Abstract
During the development of multicellular organisms and cell differentiation, the chromatin structure in the cell nucleus undergoes extensive changes, and the nucleosome structure is formed by a combination of various histone variants. Histone variants with diverse posttranslational modifications are known to play crucial roles in different regulatory functions. We have previously reported that H3t, a testis-specific histone variant, is essential for spermatogenesis. To elucidate the function of this chromatin molecule in vivo, we generated knock-in mice with a FLAG tag attached to the carboxyl terminus of H3t. In the present study, we evaluated the utility of the generated knock-in mice and comprehensively analyzed posttranslational modifications of canonical H3 and H3t using mass spectrometry. Herein, we found that H3t-FLAG was incorporated into spermatogonia and meiotic cells in the testes, as evidenced by immunostaining of testicular tissue. According to the mass spectrometry analysis, the overall pattern of H3t-FLAG posttranslational modification was comparable to that of the control H3, while the relative abundances of certain specific modifications differed between H3t-FLAG and the control bulk H3. The generated knock-in mice could be valuable for analyzing the function of histone variants in vivo.
Similar content being viewed by others
Introduction
During cell differentiation, the chromatin structure of multicellular organisms undergoes critical changes to stabilize the regulation of gene expression. It is well recognized that epigenetic regulatory mechanisms, such as posttranslational modifications of histones and microRNAs (miRNAs); chemical modifications, such as DNA methylation; and metabolites, are involved in changes in chromatin structure, and considerable supportive evidence has accumulated over the past decades1,2,3,4. Histone variants also play crucial roles in epigenetic regulation and chromatin structure formation5,6,7,8.
Although functional analysis of histone variants has been performed primarily using materials derived from cultured cells9,10, the generation of specific antibodies against histone variants has greatly advanced our knowledge and understanding of how individual histone variants can impact chromatin structure11,12,13,14. However, given that histone variants differ by only a few amino acids from canonical histones5,8, comprehensive elucidation of their functions using only specific antibodies has been challenging, and histone variants have been tagged and analyzed to overcome these issues9,15,16. In particular, the behavior of individual variants in each tissue in vivo has yet to be fully determined.
On the other hand, research on histone posttranslational modifications has progressed substantially over the past two decades, along with advancements in the development of specific antibodies against posttranslationally modified histones17,18,19. However, the precise mechanism of posttranslational modification of each histone variant has not been fully elucidated, as identifying posttranslational modifications occurring in histone variants is technically challenging. Despite the generation of specific antibodies to detect posttranslational modifications in particular histone variants and the use of mass spectrometry to identify modifications, differences in posttranslational modifications occurring for each histone variant have not been fully elucidated20,21,22,23. Moreover, although attempts have been made to address this issue by tagging individual histone variants9, there has been limited progress in terms of in vivo analysis24, which could be attributed to the presence of several histone genes (especially canonical histones) in different chromosomal locations and in multiple copies25,26. The presence of multiple genes encoding a single protein renders reverse genetics impossible.
We have previously reported that H3t, a testis-specific histone H3 variant, plays a crucial role in spermatogenesis11. We found that deletion of the H3t gene causes severe testicular shrinkage, azoospermia, and complete absence of spermatogenesis, resulting in infertility. Furthermore, the canonical histone H3.1/H3.2 was found to be replaced by H3t during spermatogonial stem cell differentiation, and H3t-containing nucleosomes assumed a more open conformation than canonical H3-containing nucleosomes. However, numerous questions remain unanswered, including the mechanism through which H3t replaces canonical H3 and how H3t-containing nucleosomes affect the higher-order structures of chromosomes. This is partly due to technical difficulties associated with the biochemical analysis of histone variants in vivo.
In the present study, we generated a mouse model in which a 3 × FLAG tag was knocked in at the carboxyl terminus of H3t to overcome these technical challenges and elucidate the functions of H3t. Using the generated mouse model, we subsequently analyzed the presence of specific posttranslational modifications in the H3t protein using mass spectrometry-based proteomics.
Methods
Animals
C57BL/6N (C57BL/6NCrSlc), DBA/2 (DBA/2CrSlc), and ICR (Slc:ICR) mice were purchased from Japan SLC, Inc. (Hamamatsu, Japan). Breeding stocks were established at the Animal Laboratory for Medical Research, Asahikawa Medical University. Specific-pathogen-free mice were maintained in an environment maintained at 22–24 °C and 40–60% humidity, with a 12/12 h light/dark cycle between 07:00 and 19:00. All the experiments were performed according to the Guidelines of Animal Experiments of Asahikawa Medical University, and efforts were made to minimize suffering. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of Asahikawa Medical University (No. R3-068, R4-115, R5-112, R6-087). The study was carried out in accordance with the ARRIVE guidelines.
