Abstract
Phosphorus and molybdenum play important roles in the formation of microbial cell structures and specific enzymes crucial for metabolic processes. Nevertheless, questions remain about the preservation of these elements within ancient microfossils. Here, we present shape-accurate ion images capturing phosphorus and molybdenum on Palaeoproterozoic filamentous microfossils by pioneering a methodology using lateral high-resolution secondary ion mass spectrometry. Introducing electrically conductive glass for mounting isolated microfossils facilitated clearer observations with increased secondary ion yields. Phosphorus was detected along the contours of microfossils, providing direct evidence of phospholipid utilization in the cell membrane. Trace amounts of molybdenum were detected within microfossil bodies, suggesting potential remnants of molybdenum-bearing proteins, such as nitrogenase. These findings align with the hypothesized cyanobacterial origin of filamentous gunflint microfossils. Our methodology introduces a groundbreaking tool for obtaining crucial insights into the cellular evolution and metabolic pathways of microorganisms, allowing comparisons of their morphological characteristics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Microfossils discovered within the 1.9 billion-year-old gunflint formation in Canada, known as “gunflint microfossils”, have been recognized as a standard for Precambrian microfossil research1,2,3,4. Predominantly characterized by filamentous, spheroid, and radial shapes, they also exhibit minor morphologies such as umbrella-like, colonial, and eukaryotic forms1,5,6,7. Variations in the morphologies, elemental distributions, coexisting minerals, and carbon isotopic compositions of these microfossils have been discussed, revealing the ecosystems and environmental conditions present at that time8,9,10,11,12. The filamentous gunflint microfossil, named Gunflintia, stands out as one of the most distinguished types of microfossils. Its morphology closely resembles that of cyanobacteria, which is supported by similarities in carbon isotopic compositions1,5,6,7,10. While the concentrations and distributions of major organic elements, such as carbon, nitrogen, and sulfur, have been examined via high-resolution ion imaging, direct imaging of other bioessential elements, such as phosphorus, has never been conducted. Its biogenicity has been inferred solely on the basis of concentration ratios of these elements to carbon by targeting bulk organic matter13,14. Molybdenum also plays an essential role in certain types of enzymatic proteins, including nitrogenase, which is necessary for biological nitrogen fixation. Consequently, the availability of molybdenum in the ocean has been a subject of discussion to elucidate palaeoenvironments and ecosystems4,15,16,17,18. Challenges in detecting trace metal elements, such as iron or manganese, in microfossil samples prepared as thin sections have been addressed in previous research using a lateral high-resolution secondary ion mass spectrometry (NanoSIMS)19. However, no study has directly detected of molybdenum in microfossils.
A recent study detected phosphorous in Archean microfossils; however, the imagery of its distribution and sporadic occurrences raise doubts about the organic origin, suggesting a mineralogical or artificial origin instead20. Notably, phosphorus and trace metal elements can also exist in sedimentary rocks in mineral forms. Therefore, to confirm their biogenic origin, observing their occurrence under an electron microscope, assessing their correlation with organic elements, and examining their compositional ratios compared with those of contemporaneous organic matter are crucial. These comprehensive analyses are essential steps in discerning the true nature and origin of these microfossils. The underlying difficulty in measuring trace elements in microfossils stems from their low concentrations, which are often in the parts-per-million (ppm) range, a concentration range that even living organisms exhibit21. In addition to these problems, the recent discovery of eukaryote-like microfossils in the Gunflint Formation has demonstrated that a combination of optical transmitted observations, combined with in situ high-resolution and high-sensitivity observations, provides more information for microfossil studies7. This integrated approach offers a more comprehensive understanding and more detailed data for microfossil studies, thereby addressing the complexities associated with their analysis. Hence, we have devised a methodology aimed at detecting trace amounts of phosphorus and molybdenum in conjunction with organic elements by using NanoSIMS while retaining optical and electrical observations targeting filamentous Gunflint microfossils. This innovative approach allows for a more holistic analysis, enabling us to unravel the intricate details of these ancient microorganisms and their surrounding environment.
Results
Sample descriptions
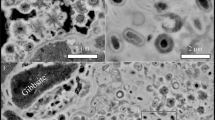
The host rock sample of the examined filamentous microfossils, with the ID number of 170908004, was collected from stromatolitic cherts occurring in the lowermost part of the Gunflint Formation; this sample is the same as that described in a previous report7. The bulk organic matter within this sample exhibited a carbon isotopic composition of -33.2‰. Microfossils were successfully isolated from bulk chert rock via an acid maceration method adapted from prior research22. Following mounting on indium‒tin oxide-coated glass slides (ITO glass, NH4-free, 100 Ω resistor, Matsunami Glass Ind., Ltd.), the naked microfossils offered significant advantages for microscale analysis. The ample electrical conductivity of these materials facilitated secondary ion analysis, whereas their transparency allowed for optical microscope observations. Six filamentous microfossils were targeted, exhibiting optical semitransparency with a brownish colour (Fig. 1a–f). Electron microscope images revealed the absence of detectable mineral crystals, such as sulfides, oxides, and fluorides, as well as the absence of any metal element accumulation in microfossils (Fig. 1g–l). Targets 1–4 and 6 displayed a narrow, smooth tubular morphology with an approximate width of 2 μm. Targets 2 and 3 exhibited a slightly rough structure, with Target 3 showing deformed ends, likely resulting from the compaction process. Targets 4 and 6 were accompanied by submicron-sized spherical organic matter adhered to the sheath, and their cell walls appeared thicker than those of the other targets. Target 5 had a wider sheath structure with an approximate width of 5 μm and featured a microscale protrusion. Detailed descriptions are available in the Supplementary Results and Discussion section.
