Abstract
Ulinastatin, a broad-spectrum inflammatory inhibitor widely employed in the management of severe pancreatitis and sepsis, has not been extensively investigated for its therapeutic potential in bacterial meningitis. This study aims to assess the neuroprotective effects of ulinastatin on bacterial meningitis and elucidate its underlying mechanism. The rat model of bacterial meningitis was established by intracerebral injection of Escherichia coli. 3-week-old SD rats were randomly divided into 5 groups with 8 rats in each group, including control group, E.coli group, E.coli + UTI group (ulinastatin 50000IU/kg), E.coli + UTI + PMA group (ulinastatin 50000IU/kg + PMA 200 ug/kg), and E.coli + PMA group(PMA 200 ug/kg). Behavioral changes were assessed by Loeffler neurobehavioral score. Histomorphologic changes and apoptosis were assessed by hematoxylin and eosin staining, Nissl staining and TUNEL staining. Immunohistochemistry and immunofluorescence and western blotting were used to detect the expression levels of zonula occludens-1 (ZO-1) and phosphorylation protein kinase C (PKCα).It was found that ulinastatin treatment in Escherichia coli meningitis rats improved neurological function, alleviated meningeal inflammatory infiltration, reduced neuronal death, promoted the integrity of the blood–brain barrier structure. Moreover, phorbol myristate acetate (PMA, a protein kinase C activator), blocked the effective action of ulinastatin. These findings suggest that ulinastatin had neuroprotective effects on bacterial meningitis by inhibiting PKCα phosphorylation and reducing ZO-1 degradation, demonstrating that ulinastatin may be a promising strategy in the treatment of bacterial meningitis.
Similar content being viewed by others
Introduction
Bacterial meningitis, including Escherichia coli meningitis, is a severe inflammatory disease of the central nervous system (CNS) with elevated mortality and morbidity rates in children. From 30 to 50 percent of survivors continue to suffer neurological sequelae despite treatment with effective antibiotics, adjuvant corticosteroids and critical care1,2. Blood–brain barrier (BBB) disruption is considered to be a major contributor to the neurological sequelae, which is a complex component that prevents pathogen from invading the CNS3,4. Neuroprotective agents may significantly reduce neurological complications and sequelae in bacterial meningitis. Unfortunately, there have been relatively few drugs that protect BBB.
Ulinastatin consists of 143 amino acid residues and two Kunitz-type protease-inhibitor domains arranged in tandem to inhibit serine proteases, granulocyte elastase, and numerous other enzymes. Therefore, it has been shown to reduce inflammatory responses and improve tissue perfusion1,5,6,7,8,9,10,11. Ulinastatin has been widely used in the treatment of acute severe pancreatitis, sepsis, acute respiratory distress syndrome (ARDS), systemic inflammatory response syndrome (SIRS), and multiple organ dysfunction syndrome (MODS). Moreover, recent studies have suggested that ulinastatin had neuroprotective effect on rats with cerebral trauma, hepatic encephalopathy, and ischemia/reperfusion injuries by reducing BBB permeability12,13,14. The tight junction protein zonula occludens-1 (ZO-1) is a major component of BBB. ZO-1 degradation induced by ischemia–reperfusion injury in rats can be ameliorated by ulinastatin. Rats with bacterial meningitis showed significant decrease in ZO-1 expression, resulting in increased vascular permeability, cerebral edema, and consequently cerebral cell death15. Phosphorylation of ZO-1 on serine, threonine, and tyrosine residues decreases transmembrane resistance and increases barrier permeability16. Protein kinase C (PKC) is a class of serine/threonine specific kinase family, which can catalyze the phosphorylation of serine or threonine residues on various protein substrates. Ulinastatin is a serine proteases inhibitor as well as may alleviate ZO-1 loss17. Therefore, we hypothesized that ulinastatin had neuroprotection in bacterial meningitis via inhibiting PKCα phosphorylation and reducing ZO-1 loss.
