Abstract
Age-related osteoporosis is a prevalent bone metabolic disorder distinguished by an aberration in the equilibrium between bone formation and resorption. The reduction in the stemness of Bone Marrow Mesenchymal Stem Cells (BMSCs) plays a pivotal role in the onset of this ailment. Comprehending the molecular pathways that govern BMSCs stemness is imperative for delineating the etiology of age-related osteoporosis and devising efficacious treatment modalities. The study utilized single-cell RNA sequencing and miRNA sequencing to investigate the cellular heterogeneity and stemness of BMSCs. Through dual-luciferase reporter assays and functional experiments, the regulatory effect of miR-183 on CTNNB1 (β-catenin) was confirmed. Overexpression and knockdown studies were conducted to explore the impact of miR-183 and β-catenin on stemness-related transcription factors Oct4, Nanog, and Sox2. Cell proliferation assays and osteogenic differentiation experiments were carried out to validate the influence of miR-183 and β-catenin on the stemness properties of BMSCs. Single-cell analysis revealed that β-catenin is highly expressed in both high stemness clusters and terminal differentiation clusters of BMSCs. Overexpression of β-catenin upregulated stemness transcription factors, while its suppression had the opposite effect, indicating a dual regulatory role of β-catenin in maintaining BMSCs stemness and promoting bone differentiation. Furthermore, the confluence of miRNA sequencing analyses and predictions from online databases revealed miR-183 as a potential modulator of BMSCs stemness and a novel upstream regulator of β-catenin. The overexpression of miR-183 effectively diminished the stemness characteristics of BMSCs by suppressing β-catenin, whereas the inhibition of miR-183 augmented stemness. These outcomes align with the observed alterations in the expression levels and functional assessments of transcription factors associated with stemness. This study provides evidence for the essential involvement of β-catenin in preserving the stemness of BMSCs, as well as elucidating the molecular mechanism through which miR-183 selectively targets β-catenin to modulate stemness. These results underscore the potential of miR-183 and β-catenin as molecular targets for augmenting the stemness of BMSCs. This strategy is anticipated to facilitate the restoration of bone microarchitecture and facilitate bone tissue regeneration by addressing potential cellular dysfunctions, thereby presenting novel targets and perspectives for the management of age-related osteoporosis.
Similar content being viewed by others
Introduction
Age-related osteoporosis is a prevalent metabolic bone disorder distinguished by an inequilibrium in the processes of bone formation and resorption1. This condition arises from an excessive resorption of bone by osteoclasts and a deficiency in bone formation due to diminished osteoblast functionality2,3. The crucial role in preserving bone mass is attributed to BMSCs, which serve as precursors to osteoblasts. Improving the stemness of BMSCs promotes the sustained generation of fully functional osteoblasts4. Numerous studies have demonstrated that the diminishment of stemness in BMSCs, encompassing their abilities to proliferate, differentiate, and self-renew, constitutes a significant determinant in the pathogenesis of age-related osteoporosis5,6. Nevertheless, the precise intrinsic and extrinsic mechanisms that contribute to the compromised stemness of BMSCs during the progression of age-related osteoporosis remain elusive. Contemporary therapeutic approaches for age-related osteoporosis predominantly center on inhibiting bone resorption and promoting bone formation utilizing pharmacological interventions including bisphosphonates, calcitonin, and selective estrogen receptor modulators. Nevertheless, these interventions frequently entail notable adverse effects and fail to target the fundamental cellular abnormalities implicated in the pathogenesis of osteoporosis7. Moreover, current methods are inadequate in fully restoring bone microarchitecture and promoting the regeneration of bone tissue, highlighting the necessity for innovative approaches that focus on the underlying cellular mechanisms crucial for bone health8. Unraveling the molecular mechanisms that govern the stemness of BMSCs will offer novel perspectives on the etiology of age-related osteoporosis and the development of cell-based therapeutic approaches.
Numerous signaling pathways and transcription factors, such as BMP, Wnt/β-catenin, and Hippo, play a crucial role in regulating the proliferation, differentiation, and preservation of stemness in BMSCs9,10. β-catenin, encoded by the CTNNB1 gene and containing 781 amino acids, is a bifunctional protein that is involved in both intercellular adhesion and transcriptional regulation. Both of these functions of β-catenin are critical in determining the cell fate of BMSCs. The Wnt/β-catenin signaling pathway is widely recognized as a crucial regulatory factor in skeletal development and the differentiation of osteoblasts. This pathway is distinguished by its role in facilitating intercellular communication and regulating gene transcription, thereby initiating the transcription of target genes that coordinate these cellular processes. This coordination is achieved through the stabilization and subsequent nuclear translocation of β-catenin11. Although β-catenin has been proven to promote the differentiation of BMSCs into osteoblasts, recent studies indicate its significant role in maintaining stemness. For example, in human and mouse embryonic stem cells, β-catenin can activate the expression of stem cell maintenance factors such as Oct4, Sox2, and Nanog, thereby enhancing the self-renewal and stemness of embryonic stem cells12. Furthermore, β-catenin exerts an impact on telomerase activity through its regulation of TEL2 and TERT expression, thereby augmenting the stemness of stem cells13,14. Nevertheless, the precise function of β-catenin in sustaining stemness in BMSCs remains ambiguous, and additional clarification is needed to determine whether it can serve as a molecular regulator bridging the maintenance of stemness and lineage differentiation.
MicroRNAs, as crucial post-transcriptional regulators of gene expression, have also been proven to regulate stem cell activity15. miR-183 belongs to the miR-183 family, which also includes miR-96 and miR-18216. These microRNAs are highly homologous in structure and function and are extensively involved in the regulation of both tumors and normal stem cells. For example, miR-183 can target and inhibit the expression of stemness transcription factors in embryonic stem cells17. Despite the acknowledged regulatory significance of miR-183 in tumors and embryonic stem cells, its precise role in the biology of BMSCs has not been definitively elucidated. Particularly, its regulatory interactions with β-catenin in this context remain entirely unknown. BMSCs are a heterogeneous group of cells, with different subpopulations exhibiting variations in proliferation and stemness18. The recent advancements in single-cell omics technologies have provided powerful tools for deciphering the heterogeneity of BMSCs. For example, Mo et al. utilized single-cell RNA sequencing to analyze the composition and molecular characteristics of mouse BMSCs, revealing that different clusters of cells with distinct expression profiles contribute differently to bone formation19. However, the current body of research examining the application of single-cell technologies for the analysis of human BMSCs heterogeneity in relation to the maintenance of stemness and the treatment of age-related osteoporosis remains somewhat limited.