Optical and electron microscopy images of six filamentous microfossils. (a–f) Optical images of six filamentous microfossils that were isolated and mounted on ITO glass. The black arrows indicate each target. The corresponding secondary electron images, denoted by (g–l), are provided in sequence. The white arrows on Targets 4 and 6 indicate spherical organic matter, and those on Target 5 indicate protrusions on microfossils. The scale bars in the optical images represent 20 μm, and those in the electron microscopy images represent 10 μm.
Secondary ion analysis of isolated microfossils
Ultrahigh-resolution secondary ion images of carbon (C), nitrogen (N), and sulfur (S) were obtained from six filamentous microfossils without a mineral matrix, achieving a spatial resolution of approximately 80 nm (CNS mode, Fig. 2). The distributions of each element were correlated with the optical images of the microfossils and were not significantly different from the background of the ITO glass surface. The mean secondary ion yields of 12C−, 12C14N−, and 32S− from the examined microfossils were 4.4 × 103, 3.1 × 103, and 4.0 × 102 cps/pA, respectively (Table 1). When the yield was restricted to only the areas where counts were obtained from the microfossil, the yields were 2.1 × 104, 1.5 × 104, and 1.9 × 103 cps/pA, respectively. These values represent a significant increase, ranging from 14 to 54 times greater than those measured for the same type of microfossils in thin section samples, as reported in a previous study (Table 1). Carbon, nitrogen, and sulfur were distributed primarily along the shape of the microfossil, although variations in count intensity were visible due to topographic effects. Notably, sulfur displayed sporadic distributions on Targets 2, 3, and 6 (Fig. 2b–d). Upon normalization to carbon, nitrogen generally exhibited a sparse distribution overall, with no observed localized concentrations, whereas a sporadic distribution of sulfur persisted (Supplementary Fig. 1). There was no difference in the distributions of nitrogen and sulfur between the main body of microfossils and the adhered spherical organic matter on Targets 4 and 6 or the microscale protrusion on Target 5. Ambiguous organic matter next to Target 3 was also imaged at the bottom of Fig. 2c, revealing different concentrations of organic elements.
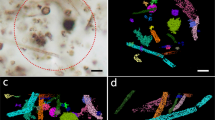
Optical and secondary ion images of six filamentous microfossils. Series (a–f) correspond to Targets 1 to 6, respectively. Three secondary ion images obtained in CNS mode (12C−, 12C14N-, and 32S- in the blue frame) and two obtained in PMo mode (31P− and 98Mo16O− in the green frame) are arranged horizontally next to the optical images. The scale bars in each ion image indicate 5 μm.
Ultrahigh-resolution secondary ion imaging of phosphorus and molybdenum
After hundreds of frames were stacked with the same spatial resolution in CNS mode, secondary ion images of phosphorus and molybdenum were acquired on the filamentous Gunflint microfossils (PMo mode, Fig. 2). Although most 31P- and 98Mo16O− images were faint, these elements were detected along the microfossil, mimicking the shape, together with 12C− and 12C14N−, rather than originating from backgrounds (Supplementary Fig. 2). The active area-based secondary ion yields of 31P− and 98Mo16O− were confirmed to be 1.41 and 0.28 cps/pA/area, respectively, surpassing the background noise levels on each detector (Table 2). A shape-dependent distribution of 31P- was observed along the wall of the sheath structure in Target 2, whereas 98Mo16O− was distributed vaguely throughout the shape (Fig. 2b). Sporadic occurrences of 98Mo16O− were observed in Target 5 without correlation with each other (Fig. 2e). In Targets 1, 3, 4, and 6, faint counts were observed throughout the entire microfossil body, whereas in Target 3, more counts appeared from ambiguous organic matter beside microfossils (Fig. 2c). In Target 6, 31P− and 98Mo16O− counts were intensified in the lower region of the microfossil, which corresponds to the area where the cell wall appears thinner in the optical image (Fig. 2f).
During the course of the measurements, temporal changes in the secondary ion images were tracked (Fig. 3). In the analysis of Target 1, 31P− was initially minorly detected in the main body of the microfossil. The detection of 31P− ions from microfossils subsequently began and intensified as the frame numbers increased. The 31P− ion count peaked at approximately 400 frames, after which it gradually decreased towards the last frame. A similar pattern was observed in the 98Mo16O− ion image. Consequently, the ion counts of both 31P− and 98Mo16O− reached their maximum in the middle of the microfossil without being localized. Comparable results were obtained for Target 4. On the other hand, a monotonic increasing trend in temporal changes was observed in Target 2 (Fig. 4, Supplementary Fig. 3). In this case, both the 31P- and 98Mo16O− counts increased steadily from the beginning to the end, together with the carbon. Similar trends were observed for Targets 3, 5, and 6. The temporal evolution of phosphorus in Target 2 exhibited distinctive features. Initially, phosphorus was detected throughout the entire sample, after which it sporadically appeared. Towards the middle to the end, it was detected along the wall of the microfossil, as observed in the stacked images in (Fig. 2b).
Temporal changes in secondary ion images of 12C-, 31P-, and 98Mo16O- of Target 1 acquired in PMo mode. The images were generated by dividing a total of 800 frames into stacks of 160 frames each, creating 5 temporal image series. Consistent with the major 12C- signal, 31P- and 98Mo16O- counts start weakly or assimilate from the surroundings, gradually intensify around the midpoint of the frame sequence, and then diminish towards the end of the images. The numbers on the upper left side of each image indicate the stacked frames used to create those images. Scale bars are 5 μm.