Materials and methods
Animals and grouping
This study was approved by the the Medical Ethics Committee of Xiamen Children’s Hospital (ethics numberXMCH2021-187). All methods were carried out in accordance with relevant regulations and ARRIVE guidelines. Three weeks old healthy male Sprague–Dawley (SD) rats (n = 40, 100–120 g) were purchased from Shanghai Slack Laboratory Animal Co. Ltd. (Shanghai, China). The rats were bred under a 12 h light/12 h dark cycle with an ad libitum access to food and water.
Forty healthy male SD rats were randomly divided into five groups, which were subjected to the following interventions: (1) Control group (n = 8): no intervention. (2) E. coli group (n = 8): the E.coli meningitis model was established by a cerebellar medulla cistern puncture18 50 ul of CSF was extracted and 50 ul of E. coli suspension was injected in the same way, immediately followed by an injection of 0.5 ml of saline in the tail vein; (3) E. coli + UTI group (n = 8): E. coli rat meningitis model was established, and UTI (50,000 U/kg,0.25ml) was injected through the tail vein, immediately followed by an injection of 0.25 ml of saline in the tail vein; (4) E. coli + UTI + PMA group (n = 8): E. coli rat meningitis model was established, and UTI (50,000 U/kg,0.25 ml) was injected through the tail vein, immediately followed by an injection of PMA (200 ug/kg,0.25ml)through the tail vein. (5) E. coli + PMA group (n = 8): E.coli meningitis of model rats was established, and PMA (200 ug/kg 0.25 ml) was injected through the tail vein, immediately followed by an injection of 0.25 ml of saline in the tail vein.
Bacteria and drugs
E. coli K1 was obtained from the Laboratory Department of Zhongshan Hospital Affiliated to Xiamen University (Xiamen, China). We inoculated Escherichia coli into Miller LB (Luria–Bertani) broth, placed it in a bacterial incubator at 37 °C, and stored it at− 80 °C for later use. E.coli strains were inoculated in LB medium, grown overnight at 37 ℃ in 5% CO2 incubator, harvested after 24 h of growth, centrifuged, rinsed with normal saline, repeated twice, centrifuged again, and diluted with saline to 106 cfu/ml. Ulinastatin (UTI) was purchased from Techpool Biopharma Co. Ltd. (Guangdong, China, 031909133). ZO-1 antibody was obtained from Santa Cruz Biotechnology, Inc., USA, (SC-33725), β-actin antibody was produced by Santa Cruz Biotechnology, Inc., USA (SC-4718), p-PKCα antibody was purchased from Affinity Biosciences company (Jiangsu, China, AF8396), t-PKCα antibody was purchased from Affinity Biosciences Co.Ltd (Jiangsu, China; AF 6196). PMA was manufactured by Merck KGaA (Darmstadt, Germany, P8139).
Neurological function assessment
Loeffler five-point neurobehavioral score was used to evaluate the neurobehavioral scores of rats at 12 h after modeling. The following point score was applied: 5 points: can hold the back during normal activity, turns over in 5 s successfully. 4 points: reduced voluntary movement, able to turn over within 5 s. 3 points: turning over can be successful but the time is more than 5 s. 2 points: cannot roll over. 1 point: no exercise. 0 points: death.
Hematoxylin and eosin (H&E) staining
The rats were administered intraperitoneal tribromoethanol (Dalian Meilun Biotechnology Co.LTD, China, MA 04781-1) at a dose of 25 ml/kg, 12 h after modeling, for anesthesia prior to euthanasia. The brain tissue was dehydrated, transparent, embedded, cut into 6-µm-thick sections, baked, dewaxed to water, stained with hematoxylin for 3 min, and rinsed with water. Then, it was stained with eosin for 3 min, rinsed with water, and placed in an oven to dry. Further, the specimen was sealed with neutral resin after drying. The pathological changes in the rat brain were observed under a light microscope.
Nissl staining
The brain sections were dehydrated with 100%, 95.0%, and 70.0% alcohol for 1 min, respectively, and washed with ultrapure water. Then, the tablets were stained with 0.1% tar violet for 30 min and washed with ultrapure water. Next, they were dehydrated with 75.0%, 85.0%, 95.0%, and 100% gradient alcohol (1–2 min each). Finally, xylene was transparent three times, 2 min each time. The slices were sealed with neutral resin, and the number of neurons was counted under a light microscope.