Due to the constraints of existing osteoporosis treatments, which predominantly focus on symptom management rather than addressing the fundamental cellular dysfunction, there is an urgent requirement for innovative therapeutic approaches that specifically target these cellular processes. This study utilizes single-cell RNA sequencing and miRNA sequencing techniques, along with Western blot analyses and other established experimental methods, to examine and confirm the molecular mechanisms that impact the stemness of BMSCs. The primary objective of these investigations is to offer new scientific perspectives on the pathogenesis of age-related osteoporosis and pinpoint potential therapeutic targets for its management.
Method
Data collection
The datasets utilized in this research were acquired from the GEO database, accessible at http://www.ncbi.nlm.nih.gov/geo. Two GEO datasets, namely GSE57127 and GSE147287, were subjected to analysis. The dataset GSE57127 contains six miRNA sequencing samples obtained from BMSCs, consisting of three samples from young mice demonstrating high stemness and three from aged mice displaying low stemness. Furthermore, the dataset GSE147287 includes two single-cell RNA sequencing samples of BMSCs, one originating from a patient diagnosed with osteoporosis and the other from an individual afflicted with osteoarthritis (Supplementary information).
MiR-183 prediction
The analysis of differential expression of miRNAs in the GSE57127 dataset was conducted using the limma package20. MiRNAs with a fold change greater than 2 and a p-value less than 0.05 were identified as potential regulators of BMSCs stemness. The identification of potential upstream regulators of CTNNB1 was achieved through the utilization of online tools including ENCORI, miRDB, and miRTarBase. A Venn diagram analysis was conducted to elucidate the intersections between miRNAs predicted by these algorithms and those identified through differential expression analysis. This comprehensive analysis definitively pinpointed miR-183 as a reliable upstream regulator of CTNNB1 and a potential regulator of BMSCs stemness.
Single-cell RNA sequencing analysis
Comprehensive single-cell RNA sequencing analysis was performed on the dataset GSE14728721. At the outset, our analysis utilized two samples from the dataset, with rigorous quality control measures being enforced to safeguard the integrity and dependability of the data. Cells with abnormally high or low numbers of detected genes, as well as cells with excessive mitochondrial gene expression, were excluded to minimize the impact of dead/dying cells and doublets. Principal component analysis (PCA) was conducted to reduce the dimensionality of the dataset before proceeding to cluster cells into distinct groups using unsupervised clustering (resolution 0.2)22. Subsequently, the identification of each cluster was accompanied by the annotation process, which relied on the expression profiles of well-known markers specific to different cell types. Notably, clusters exhibiting heightened expression of mesenchymal stem cell markers ENG, NT5E, THY1, LEPR, and NGFR, along with diminished PTPRC expression, were of particular interest. The cluster that fulfilled these criteria, namely cluster 0.10, was assigned the designation of BM-MSC cluster. Moreover, to achieve a more detailed subclustering of the BM-MSC population, the resolution at the cluster level was further increased to 0.5. To gain functional insights and infer developmental trajectories, pseudotime analysis was performed using Monocle 3, which models the progression of cells along a developmental continuum to reveal the temporal dynamics of gene expression. Differential analysis between clusters examined the expression patterns of WNT/β-catenin pathway genes to uncover factors with stemness-maintaining roles, as these play critical parts in mesenchymal stem cell biology.
Cell culture
Rat BMSCs obtained from Shanghai QiDa Biotechnology (China, CD6177) were cultured in a specialized medium for mesenchymal stem cells (Shanghai QiDa Biotechnology, China, P2001) at a density of 5 × 104 cells/cm2. The cells were incubated at 37 °C with 5% CO2. For passaging every 2 days, 0.25% trypsin–EDTA (Solarbio, China, J8150) was used to detach the cells. Experiments were conducted using BMSCs between the third and tenth passages.
Transfection assay
BMSCs were seeded at a density of 2 × 105 cells per well in 6-well plates and cultured until they reached 80–90% confluence. Subsequently, the cells were serum-starved for a duration of 30 min prior to transfection. In the case of siRNA experiments, BMSCs were transfected with 50 nM CTNNB1 siRNA (GenePharma, China), while the control groups were administered scrambled siRNA (GenePharma, China). To induce overexpression, BMSCs were transfected with 2.5 μg of the CTNNB1 plasmid (Unibio, China), while the control group received empty vectors. In miR-183 experiments, BMSCs were transfected with 50 nM of miR-183 mimics/inhibitors (GenePharma, China) along with negative control mimics/inhibitors. Transfections were carried out using Lipofectamine 3000 (Invitrogen, USA) according to the manufacturer's instructions. Following transfection, the growth medium was replaced, and cells were collected at 24 h for RNA analysis and 48 h for protein analysis. The following day, osteogenic induction medium was added for differentiation assays.
Cell proliferation assay
BMSCs were distributed in 96-well plates at a density of 2 × 103 cells per well23. Subsequently, after a period of 12–24 h, 10 μl of Cell Counting Kit-8 (CCK-8) reagent (Beyotime, C0038) was introduced into each well, and transfection was performed. The absorbance of CCK-8 at 450 nm was determined at 24-, 48-, and 72-h post-transfection utilizing a microplate reader to evaluate cellular proliferation.
Dual-luciferase assay
BMSCs were cotransfected with miR-183 mimics or miR-183 NC and wild-type or mutant CTNNB1 3′-UTR luciferase reporter plasmids (GenePharma, China) using Lipofectamine 3000. Forty-eight hours after transfection, cells were washed 1–2 times with PBS and lysed with passive lysis buffer (Beyotime, P0013). Cell lysates were centrifuged at 10,000 g for 5 min at 4 °C. Luciferase activity was measured using a dual-luciferase reporter assay system (GenePharma, China) according to the manufacturer's protocol.
Osteogenic differentiation of hBMSCs in vitro
To induce osteogenic differentiation, BMSCs were cultured in 6-well plates at a seeding density of 2 × 104 cells/cm2. Once the cells reached a confluence of 70–90%, the growth medium was substituted with osteogenic medium (OM) (QiDa Biotechnology, China, P1301), which consisted of 10 nM dexamethasone, 10 mM β-glycerophosphate, and 0.05 mM L-ascorbic acid-2-phosphate. The OM was refreshed every 48 h to maintain the differentiation process.
