Abstract
This ex vivo study devised an analytical ex vivo method for infection/disinfection of simulated lateral canals located in the middle and apical segments of the root. The antibacterial effects of supplementary approaches were tested in this model. Extracted mandibular premolars had their main root canals enlarged and then two lateral canals (100 μm in diameter) were created in the root, one in the apical and the other in the middle portion. Micro-computed tomography was used for specimen selection and to confirm the quality of the simulated ramifications. The specimens were contaminated with a mixed bacterial culture from subgingival bacterial biofilm added to pure Enterococcus faecalis strain ATCC 29212 grown overnight, using special strategies to facilitate culture medium penetration within the lateral canals. The following procedures were tested for disinfection: NaOCl/passive ultrasonic irrigation (PUI), NaOCl/XP-endo Finisher, ozonated water/continuous ultrasonic irrigation (CUI), and NaOCl/conventional irrigation with 30-G needles (control). Bacteriological samples were taken from the main canal before (S1) and after (S2) each supplementary protocol, and also from each lateral canal after treatment (S3). DNA extracted from the samples was subjected to quantitative real-time polymerase chain reaction. All S1 main canal samples were positive for bacterial presence. Bacterial counts in the main root canal substantially decreased by 99.2% after PUI, 99.1% after ozone/CUI, 99% after XP-endo Finisher, and 96% in the control group (P < 0.01 for all groups). There were no significant differences between groups (P > 0.05). The same was observed when comparing the effects of the supplementary approaches in the apical and middle lateral canals (P > 0.05). Only a few lateral canals showed no detectable bacteria. The method proposed here proved effective for ex vivo infection/disinfection studies. All supplementary approaches induced a substantial bacterial reduction in the main canal, with no significant differences between them. However, in terms of lateral canal disinfection, none of the tested approaches showed significant effects when compared to the control group.
Similar content being viewed by others
Introduction
Lateral canals and apical ramifications are routes through which bacteria and their products present in infected root canals can reach the periodontal tissues and cause inflammatory lesions1. Ramifications are very frequent in the root canal system as demonstrated by several anatomy studies using different methodologies, with higher prevalences in molars and premolars, particularly in the apical root segment2,3,4,5,6. Because of the use of magnification, histological evaluation of serial sections is a very sensitive approach to detect ramifications; a study analyzed serial sections from 493 human teeth and reported the occurrence of lateral canals/apical ramifications in 75% of the teeth5. Lateral canals are usually sinuous/curved along their extent, with diameters ranging from 10 to 200 μm6,7.
Due to their anatomical positioning and usual small and tortuous lumen, lateral canals represent a challenge for proper cleaning and disinfection. As a matter of fact, studies reporting on nonsurgical root canal treatment failure identify residual infections within lateral canals as possible causes of persistent periradicular inflammation8,9. Chemomechanical procedures have been demonstrated to be insufficient to control infection in lateral canals and apical ramifications10,11. This may be explained by the physical limitations of instruments to penetrate lateral canals and the short time available during preparation for effective irrigant diffusion to these areas.
Various methods for supplementary disinfection after chemomechanical procedures have been proposed, including finishing instruments and passive ultrasonic irrigation (PUI), with the goal to drive irrigants laterally in the root canal system, especially to difficult-to-reach areas, including lateral canals12,13,14. Ozone has been proposed as an endodontic irrigant because of its antibacterial effects and low toxicity to human tissues15,16. When used with ultrasonic activation, ozone holds potential as a supplementary approach to enhance the disinfection of the root canal system.
This study devised a novel analytical ex vivo method for infection and disinfection of simulated lateral canals located in the middle and apical segments of the root. The antibacterial effectiveness of supplementary approaches using passive ultrasonic irrigation (PUI) or mechanical XP-endo Finisher agitation of sodium hypochlorite (NaOCl), or ozonized water activated by continuous ultrasonic irrigation (CUI) was tested in this model. Bacterial reduction in the main root canal lumen promoted by these supplementary approaches was also evaluated. The null hypothesis is that there is no significant difference in the disinfecting ability of supplementary procedures in the main and lateral canals.
Methods
Specimen selection
The study protocol was approved by the Institutional Ethics Committee (#CB-176-2023) and all methods were performed following its regulation. The specimens used were mature single-rooted first and second mandibular premolars, extracted for reasons not related to this study, and available in the institutional tooth bank with the respective informed consent obtained from the donors. The exclusion criteria involved teeth presenting with open apices, pulp calcifications, root fractures or fissures, root resorption, previous endodontic treatment, root curvature greater than 20 degrees, and total length < 15 mm. Teeth with natural lateral canals or apical delta, as observed on micro-computed tomography (micro-CT, described below), were also excluded.
