Abstract
Helicobacter pylori (H. pylori) is one of the most common bacterial infections in the world, and its key virulence component CagA is the leading cause of gastric cancer. Mitophagy is a form of selective autophagy that eliminates damaged mitochondria and is essential for some viruses and bacteria to evade the immune system. However, the mechanisms by which CagA mediates H. pylori-induced mitophagy and NLRP3 inflammasome activation remain elusive. In this study, we reported that H. pylori primarily uses its CagA to induce mitochondrial oxidative damage, mitochondrial dysfunction, dynamic imbalance, and to block autophagic flux. Inhibition of mitophagy led to an increase in NLRP3 inflammasome activation and apoptosis and a decrease in the viability of H. pylori-infected cells. Our findings suggested that H. pylori induces mitochondrial dysfunction and mitophagy primarily via CagA. It reduces NLRP3 inflammasome activation to evade host immune surveillance and increases the survival and viability of infected cells, potentially leading to gastric cancer initiation and development. Our findings provide new insights into the pathogenesis of H. pylori-induced gastric cancer, and inhibition of mitophagy may be one of the novel techniques for the prevention and treatment of this disease.
Similar content being viewed by others
Introduction
Helicobacter pylori (H. pylori), one of the most common causes of bacterial infections worldwide, is a Gram-negative bacterium particularly colonized in the human stomach. It is estimated that approximately half of the world's population is infected with this pathogen, and prevalence rates of up to 90% have been reported in some developing countries1. According to the 1994 World Health Organization (WHO) classification, H. pylori is a primary carcinogen of gastric cancer as its infection can cause chronic gastritis, peptic ulcer, gastric adenocarcinoma, and mucosa-associated lymphoid tissue lymphoma2,3. Currently, the mechanism of gastric pathological damage caused by H. pylori may be associated with immune response, implantation of H. pylori in the gastric wall, and virulence proteins of H. pylori; however, the exact causative mechanism remains elusive4,5.The CagA protein, a major virulence factor of H. pylori, is translocated into host cells by the T4SS following bacterial attachment and has been implicated in the development and progression of gastric cancer6. Although CagA status is not the only contributing factor to additional gastrointestinal impairments caused by H. pylori, CagA-positive strains are associated with more severe inflammation, higher levels of atrophy and a higher risk of progression to gastric adenocarcinoma when compared to CagA-negative strains7. Therefore, further research into the pathogenesis and carcinogenic mechanism of H. pylori and its CagA protein is warranted.
Our previous study found that H. pylori CagA can increase reactive oxygen species (ROS) production and activate the NLRP3 inflammasome, thereby, promoting the inflammatory factor interleukin-1β secretion and cell pyroptosis8. Excessive activation of the NLRP3 inflammasome has been implicated in the development of inflammatory diseases and cancer9. High levels of ROS have an oxidative function that can progressively oxidise and damage mitochondria, endoplasmic reticulum and other cellular organelles. In addition, mitochondrial damage can cause a dynamic imbalance of mitochondrial fusion/division, decrease mitochondrial membrane potential, increase the rate of apoptosis and induce mitophagy10,11. Mitophagy is a selective autophagy pathway that maintains mitochondrial quality control by targeting and destroying mitochondria. Studies have also reported that mitophagy protects against several inflammatory diseases associated with the NLRP3 inflammasome12. Mitophagy can eliminate endogenous NLRP3 inflammasome signals to regulate the NLRP3 inflammasome activation, prevent changes in mitochondrial membrane permeability, and maintain cell homeostasis in addition to eliminating damaged and dysfunctional mitochondria, which is crucial for inhibiting the release of pro-apoptotic proteins and restoring cell viability and destroying intracellular pathogens13. Consequently, mitophagy is widely considered to be a key regulatory mechanism in the process of mitochondrial quality control involved in the regulation of host immune responses. However, further research into the role of H. pylori in mitophagy and its effect on NLRP3 inflammasome activation would improve our understanding of H. pylori pathogenesis and facilitate the development of new strategies for the prevention and treatment of gastric cancer.