Alizarin red staining (ARS)
Following a 21-day period of osteogenic induction, the cells were subjected to fixation using 4% paraformaldehyde (Solarbio, China, P1110) for a duration of 30 min at ambient temperature. Subsequently, the cells were rinsed twice with PBS and stained with 1% alizarin red (pH 4.2) (Solarbio, China, G1452) for a duration of 30 min at ambient temperature.
ALP staining
Following a 7-day period of osteogenic induction, the cells in 6-well plates were subjected to fixation using a 4% paraformaldehyde solution for a duration of 30 min. Subsequently, the cells were washed thrice with ddH2O and subsequently stained utilizing the BCIP/NBT Alkaline Phosphatase Color Development Kit (Beyotime, C3250s) in accordance with the manufacturer's provided protocol.
Quantitative RT-PCR
The extraction of total RNA was conducted using TRIzol Reagent (Accurate Biology, China) in accordance with the manufacturer's instructions. Subsequently, cDNA synthesis was carried out using 1 μg of RNA in a 20 μl reaction. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed on a CFX Connect Real-Time System (Bio-Rad, China) using the SYBR Premix Ex Taq II Kit (Monad, China). The relative expression of mRNA was determined using the 2-ΔΔCt method with GAPDH as the reference gene.
Western blot analysis
The cells were lysed with RIPA lysis buffer (Beyotime, P0013), supplemented with protease inhibitor (Beyotime, P1045), and phosphatase inhibitors (Beyotime, P0044, P1081), after being washed twice with cold PBS. The cell lysates were then centrifuged at 12,000 rpm for 15 min at 4 °C, and the supernatants containing total cellular proteins were collected. Protein concentrations were determined using the BCA protein assay kit (Beyotime, P0012). Equal amounts of protein samples were mixed with 5X SDS loading buffer (Beyotime, P0015) and denatured by boiling at 100 °C for 5 min. The denatured proteins were separated by SDS-PAGE using 10% Tris–glycine gels at 120 V for 2 h in a Mini-PROTEAN electrophoresis system. The separated proteins were then electrotransferred to PVDF membranes at 100 V for 1 h using a Mini Trans-Blot cell. The membranes were blocked in 5% non-fat milk prepared in TBST for 2 h at room temperature followed by incubation with primary antibodies against Actin (Proteintech, 23660-1-AP, China), Oct4 (Proteintech, 11263-1-AP, China), Nanog (Proteintech, 14295-1-AP, China), Sox2 (Proteintech, 11064-1-AP, China), and CTNNB1 (Affinity, 8480S, China) overnight at 4 °C. The membranes were then probed with HRP-conjugated secondary antibody (Beyotime, A0208, China) for 2 h at 37 °C after being washed with TBST. Protein bands were visualized using ECL reagents (Beyotime, P0018AS) and analyzed by ImageJ software (NIH, USA).
Statistical analysis
The experiments were conducted in triplicate, and the data were presented as the mean ± standard deviation (SD). Statistical analyses were performed using GraphPad Prism 9.0 (USA). Student's t-test was used for comparing two groups, while one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test was employed for comparing multiple groups. In cases involving two variables, two-way ANOVA followed by Sidak's test was utilized. Nonparametric tests were applied when the data did not meet the assumptions. A significance level of P < 0.05 was considered statistically significant. The specific statistical tests used for each analysis were indicated in the figure legends.
Result
Single-cell analysis reveals dual regulatory roles of β-catenin in maintaining BMSCs stemness and promoting osteogenic differentiation
Single-cell RNA sequencing revealed the heterogeneity of cell populations, as shown by unsupervised clustering analysis (Fig. 1A). The annotation of clustered subpopulations enabled the identification of various cell types (Fig. 1C). Differential gene expression analysis highlighted distinct molecular signatures of subpopulations 0 and 10 (Fig. 1B,D), characterized by elevated expression of NGFR, LEPR, THY1, NT5E, and ENG, along with a lack of PTPRC expression. These gene expression patterns substantiate the annotation of subpopulations 0 and 10 as BM-MSC, providing a foundation for further functional explorations. To further verify the impact of BM-MSC heterogeneity on stemness, subclustering of BM-MSC revealed seven distinct clusters (Fig. 2A). Annotation based on literature-reported marker genes validated the reliability of clustering (Fig. 2B). Differential expression analysis characterized distinct gene signatures, enabling the annotation of clusters 1 and 3 as the 'Stemness Cluster' (high CYP1B1, DPP4, LEPR, ITGB1, CXCL12), clusters 2 and 4 as the 'Transitional Cluster' (high MALAT1, STAT3, PDGFRB, S100A8), cluster 0 as the 'Early Osteogenic Cluster' (high COL1A1, ALPL, FOX1, COL11A1, COL6A1), and cluster 5 as the 'Terminal Osteogenic Cluster' (high Runx2, SP7, IBSP, MBP2K, SPTB2, BGLAP) (Fig. 2C–G). To validate the regulatory role of WNT/β-catenin signaling pathway-related genes on BMSCs stemness, this study analyzed the expression patterns of six core WNT/β-catenin genes-BAMBI, LRP8, LRP5, FZD8, FZD5, and CTNNB1—across different BMSCs clusters (Fig. 3A,B). The results showed that β-CATENIN was highly expressed in both the Stemness Cluster and Terminal Osteogenic Cluster, while the other five genes were only upregulated in the Terminal Osteogenic Cluster. Inter-cluster differential analysis corroborated these results (Fig. 3C). Pseudotime trajectory analysis revealed the differentiation trajectories of BMSCs clusters (Fig. 3D). Taken together, these results demonstrate the dual regulatory roles of β-catenin in both BMSCs stemness maintenance and osteogenic differentiation.
Single-cell RNA sequencing unveils BMSCs heterogeneity and distinct molecular signatures (A) unsupervised clustering analysis reveals distinct BMSCs clusters. Each dot represents a single cell; colors indicate different clusters. (B, D) clusters 0 and 10 exhibit elevated expression of NGFR, LEPR, THY1, NT5E, and ENG but lack PTPRC expression. (C) Annotation of clustered subpopulations allows identification of various cell types.
Reclustering and cell annotation of BM-MSC clusters (A, B) subclustering and cell annotation of BM-MSC. (C–G) Differential expression analysis characterized different gene features and could annotate clusters 1 and 3 as “stemness clusters”, clusters 2 and 4 as “transitional clusters”, and cluster 0 as “early osteogenesis cluster”. Cluster 5 is annotated as “terminal osteogenic cluster”.