For selection, the specimens were initially inspected using an operating microscope and then subjected to radiographic examination using both buccolingual and mesiodistal projections. The teeth that passed these screening approaches were then scanned in micro-CT for final selection. Micro-CT scanning was done in the SkyScan 1174v2 device (Bruker Micro-CT, Kontich, Belgium) using the following parameters: 50 kV, 800 µA, 19 µm isotropic resolution, 180° rotation around the vertical axis, 1.0° rotation step, and a 0.5 mm-thick aluminum filter. The 3D images of the roots were reconstructed using the NRecon v.1.6.10.4 program (Bruker Micro-CT) and artifact reduction was performed using the parameters ring artifact correction = 14, beam hardening correction = 50%, and smoothing = 9. Three-dimensional images of the apical root specimens were created by the CTvol software (CTvol 2.3.2; Bruker Micro-CT).
Specimen preparation
After conventional access preparation, the root canals were explored with a hand K-type file size 10 (FKG Dentaire, La Chaux-de-Fonds, Switzerland) to check their patency to the apical foramen. The length of the specimens was standardized in approximately 15 mm, from the root apex to the most coronal reference point, by sectioning part of the tooth crown.
Canals were prepared using R-Motion instruments (FKG Dentaire), in reciprocating motion driven by the VDW Silver motor (VDW, Munich, Germany). Preparation was started with the R-Motion Glider file until reaching the working length (WL), which was established by placing a hand file in the canal until its tip was visualized at the apical foramen with the aid of an operating microscope. The canal was irrigated throughout preparation using saline solution delivered through a NaviTip 30-G irrigation needle (Ultradent, South Jordan, UT). Patency of the apical foramen was confirmed with a hand file size 10 throughout the procedures. Preparation was finished with the use of a R-Motion instrument size 40.04 at the WL (Fig. 1A).
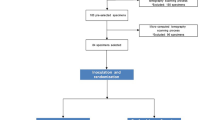
Schematics of the experiment protocol for infection and disinfection of lateral canals. (A) The root length was standardized and the root canal prepared up to the R-Motion instrument size 40/04. (B) Artificial lateral canals were created using a 0.10 mm bur operated by a drilling machine. (C) Micro-CT images confirmed the successful creation of lateral canals connecting the external root surface with the main root canal lumen. (D) Apparatus and strategy to permit penetration and circulation of culture medium in the lateral canals. A barrier was used to seal the apical foramen (blue). (E) After sealing of the apical and lateral foramina with a barrier (blue) to create a closed system, the disinfection procedures were performed (passive ultrasonic irrigation, XP-endo Finisher agitation, ozonated water/continuous ultrasonic irrigation, conventional irrigation). (F) Bacteriological sample taking from the main canal before and after preparation. (G) Bacteriological sample taking from each lateral canal.
Two artificial lateral canals were created on the mesial and distal sides of the root, one in the apical segment and the other in the middle segment of the root. Lateral canals were made using a small bur made of carbon metal and tungsten steel with 0.10 mm (100 μm) in diameter (XCAN, Hangzhou, Zhejiang, China) operated in the Discovery CEU01 machine (Romi Santa Bárbara, São Paulo, SP, Brazil) at 4000 rpm (Fig. 1B).
The specimens were again scanned in micro-CT as above to confirm the existence and path of the simulated lateral canals from the external surface of the root to the main canal lumen (Fig. 1C). Specimens with lateral canals that did not reach the main canal were excluded. The final sample consisted of 60 tooth specimens, which were randomly distributed into four groups according to the supplementary disinfection approach tested (n = 15): PUI with NaOCl, mechanical agitation of NaOCl with XP-endo Finisher, CUI with ozonated water, and a control group with conventional NaOCl irrigation. To make the detection of the exit of the lateral canals easier for further sample taking, marks were drilled on the root surface on each side and approximately 2 mm away from each lateral canal. These procedures were performed with the help of an operating microscope.
Root canals were irrigated with 17% EDTA using a NavTip 30-G needle (Ultradent, South Jordan, UT), and then the specimens were immersed in the same solution and subjected to ultrasonic agitation for 3 min to remove smear layer and other debris. In sequence, the specimens were subjected to an ultrasonic bath in distilled water for 3 min.
Specimen contamination
The apical foramen was sealed with MicroDam Blue gingival barrier (Microdont, São Paulo, SP, Brazil), leaving only the artificial lateral foramina open. The root canal was then filled with brain heart infusion (BHI) broth (Difco, Detroit, MI) using a NaviTip 30G needle. Next, the root was vertically introduced by the apex into BHI broth in a flask until the apical lateral canal was fully immersed in it. An aspiration needle connected to a vacuum pump was placed at the entrance of the main root canal, in order to drive the broth from outside the root into the main canal, via lateral canal (Fig. 1D). This guaranteed that the lateral canal was filled with culture broth. After approximately 5–10 s of broth aspiration, the whole procedure was repeated, this time with the root inserted deeper in the broth until the middle lateral canal was immersed. Afterwards, the specimens were placed in microcentrifuge tubes containing the same broth and subjected to 2 cycles of centrifugation at 10,000 rpm for 2 min, and later to ultrasonic agitation for 5 min to improve broth penetration into the main and lateral canals. The specimens completely immersed in BHI broth were sterilized in an autoclave.