Temporal changes in secondary ion images of 12C- and 31P- of Target 2 acquired in PMo mode. The images were generated by dividing a total of 700 images into stacks of 140 images each, creating 5 temporal image series. Unlike Target 1, the counts of both 12C- and 31P- increase as the frame number increases. The numbers on the upper left side of each image indicate the stacked frames used to create those images. The cartoons depicted below the ion images are schematic diagrams illustrating scanning frames passing through the tuber structure of Target 2 during NanoSIMS analysis. Scale bars are 5 μm.
Discussion
The secondary ion yields of 12C−, 12C14N−, and 32S− in CNS mode were 14 to 54 times greater than those of thin section samples, in which filamentous microfossils were targeted (Table 1). We consider that the primary ion energy was used directly and efficiently for ionization of macerated samples on ITO glass, whereas it was dispersed and weakened in the chert matrix in the case of thin section samples. This further facilitated the ionization of trace amounts of phosphorus and molybdenum in microfossils, which has never been recognized in previous microfossil studies. The secondary ion yields of 98Mo16O− were much lower than those of 31P−, reaching a minimum of 1.8 × 10−1 cps/pA (Table 2). This yield is only 70 times greater than the background yield of 2.6 × 10−3 cps/pA, underscoring its detectability solely through the enhanced ionization efficiency achieved by the methodology in this study. Analytical conditions were meticulously configured to distinguish any potential isobaric ions, and mass spectrum peaks were verified in each analysis to ensure that there was no possible effect from neighbouring ions. These trace elements were imaged along the shapes of microfossils, even in submicron spherical or ambiguous organic matter adhered to microfossils. The spherical organic matter is likely a symbiotic organism of filamentous microbes, with a similar distribution of secondary ions, providing supportive evidence for this hypothesis9. On the other hand, the high counts of 31P− and 98Mo16O− observed in the ambiguous organic matter indicate that such organic matter may have been utilized by microbes. However, as suggested by the difference in the 12C− ion image in Fig. 2c, the structure of the ambiguous organic matter appears to be distinct from that of microfossils. This indicates that such organic matter is likely composed of degraded products, which may explain the observed difference in ionization efficiency. In any case, their colocalization with carbon and nitrogen ions indicates that they were incorporated into organic matter rather than originating from surface contamination (Fig. 3). The presence of nanoscale phosphorus-bearing minerals, such as apatite, is less likely in this study, as no such crystals were observed via electron microscopic observations.
Phosphorus plays a crucial role in modern microorganisms, serving functions such as building nucleic acids, storing energy, and forming cell walls. Under specific conditions, some modern algae store phosphorus-enriched granules within their cells for energy preservation, and some cyanobacteria form spherical polyphosphates inside cells under environmental stress23,24.
The time-dependent trend observed in the 31P− ion image revealed that phosphorus was detected throughout the microfossil structures (Fig. 3). The initial 31P− images of Target 2, for example, cover the entire microfossil structure. From the middle to the end of the analytical period, the 31P− images gradually fit to the shape of the wall. This distinct imaging of 31P− for Target 2 indicates the presence of phosphorus within the microfossil wall (Fig. 4). These trends cannot be attributed to artefacts or contamination from later processes. On the other hand, other targets showed faint counts from the entire microfossil body.
Figure 4 indicates that phosphorous was connected to the organic matter of microfossil walls, most likely resulting in the phosphorylation of organic matter during the early diagenetic stage. Kerogenous organic matter is susceptible to phosphorylation because of the presence of reactive hydroxyl groups in its structure25. The mean value of the 31P−/12C− count ratio (3.6 × 10−4) can be roughly calibrated to represent the actual P/C ratio, i.e., between 3.6 × 10−5 and 3.6 × 10−4, accounting for differences in ionization potentials between 31P− and 12C−26. This value is within the same or lower order of magnitude as the reported values in the Gunflint kerogen (~ 10−4)13. These findings support the notion that the phosphorus detected in the present study is intrinsic to microfossils. Rapid silicification of gunflint stromatolites allows phosphorylated organic matter to be preserved within microfossil structures. On the other hand, the phosphorylation of organic matter is not limited to only wall sites. The distribution of phosphorus within a wall is heterogeneous and not common among the examined samples (Fig. 2). This further constrains the source of phosphorus.
A previous study reported phosphorus in microfossils from the 3.46 billion-year-old strelley pool formation20. However, several analytical problems have been identified in the phosphorus analyses. In particular, the use of polycarbonate membrane for sample mounting created ambiguities in phosphorus detection. The 31P−/12C− ratios in their study were within the same range (~ 10−4) for both the polycarbonate membrane and their microfossil samples. Several images from their study showed little distinction between the background value and the samples. The 31P−/12C− ratios of most samples were within the measurement error range of the background, and consequently, clear phosphorus imaging was not achieved. In contrast, the analyses in the present study clearly revealed differences in the 31P− counts and 31P−/12C− ratios by several orders of magnitude (~ 10−2) between the samples and the ITO glass background. This allows clear phosphorus detection because of the significant contrast from that of the background. Additionally, 31P− was often detected in areas unrelated to 12C− and 12C14N− ions. This is because of potential contamination from mineral residues, such as apatite, or interference from neighbouring isobaric ions, such as 30SiH− or 12C19F− ions. Similar 31P- detection was detected around the ejecta during presputtering. These factors were carefully tested, and ambiguous 31P− peaks were eliminated via our methodology. Therefore, our method using ITO glass is more suitable for the detection of trace amounts of phosphorus in microfossils with ultrahigh spatial resolution.