Expression patterns of WNT/β-catenin pathway genes and pseudo-time trajectory analysis reveal the dual regulatory roles of β-catenin in BM-MSC stemness and osteogenic differentiation (A, B) analysis of the expression patterns of six core WNT/β-catenin genes (BAMBI, LRP8, LRP5, FZD8, FZD5, and CTNNB1) across different BM-MSC clusters. β-catenin (CTNNB1) exhibits high expression in both the stemness clusters and terminal osteogenic cluster, while the other five genes are upregulated only in the terminal osteogenic cluster. (C) Differential expression analysis between BM-MSC clusters corroborates the distinct expression patterns of the WNT/β-catenin pathway genes. (D) Pseudo-time trajectory analysis reveals differentiation trajectories of BM-MSC clusters.
β-catenin contributes to maintaining BMSCs stemness
A key objective of this study was to validate the regulatory influence of β-catenin on BMSCs stemness using overexpression and knock-out experiments. Western blot analysis revealed increased protein levels of the stemness-associated transcription factors Oct4, Nanog, and Sox2 upon CTNNB1 overexpression, while CTNNB1 knock-out led to decreased expression (Fig. 4A–B). We further quantitated these expression changes at the mRNA level using qPCR. Consistent with the protein results, CTNNB1 overexpression increased, while its knock-out decreased, the mRNA levels of Oct4, Nanog, and Sox2 (Fig. 4C–D). In addition to regulating stemness markers, our study revealed that manipulating CTNNB1 levels had an impact on the proliferative capacity of BMSCs. Specifically, our CCK8 cell proliferation assays demonstrated increased proliferation at 24, 48, and 72 h following CTNNB1 overexpression, and decreased proliferation after CTNNB1 knock-out (Fig. 4E). Furthermore, upregulating CTNNB1 resulted in elevated alkaline phosphatase activity and matrix mineralization, as evidenced by increased alkaline phosphatase and alizarin red staining, while CTNNB1 knock-out attenuated both osteogenic phenotypes (Fig. 4F–G).
Regulation of β-catenin on BMSCs stemness (A–B) Western blot analysis showed that protein levels of stemness-related transcription factors Oct4, Nanog, and Sox2 were increased upon CTNNB1 overexpression, whereas CTNNB1 knockdown resulted in decreased expression. (C–D) qPCR reveals CTNNB1 overexpression significantly increases Oct4, Nanog, and Sox2 mRNA levels, whereas knockdown decreases their expression. Mean ± SEM (n = 3). **P < 0.01, ***P < 0.001, ****P < 0.0001. (E) CCK8 assay demonstrates CTNNB1 overexpression enhances BM-MSC proliferation at 48, and 72 h, while knockdown reduces proliferation. Mean ± SEM (n = 3). *P < 0.05, **P < 0.01. (F–G) ALP staining and quantification show CTNNB1 overexpression increases ALP activity, while knockdown attenuates it. Alizarin red staining and quantification reveal CTNNB1 overexpression enhances matrix mineralization, while knockdown reduces it. Mean ± SEM (n = 3). ****P < 0.0001.
miRNA sequencing analysis reveals regulatory relationship between CTNNB1 and miR-183
During this phase of our research, our objective was to identify miRNAs that play a role in regulating the stemness of BMSCs and also serve as upstream regulators of β-catenin. Through a differential expression analysis, we identified 109 distinct miRNAs (Fig. 5A) that have the potential to influence BMSC stemness. To further investigate which of these miRNAs could be potential upstream regulators of β-catenin, we conducted an intersection analysis between the differentially expressed miRNAs and those predicted by three online prediction platforms. This integrative analysis pinpointed miR-183 as a promising candidate for regulating β-catenin and as a key player in maintaining BMSC stemness (Fig. 5C). Further investigation revealed specific binding sites of miR-183 on the 3′UTR of β-catenin (Fig. 5B), providing a molecular basis for their interaction. Critically, the dual-luciferase reporter assay experimentally validated the direct binding relationship between miR-183 and the β-catenin 3’UTR (Fig. 5D). Together, these results identify miR-183 as a novel upstream regulator of β-catenin expression in BMSCs through direct 3’UTR binding. This finding shed new light on the post-transcriptional mechanisms governing β-catenin-mediated control of BMSCs stemness and lineage commitment.
Identification of miR-183 as an upstream regulator of CTNNB1 (A) Differential expression analysis revealed 109 distinct miRNAs. (B) Specific binding site for miR-183 on β-catenin 3′UTR. (C) Venn diagram identifies miR-183 as a strong candidate upstream regulator of β-catenin. (D) Dual-luciferase reporter assay validates the direct binding relationship between miR-183 and the β-catenin 3′UTR. Co-transfection of miR-183 mimics with wild-type (WT) β-catenin 3′UTR significantly reduces luciferase activity compared to co-transfection with mutant (MUT) 3′UTR or miR-183 NC. Mean ± SEM (n = 3). ****P < 0.0001.
miR-183 reduces BMSCs stemness by targeting and suppressing CTNNB1 expression
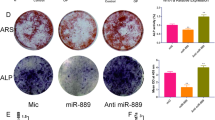
We conducted miR-183 overexpression and knock-out experiments to validate its regulatory role on BMSCs stemness. Western blot analysis showed that miR-183 overexpression decreased protein levels of β-catenin and the stemness factors Oct4, Nanog, and Sox2, while its knock-out increased these levels (Fig. 6A). qPCR experiments further demonstrated reduced mRNA levels of β-catenin, Oct4, Nanog, and Sox2 upon miR-183 overexpression, and conversely, increased levels following miR-183 knock-out (Fig. 6C). Functionally, CCK8 proliferation assays revealed impaired proliferation at 24, 48, and 72 h after miR-183 overexpression, while miR-183 knock-out enhanced proliferation (Fig. 6B). Moreover, upregulating miR-183 reduced alkaline phosphatase activity and matrix mineralization, as evidenced by decreased alkaline phosphatase and alizarin red staining, whereas miR-183 knock-out elevated both osteogenic phenotypes (Fig. 6D–E).