A mixed bacterial culture was used for inoculation. This culture was obtained by sampling the subgingival bacterial biofilm from an adult volunteer with marginal periodontitis. The sample was placed in thioglycolate broth containing agar, and then an aliquot of pure culture of Enterococcus faecalis strain ATCC 29212 grown overnight was added. This mixed culture was incubated for 7 days at 37 °C. Next, the same immersion-aspiration procedures used to drive the culture broth to the lateral canals were repeated, this time with the grown mixed culture, to make sure that bacterial cells had access to the lateral canals. The teeth were then immersed in BHI broth inoculated with the same mixed bacterial culture and incubated for 30 days under gentle agitation at 37 °C. A fresh BHI broth was replenished in the main canal weekly, by injection with a NaviTip 30-G needle. In the last week of contamination, the same bacterial culture grown for 24 h was injected into the main canal with a NaviTip 30-G needle. After incubation, the external surfaces were wiped and cleaned with sterile gauze and ready for the experiment.
Supplementary antimicrobial procedures
The apical and lateral foramina were also sealed by applying MicroDam Blue to create a closed system. The specimens were mounted vertically in a bench vise, wrapped in sterile gauze moistened with sterile saline solution.
All procedures were performed by a single operator, who is an experienced endodontic specialist. The access cavity walls were disinfected with 2.5% NaOCl and a 10% sodium thiosulfate solution was used for NaOCl inactivation. Sterile paper points were used to take a sterility control sample from the access cavity walls, placed in flasks containing Tris–EDTA buffer (10 mmol/L Tris–HCl, 1 mmol/L EDTA, pH = 7.6) and frozen at − 20 °C.
The main canal was irrigated with 1 mL sterile saline solution to displace unattached cells. A hand K-type file size 15 (FKG Dentaire) was then introduced up to 1 mm from the WL and gentle motions were applied against the canal walls to displace bacterial biofilms into the solution. An initial sample (S1) was taken from the main canal lumen by placing sterile paper points size 35 up to the WL. Each paper point was moved circumferentially, left in the canal for 1 min to absorb the fluid, and then transferred to tubes containing Tris–EDTA buffer. Additional paper points were used until no visible moisture was observed on their surfaces. All samples were frozen at − 20 °C until DNA extraction.
The root canal was irrigated with 1 mL 2.5% NaOCl for 15 s using a conventional NaviTip 30-G irrigation needle positioned 2 mm from the WL. Next, the supplementary antimicrobial approach was performed according to the assigned experimental group.
PUI group
The Irrisonic E1 insert (Helse Ultrasonic, Santa Rosa de Viterbo, SP, Brazil) was positioned in the main canal up to 2 mm short of the WL. It was operated in gentle back-and-forth movements in apical-coronal direction during ultrasonic activation using a Newtrom Booster device (Acteon, Mérignac, France) at power 6 (30%). The activation of 1 mL 2.5% NaOCl was carried out in 3 cycles of 20 s each, renewing the 2.5% NaOCl with 1 mL of fresh solution after each cycle.
XP-endo Finisher irrigation group
This instrument was placed in the root canal up to 2 mm short of the WL and operated with gentle back-and-forth movements while driven by the VDW Silver motor (VDW) with 1 Ncm torque and 1,000 rpm speed. NaOCl was mechanically agitated the same way as in the PUI group, i.e., in 3 cycles of 20 s each, irrigating with 1 mL NaOCl after each cycle.
Ozonated water + CUI group
Water ozonization was obtained using the Philozon MedPlus ozone generator equipment (Balneario Camboriú, SC, Brazil), with the following parameters: ozone concentration of 60 µg/mL in the ozone release function in continuous mode, with a workflow at 1 L/min and medical oxygen pressure of 3.5 kgf/cm2. In a glass column connected to the generator, the ozone gas was injected into water purified by reverse osmosis (Inovare RO-510-LA SMM 50GPD water purifier, Rio de Janeiro, RJ, Brazil) for 5 min. Afterwards, the ozonated water was immediately placed into the reservoir of the ultrasonic device. Subsequently, through CUI driven by the Newtrom Booster device (Acteon) at power 6 (30%), 16.5 mL ozonized water was delivered per tooth, agitated with an Irrisonic E1 insert (Helse Ultrasonic) placed 2 mm from the WL. The ultrasonic irrigation was conducted in 3 cycles of 20 s each.