Previous investigators have suggested that the phosphorus enrichment of kerogenous materials occurs via the coprecipitation of marine phosphate with iron-(oxyhydr)oxide (FeOOH)26,27. In this model, phosphate accumulates in marine sediments by adsorbing to FeOOH. Enriched phosphate in sediments is mobilized during diagenesis and then further phosphatized into fossils in sediments. However, this was not the case for our samples because of the absence of iron enrichment. In addition, this model cannot explain why the walls of microfossils were the subjects of phosphorylation.
The membrane compositions of early microbes, particularly the presence of phospholipids in cell walls, has been debated by previous investigators28. On the other hand, modern bacteria predominantly utilize phospholipids as the primary constituents of their cell membranes. It is inferred that the original bacteria for the gunflint microfossils contains phospholipids in their wall structures. These phospholipids and organic matter underwent decomposition into lipids and phosphates during early diagenesis. Local kerogen in wall structures is subjected to phosphorylation via phosphate from phospholipids. If this was indeed the case, this finding is the initial direct indication of microbial utilization of phospholipids. The rapid sedimentation of chert is recognized for its ability to conserve organic matter spanning billions of years, including the retention of delicate chemical bonds within organic molecules29,30.
Transition metals, such as molybdenum, zinc, and nickel, are important for sustaining enzymatic reactions in microorganisms. However, it is uncertain whether ancient organisms used the same transition metals to activate enzymes. A previous study successfully detected nickel and cobalt in intercellar inclusions of proterozoic cyanobacterial microfossils through synchrotron analyses31. These authors interpreted these transition metals as traces of chlorophyll degradation.
Molybdenum is another essential transition metal because molybdenum-bearing proteins are important cofactors in living microorganisms for operating metabolic systems. They have been proposed as tracers of the evolution of enzymatic proteins32,33,34,35,36,37,38,39,40,41. Molybdenum was detected in the examined microfossil samples, in the areas of carbon and phosphorus patterns but not sulfur patterns (Fig. 2). This observation suggests that molybdenum was incorporated into organic matter in microfossils rather than in the form of molybdenum sulfides. Another example of molybdenum-bearing proteins includes their involvement in common bacterial enzymes or substitution at the ribose site in adenosine-monophosphate (AMP)42,43. In these cases, molybdenum is incorporated as a molybdenum‒sulfur cluster in the structure of these enzymatic molecules. While this suggests a potential correlation between sulfur and molybdenum in this context, such a relationship was not prominently observed in the present study. Considering the isotopic abundance of 98Mo, the relative abundance of molybdenum was more than 104 orders of magnitude lower than that of sulfur (Tables 1 and 2). Similarly, a lower concentration of organic molybdenum than of sulfur is observed in modern cyanobacteria, typically less than 1 ppm, corresponding to a concentration that is 104 times lower than that of sulfur21. These findings, which are consistent with those of other studies, support the notion that the molybdenum detected in the present study was indigenous to microfossils. Targets 2 and 5 showed sporadic occurrences of molybdenum, which occurred with sulfur, suggesting a possible traces of a primitive molybdenum‒sulfur cluster modified from enzymes.
Previous investigators have suggested several pathways of molybdenum accumulation in sediments as follows: (1) molybdenum adsorption on sedimentary organic matter and bacterial cells in anoxic water column44,45,46,47; (2) precipitation of molybdenum sulfides from sulfidic water48,49; or (3) adsorption on biogenic pyrite50. The accumulated molybdenum in sediments can migrate within anoxic diagenetic fluids and react with organic matter. Tetravalent molybdenum, which is highly soluble in waters with elevated salinity, is readily absorbed by organic matter in reducing environments51. The study area was in the shallow marine region of the Gunflint ocean, and it has been established that the surface ocean water was oxic52,53,54. Therefore, possibilities (1) and (2) are less likely. Xu et al.50 reported that sulfate-reducing bacteria (SRB) can accumulate molybdenum in vitro and that pyrite produced by SRB can adsorb molybdenum. Some microfossils of the Gunflint Formation are pyritized, indicating SRB digestion of organic matter from cyanobacteria9. However, optical transparent and secondary ion images of the examined microfossils suggest that they were not replaced by pyrite and contained phosphorus in the organic matter (Fig. 2). This further indicates that the organic matter in microfossils was not significantly digested by heterotrophic microorganisms. This observation disfavours case (3). Overall, identifying a precise process for the production of molybdenum-bearing organic matter in microfossils remains challenging. Previous investigators have suggested that cyanobacteria originate from the examined filamentous type of microfossils on the basis of morphological similarity and the carbon and nitrogen isotopic compositions of bulk organic matter7,10. This is consistent with the detection of molybdenum, which is known to be used for enzymatic nitrogen fixation by cyanobacteria16,18. Redistribution of Mo from other enzymes or organic compounds cannot be excluded55. Further studies are needed to test this hypothesis.