The effects of H. pylori and CagA on mitophagy, NLRP3 inflammasome activation, and their mutual regulation are currently unknown. The aim of this study was to determine how H. pylori and its CagA influence the induction of mitochondrial oxidative damage, mitochondrial dynamic imbalance, mitochondrial dysfunction and the relationship between these processes and NLRP3 inflammasome activation. We infected gastric epithelial cells (GES) and C57BL/6 mice with the H. pylori CagA-positive strain (GZ7/cagA) and CagA gene knockout strain (GZ7/ΔcagA), respectively, to study the mitochondrial damage and mitophagy induced by H. pylori and whether CagA is primarily involved in this process. To better understand the role of mitophagy in the NLRP3 inflammasome activation and the survival and viability of H. pylori-infected cells, we demonstrated that H. pylori primarily uses its CagA to increase the level of mitophagy, block the autophagic flux, and cause mitochondrial oxidative damage, mitochondrial dynamic imbalance, and mitochondrial dysfunction. Furthermore, mitophagy regulates the activation of NLRP3 inflammasome by H. pylori to evade host immune surveillance and enhance the survival and viability of infected cells. Our findings on the selective mitophagy and the NLRP3 inflammasome in H. pylori-infected cells provide a novel perspective on gastric cancer pathogenesis induced by H. pylori infection.
Materials and methods
Cultural conditions and cell lines
The Chinese Academy of Sciences Cell Bank provided human GES-1 cells (Shanghai, China). American Type Culture Collection provided the human gastric epithelial adenocarcinoma cell line (AGS) (ATCC, USA). All cell lines were cultured in Roswell Park Memorial Institute 1640 medium supplemented with 10% fetal bovine serum (Gibco, Rockville, USA) and 100 U/mL penicillin and streptomycin (Gibco, USA) at 37 °C in a 10% CO2 humidified atmosphere.
Helicobacter pylori culture and infection
Helicobacter pylori strain GZ7/cagA is a typical East Asian strain (cagA +) that was isolated from the gastric mucosa of a gastric cancer patient in our previous research14. GZ7/ΔcagA, CagA gene knockout from the GZ7/cagA was constructed and identified by Sangon Biotech (Shanghai, China). GZ7/cagA and GZ7/ΔcagA were cultured in microaerophilic conditions at 37 °C on a Columbia agar plate (Oxoid Ltd, Basingstoke, UK) containing 10% sheep blood (BD Diagnostics) and Dent antibiotics supplement (Oxoid). After growing GES-1 and AGS cells in six-well plates with antibiotic-free medium and infecting them with H. pylori (multiplication of infection (MOI), 60:1) for 6 h, the cell culture medium was replaced with complete medium, and the cell samples from each group were collected following 42 h for subsequent experiments. The uninfected groups served as a negative control.
Measurement of adenosine triphosphate (ATP)
Following the appropriate treatment, cells were collected and an ATP assay kit (Beyotime, China, S0026B) was used to determine the ATP levels. The luminescence intensity was measured using a luminometer (ELX800UV, Bio-tec, USA). The ATP levels in the samples were determined based on the established standard curve.
Mitochondrial membrane potential (MMP) determination
MMP levels in differentially treated cells were measured using the JC-1 kit (Beyotime, China, C2006). After collecting the cells, 1000 μL of JC-1 dye solution was added. The cells were stained for 20 min in the dark at 37 °C, and 10 μL of the stained cell mixture was further collected and analyzed using flow cytometry (FACS verse, BD Bioscience, USA).
Measurement of cell apoptosis
Cells were collected and stained using the Annexin V-APC/7-AAD apoptosis kit (MultiSciences Biotech Co, China) per the manufacturer's instructions after corresponding treatment, and quantification was performed using flow cytometry (FACS verse, BD Bioscience, USA).
Transmission electron microscopy (TEM)
After co-culturing GES-1 and AGS cells with GZ7/cagA and GZ7/ΔcagA for 48 h at an MOI of 60, respectively, cells were collected and immersed in specimen preservation solution and sent to the Servicebio company (Wuhan, China) for examination using electron microscopy and obtaining digital images.