Conventional NaOCl irrigation group (control)
Irrigation was conducted with a NaviTip 30-G needle placed 2 mm short of the WL, making gentle back-and-forth movements in the apical-coronal direction. Irrigation was done in 3 cycles of 20 s each, with the irrigant remaining still in the canal, simulating the time used in the other groups.
Finally, in all groups, 1 mL 2.5% NaOCl was used to irrigate the canal using a NaviTip 30-G needle at 2 mm short of the WL, followed by inactivation using 1 mL 10% sodium thiosulfate. With the main canal lumen flooded with this inactivation solution, bacteriological sample S2 was taken as described for S1.
All procedures were conducted under strict aseptic conditions inside a previously UV-sterilized cabinet with its internal surfaces disinfected with NaOCl. The cabinet internal temperature was kept at 37 ºC by means of a heater (800-Heater; PlasLabs, Lansing, MI).
Sample taking from the lateral canals
For sampling the simulated lateral canals, the MicroDam Blue barriers were removed from the external root surfaces. The root surface surrounding the lateral canal was disinfected with 2.5% NaOCl scrubbed with a sterile microbrush (Microdont). Following this, a sterile microbrush was utilized to apply 10% sodium thiosulfate for NaOCl inactivation. A sterility control sample was taken from the disinfected root surface and placed in Tris–EDTA buffer.
Next, with the help of an operating microscope, the lateral canal foramen was visualized and a sample was taken from its entire length all the way up to the main canal using a size 2 round bur (Microdont) driven by the VDW Silver motor (VDW) at 1000 rpm. The obtained dentin filings were collected in a sterile flask, placed in Tris–EDTA buffer (S3), and frozen at − 20 °C until DNA extraction (Fig. 1E–G).
Bacterial detection and quantification
DNA was extracted from the samples using the QIAmp DNA Minikit (Qiagen, Valencia, CA) according to the manufacturer's instructions. The total bacterial levels were quantified by using a 16S rRNA gene-based quantitative real-time polymerase chain reaction (qPCR) assay conducted with the Power SYBR Green Master Mix (Thermo Fisher Scientific, Foster City, CA) in an ABI 7500 real-time PCR device (Thermo Fisher Scientific). The reaction volume of 20 µL contained 0.5 µM universal primers17 and 2 µL extracted DNA. Cycling parameters were: initial denaturation at 95 °C/10 min, and 40 repeats of denaturation at 95 °C/1 min, annealing at 58 °C/1 min and extension at 72 °C/1 min. Triplicates were used for controls and each sample. After amplification, melt curve analysis of the qPCR products was carried out to confirm specificity.
The ABI 7500 v2.0.4 software (Thermo Fisher Scientific) was used for data collection and analysis. Bacterial counts were inferred on the basis of standard curves constructed with known concentrations of Enterococcus faecalis DNA obtained from strain ATCC 29212. DNA extracted from this strain was quantified, ten-fold diluted from 107 to 102 cells in Tris–EDTA buffer and used for standard curve preparation.
Statistical methods
Data in the form of bacterial counts were collected from the main root canals before treatment (S1) and after treatment (S2). Because the data was clustered at the specimen level and showed overdispersion with the variance far exceeding the distributional mean, negative binomial (NB) regression models with individual random effects were fitted to the data. However, where significant differences in bacterial counts between the irrigation groups were observed at baseline (S1), the traditional negative binomial model, also known as NB2 model was used. The NB2 model was adjusted for S1 bacterial data. The NB2 model was also used for intergroup comparison of bacterial counts in both apical and middle lateral canal samples (S3). All analyses were performed in StataSE 17, and the significance level was set at α = 0.05.
Results
Main root canal disinfection
All S1 samples were positive in qPCR for the presence of bacteria. Two specimens from the control group were excluded because the sterility controls yielded positive results. The number of qPCR negative results for S2 samples was 2/15 (13%) in the PUI group, 3/15 (20%) in the XP-endo Finisher group, 4/15 (27%) in the ozone/CUI group, and 5/13 (33%) in the control group, with no significant differences between them (P > 0.05).
Table 1 shows the mean distribution of bacterial counts in the main root canal before (baseline) and after treatment stratified by irrigation group, as well as the Incidence Rate Ratio estimates representing intragroup comparisons. Bacterial counts in the main root canal substantially decreased by 99.2% in the PUI group, 99.1% in the ozone/CUI group, 99% in the XP-endo Finisher group, and 96% in the control group. All supplementary procedures promoted a highly statistically significant S1-to-S2 reduction in bacterial load (P < 0.01).