TUNEL staining
The sections were routinely dewaxed to water, and 20 μg/ml DNase-free proteinase K was added by drop. The reaction was carried out at 37 °C for 15–30 min, washed with PBS three times. A volume of 50 μl of TUNEL assay solution was added, and the samples were incubated at 37 ℃ for 60 min in the dark, washed three times with PBS, and sealed. Fluorescence microscopy was used to observe at 100 times field of view (excitation wavelength 450–500 nm, emission wavelength 515–565 nm). Nine fields were randomly selected for each section. Images were taken and optical density analysis was performed using Image Pro Plus 6.0 Image analysis software. The integral optical density (IOD) value of the green fluorescence area was detected, and the average value was taken as the IOD value of each section.
Immunohistochemical staining
Brain tissue sections were routinely dewaxed to water, blocked with H2O2 for 10 min, blocked with donkey serum for 10 min, and incubated with primary antibody at 4 ℃ overnight. Then secondary antibody was added, followed by incubation at 37 ℃ for 30 min, development with DAB for 3 min, and staining with hematoxylin for 3 min. All aforementioned steps were followed by washing with PBS 3 times, 1 min each time. Then, we performed routine dehydration with 95% ethanol, followed by baking and sealing. Each section was observed under a light microscope, and nine fields of view were randomly selected for each section. Image Pro Plus 6.0 Image analysis software was used for optical density analysis after images were taken. The integral optical density (IOD) value of the positive staining area was measured, and its average value was taken as the IOD value of each section.
Western blotting
A homogenate was prepared at a rate of 500 ul per 50 mg of tissue. The tissue was cut into pieces, homogenized, and its protein content was determined by using BCA Protein Assay Kit (Beyotime Biotechnology, China). Further, 2 × SDS loading buffer was added, followed by boiling in water at 100 ℃ for 3 min. SDS-PAGE was prepared for protein solution separation. Then the protein was transferred to a Polyvinylidenfluorid (PVDF) membrane. The diluted solution of primary antibody was prepared, 3% milk-TBST was prepared, and the primary antibody was added. The membrane was incubated at 4 ℃ overnight, and washed with TBST three times, for 5 min each time. Next, the membrane was incubated in 3% milk-TBST-diluted secondary antibody (1:1000) at 37 ℃ for 1.5 h. Then membrane was washed with TBST three times, for 5 min each time. The protein was detected by ECL Kit (Beyotime Biotechnology, China) and pictures were taken by image system. After scanning the bands of each group, the gray levels were measured by ImageJ.
Statistical analysis
Data were statistically compared and analyzed using SPSS 20.0 software. Data were expressed as means ± SD. One-way ANOVA was used for comparison of means between groups, and the LSD test was implemented for pairwise comparison. P < 0.05 was considered to indicate statistically significant differences.
Results
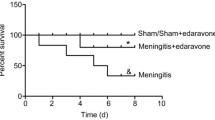
Effect of ulinastatin on Loeffler neurobehavioral score
Loeffler neurobehavioral score was used to assess behavioral changes. The E. coli was cultured in the cerebrospinal fluid of the model groups. The rats in the model groups showed lethargy, poor spirit, less movement, hemiplegia, convulsion, and ate less or no food. The Loeffler neurobehavioral score in the E.coli group was significantly lower than control group(n = 8, P < 0.001), After ulinastatin treatment, the score increased significantly(n = 8, P < 0.001), and PMA attenuated this effect (n = 8, P < 0.001) (Fig. 1).