The Gunflint microfossils boast unparalleled levels of morphological and geochemical preservation, rendering them prime subjects for investigating ancient metabolism. Moreover, they serve as benchmarks for assessing the biogenicity of other Palaeoproterozoic and Archean organic microstructures of potential biogenic origin. A recent study demonstrated the experimental formation of microbial membrane-like structures resembling filamentous Gunflint microfossils56. Our results provide compelling empirical evidence that strongly supports such experimental predictions. Our methodology, which integrates optical observations, electron microscopy, and secondary ion imaging, is particularly adept at scrutinizing samples from the Archean era, where organic matter and microfossils are seldom preserved within sedimentary rocks. This study introduces an innovative tool for obtaining pivotal insights into the evolutionary trajectories of the cellular and metabolic pathways of microorganisms while preserving their morphological characteristics. This advancement holds immense promise for elucidating the coevolution of life and the environment during the early stages of Earth’s history.
Methods
Microfossil isolation and preparation for secondary ion analysis
Approximately 20 g of rock chips from sample ID 1709080047 were immersed in 6 N hydrochloric acid (HCl) for 1–2 days to remove carbonates. The samples were subsequently macerated in a solution of HCl and hydrofluoric acid (HF) at a 1:1 volume ratio until the minerals dissolved completely; this process took at least 2 weeks, and up to one month. These acid attacks were performed at room temperature without using a shaking bath to maintain the structure of the microfossils. Afterwards, HCl was again employed to remove any precipitates that had formed due to the use of HF. Neutralization was carried out by using hot pure water and a heating block to ensure the dissolution of any remaining fluoride without using a centrifugation machine. The isolated microfossils were dispersed in ethanol and pipetted onto ITO glass. During the ethanol evaporation process, microfossils adhered to the glass surface through Coulomb’s force, and these microfossil adherences were akin to stains (Supplementary Fig. 4). This method eliminates the need for any adhesive or resin to mount microfossils, which is a common challenge in light elemental analysis of microorganic matter. Before the secondary ion analysis, the samples were examined via both a petrographic microscope (BX50, Olympus) and a field-emission scanning electron microprobe with an energy dispersive X–ray spectrometer (FE-SEM/EDX; JSM-7001F, JEOL).
NanoSIMS analysis
High-sensitivity elemental mapping with high spatial resolution was performed via a NanoSIMS 50 (AMETEK Co. Ltd.). The samples mounted on the ITO glass were coated with gold to compensate for the electric charge, eliminating the need for an electric gun. The primary Cs+ ion beam was focused on the sample surface with a submicron-metre spot size. Negatively charged secondary ions of carbon (12C−), nitrogen (12C14N−), phosphorus (31P−), sulfur (32S−), and molybdenum oxides (98Mo16O−) were obtained via electron multipliers (EMs). Since single nitrogen and molybdenum ions exhibit either no or very low ionization efficiency under Cs+ primary beam conditions, their detection relies on the molecular ions mentioned earlier. In addition, targeting oxide ions has the advantage of allowing simultaneous imaging of correlations with organic elements, such as carbon and nitrogen. The analytical procedure was as follows: initially, presputtering for 3 min was carried out using a primary beam of a 100 pA to remove surface gold coating and possible contamination. Next, 20 frames of high-resolution ion imaging were conducted via a 0.9 pA primary beam, simultaneously targeting secondary ions of 12C−, 12C14N−, and 32S− (by EMs #1, 4, and 5, respectively) with a resolution of 256 × 256 pixels. The scanning speed was 30 s per frame for a raster area from 16 to 20 μm2 (CNS mode). The spatial resolution under these analytical conditions was 78 nm for each pixel. Background counts in CNS mode were measured on several plane surfaces under the same analytical conditions, resulting in values of 1.1 × 101 cps/pA, 1.5 × 102 cps/pA, and 1.3 × 101 cps/pA, respectively. After this CNS mode, detector configurations were modified to monitor trace elements in the present study. Secondary ions of 12C−, 12C14N−, 31P−, and 98Mo16O− (by EM#1, 2, 3, and 5, respectively) were chosen for simultaneous measurement (PMo mode). To acquire enough counts, a primary beam of 1.5 pA was used, and 600 to 800 frames were accumulated, with a scanning speed of 30 s per frame in this mode. The raster area and pixels were appropriately adjusted to conform to the shape of each target to maximize the ion collection efficiency. During the PMo mode measurement, EM #4 was positioned at mass number of 94 to monitor potential spurious counts, resulting in a value of 1.03 × 10−3 cps/pA (Table 2). Possible background noise in this mode was carefully monitored and calculated. In static mode, background count rates on EMs #3 and #5 were checked without using the primary beam, resulting in less than 3.33 × 10−4 cps. Active background counts on EMs 1, 2, 3, and #5 were measured on 4 plane surfaces of ITO glass surrounding the examined targets (Targets 1 and 5) under the same analytical conditions, resulting in values of 1.0 cps/pA, 2.5 × 101 cps/pA, 5.4 × 10−2 cps/pA, and 2.6 × 10−3 cps/pA, respectively (Supplementary Table 1). These values are higher than those determined in the blanked EMs #4 or static mode. Thus, we treated these active background values as analytical background noise for each EM in the PMo method.
The identification of target secondary ions was carried out via the use of epoxy-mounted reference minerals, which were separately arranged with microfossil samples on the NanoSIMS holder. The secondary ions of the organic elements (C, N, and S) were adjusted on epoxy resin. The 13C13C− ions next to the 12C14N− ion and the 16O2− ions next to the 32S− ion were safely separated under a mass resolution power (MRP) of 8000. Apatite (Ca5(PO4)3(Cl, F, OH)), wulfenite (PbMoO4) and trace element-doped glass (SRM610, products of the National Institute of Standards and Technology) were used for the identification of 31P− and 98Mo16O− ions. For the identification of 98Mo16O− ions, the isotope abundances of 92Mo16O−, 95Mo16O−, and 96Mo16O− ions on wulfenite were carefully confirmed (Supplementary Fig. 5).