Intergroup comparisons of S1 and S2 canal samples are presented in Table 2. Findings showed no statistically significant differences between groups for S1 samples (P > 0.05). The same was observed when comparing S2 samples (P > 0.05).
Lateral canal disinfection
Data on intergroup comparisons of the bacterial counts in the apical and middle lateral canal samples are presented in Table 3. There were no statistically significant differences between the supplementary disinfection approaches for either the apical or the middle lateral canals (P > 0.05). There were no differences in results between apical and middle lateral canals either (P > 0.05). The numbers of apical lateral canals with negative results for bacteria were 7/15 (47%) for the PUI, 4/15 (27%) for the XP-endo Finisher, 7/15 (47%) for the ozone/CUI, and 4/13 (31%) for the control. Corresponding figures for the middle lateral canal were 3 (20%), 8 (53%), 6 (40%), and 3 (23%).
Discussion
This ex vivo study aimed to evaluate the disinfection of lateral canals using an innovative methodology for creating and contaminating simulated ramifications. Supplementary disinfection approaches were tested for their efficacy in erradicating or reducing bacterial counts not only in the simulated lateral canals but also in the main root canal. Three procedures were compared with a conventional irrigation approach and the results demonstrated that all of them were significantly effective in reducing the bacterial counts in the instrumented main root canal lumen, with no significant difference between them. As for their effectiveness in disinfecting apical and middle lateral canals, no significant differences were observed between them, with only a few specimens showing a complete absence of detectable bacteria. The amount of lateral canals with negative bacteria results ranged from 27 to 47% in the apical root segment and 20–53% in the middle portion.
The substantial bacterial reduction in the main canal by all the methods tested is in agreement with previous reports18,19,20. Mean counting reduction exceeded 99% in all experimental groups, while the control group, employing conventional NaOCl irrigation, demonstrated 96% reduction. However, these differences were not statistically significant. This suggests that, for the groups in which NaOCl was used with or without agitation (mechanical or ultrasonic), the major antibacterial effects in the main canal are mostly reliant on the time, renewal, and concentration of NaOCl irrigant, as pointed out in several previous studies21,22,23,24,25.
The antibacterial effects of PUI are attributed to the generation of cavitation and acoustic streaming phenomena, which are expected to improve both the irrigant distribution throughout the root canal system and the mechanical removal of biofilms13,26,27. Nonetheless, the results of different studies regarding the effectiveness of PUI in enhancing disinfection after chemomechanical preparation are conflicting20,28,29,30,31,32. The XP-endo Finisher instrument was also used in this study for NaOCl agitation. When activated, this instrument has an expanded action in the root canal assuming a spoon-shape under the body temperature (which was simulated in the present model). This shape allows it to touch more canal walls and displace bacterial biofilms therefrom31,33,34. Although both approaches significantly eliminated bacteria from the main canal, they showed no significant effects in lateral canals when compared to the control group. This suggests that irrigant distribution to these areas, if any, was not sufficient to cause a significant impact on bacterial counts. Consistent with these findings, a previous study also demonstrated the limited antibacterial effects of both approaches in the isthmus area of mesial roots of mandibular molars35. Therefore, although promising, the present results indicate that further improvements are necessary when using these strategies to disinfect remote areas of the root canal system.
The present study also included a protocol with ozonated water agitated with CUI. Ozone therapy has emerged as a potential supplementary approach in endodontic treatment, because of its antimicrobial properties and relatively low toxicity16,36. Ozone has an antimicrobial mechanism based on the strong oxidant effects on bacterial membranes, enzymes, and DNA37. However, there is a lack of well-controlled ex vivo and clinical studies evaluating the effects of ozone in endodontics and the available data are not yet sufficient to support its clinical application38. The use of ozone agitated with CUI in this ex vivo study showed antibacterial results in the main and lateral canals comparable to the groups in which NaOCl was used with or without agitation. While promising, further studies are required to test other parameters and applications of ozone during root canal treatment.
During irrigation with systems that produce an irrigant flow parallel to the canal walls, convection is generated into a lateral ramification only within a distance twice the size of its opening39. For the antibacterial irrigant to properly reach beyond this point and affect the whole extent of the lateral canal, it should diffuse, which takes time. To improve convection and expedite penetration for intravisit antibacterial effectiveness, mechanical, sonic, ultrasonic, and laser activation of the irrigant have been recommended12,39. Proper irrigant refreshment is required to renew the amount of the active agent in the target area, which was done in all groups here by using cycles of irrigation/activation. Even so, the results demonstrated that none of the tested approaches effectively disinfected the lateral canals. Further studies should explore different irrigation/activation parameters (including irrigant type, concentration, and retention time), devices, and other supplementary activating procedures to find a protocol that can predictably disinfect lateral canals.