Generation of H3t knock-in mice via the CRISPR/Cas9 gene editing method
C57BL/6N × DBA/2 F1 (BDF1) female mice were superovulated and fertilized in vitro with BDF1 spermatozoa in modified Krebs–Ringer bicarbonate (TYH) medium. The mixture comprising gRNA, Cas9 protein (A36497; Thermo Fisher Scientific, Inc.), and the targeting vector was injected into the male pronuclei of zygotes at 20 ng/μL, 27 ng/μL and 10 ng/μL, respectively27,28. The embryos were cultured in potassium simplex optimized medium (KSOM) overnight, after which 2-cell stage embryos were transferred into the oviducts of pseudopregnant female ICR mice. The following sequence was used for guide (g) RNA: 5′–GAG CCT AAG CCC GTT CTC CG–3′. The primers used to detect the 5′-homology arm (917 bp, pink arrows in Fig. 1A) were as follows: ‘H3t knock-in genotype-Fwd_4′ 5′–CTT CCA CAT GCT AGA GAA CCC ATG CCA TGC–3′ and ‘H3t knock-in genotype-Rev’ 5′–AGA GCC GTT GGG TTC ATG AGC GGT GCC TGT–3′. The primers used to detect the 3′-homology arm (1880 bp, purple arrows in Fig. 1A) were as follows: ‘C-term-FLAG-Fwd’ 5′–GAC TAC AAA GAC CAT GAC GGT GAT TAT AAA–3′ and ‘H3t knock-in genotype-Rev_2′ 5′–CAC AGG GCA TTA CCT TCT CCC GAC CCA ATC–3′. Genomic DNA was extracted from the tail tips, and PCR was performed using the following primers (light blue and right to left pink arrows in Fig. 1A): ‘H3t knock-in genotype-Fwd’ 5′–TAC CTC GTG GGG CTG TTC GAA GAC ACG AAC–3′ and ‘H3t knock-in genotype-Rev’ 5′–AGA GCC GTT GGG TTC ATG AGC GGT GCC TGT–3′.
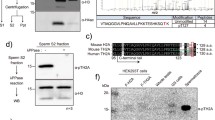
Generation of H3t-3 × FLAG knock-in mice. (A) Knock-in mice were generated using the CRISPR/Cas9 method with the targeting vectors indicated in the diagram. For more information on the targeting vectors, see Supplementary Fig. S1. (B) Validation of genotypes by PCR. The presence of 5′- and 3′-homology arms was confirmed by PCR using the primers indicated in (A). WT, wild-type; KI Het, heterozygous knock-in. The original electrophoretic images are shown in Supplementary Fig. S2A,B. (C) Validation of genotypes by PCR using the primers indicated in (A). WT, wild-type; KI Het, heterozygous knock-in. The original electrophoretic image is shown in Supplementary Fig. S2C. (D) Confirmation of H3t-3 × FLAG protein expression in heterozygous knock-in mouse testes. Immunoblot analysis of H3t in the testes of wild-type (WT) and FLAG-tagged H3t knock-in (KI) mice using an anti-H4 antibody (top left), anti-H3 antibody (top right), anti-FLAG antibody (bottom left) and anti-H3t antibody (bottom right). The original blots are shown in Supplementary Fig. S3. (E) Immunoblot analysis of FLAG-tagged H3t protein in various mouse tissues collected from heterozygous knock-in mice. An anti-H3 antibody was used as a loading control (bottom). The original blots are shown in Supplementary Fig. S6.
Histological analyses
For staining of testicular sections with antibodies, the samples were first fixed with 4% paraformaldehyde at 4 °C overnight, equilibrated in phosphate-buffered saline (PBS) (–) with 30% sucrose, embedded in O.C.T. compound (Sakura Finetek, Tokyo, Japan), frozen, and then sectioned at 4 μm thickness using a CM3050S cryostat (Leica). All sections were stained with Hoechst 33,342 (Thermo Fisher Scientific) and imaged using a BZ-X700 microscope (Keyence). All the fixed sections were activated using boiling citrate buffer (pH 6.0). To stain the sperm acrosomes, the lectin PNA tagged with Alexa Fluor 488 (L21409; Thermo Fisher Scientific, Inc.) was used. For hematoxylin and eosin staining, the slides were dehydrated and stained with hematoxylin and eosin solutions. Images were taken using a BZ-X700 microscope (Keyence).
Antibodies
The following antibodies were used in this study: anti-H3 (ab1791; Abcam), anti-H4 (05-858; Merck Millipore), FLAG-M2-HRP (A8592; Sigma), anti-FLAG (F7425; Merck Millipore), anti-PLZF (2A9; Active Motif), anti-SCP3 (ab97672; Abcam), anti-γ H2AX (05-636; Merck Millipore), HRP-conjugated anti-mouse IgG (115-035-146; Jackson ImmunoResearch), HRP-conjugated anti-rabbit IgG (A6667; Sigma) and anti-H3t11.
Nuclei isolation from tissue samples
Briefly, mouse testes were frozen in 10 mL of N buffer (5 mM Tris–HCl [pH 7.5], 60 mM KCl, 15 mM NaCl, 5 mM MgCl2, 1 mM CaCl2, 250 mM sucrose, 1 mM dithiothreitol [DTT], 2 mM sodium vanadium [NaV], 1 mM phenylmethylsulfonyl fluoride [PMSF], and 0.3% Nonidet P-40 [NP-40]) supplemented with protease inhibitors (Complete™, Roche). The thawed testes were homogenized using a Dounce homogenizer and then centrifuged at 2000 × g for 5 min at 4 °C. The pellets were washed with 1 mL of N buffer, followed by resuspension in 1 mL of N buffer. The resuspended pellets were filtered through a 100 μm cell strainer (BD Falcon) to remove debris and centrifuged at 2000 × g for 5 min at 4 °C. The resulting pellets were subjected to histone isolation.
Extraction of histones from testes nuclei
Briefly, histones were extracted from nuclei isolated from mouse testes. The isolated nuclei were divided and transferred to 5 × 1.5 mL tubes and then dissolved in 200 μL of 0.4 N sulfuric acid. The dissolved nuclei were incubated for 2 h at 4 °C with rotation and then centrifuged at 5470 × g for 5 min at 4 °C. Soluble supernatants were precipitated using ethanol. The pellets were dissolved in ice-cold sterile water. The isolated histones were stored at − 80 °C until use.