Effect of ulinastatin on brain histomorphological analysis
Hematoxylin and eosin (H&E) staining was used to visualize the cellular morphology of the meninges and brain tissues. The E.coli group showed typical inflammatory manifestations, in which necrosis of the nerve cells was obvious near the meninges. Meanwhile, the meninges were edematous and congested, with a large number of inflammatory cells. The E. coli + UTI Group had decreased neural necrosis in the cerebral cortex and a slightly lower number of the inflammatory cells under the meninges than the E.coli group. However, The pathological manifestations were aggravated when added PMA, more gray matter necrosis was present in the cerebral cortex, meningeal congestion, swelling, and inflammatory cell infiltration were increased, and even submeningeal cortical hemorrhage had occurred (Fig. 2a). Furthermore, Nissl staining was used to observe neuronal damage. The control group showed a large number of nissl bodies arranged regularly under the microscope, stained nuclei were intact and with normal morphology. The number of nissl bodies in E. coli group decreased (n = 4, P < 0.001), nuclear pyknosis, deformation, lysis, and other morphological abnormalities were observed. After ulinastatin treatment, nissl bodies increased significantly (n = 4, P < 0.01), increased nuclei with normal morphology, and fewer cells with abnormal morphology. Howerer, When combined with PMA, nissl bodies decreased again (n = 4, P < 0.05) (Fig. 2b,c). These findings suggested that ulinastatin reduced meningeal inflammatory infiltration, while PMA can aggravate the progression of inflammation.
H&E and Nissl staining of brain tissue in rats of each group Prcm medial precentral area, Prcl lateral precentral area, SI sensorimotor cortex I, ACd anterior cingulate cortex, dorsal part, CPu caudate putamen, acb accubens nucleus. (a). H&E staining of brain tissue in rats of each group; Black Up-Pointing Traingle Indicates degeneration, necrosis and lysis of nerve cells; Black Diamond Indicates meningeal congestion and inflammatory cell aggregation; (b). Nissl staining of brain tissue in rats of each group; Black Star Indicates Nissl bodies of nerve cells; (c). Statistics of Nissl bodies count in brain tissue of rats in each group; Data were expressed as Mean ± SD, (n = 4); *P < 0.05, **P < 0.01, ***P < 0.001.
Effect of ulinastatin on cerebral cell apoptosis
TdT-mediated dUTP nick end labeling (TUNEL) staining was used to detect cerebral cell apoptosis. It could be seen that compared with the control group, The integral optical density (IOD) value of TUNEL positive staining area in E.coli group rats was significantly increased (n = 4, P < 0.001). However, the IOD value was significantly decreased after ulinastatin treatment (n = 4, P < 0.001). Nevertheless, the IOD value was significantly increased (n = 4, P < 0.001) when PMA were combined to treat E.coli rats. E.coli + PMA group had the most apoptotic cells. These datas suggested that ulinastatin reduced apoptosis, while PMA did the opposite (Fig. 3a,b).
TUNEL staining of brain tissue in rats of each group Prcm medial precentral area, Prcl lateral precentral area, SI sensorimotor cortex I, ACd anterior cingulate cortex,dorsal part, CPu caudate putamen, acb accubens nucleus, TUNEL Terminal Deoxynucleotidyl Transferase Mediated dUTP Nick End Labeling. (a). TUNEL staining of brain tissue in rats of each group; White diamond indicates TUNEL positive staining, it appears as a bright green fluorescence, located in the nucleus, indicating DNA breakage and apoptosis. (b). Statistics of TUNEL positive staining in brain tissue of rats in each group; Data were expressed as Mean ± SD, (n = 4); *P < 0.05, ***P < 0.001.
Effect of ulinastatin on expressions of PKCα phosphorylation and ZO-1
Immunohistochemistry and immunofluorescence staining was used to detect the expressions of p-PKCα and ZO-1. The expression of ZO-1 in the E. coli group was significantly reduced compared with control group (n = 4, P < 0.001), but increased significantly after ulinastatin treatment (n = 4, P < 0.001). However, compared with the E.coli + UTI group, the expression of ZO-1 in E.coli + UTI + PMA group was significantly reduced (n = 4, P < 0.001) (Fig. 4a,b). Compared with the control group, the expression of p-PKCα in the E. coli group was significantly increased (n = 4, P < 0.001). The number was significantly decreased in the E.coli + UTI group (n = 4, P < 0.001). PMA aggravated PKCα phosphorylation and attenuated the effect of ulinastatin (n = 4, P < 0.001) (Fig. 5a,b). Western blotting was used to the detect the relative expression levels of ZO-1 and p-PKCα, verifying the above immunohistochemical results (Fig. 6). These datas suggested that ulinastatin may increase ZO-1 expression by inhibiting PKCα phosphorylation.