This study used a devised method to evaluate the disinfection of simulated lateral canals in an ex vivo condition. The main strengths of this study are related to the fact that the artificial lateral canals were standardized in diameter (100 μm) and location, and were made in natural human teeth, better replicating the clinical condition for bacterial colonization and irrigant interactions. The diameter was thin and within the range reported for natural lateral canals6,7. The quality of the specimens was meticulously evaluated with micro-CT, to confirm that the simulated lateral canals were patent and were in direct contact with the main canal. The new method also included a modified approach that managed each tooth specimen individually to guarantee adequate contamination, by actively driving the fresh culture medium and later the mixed bacterial culture into the lateral canals. The approach commonly used for contaminating tooth specimens in the laboratory might not suffice to take the bacterial culture to these thin lateral canals. A mixed culture was used because it represents what is commonly observed in endodontic infections40, and E. faecalis was added to the mixed culture because it has been commonly reported to occur in teeth with posttreatment infections41, effectively colonize dentin42, and be resistant to treatment procedures43. Finally, round burs larger than the lateral canal diameter were used to exclusively sample the entire extent of the lateral canal.
This study has its limitations. One refers to the ex vivo nature, which is good on one hand to permit proper sample standardization, but on the other hand, cannot simulate all the clinical conditions that might influence the results. Canal preparation was performed before contamination to increase room for proper contamination of the whole system as well as to make the confection of the simulated lateral canals easier. However, the supplementary approaches were tested against a higher bacterial load than usually observed in the clinical setting immediately after preparation, which is when the supplementary approach is applied. Preparation was not performed after contamination and immediately before application of the supplementary approach in order to standardize the bacterial load subjected to supplementary antibacterial procedures and also to avoid the formation of dentinal debris that could block the lateral canals and introduce a critical source of bias. Another limitation was that the simulated lateral canals were straight and round, contrasting to the natural ones that are usually curved/sinuous and irregular5,44 and conceivably more difficult to disinfect. Finally, the method used to take samples from the lateral canals is destructive, precluding a longitudinal analysis before and after disinfection.
In conclusion, the method proposed here for creating and contaminating simulated lateral canals proved to be effective for ex vivo disinfection studies. All supplementary antibacterial approaches induced a substantial reduction in bacterial counts against a mixed bacterial culture in the main canal, with no significant differences between them. However, regarding lateral canal disinfection, none of the tested supplementary approaches showed significant effects when compared with the control group. Future studies using the methodology described and validated here should explore other methods or protocols for effectively disinfecting lateral canals.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
References
Weine, F. S. The enigma of the lateral canal. Dent. Clin. N. Am. 28, 833–852 (1984).
Pineda, F. & Kuttler, Y. Mesiodistal and buccolingual roentgenopraphic investigation of 7275 root canals. Oral Surg. Oral Med. Oral Pathol. 33, 101–110 (1972).
Vertucci, F. J. Root canal anatomy of the human permanent teeth. Oral Surg. Oral Med. Oral Pathol. 58, 589–599 (1984).
De Deus, Q. D. Frequency, location, and direction of the lateral, secondary, and accessory canals. J. Endod. 1, 361–366 (1975).
Ricucci, D. & Siqueira, J. F. Jr. Fate of the tissue in lateral canals and apical ramifications in response to pathologic conditions and treatment procedures. J. Endod. 36, 1–15. https://doi.org/10.1016/j.joen.2009.09.038 (2010).
Xu, T., Fan, W., Tay, F. R. & Fan, B. Micro-computed tomographic evaluation of the prevalence, distribution, and morphologic features of accessory canals in Chinese permanent teeth. J. Endod. 45, 994–999. https://doi.org/10.1016/j.joen.2019.04.001 (2019).
Dammaschke, T., Witt, M., Ott, K. & Schafer, E. Scanning electron microscopic investigation of incidence, location, and size of accessory foramina in primary and permanent molars. Quintessence Int. 35, 699–705 (2004).
Ricucci, D., Loghin, S. & Siqueira, J. F. Jr. Exuberant biofilm infection in a lateral canal as the cause of short-term endodontic treatment failure: Report of a case. J. Endod. 39, 712–718. https://doi.org/10.1016/j.joen.2012.12.008 (2013).
Ricucci, D., Siqueira, J. F., Bate, A. L. & Pitt-Ford, T. R. Histologic investigation of root canal-treated teeth with apical periodontitis: A retrospective study from twenty-four patients. J. Endod. 35, 493–502 (2009).
Vera, J. et al. One- versus two-visit endodontic treatment of teeth with apical periodontitis: A histobacteriologic study. J. Endod. 38, 1040–1052. https://doi.org/10.1016/j.joen.2012.04.010 (2012).