Preparation of mononucleosomes from testes
Briefly, frozen testes were crushed using a mortar and pestle. The crushed testes were resuspended in 5 mL of Buffer I (15 mM Tris–HCl [pH 7.5], 0.3 M sucrose, 60 mM KCl, 15 mM NaCl, 5 mM MgCl2, 0.1 mM EGTA, 0.5 mM dithiothreitol [DTT], and 0.1 mM phenylmethylsulfonyl fluoride [PMSF]) supplemented with protease inhibitors (Complete™, Roche). Resuspended testes were homogenized using a Dounce homogenizer and then filtered through a 100 μm cell strainer (BD Falcon) to remove debris. The filtered testis lysates were centrifuged at 4,000 × g for 15 min at 4 °C. The pellets were resuspended in 4 mL of Buffer II (buffer I with 0.2% NP-40) and then incubated on ice for 20 min. The incubated samples were layered on top of 8 mL of Buffer III (15 mM Tris–HCl [pH 7.5], 1.2 M sucrose, 60 mM KCl, 15 mM NaCl, 5 mM MgCl2, 0.1 mM EGTA, 0.5 mM DTT and 0.1 mM PMSF) supplemented with protease inhibitors (Complete™, Roche) and then centrifuged at 4,000 × g for 20 min at 4 °C. The resulting pellets were collected as nuclei and subjected to nucleosome purification.
Briefly, nuclei were dissolved in 2 mL of MNase digestion buffer (20 mM Tris–HCl [pH 8.0], 0.32 M sucrose, 5 mM NaCl, 2.5 mM CaCl2, 4 mM MgCl2 and 0.1 mM PMSF). The lysed nuclei were incubated with 40 U of MNase (Takara) for 10 min at 37 °C, after which 80 µL of 0.5 M EDTA was added to stop the reactions. The incubated nuclei were dialyzed against dialysis buffer (20 mM Tris–HCl [pH 7.5], 50 mM KCl, 0.5 mM EDTA, 0.5 mM DTT and 0.1 mM PMSF). The dialyzed samples were layered onto 14 mL of continuous sucrose gradient buffer (15 mM Tris–HCl [pH 7.5], 15–55% sucrose, 50 mM KCl, 0.5 mM EDTA, 0.5 mM DTT and 0.1 mM PMSF) and then centrifuged at 100,000 × g for 22 h at 4 °C. A total of 600 µL of each fraction was collected, and DNA was subsequently purified from 50 µL of each fraction by using a NucleoSpin Gel and PCR Clean-up Kit (Machley) to analyze the nucleosomal DNA on agarose gels. Fractions containing mononucleosomes were pooled and subjected to immunoblotting.
Immunoblotting
For immunoblotting, harvested mouse tissues were frozen in liquid nitrogen and crushed using a mortar and pestle. The crushed tissues were resuspended in 1 × sodium dodecyl sulfate (SDS) sample buffer. Proteins were separated by SDS‒polyacrylamide gel electrophoresis and subjected to immunoblotting.
Mass spectrometry
For mass spectrometry analysis, isolated histones were resolved on 4–20% gradient gels (414,879, Cosmobio) and stained with Coomassie brilliant blue (04,543–51; CBB Stain One, Nacalai Tesque). The proteins in each gel slice were subjected to reduction with 10 mM DTT at 56 °C for 1 h, alkylation with 55 mM iodoacetamide at room temperature for 45 min in the dark, and digestion with 400 ng of trypsin (Thermo Fisher Scientific, Inc.) at 37 °C for 16 h. The resulting peptides were extracted using 1% trifluoroacetic acid and 50% acetonitrile, dried under vacuum, and dissolved in 2% acetonitrile and 0.1% formic acid.
Mass spectra were obtained using an Orbitrap Eclipse (Thermo Fisher Scientific, Inc.) coupled with a nanoflow UHPLC system (Vanquish; Thermo Fisher Scientific, Inc.). The peptide mixtures were loaded onto a C18 trap column (PepMap Neo Trap Cartridge, ID 0.3 mm × 5 mm, particle size 5 μm; Thermo Fisher Scientific, Inc.) and then fractionated using a C18 analytical column (Aurora, ID 0.075 × 250 mm, particle size 1.7 μm; IonOpticks). The peptides were eluted at a flow rate of 300 nL/min using the following gradient, with the indicated percentage of solvent B: a linear gradient from 0 to 2% over 1 min, followed by a linear increase from 2 to 5% over 1 min; a linear gradient from 5 to 16% over 19.5 min; another linear gradient from 16 to 25% over 10 min; a linear gradient from 25 to 35% over 4.5 min; a sharp increase to 95% over 1 min, which was maintained for 5 min; and finally re-equilibration at 5%. Solvent A comprised 100% H2O and 0.1% formic acid, while solvent B comprised 100% acetonitrile and 0.1% formic acid. The Orbitrap analyzer was operated in data-dependent mode with a cycle time of 3 s. The MS1 scan was collected at a resolution of 60,000 with a mass range of 375–1500 m/z using standard AGC. An intensity threshold of 20,000 and charge states of 2–7 were used for MS/MS, starting from high-intensity precursors. A quadrupole was used for precursor isolation with a 1.6 m/z isolation width and fragmentation with a normalized HCD energy of 30%; the resulting fragment ions were recorded using an ion-trap analyzer. The MS1 scan was collected at 15,000 resolution using a standard AGC target and a maximum injection time of 54 ms. Dynamic exclusion was set to 20 s.
The raw data files were searched against the UniProt Mouse Proteome Database (2024.3.4 downloaded), which includes the cRAP contaminant protein dataset, using the MASCOT program (version 2.8; Matrix Science) via Proteome Discoverer 2.5 (Thermo). The search was performed using carbamidomethylation of cysteine as a fixed modification; oxidation of methionine; acetylation of protein N-termini; and acetylation, methylation, dimethylation, trimethylation, and diglycine of lysine as variable modifications. The number of missed cleavage sites was set to 2.