IF staining of ZO-1 of brain tissue in rats of each group IF Immunofluorescence; DAPI 4’,6-diamidino-2-phenylindole; (a) IF staining of ZO-1 of brain tissuein rats of each group; DAPI staining shows the nucleus, it appears as a blue fluorescence; White diamond Indicates ZO-1 positive staining, it appears as a bright red fluorescence, located in the cytoplasm of vascular endothelial cell. Merge was merged from DAPI staining and ZO-1IF staining. (b) Statistics of ZO-1 positive staining in brain tissue of rats in each group; Data were expressed as Mean ± SD, (n = 4); ***P < 0.001.
IHC staining of p-PKCαof brain tissue in rats of each group Prcm medial precentral area, Prcl lateral precentral area, SI sensorimotor cortex I, ACd anterior cingulate cortex, dorsal part, CPu caudate putamen, acb accubens nucleus; (a) IHC staining of p-PKCα of brain tissue in rats of each group; Black Up-Pointing Traingle Indicates p-PKCα positive staining; p-PKCα positive staining sites were brownish yellow, located near the nucleus in the cytoplasm; (b) Statistics of p-PKCα positive staining in brain tissue of rats in each group; Data were expressed as Mean ± SD, (n = 4); ***P < 0.001.
Expression of p-PKCα& ZO-1of brain tissue in rats of each group (a). western blotting of p-PKCα& ZO-1of brain tissue in rats of each group; (b). Statistics of p-PKCαin brain tissue of rats in each group; (c). Statistics of ZO-1in brain tissue of rats in each group; Data were expressed as Mean ± SD, (n = 4); **P < 0.01, ***P < 0.001. Fig. 6 is a representative image of the Western blot results for each group in this experiment. The original Western blot bands and membrane photos for all experimental rats can be found in Supplementary Image S1 online.
Discussion
Our findings demonstrated that intravenous ulinastatin administration immediately after E.coli meningitis modeling in rats is neuroprotective, as assessed by neurobehavioral scores, inflammatory infiltration, histomorphological results, and brain cell apoptosis. One possible protective mechanism was that ulinastatin inhibits PKCɑ phosphorylation and thus reduces ZO-1 damage (Fig. 7).
Hematoxylin and eosin (H&E) staining, Nissl staining and TUNEL staining showed that ulinastatin attenuated inflammatory response and neuronal apoptosis in cerebral cells,similar to the results in lung, heart, liver and kidney cells subjected to lipopolysaccharide (LPS)-induced sepsis. LPS is a principal component of the outer membranes of gram-negative bacteria which is known to induce the production of several inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β(IL-1β), IL-6, and IL-2, which recruit and activate neutrophils and lead to increase neutrophil-endothelial adhesion, neutrophil accumulation, transendothelial migration of neutrophil6. Ulinastatin could alleviate these inflammatory cytokines, reduced inflammatory cell infiltration and apoptosis19,20. It inhibited apoptosis via downloading incRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) and Enhancer of Zeste Homolog 2 (EZH2), decreasing pAkt, NF-κB and Bcl-2 in cardiac microvascular endothelial cells (CMVECs)21,22, or blocked High Mobility Group Protein 1(HMGB1)/Toll Like Receptor 4(TLR4) NF-KB signal transduction in kidney23,24. In addition, ulinastatin also has anti-inflammatory and anti-apoptotic effects in the central nervous system (CNS). It could inhibit TNF-a, IL-1β, IL-6, translocation of NF-kB p65, oxidative stress, reduced the expression of caspase-3 and Bax, and increased Bcl-2 in cerebral cortex cells after cardiopulmonary resuscitation and brain trauma rats, The same effect was seen in hippocampal damage caused by paraquat poisoning, isoflurane anesthesia, and ischemia-reperfusion25,26,27,28. In this study, the anti-inflammatory and anti-apoptotic effects of ulinastatin on cerebral cells were demonstrated in Escherichia coli meningitis rats.