Perez, A. R. et al. Cleaning, shaping, and disinfecting abilities of 2 instrument systems as evaluated by a correlative micro-computed tomographic and histobacteriologic approach. J. Endod. 46, 846–857. https://doi.org/10.1016/j.joen.2020.03.017 (2020).
Siqueira, J. F. Jr. & Rôças, I. N. Optimising single-visit disinfection with supplementary approaches: A quest for predictability. Aust. Endod. J. 37, 92–98. https://doi.org/10.1111/j.1747-4477.2011.00334.x (2011).
van der Sluis, L. W., Versluis, M., Wu, M. K. & Wesselink, P. R. Passive ultrasonic irrigation of the root canal: A review of the literature. Int. Endod. J. 40, 415–426 (2007).
Debelian, G. & Trope, M. Cleaning the third dimension. Endodont. Pract. 8, 22–24 (2015).
Moraes, M. M. et al. The antimicrobial effect of different ozone protocols applied in severe curved canals contaminated with Enterococcus faecalis: Ex vivo study. Odontology 109, 696–700. https://doi.org/10.1007/s10266-021-00592-6 (2021).
Nogales, C. G. et al. Ozone therapy as an adjuvant for endondontic protocols: Microbiological—ex vivo study and citotoxicity analyses. J. Appl. Oral Sci. 24, 607–613. https://doi.org/10.1590/1678-775720160029 (2016).
Martin, F. E., Nadkarni, M. A., Jacques, N. A. & Hunter, N. Quantitative microbiological study of human carious dentine by culture and real-time PCR: Association of anaerobes with histopathological changes in chronic pulpitis. J. Clin. Microbiol. 40, 1698–1704 (2002).
Paiva, S. S. et al. Molecular microbiological evaluation of passive ultrasonic activation as a supplementary disinfecting step: A clinical study. J. Endod. 39, 190–194. https://doi.org/10.1016/j.joen.2012.09.014 (2013).
Nakamura, V. C. et al. Effect of ultrasonic activation on the reduction of bacteria and endotoxins in root canals: A randomized clinical trial. Int. Endod. J. 51(Suppl 1), e12–e22. https://doi.org/10.1111/iej.12783 (2018).
Loyola-Fonseca, S. C. et al. Disinfection and shaping of Vertucci class ii root canals after preparation with two instrument systems and supplementary ultrasonic activation of sodium hypochlorite. J. Endod. 49, 1183–1190. https://doi.org/10.1016/j.joen.2023.06.017 (2023).
Gazzaneo, I. et al. Root canal disinfection by single- and multiple-instrument systems: Effects of sodium hypochlorite volume, concentration, and retention time. J. Endod. 45, 736–741. https://doi.org/10.1016/j.joen.2019.02.017 (2019).
Ulin, C., Magunacelaya-Barria, M., Dahlen, G. & Kvist, T. Immediate clinical and microbiological evaluation of the effectiveness of 0.5% versus 3% sodium hypochlorite in root canal treatment: A quasi-randomized controlled trial. Int. Endod. J. 53, 591–603. https://doi.org/10.1111/iej.13258 (2020).
Siqueira, J. F. Jr., Rôças, I. N., Favieri, A. & Lima, K. C. Chemomechanical reduction of the bacterial population in the root canal after instrumentation and irrigation with 1%, 2.5%, and 5.25% sodium hypochlorite. J. Endod. 26, 331–334 (2000).
Boutsioukis, C. & Arias-Moliz, M. T. Present status and future directions—irrigants and irrigation methods. Int. Endod. J. 55(Suppl 3), 588–612. https://doi.org/10.1111/iej.13739 (2022).
Macedo, R. G., Wesselink, P. R., Zaccheo, F., Fanali, D. & van der Sluis, L. W. Reaction rate of NaOCl in contact with bovine dentine: Effect of activation, exposure time, concentration and pH. Int. Endod. J. https://doi.org/10.1111/j.1365-2591.2010.01785.x (2010).
Ahmad, M., Pitt-Ford, T. R. & Crum, L. A. Ultrasonic debridement of root canals: An insight into the mechanisms involved. J. Endod. 13, 93–101 (1987).
Pereira, T. C. et al. Chemical and mechanical influence of root canal irrigation on biofilm removal from lateral morphological features of simulated root canals, dentine discs and dentinal tubules. Int. Endod. J. 54, 112–129. https://doi.org/10.1111/iej.13399 (2021).
Silva, E. et al. Effectiveness of passive ultrasonic irrigation on periapical healing and root canal disinfection: A systematic review. Br Dent. J. 227, 228–234. https://doi.org/10.1038/s41415-019-0532-z (2019).
