino functional groups in the chitosan structure can provide adsorption capacity for metal ions to form complexes via physical complexation, chelation, hydrogen bonding, or electrostatic attraction39,40. Therefore, significant amounts of calcium, phosphate, and fluoride ions can be trapped inside the chitosan structure. According to the EDS findings, NaF/CS-based hydrogel demonstrated the highest F content of all intervention groups. Low molecular weight (41.99 g/mol) and high water solubility (4.3 g/100 ml at 25 °C) of sodium fluoride affect the ionic strength of the solution and facilitate the formation of nanoparticles41. Fluorine, as an element with the highest reactivity in the periodic table, has a high affinity to bind to cations. These high attraction forces provide an appropriate medium for TPP to crosslink the CS chains, leading to a tighter matrix17. The EDS-elemental mapping confirmed the uniform distribution of F, Ca, and P elements on the treated enamel surface with the nHA/NaF/CS-based hydrogel compared to the negative control group (Fig. 2).
The rationale behind enamel remineralization is to redeposit the minerals and seal the demineralized pores using an appropriate vehicle to transport remineralizing agents into the tooth structure2. As a non-living tissue, enamel lacks the ability to remineralize naturally. Thus, the development of biomimetic materials that mimic enamel is considered the future of preventive dentistry2. However, the protein-free and acellular composition of natural enamel prevents the actual regeneration of its highly organized structure and morphology. In fact, biomimetic regeneration mirrors the nucleation and organized growth of HA crystals on the demineralized tooth surface9.
We have previously identified and confirmed the physicochemical, morphological, textural, rheological characteristics, and physical stability of the fabricated hydrogels with the Fourier transform infrared spectroscopy (FTIR), FESEM-EDS, texture analyzer device, rheometer, and centrifugation, respectively17. To our knowledge, this is the first study to assess the ability of nHA/NaF/CS-based in situ forming gel using a more clinically relevant biological model. The artificial saliva and pH cycling model were intended for this purpose. This study demonstrated a successful in vitro method for the creation of enamel-like apatite layers using a CS hydrogel matrix enriched with nHA and NaF. The current investigation has some limitations. Due to the lack of comparable studies, it was not possible to make relevant comparisons. Besides, it is necessary to assess the Ca, P, and F release pattern at acidic challenges, the mineral absorption capacity, the depth of penetration into the intact and demineralized enamel surface, and the adhesive properties of the formulation on the tooth surface. We also recommend a long-term clinical study to understand the role of nHA and NaF-containing CS-based hydrogel on remineralization.
Conclusion
The CS-based hydrogel could repair demineralized enamel simply by providing a remineralization environment that promotes the formation of a new apatite layer. All intervention groups showed enhanced microhardness values in comparison to the demineralized enamel. Despite the microhardness recovery of the demineralized enamel layers treated with NaF varnish, nHA/NaF/CS-based in situ-forming gel showed the highest remineralization effect. Our results confirmed that CS could facilitate HA formation. Therefore, CS can serve as a bioactive polymer in the biomimetic regeneration of enamel structure for enamel repair.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Schwendicke, F. et al. When to intervene in the caries process? An expert Delphi consensus statement. Clin. Oral Investig. 23, 3691–3703. https://doi.org/10.1007/s00784-019-03058-w (2019).
Nimbeni, S. B., Nimbeni, B. S. & Divakar, D. D. Role of chitosan in remineralization of enamel and dentin: A systematic review. Int. J. Clin. Pediatr. Dent. 14, 562–568. https://doi.org/10.5005/jp-journals-10005-1971 (2021).
Selwitz, R. H., Ismail, A. I. & Pitts, N. B. Dental caries. Lancet 369, 51–59. https://doi.org/10.1016/s0140-6736(07)60031-2 (2007).
Pichaiaukrit, W., Thamrongananskul, N., Siralertmukul, K. & Swasdison, S. Fluoride varnish containing chitosan demonstrated sustained fluoride release. Dent. Mater. J. 38, 1036–1042. https://doi.org/10.4012/dmj.2018-112 (2019).