Protein kinase C alpha (PKCα) is a member of the PKC family of serine/threonine kinases and has been implicated in the regulation of cell survival and apoptosis in neurons.The effects of PKC activation can vary significantly depending on the specific PKC isoform involved. While isoforms such as PKCεare generally associated with anti-apoptotic effects29, other isoforms like PKCδ and PKCα can promote apoptosis30. PKCα can influence apoptotic pathways by affecting various signaling cascades. It may activate or enhance the activity of pro-apoptotic proteins while inhibiting anti-apoptotic factors, leading to cell death. PKCα has been reported to alter the balance between pro-apoptotic and anti-apoptotic members of the Bcl-2 family. For example, PKCα may increase the expression of pro-apoptotic proteins like Bax while decreasing anti-apoptotic proteins like Bcl-2. PKCα’s role in cell cycle regulation can also affect apoptosis. By disrupting cell cycle checkpoints or promoting cell cycle arrest, PKCα can contribute to apoptotic processes31.
Immunohistochemical staining immunofluorescence and western blotting results showed decreased PKCα phosphorylation (p-PKCα) and increased ZO-1 expression after ulinastatin intervention, with PKC agonists blocking ulinastatin action. Another animal experiment confirmed that ulinastatin increased ZO-1 expression in LPS -induced lung injury32. ZO-1 was initially identified as part of the tight junction in 198633, linking occludin and claudins to the actin cytoskeleton34. ZO-1 expression was significantly reduced in cerebral cells of rats with E.coli meningitis15,35, ZO-1 serine/threonine phosphorylation disrupts BBB integrity and permeability. PKC is an important signaling molecule in the regulation of life activities, which is divided into multiple isoforms and plays an important role in inflammation. PKC activation is mainly divided into two parts, one is the release of pseudosubstrate from the substrate binding lumen, and the other is the phosphorylation of the catalytic region. PKC is a classical serine/threonine kinase, and ulinastatin is a kuntiz domain that inhibits phosphorylation of serine/threonine. PKCα could be activated by LPS and alter brain microvascular endothelial cells (BMECs) tight junctions, with greater activity of PKCα, the tight junctions lose their ability to form a tight barrier36. The host cell signal transduction pathways of PKC were involved in meningitis-causing E. coli invasion of HBMECs37. our Immunohistochemical and western results suggested that ulinastatin inhibited the phosphorylation of PKCα and reduced the degradation of ZO-1 in E.coli meningitis rats, what’s more, the affection for ulinastatin is blocked by PKC agonists. In this study, ulinastatin acted as a PKC-like inhibitor, inhibiting PKCα activity and likely inhibiting E.coli invasion into the HMEC and protecting ZO-1 expression, thus protecting the blood–brain barrier and having neuroprotective effects on the brain.
This study provides valuable insights into the effects of ulinastatin, but several limitations should be acknowledged. Firstly, the sample size used in our study was relatively small, which may limit the generalizability of our results. Future studies with larger cohorts are needed to validate our findings. Secondly, the study primarily assessed the short-term effects of ulinastatin. Long-term effects and potential side effects were not evaluated, which are important for understanding the overall efficacy and safety of the treatment.
Conclusion
In conclusion, our findings suggested that ulinastatin achieved neuroprotection by inhibiting PKCα phosphorylation and increasing ZO-1 expression, reducing brain cell apoptosis in a rat model of E. coli meningitis. Therefore, Ulinastatin can be considered as a strategy for therapeutic intervention in bacterial meningitis.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Kim, K. S. Pathogenesis of bacterial meningitis: From bacteraemia to neuronal injury. Nat. Rev. Neurosci. 4, 376–385 (2003).
Lucas, M. J., Brouwer, M. C. & van de Beek, D. Neurological sequelae of bacterial meningitis. J. Infect. 73, 18–27 (2016).
Tsukita, S., Furuse, M. & Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2, 285–293 (2001).