Caputa, P. E., Retsas, A., Kuijk, L., Chavez-de-Paz, L. E. & Boutsioukis, C. Ultrasonic irrigant activation during root canal treatment: A systematic review. J. Endod. 45, 31–44. https://doi.org/10.1016/j.joen.2018.09.010 (2019).
de Oliveira, H. F. et al. Influence of different agitation techniques on bacterial reduction in curved root canals. Aust. Endod. J. 49, 104–110. https://doi.org/10.1111/aej.12623 (2023).
Teves, A. et al. Multispecies biofilm removal by XP-endo Finisher and passive ultrasonic irrigation: A scanning electron microscopy study. Aust. Endod. J. 48, 91–97. https://doi.org/10.1111/aej.12549 (2022).
Beus, C., Safavi, K., Stratton, J. & Kaufman, B. Comparison of the effect of two endodontic irrigation protocols on the elimination of bacteria from root canal system: A prospective, randomized clinical trial. J. Endod. 38, 1479–1483. https://doi.org/10.1016/j.joen.2012.07.005 (2012).
Campello, A. F. et al. Enhancing the intracanal antibacterial effects of sodium hypochlorite with etidronic acid or citric acid. J. Endod. 48, 1161–1168. https://doi.org/10.1016/j.joen.2022.06.006 (2022).
Amaral, R. R. et al. Quantitative assessment of the efficacy of two different single-file systems in reducing the bacterial load in oval-shaped canals: A clinical study. J. Endod. 46, 1228–1234. https://doi.org/10.1016/j.joen.2020.06.007 (2020).
Alves, F. R. et al. Adjunctive steps for disinfection of the mandibular molar root canal system: A correlative bacteriologic, micro-computed tomography, and cryopulverization approach. J. Endod. 42, 1667–1672. https://doi.org/10.1016/j.joen.2016.08.003 (2016).
Hubbezoglu, I., Zan, R., Tunc, T. & Sumer, Z. Antibacterial efficacy of aqueous ozone in root canals infected by Enterococcus faecalis. Jundishapur J. Microbiol. 7, e11411. https://doi.org/10.5812/jjm.11411 (2014).
Barczyk, I. et al. Potential clinical applications of ozone therapy in dental specialties—a literature review, supported by own observations. Int. J. Environ. Res. Public Health 2023, 20. https://doi.org/10.3390/ijerph20032048 (2023).
Silva, E. et al. The effect of ozone therapy in root canal disinfection: A systematic review. Int. Endod. J. 53, 317–332. https://doi.org/10.1111/iej.13229 (2020).
Petridis, X. & van der Sluis, L. In Treatment of Endodontic Infections (eds. Siqueira, J. F. Jr & Rôças, I. N.) 307–337 (Quintessence Verlags-GmbH, 2022).
Siqueira, J. F. Jr. & Rôças, I. N. Present status and future directions: Microbiology of endodontic infections. Int. Endod. J. 55(Suppl 3), 512–530. https://doi.org/10.1111/iej.13677 (2022).
Rôças, I. N., Siqueira, J. F. Jr. & Santos, K. R. Association of Enterococcus faecalis with different forms of periradicular diseases. J. Endod. 30, 315–320 (2004).
Haapasalo, M. & Ørstavik, D. In vitro infection and disinfection of dentinal tubules. J. Dent. Res. 66, 1375–1379 (1987).
Portenier, I., Waltimo, T. M. T. & Haapasalo, M. Enterococcus faecalis—the root canal survivor and “star” in post-treatment disease. Endod. Top. 6, 135–159 (2003).
Xu, T. et al. Micro-computed tomography assessment of apical accessory canal morphologies. J. Endod. 42, 798–802. https://doi.org/10.1016/j.joen.2016.02.006 (2016).
Acknowledgements
This study was supported by grants from Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazilian Governmental Institutions.
Author information
Authors and Affiliations
Contributions
K.B.S., F.R.F.A., and J.F.S.Jr., designed research; K.B.S., A.F.C., F.R.F.A., R.C.V.R., D.D.V., K.R., S.C.L.F., F.L.H., and I.N.R.S., performed research; J.F.S.Jr., I. M., and I.N.R.S analyzed data; J.F.S.Jr. and F.R.F.A. contributed new analytic tools and assisted with writing; K.B.S., J.F.S.Jr., and F.R.F.A., wrote the paper. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Research involving human participants and/or animals
This study was conducted with the approval of the Research Ethics Committee from Central University of Venezuela (approval number #CB-176-2023) and followed medical protocol and ethics as outlined in the Declaration of Helsinki.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Brisson-Suárez, K., Siqueira, J.F., Alves, F.R.F. et al. Effectiveness of supplementary antimicrobial procedures in disinfecting lateral canals as evaluated by a novel ex vivo analytical approach. Sci Rep 14, 21840 (2024). https://doi.org/10.1038/s41598-024-72041-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72041-7
- Springer Nature Limited