Statistical analysis
Statistical significance was determined using the Steel–Dwass test, with P < 0.05 considered to indicate statistical significance.
Results
Generation of H3t-3 × FLAG knock-in mice
We first performed a knock-in in C57BL/6N × DBA/2 F1 (BDF1) mouse embryos using genome editing technology27,28 and successfully generated mice with a knock-in of a 3 × FLAG tag on the carboxyl terminus of H3t (Fig. 1A–C, Supplementary Fig. S1 and S2). Immunoblotting of testes harvested from wild-type and heterozygous knock-in mice confirmed that full-length 3 × FLAG-tagged H3t (H3t-FLAG) was expressed as a protein in the testes (Fig. 1D and Supplementary Fig. S3). More importantly, H3t-FLAG was incorporated into mononucleosomes isolated from heterozygous knock-in testes, although the N-terminal tails of both H3 and H3t-FLAG appeared to be cleaved by testicular proteinases during nucleosome preparation (Supplementary Fig. S4A,B). Thus, the H3t-FLAG protein was expressed and integrated into the genome.
Next, we backcrossed the founder BDF1 male mice with C57BL/6N female mice. However, when the knock-in mice were converted to the C57BL/6N genetic background, the males had smaller testes and were infertile, and fertility was restored when the mice were converted back to the BDF1 background (Supplementary Fig. S5A–C; average testis weight ± standard deviation: wild-type [C57BL/6N] 115.2 ± 12.5 mg vs. heterozygous knock-in [C57BL/6N] 22.8 ± 2.5 mg). In addition, heterozygous knock-in male mice on a C57BL/6N background were infertile because of the absence of sperm in the testes (Supplementary Fig. S5D). Interestingly, the testes of the heterozygous knock-in mice were slightly larger than those of the H3t homozygous knockout mice (Supplementary Fig. S5A,B; average testis weight ± standard deviation: H3t homozygous knockout [C57BL/6N] 18.0 ± 1.7 mg). Although changing the background to BDF1 restored fertility in heterozygous knock-in mice (Supplementary Fig. S5C), the testis size did not return to the wild-type level (Supplementary Fig. S5A,B; average testis weight ± standard deviation: wild-type [BDF1] 110.2 ± 11.2 mg vs. heterozygous knock-in [BDF1] 44.4 ± 5.0 mg). Accordingly, this suggests that even small tags, such as FLAG tags, may have a dominant negative effect on molecules incorporated into the genome in large quantities, such as histones, as discussed later. Herein, considering the lesser effects observed on the testes of the knock-in mice, we conducted a subsequent analysis using the testes of fertile male BDF1 mice.
Characterization of tagged H3t expressed in testicular tissue
Initially, we determined whether H3t-FLAG was properly expressed in the testes and incorporated into the cell nucleus. Histone-enriched protein samples were prepared from multiple organs and subjected to immunoblotting. As previously observed for endogenous H3t11, H3t-FLAG was expressed only in the testes (Fig. 1E and Supplementary Fig. S6). Immunostaining of testicular tissue was performed to examine the subcellular localization of the tagged proteins, confirming that H3t-FLAG was incorporated into the spermatogonia and meiotic cells in the testes (Fig. 2A–C). These results also indicated that tagged H3t functions in vivo. However, the H3t localization pattern in the cell nucleus was not consistent with the immunostaining results obtained using anti-H3t and anti-FLAG antibodies (Fig. 2A–C). For example, no signal was detected in early elongating spermatids with an anti-H3t antibody, but a clear signal was detected with an anti-FLAG antibody (Fig. 2C). This difference may be attributed to the inability of anti-H3t antibodies to detect certain H3t proteins owing to posttranslational modifications near the antigen (valine residue at the 24th position) and/or binding of the posttranslational modification reader protein to the vicinity of the antigen, which makes the anti-H3t antibody inaccessible to the antigen.
Colocalization of H3t and FLAG-tagged H3t proteins during spermatogenesis. (A) Immunostaining of testicular sections using antibodies against H3t (green) and FLAG (red). Seminiferous tubules at stage XII are presented. The area framed by the dotted lines is enlarged in the upper left corners of each panel. Note that elongating spermatids are stained with an anti-FLAG antibody but not with an anti-H3t antibody. Scale bar, 20 μm. (B) Comparison of the localization of the H3t protein and FLAG-tagged H3t protein in the cell nuclei during spermatogenesis (pachytene stage). The graph shows the intensity of the green (H3t) and red (FLAG) signals in the dashed yellow line in the upper right panel (from upper left to lower right). Scale bar, 10 μm. (C) Comparison of the localization of the H3t protein and FLAG-tagged H3t protein in the cell nuclei during meiotic metaphase, round spermatids and early elongating spermatids. Scale bar, 10 μm.
We also confirmed the expression pattern of H3t-FLAG within seminiferous tubules by costaining with various markers (Fig. 3A–D), which was consistent with our previous immunostaining results obtained using specific antibodies against H3t11,29. We observed that H3t-FLAG was expressed in meiotic spermatocytes and haploid round spermatids (Fig. 3B,C). However, similar to immunostaining with the anti-H3t antibody, no colocalization of XY bodies formed during the pachytene stage or H3t-FLAG was observed (Fig. 3D). Interestingly, when an anti-H3t antibody was used, no signal was detected in PLZF-positive cells11; whereas, when an anti-FLAG antibody was used, a weak signal was detected (Fig. 3A), indicating that H3t is weakly expressed in undifferentiated spermatogonia. Additionally, as mentioned above, no signal was detected in early elongating spermatids with the anti-H3t antibody, whereas a weak signal was detected with the anti-FLAG antibody (Fig. 2A,C; see enlarged figure enclosed in the square)11. These findings suggested that the anti-FLAG antibody is more sensitive than the anti-H3t antibody for capturing the behavior and localization of the H3t protein in vivo. Importantly, the presence of the 3 × FLAG tag at the carboxyl terminus did not impair immunostaining efficiency.