He, F. et al. PKC and RhoA signals cross-talk in Escherichia coli endotoxin induced alterations in brain endothelial permeability. Biochem. Biophys. Res. Commun. 425, 182–188 (2012).
Onai, H. & Kudo, S. Suppression of superantigen-induced lung injury and vasculitis by preadministration of human urinary trypsin inhibitor. Eur. J. Clin. Invest. 31, 272–280 (2001).
Yano, T., Anraku, S., Nakayama, R. & Ushijima, K. Neuroprotective effect of urinary trypsin inhibitor against focal cerebral ischemia-reperfusion injury in rats. Anesthesiology 98, 465–473 (2003).
Koga, Y. et al. Urinary trypsin inhibitor suppresses excessive superoxide anion radical generation in blood, oxidative stress, early inflammation, and endothelial injury in forebrain ischemia/reperfusion rats. Neurol. Res. 32, 925–932 (2010).
Park, J. H., Kwak, S. H., Jeong, C. W., Bae, H. B. & Kim, S. J. Effect of ulinastatin on cytokine reaction during gastrectomy. Korean J. Anesthesiol. 58, 334–337 (2010).
Luo, H. M. et al. The effects of ulinastatin on systemic inflammation, visceral vasopermeability and tissue water content in rats with scald injury. Burns 39, 916–922 (2013).
Shu, H. et al. Ulinastatin, a protease inhibitor, may inhibit allogeneic blood transfusion-associated pro-inflammatory cytokines and systemic inflammatory response syndrome and improve postoperative recovery. Blood Transfus 12(Suppl 1), s109-118 (2014).
Liu, M. et al. Effect of ulinastatin on the permeability of the blood-brain barrier on rats with global cerebral ischemia/reperfusion injury as assessed by MRI. Biomed. Pharmacother. 85, 412–417 (2017).
Xiong, L. et al. The protective roles of urinary trypsin inhibitor in brain injury following fat embolism syndrome in a rat model. Cell Transplant. 28, 704–712 (2019).
Abo El Gheit, R. E. et al. Role of serine protease inhibitor, ulinastatin, in rat model of hepatic encephalopathy: Aquaporin 4 molecular targeting and therapeutic implication. J. Physiol. Biochem. 76, 573–586 (2020).
Liu, T., Liao, X. Z. & Zhou, M. T. Ulinastatin alleviates traumatic brain injury by reducing endothelin-1. Transl. Neurosci. 12, 1–8 (2021).
Yang, R. et al. circ_2858 helps blood-brain barrier disruption by increasing VEGFA via sponging miR-93-5p during Escherichia coli meningitis. Mol. Ther. Nucl. Acids 22, 708–721 (2020).
Cruzalegui, F. H. & Bading, H. Calcium-regulated protein kinase cascades and their transcription factor targets. Cell. Mol. Life Sci. 57, 402–410 (2000).
Li, X. F. et al. Ulinastatin protects brain against cerebral ischemia/reperfusion injury through inhibiting MMP-9 and alleviating loss of ZO-1 and occludin proteins in mice. Exp. Neurol. 302, 68–74 (2018).
Koedel, U. & Pfister, H. W. Models of experimental bacterial meningitis. Role and limitations. Infect. Dis. Clin. North Am. 13, 549–577 (1999).
Cao, C. et al. Ulinastatin protects against LPS-induced acute lung injury by attenuating TLR4/NF-κB pathway activation and reducing inflammatory mediators. Shock 50, 595–605 (2018).
Zhao, P. et al. Ulinastatin attenuates lipopolysaccharide-induced cardiac dysfunction by inhibiting inflammation and regulating autophagy. Exp. Ther. Med. 20, 1064–1072 (2020).
Yu, Z., Rayile, A., Li, Y., Zhao, Q. & Zhang, X. Ulinastatin alleviated Lps-induced injury of cardiac micro vascular endothelial Cell via NF-κB pathway. Pak. J. Pharm. Sci. 30, 983–988 (2017).
Feng, X., Ma, W., Chen, J., Jiao, W. & Wang, Y. Ulinastatin alleviates early brain injury after traumatic brain injury by inhibiting oxidative stress and apoptosis. Acta Cir. Bras. 37, e370108 (2022).