Regulation of stemness by miR-183 (A) Western blot analysis reveals the impact of miR-183 overexpression and knockdown on the protein levels of β-catenin and stemness factors Oct4, Nanog, and Sox2. miR-183 overexpression decreases the expression of these proteins, while miR-183 knockdown increases their levels. β-actin serves as a loading control. (B) CCK8 proliferation assay demonstrates the effect of miR-183 overexpression and knockdown on BM-MSC proliferation. miR-183 overexpression significantly impairs proliferation at 24, 48, and 72 h, whereas miR-183 knockdown enhances proliferation at these time points. Mean ± SEM (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001. (C) qPCR analysis shows the effect of miR-183 overexpression and knockdown on the mRNA levels of β-catenin, Oct4, Nanog, and Sox2. miR-183 overexpression significantly reduces the mRNA levels of these genes, while miR-183 knockdown significantly increases their expression. Mean ± SEM (n = 3). ***P < 0.001, ****P < 0.0001. (D–E) ALP staining and quantification demonstrate the impact of miR-183 overexpression and knockdown on the osteogenic differentiation potential of BM-MSCs. miR-183 overexpression significantly reduces ALP activity, whereas miR-183 knockdown significantly elevates ALP activity. Alizarin red staining and quantification show that miR-183 overexpression decreases matrix mineralization, while miR-183 knockdown enhances mineralization. Mean ± SEM (n = 3). **P < 0.01, ****P < 0.0001.
To further demonstrate that miR-183 regulates BMSCs stemness through β-catenin, we performed rescue experiments by modulating both miR-183 and β-catenin. Western blot analysis showed that knockdown of CTNNB1 effectively inhibited the stemness-promoting effect of miR-183 inhibition (Fig. 7A). This suggests that β-catenin is required for miR-183 inhibition to maintain stemness. Conversely, overexpression of CTNNB1 could rescue the stemness-suppressing effect of miR-183 mimics (Fig. 7B). Together, these rescue experiments provide compelling evidence that miR-183 specifically controls BMSCs stemness through regulating β-catenin levels.
Function recovery and loss (A) Western blot analysis indicates that knockdown of CTNNB1 inhibits the stemness-promoting effect associated with miR-183 inhibition. This is evidenced by decreased expression levels of Oct4, Nanog, and Sox2 in the miR-183 inhibitor + siCTNNB1 group compared to the miR-183 inhibitor + siControl group. The miR-183 NC + siControl group serves as a negative control. Mean ± SEM (n = 3). *P < 0.05, **P < 0.01. (B) Western blot analysis reveals that CTNNB1 overexpression rescues the stemness-suppressing effect of miR-183 mimics, as demonstrated by increased Oct4, Nanog, and Sox2 levels in the miR-183 mimics + pcDNA3.1-CTNNB1 group compared to the miR-183 mimics + vector group. The miR-183 NC + Vector group serves as a negative control. Mean ± SEM (n = 3). *P < 0.05, **P < 0.01.
Discussion
This research emphasizes the significant contributions of miR-183 and β-catenin in the regulation of stemness of BMSCs, a key aspect in comprehending the development of age-related osteoporosis. Through the application of single-cell RNA sequencing, we have illustrated a high expression level of β-catenin in both stem and terminal osteogenic clusters of BMSCs. This discovery provides a foundation for future investigations into the mechanisms by which β-catenin supports the maintenance of stemness of BMSCs. The findings from in vitro experiments provide evidence that the modulation of β-catenin expression plays a crucial role in regulating the stemness and osteogenic capabilities of BMSCs. Furthermore, the integration of miRNA sequencing analyses and data from online databases has identified miR-183 as a potential regulator of BMSCs stemness and a novel upstream modulator of β-catenin. Subsequent in vitro experiments have demonstrated that the upregulation of miR-183 significantly reduces the stemness of BMSCs through the downregulation of β-catenin, while inhibition of miR-183 enhances the stemness of BMSCs.
β-Catenin serves as a pivotal mediator within the intracellular Wnt signaling pathway, establishing connections with cytoskeletal proteins and operating as a transcriptional regulator within the nucleus24. Following activation of the Wnt pathway, β-catenin translocates into the nucleus and interacts with the TCF/LEF family of transcription factors, thereby initiating the transcriptional activation of downstream target genes25. Substantial investigation has substantiated the crucial involvement of the Wnt/β-catenin signaling pathway in the regulation of skeletal development and the differentiation of osteoblasts26. The activation of β-catenin's nuclear entry and its collaboration with transcription factors, such as Runx2, following stimulation by Wnt ligands, leads to the upregulation of bone-specific genes, including OCN and OPN, thereby facilitating the process of osteogenic differentiation27. While the majority of studies affirm the involvement of the Wnt/β-catenin pathway in facilitating the transformation of BMSCs into osteoblasts, our analysis utilizing single-cell RNA sequencing presents a contrasting outcome. Unlike the other five fundamental genes associated with the Wnt signaling pathway, β-catenin exhibits significant expression not only in the cluster of fully differentiated osteoblasts but also in the cluster of BMSCs possessing heightened stemness. This discovery implies that β-catenin may have a dual function, both in preserving the stemness of BMSCs and in promoting their osteogenic differentiation. In order to provide additional support for this hypothesis, we performed experiments involving the manipulation of β-catenin expression through overexpression and knockdown techniques. The findings of these experiments indicate that the augmentation of β-catenin expression leads to an upregulation of stemness transcription factors, including Oct4, Nanog, and Sox2, consequently enhancing the stemness properties. Furthermore, the results obtained from CCK8 assays corroborate the notion that the overexpression of β-catenin amplifies the proliferative capacity of BMSCs, aligning with the established role of β-catenin in other types of stem cells. According to a recent study utilizing single-cell transcriptomics, it was observed that the activation of the Wnt/β-catenin pathway can lead to the retention of progenitor characteristics in a subset of adipose mesenchymal stem cells. This retention is specifically characterized by the overexpression of β-catenin, which is consistent with our own research findings28. Furthermore, the maintenance of stem cell stemness is facilitated by β-catenin through the activation of a network of transcription factors associated with stemness, such as Oct4, Sox2, and Nanog. Research conducted by Chatterjee et al. indicates that β-catenin plays a crucial role in governing the self-renewal and preservation of stemness in mouse embryonic stem cells. Inhibition of β-catenin has been found to impede both proliferation and differentiation processes29.