Possible neighboring ions of 31P−, including 12C19F−, 14N16OH−, and 12C18OH−, were effectively separated with an MRP of 8000. The nearest neighboring ion, 30Si1H−, which requires an MRP of 4000, was observed next to 31P−. As this spectrum was observed only in microfossil sample imaging, which includes ITO glass in the raster area, we considered that 30Si1H− was solely generated from the ITO glass. To avoid possible tailing from 30Si1H−, the centre of 31P- was determined to be 0.002 amu lower than the exact centre position (Supplementary Fig. 5). The molecular ion of 98Mo16O− remained identical even when it was detected in organic matter samples on ITO glass in the present study. Such a large mass of secondary ions, with an accurate molecular weight of 113.9003, often encounters challenges with isobaric ions formed through ambiguous combinations of other light elements originating from organic matter. Molecules with the formulas C8H18 and C6H10O2 typically have molecular weights near 113.9003. However, organic molecules with similar molecular weight typically exceed 114, suggesting that they can be effectively separated from 98Mo16O− under MRP in this study. Thus, the isobaric ions from organic molecules are not the case. Molecular ions composed of metal ions and light elements are also less likely. The generation of molecular ions occurs through the collision of sputtered single ions near the sample surface57. Therefore, it is necessary to consider any potential combinations of molecular ions, even if such molecules were not initially present in the original material. Metal oxides, such as MO2 or MO3, are other candidates for isobaric ions; however, there is no single stable isotope element whose total molecular weight matches 113.9003. Unstable isotopes of other single elements or their oxides, as well as half charged 57Fe, are all below detection limits under Cs+ conditions. The overlap of the 113In1H− and 114Sn− ions, generated from the ITO coating, is also negligible, which is attributed to the low ionization efficiency of both ions under Cs+ primary beam conditions58. Additionally, background measurements conducted on multiple plane surfaces of ITO glass revealed no significant counts from the surface, resulting in a yield of 8.9 × 10−3 cps/pA for mass number 113.9003 (Supplementary Table 1). This suggests that 113In1H− and 114Sn− ions are negligible during analyses. We conclude that use of 98Mo16O− ions is advantageous for visualizing the distribution of molybdenum in microfossils via this method.
The secondary ion images were processed via the open-source imaging software FIJI57 and a plugin application of OpenMIMS (http://nano.bwh.harvard.edu). Drift and artificial signal noise were removed or corrected from the original images. In ratio images compared with carbon images, any pixel data with an original carbon count below the background threshold were removed. The total counts and ratio values summarized in Table 1 do not include data from those noises or background counts. The active areas where carbon counts were observed were calculated in CNS mode, and yields were also calculated in the basis of the active areas (Table 1). This suggests that differences in ionization efficiency between our method and thin section samples were not caused by differences in target size.
Data availability
All the data are available in the main text and supplementary information.
References
Tyler, S. A. & Barghoorn, E. S. Occurrence of structurally preserved plants in pre-Cambrian rocks of the Canadian shield. Science 119, 606–608 (1954).
Barghoorn, E. S. & Tyler, S. Microorganisms from the gunflint chert. Science 147, 3658 (1965).
Javaux, E. J. & Lepot, K. The paleoproterozoic fossil record: implications for the evolution of the biosphere during Earth’s middle-age. Earth Sci. Rev. 176, 68–86 (2018).
Lepot, K. Signatures of early microbial life from the archean (4 to 25° Ga) eon. Earth Sci. Rev. 209, 103296 (2020).
Awramik, S. M. & Barghoorn, E. S. The gunflint microbiota. Precam. Res. 5, 121–142 (1977).
Planavsky, N. et al. Iron-oxidizing microbial ecosystems thrived in late paleoproterozoic redox-stratified oceans. Earth Planet. Sci. Lett. 286, 230–242 (2009).
Sasaki, K., Ishida, A., Takahata, N., Sano, Y. & Kakegawa, T. Evolutionary diversification of paleoproterozoic prokaryotes: New microfossil records in 1.88 Ga gunflint formation. Precam. Res. 380, 106798 (2022).
House, C. H. et al. Carbon isotopic composition of individual Precambrian microfossils. Geology 28(8), 707–710 (2000).
Wacey, D. et al. Nanoscale analysis of pyritized microfossils reveals differential heterotrophic consumption in the ̃1 9 Ga gunflint chert. Proc. Nat. Acad. Sci. USA 110(20), 8020–8024 (2013).
Williford, K. H. et al. Preservation and detection of microstructural and taxonomic correlations in the carbon isotopic compositions of individual precambrian microfossils. Geochim. Cosmochim. Acta 104, 165–182 (2013).
Wacey, D., Saunders, M., Kong, C., Brasier, A. & Brasier, M. 3.46 Ga apex chert ‘microfossils’ reinterpreted as mineral artefacts produced during phyllosilicate exfoliation. Gondwana Res. 36, 296–313 (2016).
Lepot, K. et al. Iron minerals within specific microfossil morphospecies of the 1.88 Ga Gunflint Formation. Nat. Commun. 8, 14890 (2017).
De Gregorio, B. T., Sharp, T. G., Flynn, G. J., Wirick, S. & Hervig, R. L. Biogenic origin for earth’s oldest putative microfossils. Geology 37(7), 631–634 (2009).