Chen, F., Zhu, J. & Wang, W. Ulinastatin attenuates LPS-induced inflammation and inhibits endoplasmic reticulum stress-induced apoptosis in renal tubular epithelial cells via regulation of the TLR4/NF-κB and Nrf2/HO-1 pathways. Inflammation 44, 2323–2332 (2021).
Zhang, X., Su, C., Zhao, S., Li, J. & Yu, F. Combination therapy of Ulinastatin with Thrombomodulin alleviates endotoxin (LPS)—Induced liver and kidney injury via inhibiting apoptosis, oxidative stress and HMGB1/TLR4/NF-κB pathway. Bioengineered 13, 2951–2970 (2022).
Cho, Y. S. et al. Ulinastatin inhibits cerebral ischemia-induced apoptosis in the hippocampus of gerbils. Mol. Med. Rep. 12, 1796–1802 (2015).
Li, H. F., Zhao, S. X., Xing, B. P. & Sun, M. L. Ulinastatin suppresses endoplasmic reticulum stress and apoptosis in the hippocampus of rats with acute paraquat poisoning. Neural Regen. Res. 10, 467–472 (2015).
Jiang, X. M. et al. Ulinastatin alleviates neurological deficiencies evoked by transient cerebral ischemia via improving autophagy, Nrf-2-ARE and apoptosis signals in hippocampus. Physiol. Res. 67, 637–646 (2018).
Guo, Y. et al. Effect of ulinastatin on isoflurane-induced neuronal apoptosis in the hippocampus of rats. Nan Fang Yi Ke Da Xue Xue Bao 39, 850–854 (2019).
Muscella, A. et al. Anti-apoptotic effects of protein kinase C-delta and c-fos in cisplatin-treated thyroid cells. Br. J. Pharmacol. 156, 751–763 (2009).
Li, Z. et al. Role of PKC-ERK signaling in tamoxifen-induced apoptosis and tamoxifen resistance in human breast cancer cells. Oncol. Rep. 27, 1879–1886 (2012).
Lee, S. J. et al. Vibrio vulnificus VvhA induces NF-κB-dependent mitochondrial cell death via lipid raft-mediated ROS production in intestinal epithelial cells. Cell Death Dis. 6, 1655 (2015).
Jiang, Y. X. & Huang, Z. W. Ulinastatin alleviates pulmonary edema by reducing pulmonary permeability and stimulating alveolar fluid clearance in a rat model of acute lung injury. Iran J. Basic Med. Sci. 25, 1002–1008 (2022).
Stevenson, B. R., Siliciano, J. D., Mooseker, M. S. & Goodenough, D. A. Identification of ZO-1: A high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J. Cell Biol. 103, 755–766 (1986).
Itoh, M. et al. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell Biol. 147, 1351–1363 (1999).
Chi, F. et al. Meningitic Escherichia coli K1 penetration and neutrophil transmigration across the blood-brain barrier are modulated by alpha7 nicotinic receptor. PLoS One 6, e25016 (2011).
Rosson, D. et al. Protein kinase C-alpha activity modulates transepithelial permeability and cell junctions in the LLC-PK1 epithelial cell line. J. Biol. Chem. 272, 14950 (1997).
Kim, K. S. Human meningitis-associated Escherichia coli. EcoSal Plus https://doi.org/10.1128/ecosalplus.esp-0015-2015 (2016).
Funding
This work was supported by the Xiamen Children’s Hospital and Xiamen Municipal Bureau of Science and Technology (Grant no.3502Z20209211).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by XLZ, JSW, HHS, LLW, YZ, QQ T, TTB, and KX. The first draft of the manuscript was written by XLZ ,CMW, and CML, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zheng, X., Wang, J., Su, H. et al. Neuroprotective effects of ulinastatin on Escherichia coli meningitis rats through inhibiting PKCα phosphorylation and reducing zonula occludens-1 degradation. Sci Rep 14, 21236 (2024). https://doi.org/10.1038/s41598-024-72097-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72097-5
- Springer Nature Limited