Abufarwa, M., Noureldin, A., Campbell, P. M. & Buschang, P. H. The longevity of casein phosphopeptide-amorphous calcium phosphate fluoride varnish’s preventative effects: Assessment of white spot lesion formation. Angle Orthod. 89, 10–15. https://doi.org/10.2319/021718-127.1 (2019).
Hanafy, R. A., Mostafa, D., Abd El-Fattah, A. & Kandil, S. Biomimetic chitosan against bioinspired nanohydroxyapatite for repairing enamel surfaces. Bioinspir. Biomim. Nanobiomater. 9, 85–94 (2019).
Butera, A. et al. SEM/EDS evaluation of the mineral deposition on a polymeric composite resin of a toothpaste containing biomimetic Zn-carbonate hydroxyapatite (microRepair(®)) in oral environment: A randomized clinical trial. Polymers https://doi.org/10.3390/polym13162740 (2021).
Muşat, V. et al. A chitosan–agarose polysaccharide-based hydrogel for biomimetic remineralization of dental enamel. Biomolecules https://doi.org/10.3390/biom11081137 (2021).
Zaharia, A. et al. Biomimetic chitosan-hydroxyapatite hybrid biocoatings for enamel remineralization. Ceram. Int. 43, 11390–11402. https://doi.org/10.1016/j.ceramint.2017.05.346 (2017).
Fan, Y. et al. Amelogenin-assisted ex vivo remineralization of human enamel: Effects of supersaturation degree and fluoride concentration. Acta Biomater. 7, 2293–2302. https://doi.org/10.1016/j.actbio.2011.01.028 (2011).
Busch, S. Regeneration of human tooth enamel. Angew. Chem. Int. Ed. 43, 1428–1431. https://doi.org/10.1002/anie.200352183 (2004).
Li, L. et al. Bio-inspired enamel repair via Glu-directed assembly of apatite nanoparticles: An approach to biomaterials with optimal characteristics. Adv. Mater. 23, 4695–4701. https://doi.org/10.1002/adma.201102773 (2011).
Wu, D. et al. Hydroxyapatite-anchored dendrimer for in situ remineralization of human tooth enamel. Biomaterials 34, 5036–5047. https://doi.org/10.1016/j.biomaterials.2013.03.053 (2013).
El-Fattah, A. A. & Mansour, A. Viscoelasticity, mechanical properties, and in vitro biodegradation of injectable chitosan-poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/nanohydroxyapatite composite hydrogel. Bull. Mater. Sci. 41, 141 (2018).
Róna, V. et al. Effect of chitosan on the number of Streptococcus mutans in saliva: A meta-analysis and systematic review. Int. J. Mol. Sci. https://doi.org/10.3390/ijms242015270 (2023).
Qu, S., Ma, X., Yu, S. & Wang, R. Chitosan as a biomaterial for the prevention and treatment of dental caries: Antibacterial effect, biomimetic mineralization, and drug delivery. Front. Bioeng. Biotechnol. 11, 1234758. https://doi.org/10.3389/fbioe.2023.1234758 (2023).
Rafiee, A., Mozafari, N., Fekri, N., Memarpour, M. & Azadi, A. Preparation and characterization of a nanohydroxyapatite and sodium fluoride loaded chitosan-based in situ forming gel for enamel biomineralization. Heliyon 10, e24217. https://doi.org/10.1016/j.heliyon.2024.e24217 (2024).
Santoso, T., Djauharie, N. K., Ahdi, W., Latief, F. D. E. & Suprastiwi, E. Carboxymethyl chitosan/amorphous calcium phosphate and dentin remineralization. J. Int. Dent. Med. Res. 12, 84–87 (2019).