Lukmanov, R. A. et al. Multiwavelength ablation/ionization and mass spectrometric analysis of 1.88 Ga gunflint chert. Astrobiology 22(4), 369–386 (2022).
Tribovillard, N., Algeo, T. J., Lyons, T. & Riboulleau, A. Trace metals as paleoredox and paleoproductivity proxies: An update. Chem. Geol. 232(1–2), 12–32 (2006).
Zerkle, A. L., House, C. H., Cox, R. P. & Canfield, D. E. Metal limitation of cyanobacterial N2 fixation and implications for the precambrian nitrogen cycle. Geobiology 4(4), 285–297 (2006).
Anbar, A. D. et al. A whiff of oxygen before the great oxidation event?. Science 317, 1903–1906 (2007).
Parnell, J., Spinks, S., Andrews, S., Thayalan, W. & Bowden, S. High molybdenum availability for evolution in a mesoproterozoic lacustrine environment. Nat. Commun. 6, 6996 (2015).
Guo, Z., Peng, X., Czaja, A. D., Chen, S. & Ta, K. Cellular taphonomy of well-preserved Gaoyuzhuang microfossils: A window into the preservation of ancient cyanobacteria. Precam. Res. 304, 88–98 (2018).
Delarue, F. et al. Out of rock: A new look at the morphological and geochemical preservation of microfossils from the 3.46 Gyr-old strelley pool formation. Precam. Res. 336, 105472 (2020).
Pett-Ridge, J. & Weber, P. K. NanoSIP: NanoSIMS applications for microbial biology. Microb. Syst. Biol. Methods Protoc. https://doi.org/10.1007/978-1-61779-827-6_13 (2012).
Grey, K. & Sugitani, K. Palynology of archean microfossils (ca. 3.0 Ga) from the Mount grant area, pilbara craton, Western Australia: Further evidence of biogenicity. Precam. Res. 173, 60–69 (2009).
Slaveykova, V. I., Guignard, C., Eybe, T., Migeon, H. N. & Hoffmann, L. Dynamic NanoSIMS ion imaging of unicellular freshwater algae exposed to copper. Anal. Bioanal. Chem. 393, 583–589 (2009).
Sanz-Luque, E., Bhaya, D. & Grossman, A. R. Polyphosphate: A multifunctional metabolite in cyanobacteria and algae. Front. Plant Sci. 11, 93 (2020).
Jackson, M. D. & Denu, J. M. Molecular reactions of protein phosphatases—Insights from structure and chemistry. Chem. Rev. 101, 2313–2340 (2001).
Edwards, C. T., Pufahl, P. K., Hiatt, E. E. & Kyser, T. K. Paleoenvironmental and taphonomic controls on the occurrence of paleoproterozoic microbial communities in the 1.88 Ga ferriman group labrador trough Canada. Precam. Res 212, 91–106 (2012).
Muscente, A. D., Hawkins, A. D. & Xiao, S. Fossil preservation through phosphatization and silicification in the ediacaran doushantuo formation (South China): A comparative synthesis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 434, 46–62 (2015).
Hanczyc, M. M., Fujikawa, S. M. & Szostak, J. W. Experimental models of primitive cellular compartments: encapsulation, growth, and division. Science 302(5645), 618–622 (2003).
Alleon, J. et al. Molecular preservation of 1.88 Ga gunflint organic microfossils as a function of temperature and mineralogy. Nat. Commun. https://doi.org/10.1038/ncomms11977 (2016).
Igisu, M. et al. Changes of aliphatic C–H bonds in cyanobacteria during experimental thermal maturation in presence or absence of silica as evaluated by FTIR microspectroscopy. Geobiology 16, 412–428 (2018).
Demoulin, C. F. et al. Polysphaeroids filiformis, a proterozoic cyanobacterial microfossil and implications for cyanobacteria evolution. iScience 27, 108865 (2024).
Jonkers, H. M., van der Maarel, M. J. E. C., Gemerden, H. V. & Hansen, T. A. Dimethylsulfoxide reduction by marine sulfate-reducing bacteria. FEMS Microbiol. Lett. 136, 283–287 (1996).
Tucker, M. D., Barton, L. L. & Thomson, B. M. Reduction and immobilization of molybdenum by desulfovibrio desulfuricans. J. Environ. Qual. 26, 1146–1152 (1997).
Tucker, M. D., Barton, L. L. & Thomson, B. M. Reduction of Cr, Mo, Se and U by Desulfovibrio desulfuricans immobilized in polyacrylamide gels. J. Ind. Microbiol. Biotechnol. 20, 13–19 (1998).
Hensel, M., Hinsley, A. P., Nikolaus, T., Sawers, G. & Berks, B. C. The genetic basis of tetrathionate respiration in Salmonella typhimurium. Mol. Microbiol. 32, 275–287 (1999).
Kappler, U. et al. Sulfite: Cytochrome c oxidoreductase from Thiobacillus novellus. Purification, characterization, and molecular biology of a heterodimeric member of the sulfite oxidase family. J. Biol. Chem. 275, 13202–13212 (2000).
Burns, J. L. & DiChristina, T. J. Anaerobic respiration of elemental sulfur and thiosulfate by Shewanella oneidensis MR-1 requires psrA, a homolog of the phsA gene of Salmonella enterica serovar typhimurium LT2. Appl. Environ. Microbiol. 75, 5209–5217 (2009).