The precise mechanism by which β-catenin sustains the stemness of BMSCs remains elusive; however, previous investigations have yielded valuable insights. For instance, Suman et al. demonstrated that exposure to fluid shear stress can induce nuclear translocation of β-catenin, leading to the activation of the downstream gene c-Myc. This, in turn, augments the expression of stemness-associated genes such as Oct4, Sox2, and Nanog, thereby conferring "mechanical pluripotency" upon embryonic stem cells in mice. These findings suggest that β-catenin may uphold the stemness of BMSCs through the mediation of mechanical signals30. Furthermore, Hoffmeyer et al. conducted a study that revealed the positive regulation of the telomerase Tert and the maintenance of telomere length in mouse ES cells through the Wnt/β-catenin pathway and the transcription factor Klf4. This suggests that β-catenin may play a role in maintaining the stemness of BMSCs by controlling the expression of telomerase and the length of telomeres31. Moreover, Chiara et al. employed LiCl treatment as a means to induce the nuclear translocation of β-catenin, resulting in a transient upregulation of Sox2 and Oct4 expression32. In a separate investigation, it was discovered that Forkhead box D1 has the ability to directly bind to β-catenin, facilitating its entry into the nucleus. This process subsequently enhances the stemness and chemotherapy resistance of colon cancer cells, underscoring the significance of β-catenin nuclear translocation in the preservation of BMSCs stemness33. Furthermore, a study conducted by Chen et al. revealed that the nuclear accumulation of β-catenin plays a crucial role in sustaining the stemness of liver tumor stem cells, which is closely associated with deacetylation mechanisms. These findings suggest that β-catenin potentially preserves the stemness of BMSCs through its involvement in mediating deacetylation processes34. Our research team, along with other researchers, has discovered that β-catenin plays a crucial role in both maintaining stemness and facilitating stem cell proliferation. Lin et al. conducted a study demonstrating that the overexpression of β-catenin promotes the proliferation of Pulmonary vascular endothelial cells, partially through the activation of downstream proliferative genes, including c-myc and cyclin D1. This finding aligns with our own observation that the overexpression of β-catenin enhances the proliferation of BMSCs35. In conclusion, our proposition posits that β-catenin potentially exerts an initial influence on the stemness and proliferative potential of BMSCs, subsequently facilitating their differentiation into osteoblasts. This hypothesis is further substantiated by the findings of single-cell pseudo-time analysis, which reveal distinct developmental trajectories within various BMSCs clusters and provide evidence for the dual regulatory role of β-catenin in preserving BMSCs stemness while concurrently fostering osteogenic differentiation. Through single-cell transcriptomics and functional validation experiments, we have demonstrated that β-catenin not only promotes BMSCs osteogenic differentiation but is also a key regulator in maintaining their stemness, providing a novel perspective for the treatment of age-related osteoporosis.
The modulation of osteoblasts and osteoclasts by miRNAs, particularly through the influence on the stemness of BMSCs, suggests their potential as therapeutic targets for age-related osteoporosis. A study conducted by Xu and colleagues observed a notable elevation in mir-31a-5p levels in BMSCs of aged rats, concomitant with a reduction in stemness36. This modification impacts the differentiation of osteoblasts and osteoclasts, serving as a crucial factor in the regulation of the bone marrow microenvironment linked to the aging process. Suppression of miR-31a-5p has the potential to mitigate bone loss and decrease osteoclast function in elderly rats. Furthermore, their other research has highlighted the traditional and non-traditional functions of miR-143/145 in governing the stemness of BMSCs, unveiling their dual influence on both bone formation and resorption37. miR-183 has been found to exert its inhibitory effects on the expression of CTNNB1, thereby reducing the stemness of BMSCs. This discovery suggests that miR-183 could potentially serve as a therapeutic target for the treatment of age-related osteoporosis. Our examination of miRNA sequencing data revealed 109 differentially expressed miRNAs that have the potential to modulate the stemness of BMSCs. Upon cross-referencing these findings with predictions from online databases, we identified miR-183 as a potential regulator of β-catenin. The validity of this regulatory relationship was confirmed through dual-luciferase reporter assays, which demonstrated that miR-183 directly binds to the 3'UTR of CTNNB1, leading to the inhibition of its expression. In order to provide additional evidence for the regulatory relationship, experiments involving the overexpression and knockdown of miR-183 were conducted. The findings indicated that the overexpression of miR-183 resulted in the inhibition of β-catenin expression and downstream stemness factors, as well as the suppression of both proliferation and osteogenic differentiation in BMSCs. Conversely, the knockdown of miR-183 yielded contrasting effects. These observations are consistent with the functional alterations observed in β-catenin, suggesting that miR-183 may regulate BMSCs by targeting β-catenin. This regulatory mechanism was further confirmed through dual intervention experiments.
miR-183 is a member of the miR-183 family miRNA cluster, which consists of miR-96 and miR-182 as well. These three miRNA genes are closely situated within the same cluster region and exhibit a high degree of evolutionary conservation16,38. Although there are minor variations in their seed sequences, the members of the miR-183 family collectively contribute to the regulation of diverse biological processes, including cell proliferation, differentiation, and the maintenance of stemness38. Prior research has confirmed the ability of miR-183 to selectively target and regulate the expression of β-catenin. For instance, Ling et al. observed that miR-183 effectively impedes the mRNA levels and nuclear translocation of β-catenin, consequently suppressing the proliferation of vascular smooth muscle cells39. Previous investigations have consistently demonstrated that the miR-183 family predominantly functions in inhibiting tumor stemness, exhibiting reduced expression in diverse tumor types40. Elucidating the underlying mechanisms, it has been discovered that miR-183 exerts its inhibitory effects on tumor progression by targeting genes associated with tumorigenesis. For example, in the context of gastric cancer, miR-183 exhibits the ability to target ITGB1, thereby impeding tumor invasion and metastasis41. Similarly, in nasopharyngeal carcinoma, miR-183 functions by suppressing the NOTCH signaling pathway, consequently attenuating tumor stemness42. Furthermore, Wellner et al. have elucidated that the miR-183 family possesses the capacity to suppress the expression of stemness transcription factors in both neoplastic conditions and embryonic stem cells. Moreover, ZEB1 demonstrates the capacity to establish a connection between epithelial-mesenchymal transition (EMT) and the preservation of stemness by impeding the activity of stemness-inhibitory miRNAs, including miR-18343. Consequently, miR-183 may exhibit its inhibitory effects on tumor stemness through various pathways in a synergistic manner. However, the involvement of miR-183 in the maintenance of stemness in BMSCs remains unexplored. Focusing on miR-183 as a therapeutic target shows promise for addressing age-related osteoporosis. The creation of small molecule inhibitors that modulate miR-183 levels may increase β-catenin activity, ultimately boosting the stemness of BMSCs. This enhancement is expected to stimulate bone formation and potentially counteract the bone loss linked to osteoporosis.