Zhu, Y. et al. The dual anti-caries effect of carboxymethyl chitosan nanogel loaded with chimeric lysin ClyR and amorphous calcium phosphate. Eur. J. Oral Sci. 129, e12784. https://doi.org/10.1111/eos.12784 (2021).
Simeonov, M. et al. Novel hybrid chitosan/calcium phosphates microgels for remineralization of demineralized enamel—A model study. Eur. Polym. J. https://doi.org/10.1016/j.eurpolymj.2019.07.005 (2019).
Sato, T. P. et al. The role of nanohydroxyapatite on the morphological, physical, and biological properties of chitosan nanofibers. Clin. Oral Investig. 25, 3095–3103. https://doi.org/10.1007/s00784-020-03633-6 (2021).
Vagropoulou, G. et al. Hybrid chitosan/gelatin/nanohydroxyapatite scaffolds promote odontogenic differentiation of dental pulp stem cells and in vitro biomineralization. Dent. Mater. 37, e23–e36. https://doi.org/10.1016/j.dental.2020.09.021 (2021).
Souza, B. M., Machado, P. F., Vecchia, L. R. & Magalhães, A. C. Effect of chitosan solutions with or without fluoride on the protection against dentin erosion in vitro. Eur. J. Oral Sci. 128, 495–500. https://doi.org/10.1111/eos.12740 (2020).
Rafiee, A., Memarpour, M. & Benam, H. Evaluation of bleaching agent effects on color and microhardness change of silver diamine fluoride-treated demineralized primary tooth enamel: An in vitro study. BMC Oral Health 22, 347. https://doi.org/10.1186/s12903-022-02371-3 (2022).
Patil, N., Choudhari, S., Kulkarni, S. & Joshi, S. R. Comparative evaluation of remineralizing potential of three agents on artificially demineralized human enamel: An in vitro study. J. Conserv. Dent. 16, 116–120. https://doi.org/10.4103/0972-0707.108185 (2013).
Mohd Said, S. N., Ekambaram, M. & Yiu, C. K. Effect of different fluoride varnishes on remineralization of artificial enamel carious lesions. Int. J. Paediatr. Dent. 27, 163–173. https://doi.org/10.1111/ipd.12243 (2017).
Comar, L. P. et al. TiF4 and NaF varnishes as anti-erosive agents on enamel and dentin erosion progression in vitro. J. Appl. Oral Sci. 23, 14–18. https://doi.org/10.1590/1678-775720140124 (2015).
Pilar, G.-S. & Reyes-Gasga, J. Microhardness and chemical composition of human tooth. Mater. Res. https://doi.org/10.1590/S1516-14392003000300011 (2003).
Kim, M. Y., Kwon, H. K., Choi, C.-H. & Kim, B.-I. Combined effects of nano-hydroxyapatite and NaF on remineralization of early Caries lesion. Key Eng. Mater. 1347, 1347–1350. https://doi.org/10.4028/www.scientific.net/KEM.330-332.1347 (2007).
Ebadifar, A., Nomani, M. & Fatemi, S. A. Effect of nano-hydroxyapatite toothpaste on microhardness of artificial carious lesions created on extracted teeth. J. Dent. Res. Dent. Clin. Dent. Prospects 11, 14–17. https://doi.org/10.15171/joddd.2017.003 (2017).
Juntavee, N., Juntavee, A. & Plongniras, P. Remineralization potential of nano-hydroxyapatite on enamel and cementum surrounding margin of computer-aided design and computer-aided manufacturing ceramic restoration. Int. J. Nanomed. 13, 2755–2765. https://doi.org/10.2147/ijn.S165080 (2018).
Vagropoulou, G. et al. Hybrid chitosan/gelatin/nanohydroxyapatite scaffolds promote odontogenic differentiation of dental pulp stem cells and in vitro biomineralization. Dent. Mater. J. 37, e23–e36. https://doi.org/10.1016/j.dental.2020.09.021 (2021).