Localization of FLAG-tagged H3t protein during spermatogenesis. (A) The FLAG-tagged H3t protein is weakly expressed in undifferentiated spermatogonia. Coimmunostaining with PLZF (green, arrowheads), a marker of undifferentiated spermatogonia, and FLAG (red) antibodies was performed. Scale bar, 50 μm. (B) FLAG-tagged H3t protein expression during meiosis. Coimmunostaining with antibodies against SCP3 (green), a marker of meiosis, and FLAG (red) was performed. Scale bar, 10 μm. (C) The FLAG-tagged H3t protein (red) and H3t protein (green) colocalize from the pachytene stage to the round spermatid stage. Scale bar, 50 μm. (D) The FLAG-tagged H3t protein was excluded from the XY body. Sections of testicular tissue were stained with anti-FLAG (red) and anti–γH2AX (green) antibodies, and pachytene stage nuclei are indicated. FLAG-tagged H3t was also excluded from the XY body. Scale bar, 10 μm.
Analysis of posttranslational modifications of tagged H3t
Next, using testes harvested from the knock-in mice, histones present in the chromatin fraction (those forming nucleosomes) were acid-extracted and separated via electrophoresis. Importantly, an H3t-FLAG band (approximately 18–19 kDa) was clearly detected at a position sufficiently distinct from that of endogenous H3t (Fig. 4A, indicated by a box with H3t-FLAG and Supplementary Fig. S7). Taking advantage of the distinct differences in mobility, bulk histone H3 (H3) from both the wild-type and H3t-FLAG knock-in strains (Fig. 4A, indicated by boxes) and H3t-FLAG were recovered from the gel and subjected to extensive mass spectrometry analysis to compare the posttranslational modifications of H3 and H3t-FLAG. The mass spectrometry results are summarized in Fig. 4B and Supplementary Table S1.
Comprehensive analysis of posttranslationally modified lysine residues on FLAG-tagged H3t. (a) SDS‒PAGE analysis of purified histones from mouse testes after Coomassie brilliant blue staining. The gel slices indicated by H3t-FLAG and the control were excised and subjected to LC‐MS/MS analysis. H3t-FLAG was detected as a clear single band (also shown in Fig. 1D). A control band comprising canonical H3, H3.3, and endogenous H3t was used for comparison. The original CBB-stained gel image is shown in Supplementary Fig. S7. (B) The relative abundance of posttranslationally modified lysine residues of histone H3 and FLAG-tagged H3t identified by mass spectrometry is shown as a 100% stacked horizontal bar chart. The source data are shown in Supplementary Table S1. SDS‒PAGE, sodium dodecyl sulfate‒polyacrylamide gel electrophoresis; LC‒MS/MS, liquid chromatography with tandem mass spectrometry.
Mass spectrometry analysis detected the majority of posttranslational modifications previously identified in canonical histone H3, except for modifications to K4, presumably due to a technical problem, with mass spectrometry unable to detect the markedly shortened peptides covering K4 after proteinase digestion. The overall pattern of H3t-FLAG posttranslational modifications was comparable to that of the control H3. Although we determined the posttranslational modifications of H3t by using the differences in migration between H3t-FLAG and other histone H3, we could distinguish the peptide derived from H3t by the presence of V24, and the most frequently detected peptide containing V24 was K18-R26 (KQLATKVAR). We found that the overall pattern of modifications at K18 and K23 was not significantly different between H3t and canonical H3.1/H3.2, confirming that the overall pattern of posttranslational modifications of H3t was comparable to that of canonical H3 (Supplementary Table S1, indicated by asterisks). However, the relative abundances of certain specific modifications differed between H3t-FLAG and the control bulk H3. For example, the relative abundance of K9 acetylation was high in H3t-FLAG (Fig. 4B), whereas the relative abundances of K9 mono-, di-, and trimethylation were low compared with those in the control bulk H3 (Fig. 4B). Although H3K9 methylation levels have been shown to differ between H3 and H3t22, our study detected a distinct pattern. Interestingly, K27 exhibited the opposite pattern: the relative abundance of K27 acetylation was low, whereas that of mono- and dimethylation was high in H3t-FLAG compared with that of the control bulk H3. Furthermore, the relative amount of K37 monomethylation was lower in H3t-FLAG than in bulk H3, and no dimethylation was observed. This difference may be attributed to K37 monomethylation occurring at a specific time point during spermatogenesis, and the timing of H3t deposition may differ from this time point. Taken together, these results suggested that H3t is incorporated into nucleosomes and undergoes a series of posttranslational modifications in a manner comparable to that of canonical H3.
Discussion
Previous studies have attempted to reveal posttranslational modifications of H3t via the combined utilization of liquid chromatography and mass spectrometry29,30. However, distinguishing between the canonical histones H3.1/H3.2 and H3.3, known histone H3 variants, and H3t presents a considerable challenge, given that they vary by only a few amino acids. Previous investigations of posttranslational histone modifications were conducted using specific antibodies against posttranslational modifications; therefore, the substrate histones themselves were not distinguished18. Furthermore, because specific antibodies against various variants use different amino acids as antigens, there are concerns that posttranslational modifications of nearby amino acids may reduce antibody specificity, and such a problem has been reported with posttranslationally modified antibodies for histones19,31,32. On the other hand, if a reader protein that recognizes posttranslational modifications of histone proteins binds in the vicinity of the antigen, specific antibodies may not be able to access the antigen and consequently fail to capture the target protein. It is also possible that the H3t-FLAG may have changed its localization in the genome compared to the endogenous H3t. To investigate this possibility, ChIP-seq analysis of H3t-FLAG will be necessary.