Generation of a C57BL/6 model
Eighteen SPF male C57BL/6 mice, 4–5-week-old, weighing 18–22 g, were purchased from Zhejiang Provincial Laboratory Animal Center, Zhejiang, China (license number: SYXK 2018-0001). C57BL/6 mice were orally inoculated with the GZ7/cagA and GZ7/ΔcagA, respectively, thrice with 1 mL each (15 × 108/mL) at 48-h intervals. C57BL/6 mice orally inoculated with saline were used as a negative control and observed for 3 months. After a 24-h fasting and 12-h deprivation from water, the mice were euthanized in accordance with the 2020 Animal Euthanasia Guidelines of the American Veterinary Medical Association (AVMA) in a humane manner, and the gastric tissue was removed and divided into two parts, one of which was fixed in 4% formaldehyde and the other in liquid nitrogen. The data represented the average of five mice per group. All animal experimental protocols and procedures in this study were approved by the Animal Care Welfare Committee of Guizhou Medical University (Guiyang, China; approval No. 1900801). The study is reported in accordance with the ARRIVE guidelines (https://arriveguidelines.org).
Immunochemistry
Tissue sections from C57BL/6 mice were deparaffinized, rehydrated, and incubated for 15 min in 3% H2O2 and methanol. To perform antigenic repair, the slides were placed in 0.01 M sodium citrate buffer (pH 6.0) and incubated at 95 °C for 20 min. After cooling in 3% H2O2 for 10 min, sections were rinsed with phosphate-buffered saline (PBS) for 10 min and then incubated with the primary antibody overnight at 4 °C. Sections were further rinsed thrice with PBS at 10-min intervals before being incubated for 1 h at room temperature with the secondary antibody labeled with horseradish peroxidase (1:10,000; Proteintech Group, Inc.; Wuhan Sanying). After incubating with 3,3′-diaminobenzidine, immunostaining was performed, and the sections were counter-stained with hematoxylin. Using an optical microscope, the level of protein expression in each sample was assessed based on the number of stained cells and the depth of staining. The average integrated optical density of active areas in each group was calculated using Image-Pro Plus 6.0 software (Media Cybernetics, Inc.USA), and the average optical density was used for analysis. Primary antibodies were as follows: Anti-P62 (1:100, 18420-1-AP), anti-LC3 (1:100, 14600-1-AP), anti-phosphatase and tensin homolog-induced kinase 1 (PINK1) (1:200, 23274-1-AP), anti-PARKIN (1:200, 14060-1-AP) were obtained from Proteintech, USA; Anti-H. pylori (1:100, ab7788) were obtained from Abcam, USA.
Immunofluorescence demonstrates LC3 and mitochondrial co-localization
We co-cultured GES-1 and AGS cells with GZ7/cagA and GZ7/ΔcagA for 48 h at an MOI of 60, respectively. The cells in the wells were fixed with 4% paraformaldehyde for 15 min at 4 °C before being permeabilized for 15 min with 0.5% Triton X-100 in PBS at room temperature. After blocking the cells for 2 h with 1% bovine serum albumin, they were incubated overnight at 4 °C with LC3 primary antibodies (1:200, 14600-1-AP, Proteintech, USA) and washed thrice with PBS. Afterward, the fluorescein-isothiocyanate-labeled secondary antibodies (1:300, F-2765, Proteintech, USA) were incubated at room temperature for 2 h before being washed with PBS. The mitochondria were then labeled with Mito Tracker Green (100 nmol/L) for 30 min at room temperature before being rinsed with PBS. Finally, DAPI was used to counterstain the nuclear staining. The stained slides were photographed and analyzed using a confocal microscope (Carl Zeiss LSM 880) and Image J software (version 1.41; National Institutes of Health, Bethesda, MD, USA).