Featherstone, J. D. Delivery challenges for fluoride, chlorhexidine and xylitol. BMC Oral Health 6, S8. https://doi.org/10.1186/1472-6831-6-s1-s8 (2006).
Venter, J. P., Kotzé, A. F., Auzély-Velty, R. & Rinaudo, M. Synthesis and evaluation of the mucoadhesivity of a CD-chitosan derivative. Int. J. Pharm. 313, 36–42. https://doi.org/10.1016/j.ijpharm.2006.01.016 (2006).
Bulanov, E. et al. Study of physicochemical properties of nanohydroxyapatite–chitosan composites. Bull. Mater. Sci. 43, 91. https://doi.org/10.1007/s12034-020-2065-0 (2020).
Arnaud, T. M. S., De Barros Neto, B. & Diniz, F. B. Chitosan effect on dental enamel de-remineralization: An in vitro evaluation. J. Dent. 38, 848–852. https://doi.org/10.1016/j.jdent.2010.06.004 (2010).
Sakr, A. H., Nassif, M. S. & El-Korashy, D. I. Amelogenin-inspired peptide, calcium phosphate solution, fluoride and their synergistic effect on enamel biomimetic remineralization: An in vitro pH-cycling model. BMC Oral Health 24, 279. https://doi.org/10.1186/s12903-024-04008-z (2024).
Liu, X., Wu, Y., Zhao, X. & Wang, Z. Fabrication and applications of bioactive chitosan-based organic–inorganic hybrid materials: A review. Carbohydr. Polym. 267, 118179. https://doi.org/10.1016/j.carbpol.2021.118179 (2021).
Gamage, A. et al. Recent application prospects of chitosan based composites for the metal contaminated wastewater treatment. Polymers https://doi.org/10.3390/polym15061453 (2023).
Wei, X. et al. Improving the Ca(II) adsorption of chitosan via physical and chemical modifications and charactering the structures of the calcified complexes. Polym. Test. 98, 107192. https://doi.org/10.1016/j.polymertesting.2021.107192 (2021).
Nguyen, S., Escudero, C., Sediqi, N., Smistad, G. & Hiorth, M. Fluoride loaded polymeric nanoparticles for dental delivery. Eur. J. Pharm. Sci. 104, 326–334. https://doi.org/10.1016/j.ejps.2017.04.004 (2017).
Acknowledgements
The authors thank the Vice-Chancellery of Research of Shiraz University of Medical Sciences, Shiraz, Iran, for supporting this research (Grant No. 27355). This article is based on thesis by Dr. Milad Amiri.
Funding
This work was supported the Vice-Chancellery of Research of Shiraz University of Medical Sciences, Shiraz, Iran (Grant No. 27355).
Author information
Authors and Affiliations
Contributions
AR, MM, and AA: conceptualized and designed the study, supervised data collection, interpreted the data, drafted the manuscript, and approved the final manuscript as submitted. NF, NM, FR: conceptualized the study, supervised data collection and interpretation, reviewed the manuscript, and approved the final manuscript as submitted. MA: assisted in data collection, carried out the initial analyses and interpretation of data, reviewed the manuscript, and approved the final manuscript as submitted. All authors agreed to be accountable for all aspects of this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by the Ethics Review Committee of the School of Dentistry, Shiraz University of Medical Sciences (IR.SUMS.DENTAL.REC.1401.120). All methods were performed in accordance with the relevant guidelines and regulations (Declaration of Helsinki). Written informed consents for the use of the teeth are available from the corresponding author upon reasonable request.
Declaration of Generative AI and AI-assisted technologies in the writing process
During the preparation of this work the author(s) used QuillBot in order to improve the language. After using this service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Rafiee, A., Memarpour, M., Amiri, M. et al. Comparison of various chitosan-based in situ forming gels with sodium fluoride varnish for enamel biomineralization: an in-vitro pH cycling model. Sci Rep 14, 21100 (2024). https://doi.org/10.1038/s41598-024-71993-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-71993-0
- Springer Nature Limited