Histone H3 is known to undergo numerous posttranslational modifications at its amino terminus31. Given that histone tail clipping has been described previously33 and with reference to previous literature on histone variants9,15,34, we knocked in a 3 × FLAG tag at the carboxyl terminus of H3t to avoid the tag being cleaved. However, the immunoblotting results clearly indicated the absence of a small molecular weight H3t protein within the testis (Fig. 1D). Although further analysis is needed, our results suggest that histone tail clipping at the macroscopic level does not occur during spermatogenesis.
Initially, we expected the presence of posttranslational modifications specific to H3t, as observed in H3.321,23. However, a different pattern of posttranslational modifications was found for H3t-FLAG than for canonical H3, with a higher percentage of mono- and dimethylation of K27 and lower percentage of methylation of K37. Given that the methylation of H3K9 and H3K27 plays distinct roles in establishing a repressive chromatin structure and that H3K27 monomethylation reportedly labels enhancers of actively transcribed genes35, these differences may be related to H3t-specific functions during spermatogenesis. Although the role of H3K37 methylation in mammalian cells has not been extensively explored, its involvement in gametogenesis has been reported in Schizosaccharomyces pombe36. With a more detailed analysis in the future, it may be possible to identify H3t-specific posttranslational modifications in local regions in the genome or during the progression of spermatogenesis. Our analysis revealed that those residues replaced in canonical H3 and H3t during spermatogenesis undergo similar posttranslational modifications. Notably, H3t had a larger number of transcriptionally active posttranslational modifications and fewer transcriptionally repressive posttranslational modifications than did the control H3 (Figs. 4B, 5). Given that H3t-containing nucleosomes form an open conformation in vitro11, further studies are necessary to elucidate how these transcriptionally active posttranslational modifications work in conjunction to impact the formation of higher-order chromosome structures in vivo. In addition, the observation that tagged H3t undergoes posttranslational modification also indicated that H3t-FLAG is incorporated into the nucleosome (Fig. 4B). This is because H3, the substrate for posttranslational modification, must be incorporated into nucleosomes to be recognized by modifying enzymes37.
Summary of the results for the presence ratios of H3t-FLAG and H3 posttranslational modifications. A visual representation of the results is shown in Fig. 4B and Supplementary Table S1, where the circle indicates the posttranslational modifications at each lysine residue. The larger the circle is, the greater the abundance of that posttranslational modification at the lysine residue.
The current results strongly suggest that the 3 × FLAG tag, although small among the tags, may inhibit chromatin structure formation during spermatogenesis and affect fertility. It was particularly surprising that heterozygous knock-in mice and H3t homozygous knockout mice exhibited azoospermia11. Since H3t-FLAG protein is expressed and properly integrated into chromatin (Supplementary Fig. S4), the severe phenotype in the knock-in mice was not due to a “failure of H3t-FLAG deposition” but to the effect of H3t-FLAG incorporated into chromatin. Tagging of the carboxyl terminus of TH2B, a testis-specific histone variant, has been shown to induce infertility38. Thus, even if the tag attached to histones is small, histones are more likely to influence spermatogenesis than other factors because they are incorporated into chromatin in greater amounts than other proteins are. Intriguingly, although we demonstrated that H3t-FLAG was incorporated into chromatin and nucleosomes in the testes of H3t-FLAG knock-in mice (Supplementary Figure S4), the total amount of H3t in the knock-in mice, as assessed by immunoblotting signals for endogenous H3t and H3t-FLAG, appears to be lower compared to that of endogenous H3t in wild-type mice (Fig. 1D). This suggests that H3t-FLAG may have a dominant negative effect on H3t deposition or destabilize the structure of H3t-containing nucleosomes, which may result in the reduced testis size in the knock-in mice (Supplementary Figure S5A,B). At present, the possible reasons for this potential effect are not clear, but future studies comparing the nucleosome structure containing H3t or H3t-FLAG may help to explore the reason for such an effect. Furthermore, the carboxyl terminal FLAG tag may affect the balance of post-translational modifications on H3t, which may interfere with H3t function during spermatogenesis. In any case, this may also explain why studies exploring the generation of histone-tagged knock-in mice have failed to progress. Moreover, the recovery of fertility upon altering the mouse background to BDF1 has yet to be determined (Supplementary Fig. S5C). One possible reason is that BDF1 mice are larger and more robust than C57BL/6N mice are39,40, and this genetic diversity may account for the observed variation in fertility. Nevertheless, the fact that heterozygous knock-in mice exhibited the same infertility phenotype as knockout mice suggested that the H3t-3 × FLAG protein was indeed incorporated into the nucleosome.
Finally, the knock-in mice used in the present study will facilitate the analysis of interaction factors, such as chaperones specific to H3t, which have been difficult to analyze with monoclonal antibodies against H3t29. In addition, ChIP-seq analysis of the H3t localization pattern in the genome, as well as histone code analysis of specific genomic loci, will enable the elucidation of the roles of histone variants in spermatogenesis and spermiogenesis. In particular, the immunostaining patterns obtained using anti-H3t and anti-FLAG antibodies in this paper were quite different (Fig. 2A–C), and detailed ChIP-seq analysis using each antibody will be necessary in the future. Moreover, as various histone variants are known to be expressed during spermatogenesis7,41, our knock-in mouse model will be valuable for elucidating the crosstalk between these molecules.