Despite the significant findings of our study, there are several limitations that need to be acknowledged. Firstly, our research primarily focuses on the in vitro analysis of BMSCs, which may not fully replicate the complex in vivo environment where multiple cellular interactions and signaling pathways coexist. Therefore, further in vivo studies are required to validate our findings and to understand the broader implications of miR-183 and β-catenin interactions in age-related osteoporosis. Secondly, while our research suggests a novel regulatory mechanism of BMSCs stemness by miR-183 and β-catenin, the complete signaling cascade and interaction with other pathways remain to be elucidated. Future research should aim to explore the in vivo effects of miR-183 and β-catenin modulation on bone health, particularly in age-related osteoporosis models. This will help in understanding the potential therapeutic applications of targeting these molecules.
Conclusion
This study validates the role of β-catenin in promoting stemness of BMSCs and identifies miR-183 as a regulatory factor that suppresses this stemness by targeting β-catenin. These results underscore the significance of miR-183 and β-catenin in preserving stemness of BMSCs, offering novel perspectives on the development of age-related osteoporosis. The clinical implications of targeting miR-183 and β-catenin as potential therapeutic interventions for age-related osteoporosis are significant. By addressing the underlying cellular abnormalities, these targets have the potential to enhance bone microarchitecture and facilitate bone tissue regeneration, presenting innovative approaches to managing this condition.
Data availability
Data is provided within the manuscript or supplementary information files.
References
Rachner, T. D., Khosla, S. & Hofbauer, L. C. Osteoporosis: Now and the future. Lancet (London, England) 377, 1276–1287. https://doi.org/10.1016/s0140-6736(10)62349-5 (2011).
Ikebuchi, Y. et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature 561, 195–200. https://doi.org/10.1038/s41586-018-0482-7 (2018).
Brown, C. Osteoporosis: Staying strong. Nature 550, S15-s17. https://doi.org/10.1038/550S15a (2017).
Uccelli, A., Moretta, L. & Pistoia, V. Mesenchymal stem cells in health and disease. Nature reviews. Immunology 8, 726–736. https://doi.org/10.1038/nri2395 (2008).
Ma, Y. et al. Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging cell https://doi.org/10.1111/acel.12709 (2018).
Yi, L. et al. Intraperitoneal injection of Desferal® alleviated the age-related bone loss and senescence of bone marrow stromal cells in rats. Stem Cell Res Ther 12, 45. https://doi.org/10.1186/s13287-020-02112-9 (2021).
Khosla, S. & Hofbauer, L. C. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol 5, 898–907. https://doi.org/10.1016/s2213-8587(17)30188-2 (2017).
Gennari, L. et al. Appropriate models for novel osteoporosis drug discovery and future perspectives. Expert Opin Drug Discov 10, 1201–1216. https://doi.org/10.1517/17460441.2015.1080685 (2015).
Zhang, Y. et al. Activating Wnt/β-catenin signaling in osteocytes promotes osteogenic differentiation of bmscs through BMP-7. Int J Mol Sci https://doi.org/10.3390/ijms232416045 (2022).
Wang, Y. Q., Wang, N. X., Luo, Y., Yu, C. Y. & Xiao, J. H. Ganoderal A effectively induces osteogenic differentiation of human amniotic mesenchymal stem cells via cross-talk between Wnt/β-catenin and BMP/SMAD signaling pathways. Biomed. Pharmacother. 123, 109807. https://doi.org/10.1016/j.biopha.2019.109807 (2020).
Jeong, W. & Jho, E. H. Regulation of the low-density lipoprotein receptor-related protein lrp6 and its association with disease: Wnt/β-catenin signaling and beyond. Front. Cell Dev. Biol. 9, 714330. https://doi.org/10.3389/fcell.2021.714330 (2021).
Wei, X. et al. Bach1 regulates self-renewal and impedes mesendodermal differentiation of human embryonic stem cells. Sci. Adv. 5, eaau7887. https://doi.org/10.1126/sciadv.aau7887 (2019).
Cai, Y. et al. The Wnt/β-catenin signaling pathway inhibits osteoporosis by regulating the expression of TERT: an in vivo and in vitro study. Aging 15, 11471–11488. https://doi.org/10.18632/aging.205136 (2023).
Kotiyal, S. & Evason, K. J. Exploring the interplay of telomerase reverse transcriptase and β-catenin in hepatocellular carcinoma. Cancers https://doi.org/10.3390/cancers13164202 (2021).
Shi, D. L. & Grifone, R. RNA-binding proteins in the post-transcriptional control of skeletal muscle development, regeneration and disease. Front. Cell Dev. Biol. 9, 738978. https://doi.org/10.3389/fcell.2021.738978 (2021).
Ichiyama, K. & Dong, C. The role of miR-183 cluster in immunity. Cancer Lett 443, 108–114. https://doi.org/10.1016/j.canlet.2018.11.035 (2019).
Moradi, S. et al. Time-resolved small-RNA Sequencing identifies microRNAs critical for formation of embryonic stem cells from the inner cell mass of mouse embryos. Stem Cell Rev. Rep. 19, 2361–2377. https://doi.org/10.1007/s12015-023-10582-6 (2023).
Zhang, P. et al. Characterization of mesenchymal stem cells in human fetal bone marrow by single-cell transcriptomic and functional analysis. Signal Transduct. Target. Ther. 8, 126. https://doi.org/10.1038/s41392-023-01338-2 (2023).
Mo, M., Wang, S., Zhou, Y., Li, H. & Wu, Y. Mesenchymal stem cell subpopulations: Phenotype, property and therapeutic potential. Cell. Mol. Life Sci.: CMLS 73, 3311–3321. https://doi.org/10.1007/s00018-016-2229-7 (2016).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47. https://doi.org/10.1093/nar/gkv007 (2015).
Wang, Z. et al. Single-cell RNA sequencing deconvolutes the in vivo heterogeneity of human bone marrow-derived mesenchymal stem cells. Int. J. Biol. Sci. 17, 4192–4206. https://doi.org/10.7150/ijbs.61950 (2021).
Tikhonova, A. N. et al. The bone marrow microenvironment at single-cell resolution. Nature 569, 222–228. https://doi.org/10.1038/s41586-019-1104-8 (2019).
Hou, Y. et al. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581. https://doi.org/10.1038/s41582-019-0244-7 (2019).