Wilson, J. J. & Kappler, U. Sulfite oxidation in Sinorhizobium meliloti. Biochim. Biophys. Acta 1787, 1516–1525 (2009).
Lenk, S. et al. Novel groups of gammaproteobacteria catalyse sulfur oxidation and carbon fixation in a coastal, intertidal sediment. Environ. Microbiol. 13, 758–774 (2011).
Wasmund, K., Mußmann, M. & Loy, A. The life sulfuric: Microbial ecology of sulfur cycling in marine sediments. Environ. Microbiol. Rep. 9, 323–344 (2017).
Crichton, R. Molybdenum, tungsten, vanadium and chromium. In Biological Inorganic Chemistry (ed. Crichton, R.) (Elsevier, UK, 2019).
Dahl, T. W., Chappaz, A., Fitts, J. P. & Lyons, T. W. Molybdenum reduction in a sulfidic lake: Evidence from x-ray absorption fine structure spectroscopy and implications for the Mo paleoproxy. Geochim. Cosmochim. Acta 103, 213–231 (2013).
Nair, R. R., Silveira, C. M. & Diniz, M. S. Changes in metabolic pathways of desulfovibrio alaskensis G20 cells induced by molybdate excess. J. Biol. Inorg. Chem. 20, 311–322 (2015).
Bellenger, J., Arnaud-Neu, F. & Asfari, Z. Complexation of oxoanions and cationic metals by the biscatecholate siderophore azotochelin. J. Biol. Inorg. Chem. 12, 367–376 (2007).
Dahl, T. W. et al. Evidence of molybdenum association with particulate organic matter under sulfidic conditions. Geobiology 15(2), 311–323 (2017).
Wagner, M., Chappaz, A. & Lyons, T. W. Molybdenum speciation and burial pathway in weakly sulfidic environments: Insights from XAFS. Geochim. Cosmochim. Acta 206, 18–29 (2017).
Bao, P. et al. The role of sulfate- reducing prokaryotes in the coupling of element biogeochemical cycling. Sci. Total Environ. 613–614, 398–408 (2018).
Poulson-Brucker, R. L., McManus, J., Severmann, S. & Berelson, W. M. Molybdenum behavior during early diagenesis: Insights from Mo isotopes. Geochem. Geophys. Geosyst. https://doi.org/10.1029/2008GC002180 (2009).
Poulson-Brucker, R. L., McManus, J. & Poulton, S. W. Molybdenum isotope fractionations observed under anoxic experimental conditions. Geochem. J. 46, 201–209 (2012).
Xu, N., Christodoulatos, C. & Braida, W. Adsorption of molybdate and tetrathiomolybdate onto pyrite and goethite: Effect of pH and competitive ions. Chemosphere 62, 1726–1735 (2006).
Mohajerin, T. J., Helz, G. R. & Johannesson, K. H. Tungstem-molybdenum fractionation in estuarine environment. Geochim. Cosmochim. Acta 177, 105–119 (2016).
Poulton, S. W., Fralick, P. W. & Canfield, D. E. The transition to a sulphidic ocean, ~1.84 billion years ago. Nature 431, 173–177 (2004).
Rasmussen, B. et al. Deposition of 1.88-billion-year-old iron formations as a consequence of rapid crustal growth. Nature 484, 498–501 (2012).
Ishida, A., Hashizume, K. & Kakegawa, T. Microbial nitrogen cycle enhanced by continental input recorded in the gunflint formation. Geochem. Persp. Let. 4, 13–18 (2017).
Hemkemeyer, M., Schwalb, S. A., Heinze, S., Joergensen, R. G. & Wichern, F. Functions of elements in soil microorganisms. Microbiol. Res. 252, 126832 (2021).
Jordan, S. F., Zuilen, M. A., Rouilland, J., Martins, Z. & Lane, N. Prebiotic membrane structures mimic the morphology of alleged early traces of life on Earth. Commun. Earth Envion. https://doi.org/10.1038/s43247-024-01372-0 (2024).
Schindelin, J. et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Wilson, R. SIMS quantification in Si, GaAs, and diamond—An update. Int. J. Mass Spec. Ion Procs. 143, 43–49 (1995).
Acknowledgements
We appreciate the associate editor and two reviewers for their constructive comments on our manuscript. This research was supported by JSPS KAKENHI to A.I. (22K03790), K.S. (23K19065), and T.K. (22H00163) and was also supported by the Cooperative Program of Atmosphere and Ocean Research Institute, The University of Tokyo. We are grateful to Dr. Miyuki Takeuchi for her technical support using NanoSIMS50L in the fundamental development of this methodology, as provided by the Nanotechnology Platform project by the Ministry of Education, Culture, Sports, Science and Technology of Japan (JPMXP09A21UT0383).
Author information
Authors and Affiliations
Contributions
A.I. designed and supervised the study. K.S. performed the microfossil isolation and basic description of the samples. A.I., K.S., and N.T. developed, designed and performed the secondary ion analyses via NanoSIMS. A.I. and K.S. wrote the manuscript. T.K. contributed to the sample selection and major discussion on Palaeoproterozoic microbiology and geology. Y.S. contributed to the supervision of the ground design of secondary ion analysis and the discussion of trace element distributions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Sasaki, K., Ishida, A., Kakegawa, T. et al. Ultrahigh-resolution imaging of biogenic phosphorus and molybdenum in palaeoproterozoic gunflint microfossils. Sci Rep 14, 21780 (2024). https://doi.org/10.1038/s41598-024-72191-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72191-8
- Springer Nature Limited