Data availability
The mass spectrometry datasets analyzed during the current study have been deposited to the ProteomeXchange Consortium via the jPOST. The accession numbers are PXD052428 for ProteomeXchange and JPST003119 for jPOST. (Preview URL : https://repository.jpostdb.org/preview/368100760664c374cc6b13, Access key : 7521).
References
Cavalli, G. & Heard, E. Advances in epigenetics link genetics to the environment and disease. Nature 571, 489–499. https://doi.org/10.1038/s41586-019-1411-0 (2019).
Dai, Z., Ramesh, V. & Locasale, J. W. The evolving metabolic landscape of chromatin biology and epigenetics. Nat. Rev. Genet. 21, 737–753. https://doi.org/10.1038/s41576-020-0270-8 (2020).
Boon, R., Silveira, G. G. & Mostoslavsky, R. Nuclear metabolism and the regulation of the epigenome. Nat. Metab. 2, 1190–1203. https://doi.org/10.1038/s42255-020-00285-4 (2020).
Allis, C. D. & Jenuwein, T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 17, 487–500. https://doi.org/10.1038/nrg.2016.59 (2016).
Martire, S. & Banaszynski, L. A. The roles of histone variants in fine-tuning chromatin organization and function. Nat. Rev. Mol. Cell Biol. 21, 522–541. https://doi.org/10.1038/s41580-020-0262-8 (2020).
Maze, I., Noh, K.-M., Soshnev, A. A. & Allis, C. D. Every amino acid matters: Essential contributions of histone variants to mammalian development and disease. Nat. Rev. Genet. 15, 259–271. https://doi.org/10.1038/nrg3673 (2014).
Talbert, P. B. & Henikoff, S. Histone variants at a glance. J. Cell Sci. https://doi.org/10.1242/jcs.244749 (2021).
Kurumizaka, H., Kujirai, T. & Takizawa, Y. Contributions of histone variants in nucleosome structure and function. J. Mol. Biol. 433, 166678. https://doi.org/10.1016/j.jmb.2020.10.012 (2021).
Tagami, H., Ray-Gallet, D., Almouzni, G. & Nakatani, Y. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 116, 51–61. https://doi.org/10.1016/s0092-8674(03)01064-x (2004).
Ray-Gallet, D. et al. Dynamics of histone H3 deposition in vivo reveal a nucleosome gap-filling mechanism for H3.3 to maintain chromatin integrity. Mol. Cell 44, 928–941. https://doi.org/10.1016/j.molcel.2011.12.006 (2011).
Ueda, J. et al. Testis-specific histone variant h3t gene is essential for entry into spermatogenesis. Cell Rep. 18, 593–600. https://doi.org/10.1016/j.celrep.2016.12.065 (2017).
Lacoste, N. et al. Mislocalization of the centromeric histone variant CenH3/CENP-A in human cells depends on the chaperone DAXX. Mol. Cell 53, 631–644. https://doi.org/10.1016/j.molcel.2014.01.018 (2014).
Ibarra-Morales, D. et al. Histone variant H2A.Z regulates zygotic genome activation. Nat. Commun. 12, 7002. https://doi.org/10.1038/s41467-021-27125-7 (2021).
Udugama, M. et al. Histone variant H3.3 provides the heterochromatic H3 lysine 9 tri-methylation mark at telomeres. Nucleic Acids Res. 43, 10227–10237. https://doi.org/10.1093/nar/gkv847 (2015).
Goldberg, A. D. et al. Distinct factors control histone variant H3.3 localization at specific genomic regions. Cell 140, 678–691. https://doi.org/10.1016/j.cell.2010.01.003 (2010).
Santenard, A. et al. Heterochromatin formation in the mouse embryo requires critical residues of the histone variant H3.3. Nat. Cell Biol. 12, 853–862. https://doi.org/10.1038/ncb2089 (2010).
Kimura, H., Hayashi-Takanaka, Y., Goto, Y., Takizawa, N. & Nozaki, N. The organization of histone H3 modifications as revealed by a panel of specific monoclonal antibodies. Cell Struct. Funct. 33, 61–73. https://doi.org/10.1247/csf.07035 (2008).
Egelhofer, T. A. et al. An assessment of histone-modification antibody quality. Nat. Struct. Mol. Biol. 18, 91–93. https://doi.org/10.1038/nsmb.1972 (2010).
Bock, I. et al. Detailed specificity analysis of antibodies binding to modified histone tails with peptide arrays. Epigenetics 6, 256–263. https://doi.org/10.4161/epi.6.2.13837 (2011).
McKittrick, E., Gafken, P. R., Ahmad, K. & Henikoff, S. Histone H3.3 is enriched in covalent modifications associated with active chromatin. Proc. Natl. Acad. Sci. 101, 1525–1530. https://doi.org/10.1073/pnas.0308092100 (2004).
Hake, S. B. et al. Serine 31 phosphorylation of histone variant H3.3 is specific to regions bordering centromeres in metaphase chromosomes. Proc. Natl. Acad. Sci. U S A 102, 6344–6349. https://doi.org/10.1073/pnas.0502413102 (2005).
Kwak, H.-G. & Dohmae, N. Proteomic characterization of histone variants in the mouse testis by mass spectrometry-based top-down analysis. BioScience Trends 10, 357–364. https://doi.org/10.5582/bst.2016.01090 (2016).
Armache, A. et al. Histone H3.3 phosphorylation amplifies stimulation-induced transcription. Nature 583, 852–857. https://doi.org/10.1038/s41586-020-2533-0 (2020).