Observation of the autophagic flux
The AGS and GES-1 cells were transfected with a monomeric red fluorescent protein (mRFP)-green fluorescent protein (GFP)-LC3 lentivirus (Hanbio Co. LTD, HB-LP2100001) for 48 h and further infected with H. pylori (MOI, 60:1) for an additional 24 h. Afterward, cells were imaged under a confocal microscope to observe autophagosomes and autolysosomes. mRFP is used to identify and track LC3. Because GFP fluorescent protein is sensitive to acidity, GFP fluorescence decreases during autophagosome-lysosome fusion, leaving only red fluorescence detectable at this stage. As a result, GFP attenuation serves as a signal of lysosome-autophagosome fusion, resulting in the creation of autophagolysosomes. After microscopic imaging, the red and green fluorescence signals are merged, with yellow spots indicating autophagosomes and red spots indicating autophagolysosomes. The intensity of the autophagy flux can be effectively assessed by quantifying these distinct coloured spots. Red and yellow puncta were carefully counted and noted. At least 10 cells were counted to determine the number of yellow and red puncta.
Western blotting analyses
Cells were harvested and lysed in radioimmunoprecipitation assay lysis buffer containing fresh protease and phosphate inhibitor mixture and further boiled for denaturation to determine protein concentrations using the Bicinchoninic Acidic Assay Protein Assay Kit (Beyotime, China). Sodium dodecyl sulfate–polyacrylamide gel electrophoresis was used to separate the cell lysate, which was then transferred to polyvinylidene difluoride membranes (Millipore Corporation, MA, USA). Membranes were blocked for 1 h with 5% non-fat milk before being incubated overnight at 4 °C with primary antibodies. Primary antibodies were as follows: Anti-P62 (1:800, 18420-1-AP), anti-LC3 (1:800, 14600-1-AP), anti-phosphatase and tensin homolog-induced kinase 1 (PINK1) (1:1000, 23274-1-AP), anti-PARKIN (1:1000, 14060-1-AP), anti-mitofusin 1 (MFN1) (1:1000, 13798-1-AP), anti-Mfn2 (1:1000, 12186-1-AP), anti-dynamin-related protein 1 (DRP1) (1:800, 12957-1-AP). Anti-mitochondrial fission 1 (FIS1) (1:1000, 10956-1-AP), anti-NLRP3 (1:1000, 19771-1-AP), anti-Caspase-1 (1:1000, 22915-1-AP), and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:5000, 10494-1-AP) were obtained from Proteintech, USA. Antibodies against CagA (1:500, sc-28368) were obtained from Santa Cruz Biotechnology. After being washed with Tris-buffered saline with 0.1% Tween-20 detergent thrice, the membranes were incubated for 1 h with peroxidase-conjugated secondary antibody (1:10,000 dilution, Santa Cruz, CA, USA). Bands were imaged using a chemiluminescence imager (Amersham Biosciences, USA) and ECL Plus chemiluminescence (Thermo Fisher Scientific, MA, USA). The western blot band intensities were quantified using ImageJ software, with GAPDH protein serving as a loading control.
Cell counting kit (CCK)-8 assay
The cell viability was determined using the CCK-8 assay (Beyotime, China, C0038). Cell suspensions were prepared and seeded into 96-well culture plates. The cells were pretreated with Bafilomycin A1 (BafA1) (10 M, MCE, HY-100558) for 24 h before being infected with GZ7/cagA and GZ7/ΔcagA (MOI, 1: 60) at four different time points (0, 6, 24, and 48 h). Further, 10 μL of CCK-8 reagent was added to each well and incubated in the dark for 2 h at 37 °C with 5% CO2. After shaking for 10 s, the optical density (O.D.) at 450 nm was measured with a microplate reader to determine the relative number of surviving cells (ELX800UV, Bio-tec, USA).The cell viability of the cells in the 6 h control group is calibrated as 100%, and the data of the other groups are relative values compared to the control.
Statistical analysis
The data from at least three independent experiments were presented as mean ± SD, and statistical analyses were performed using GraphPad Prizm 5.0. (GraphPad Software Inc., San Diego, CA, USA). The difference between two groups was analyzed using two-tailed Student t-test. One-way ANOVA plus Bonferroni post-hoc test were used for over more than two groups. P < 0.05 was considered significant. The P values are indicated in the figures with asterisks: **P < 0.01; *P < 0 0.05.