Moparthi, L. & Koch, S. FOX transcription factors are common regulators of Wnt/β-catenin-dependent gene transcription. J. Biol. Chem. 299, 104667. https://doi.org/10.1016/j.jbc.2023.104667 (2023).
Danek, P. et al. β-Catenin-TCF/LEF signaling promotes steady-state and emergency granulopoiesis via G-CSF receptor upregulation. Blood 136, 2574–2587. https://doi.org/10.1182/blood.2019004664 (2020).
Bahlakeh, G., Rahbarghazi, R., Abedelahi, A., Sadigh-Eteghad, S. & Karimipour, M. Neurotrophic factor-secreting cells restored endogenous hippocampal neurogenesis through the Wnt/β-catenin signaling pathway in AD model mice. Stem Cell Res. Ther. 13, 343. https://doi.org/10.1186/s13287-022-03024-6 (2022).
Chen, X. J. et al. Polydatin promotes the osteogenic differentiation of human bone mesenchymal stem cells by activating the BMP2-Wnt/β-catenin signaling pathway. Biomed. Pharmacother. 112, 108746. https://doi.org/10.1016/j.biopha.2019.108746 (2019).
Yang Loureiro, Z. et al. Wnt signaling preserves progenitor cell multipotency during adipose tissue development. Nat. Metab. 5, 1014–1028. https://doi.org/10.1038/s42255-023-00813-y (2023).
Chatterjee, S. S. et al. Inhibition of β-catenin-TCF1 interaction delays differentiation of mouse embryonic stem cells. J. Cell Boil. 211, 39–51. https://doi.org/10.1083/jcb.201503017 (2015).
Nath, S. C. et al. Fluid shear stress promotes embryonic stem cell pluripotency via interplay between β-catenin and vinculin in bioreactor culture. Stem Cells (Dayton, Ohio) 39, 1166–1177. https://doi.org/10.1002/stem.3382 (2021).
Hoffmeyer, K. et al. Wnt/β-catenin signaling regulates telomerase in stem cells and cancer cells. Science (New York, N.Y.) 336, 1549–1554. https://doi.org/10.1126/science.1218370 (2012).
Verdelli, C. et al. The core stem genes SOX2, POU5F1/OCT4, and NANOG are expressed in human parathyroid tumors and modulated by MEN1, YAP1, and β-catenin pathways activation. Biomedicines https://doi.org/10.3390/biomedicines9060637 (2021).
Feng, W. Q. et al. FOXD1 promotes chemotherapy resistance by enhancing cell stemness in colorectal cancer through β-catenin nuclear localization. Oncol. Rep. https://doi.org/10.3892/or.2023.8571 (2023).
Chen, X. et al. Deacetylation of β-catenin by SIRT1 regulates self-renewal and oncogenesis of liver cancer stem cells. Cancer Lett. 463, 1–10. https://doi.org/10.1016/j.canlet.2019.07.021 (2019).
Lin, S. et al. Overexpression of HOXB4 promotes protection of bone marrow mesenchymal stem cells against lipopolysaccharide-induced acute lung injury partially through the activation of Wnt/β-catenin signaling. J. Inflamm. Res. 14, 3637–3649. https://doi.org/10.2147/jir.S319416 (2021).
Xu, R. et al. MicroRNA-31a-5p from aging BMSCs links bone formation and resorption in the aged bone marrow microenvironment. Aging Cell 17, e12794. https://doi.org/10.1111/acel.12794 (2018).
Xu, R. et al. Identification of the canonical and noncanonical role of miR-143/145 in estrogen-deficient bone loss. Theranostics 11, 5491–5510. https://doi.org/10.7150/thno.55041 (2021).
Dambal, S., Shah, M., Mihelich, B. & Nonn, L. The microRNA-183 cluster: the family that plays together stays together. Nucleic Acids Res. 43, 7173–7188. https://doi.org/10.1093/nar/gkv703 (2015).
Ling, C. et al. Phoenixin-14 regulates proliferation and apoptosis of vascular smooth muscle cells by modulation of KCNQ1OT1/miR-183-3p/CTNNB1 axis. Environ. Toxicol. Pharmacol. 86, 103655. https://doi.org/10.1016/j.etap.2021.103655 (2021).
Han, T. et al. Downregulation of MUC15 by miR-183-5p.1 promotes liver tumor-initiating cells properties and tumorigenesis via regulating c-MET/PI3K/AKT/SOX2 axis. Cell Death Dis. 13, 200. https://doi.org/10.1038/s41419-022-04652-9 (2022).
Cao, C. et al. Downregulated circular RNA hsa_circ_0000291 suppresses migration and proliferation of gastric cancer via targeting the miR-183/ITGB1 axis. Cancer Manag. Res. 11, 9675–9683. https://doi.org/10.2147/cmar.S213830 (2019).
Cheung, C. C. et al. MicroRNA-183 suppresses cancer stem-like cell properties in EBV-associated nasopharyngeal carcinoma. BMC cancer 16, 495. https://doi.org/10.1186/s12885-016-2525-5 (2016).
Wellner, U. et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 11, 1487–1495. https://doi.org/10.1038/ncb1998 (2009).
Funding
This work was supported by Dalian Medical Science Research Program Project (Grant No. 2111005), Guangzhou Science and Technology Plan Project (grant number 202102020033), Natural Science Foundation of Liaoning Province (grant number 2022-MS-443) and the Dalian University of Technology and Affiliated Central Hospital joint research fund (2022ZXYG45).
Author information
Authors and Affiliations
Contributions
Conceptualization, N.J., J.J., Q.W. Methodology, N.J., J.J., Q.W., H.W. Data curation, N.J., J.H., R.Y., Q.W. Data analysis, J.H., R.Y., X.T. Writing—Original Draft, N.J. Writing—Review & Editing, J.J., H.W. Funding acquisition, J.J., H.W. Project administration, J.J., H.W. Supervision, J.J., H.W. Investigation, N.J., J.J., Q.W., J.H., R.Y., X.T., H.W. Formal analysis, N.J., J.J., Q.W., J.H., R.Y., X.T., H.W. Visualization, N.J., Q.W. Resources, J.J., H.W. Intellectual input, J.J., H.W.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jiang, N., Jiang, J., Wang, Q. et al. Strategic targeting of miR-183 and β-catenin to enhance BMSC stemness in age-related osteoporosis therapy. Sci Rep 14, 21489 (2024). https://doi.org/10.1038/s41598-024-72474-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72474-0
- Springer Nature Limited