Bachu, M. et al. A versatile mouse model of epitope-tagged histone H3.3 to study epigenome dynamics. J. Biol. Chem. 294, 1904–1914. https://doi.org/10.1074/jbc.RA118.005550 (2019).
Heintz, N., Zernik, M. & Roeder, R. G. The structure of the human histone genes: Clustered but not tandemly repeated. Cell 24, 661–668. https://doi.org/10.1016/0092-8674(81)90092-1 (1981).
Marzluff, W. F., Gongidi, P., Woods, K. R., Jin, J. & Maltais, L. J. The human and mouse replication-dependent histone genes. Genomics 80, 487–498. https://doi.org/10.1006/geno.2002.6850 (2002).
Mashiko, D. et al. Generation of mutant mice by pronuclear injection of circular plasmid expressing Cas9 and single guided RNA. Sci. Rep. 3, 3355. https://doi.org/10.1038/srep03355 (2013).
Raveux, A., Vandormael-Pournin, S. & Cohen-Tannoudji, M. Optimization of the production of knock-in alleles by CRISPR/Cas9 microinjection into the mouse zygote. Sci. Rep. 7, 42661. https://doi.org/10.1038/srep42661 (2017).
Dong, C. et al. Structural basis for histone variant H3tK27me3 recognition by PHF1 and PHF19. Elife https://doi.org/10.7554/eLife.58675 (2020).
Kwak, H.-G., Suzuki, T. & Dohmae, N. Global mapping of posttranslational modifications on histone H3 variants in mouse testes. Biochem. Biophys. Rep. 11, 1–8. https://doi.org/10.1016/j.bbrep.2017.05.003 (2017).
Kimura, H. Histone modifications for human epigenome analysis. J. Hum. Genet. 58, 439–445. https://doi.org/10.1038/jhg.2013.66 (2013).
Fuchs, S. M., Krajewski, K., Baker, R. W., Miller, V. L. & Strahl, B. D. Influence of combinatorial histone modifications on antibody and effector protein recognition. Curr. Biol. 21, 53–58. https://doi.org/10.1016/j.cub.2010.11.058 (2011).
Santos-Rosa, H. et al. Histone H3 tail clipping regulates gene expression. Nat. Struct. Mol. Biol. 16, 17–22. https://doi.org/10.1038/nsmb.1534 (2008).
Tagami, H. in Histone Variants Methods in Molecular Biology Ch. Chapter 3, 51–60 (2018).
Ferrari, K. J. et al. Polycomb-dependent H3K27me1 and H3K27me2 regulate active transcription and enhancer fidelity. Mol. Cell 53, 49–62. https://doi.org/10.1016/j.molcel.2013.10.030 (2014).
Shen, Y. et al. Set7 Is a H3K37 methyltransferase in Schizosaccharomyces pombe and is required for proper gametogenesis. Structure 27, 631–638. https://doi.org/10.1016/j.str.2019.01.011 (2019).
Almouzni, G. & Cedar, H. Maintenance of epigenetic information. Cold Spring Harb. Perspect. Biol. https://doi.org/10.1101/cshperspect.a019372 (2016).
Montellier, E. et al. Chromatin-to-nucleoprotamine transition is controlled by the histone H2B variant TH2B. Genes Dev. 27, 1680–1692. https://doi.org/10.1101/gad.220095.113 (2013).
Suto, J. Genetic dissection of testis weight in mice: Quantitative trait locus analysis using F(2) intercrosses between strains with extreme testis weight, and association study using Y-consomic strains. Mamm Genome 22, 648–660. https://doi.org/10.1007/s00335-011-9353-3 (2011).
Harbison, C. E., Lipman, R. D. & Bronson, R. T. Strain- and Diet-Related Lesion Variability in Aging DBA/2, C57BL/6, and DBA/2xC57BL/6 F1 Mice. Vet. Pathol. 53, 468–476. https://doi.org/10.1177/0300985815612152 (2016).
Rathke, C., Baarends, W. M., Awe, S. & Renkawitz-Pohl, R. Chromatin dynamics during spermiogenesis. Biochim Biophys Acta 155–168, 2014. https://doi.org/10.1016/j.bbagrm.2013.08.004 (1839).
Acknowledgements
We thank Dr. Yasuyuki Ohkawa for providing us with the anti-H3t antibody. The authors thank Saki Nishioka for the technical assistance.
Funding
This research was funded by the Japan Society for the Promotion of Science, Kakenhi (Grant Numbers JP16H06276 [AdAMS], JP19K06452, and JP22K06063 to J.U.; JP18H05532 and JP24H02324 to J.N.); the Agency for Medical Research and Development (Grant Number JP22ym0126801); and the National Institute for Basic Biology, Collaborative Research Program (Grant Numbers 21NIBB229, 22NIBB330, 22NIBB325, 23NIBB319, and 24NIBB333).
Author information
Authors and Affiliations
Contributions
Conceptualization, T.K., J.N., and J.U.; investigation, T.K., M.H., R.N., R.M., M.I., J.N., and J.U.; data curation, T.K., M.H., R.N., R.M., M.I., J.N., and J.U.; writing—original draft preparation, T.K., J.N., and J.U.; writing—review and editing, T.K., M.H., R.N., R.M., J.N., and J.U.; visualization, T.K., J.N., and J.U.; supervision, J.N. and J.U.; project administration, J.N. and J.U.; funding, J.N. and J.U. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Kawaguchi, T., Hashimoto, M., Nakagawa, R. et al. Comprehensive posttranslational modifications in the testis-specific histone variant H3t protein validated in tagged knock-in mice. Sci Rep 14, 21305 (2024). https://doi.org/10.1038/s41598-024-72362-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72362-7
- Springer Nature Limited