Results
Helicobacter pylori CagA induces mitochondrial dysfunction and apoptosis of infected cells
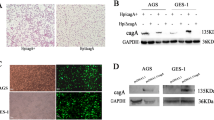
We have previously found that H. pylori CagA can increase ROS production and activate the NLRP3 inflammasome8. High levels of ROS can undergo oxidation and cause oxidative damage to cell mitochondria, leading to cell dysfunction and apoptosis15. To investigate on the effect of H. pylori and its CagA on mitochondrial function and cell apoptosis, we co-cultured GES-1 and AGS cells with GZ7/cagA and GZ7/ΔcagA, respectively, for 48 h at an MOI of 60 followed by detection of mitochondrial membrane potential, ATP production levels and cell apoptosis. GZ7/cagA and GZ7/ΔcagA strains were cultured and observed under a microscope following Gram staining and depicted a short rod-shaped structure and round blunt Gram-negative bacteria, respectively. Western blotting was performed to confirm CagA protein expression in GZ7/cagA infected cells, but no expression was observed in GZ7/ΔcagA infected cells (see Fig. 1A). The results showed that GZ7/cagA infection significantly decreased the mitochondrial membrane potential and ATP production levels and promoted cell apoptosis compared to the control cells and GZ7/ΔcagA-infected cells (see Fig. 1B,C,D). These data indicate that CagA mediates H. pylori to induce mitochondrial dysfunction and apoptosis of gastric epithelial cells.
H. pylori CagA induces mitochondrial dysfunction, apoptosis and mitochondrial dynamic imbalance of gastric epithelial cells. GES-1 and AGS cells infected with H. pylori for 48 h at an MOI of 60, respectively. (A) Morphological observation of GZ7/cagA and GZ7/Δ cagA after Gram staining, and western blotting was used to detect the expression of intracellular CagA protein.Scale bar: 200 μm. The membrane strips were cut before hybridization with antibodies, which resulted in the inability to provide the original full-length blot images. (B) Effect of H. pylori infection on cellular ATP production the ATP production levels were determined using an ATP assay kit. (C) Effect of H. pylori infection on Mitochondrial Membrane Potential, the Mitochondrial Membrane Potential were determined using the JC-1 kit, (D) Effect of H. pylori infection on cell apoptosis, the rates of cell apoptosis were measured by flow cytometry. The figure presents the average of three independent experiments. Data are presented as the means ± SD. Error bars represent the standard deviation. control, the uninfected cells; GZ7/ΔcagA, the GZ7/ΔcagA strains infected cells; GZ7/cagA, the GZ7/cagA strains infected cells; *p < 0.05, **p < 0.01; GZ7/cagA vs control, GZ7/cagA vs GZ7/ΔcagA.
Helicobacter pylori CagA induces a mitochondrial dynamic imbalance in infected cells
Mitochondria constantly divide and fuse to maintain a balance in mitochondrial dynamics. The ideal balance of mitochondrial dynamics is considered necessary for mitochondrial homeostasis, cell stability, and cell survival16. An imbalance in mitochondrial dynamics can lead to the accumulation of damaged mitochondria and mitophagy. Mitochondrial fusion can repair damaged mitochondria, whereas mitochondrial fission can activate mitophagy and regulate mitochondrial clearance17. Western blotting was performed to detect the expression of proteins associated with mitochondrial fusion and fission in gastric epithelial cells, and the results showed that GZ7/cagA-infected cells significantly decreased the expression of mitochondrial fusion-related proteins MFN1 and Mfn2 while significantly increased the expression of mitochondrial fission-related proteins DRP1 and FIS1 when compared to uninfected control cells and GZ7/ΔcagA-infected cells (refer to Fig. 2). Mitophagy is induced by the expression of factors associated with mitochondrial fission or the degradation of mitochondrial fusion. These factors regulate the process by interacting with LC3 junction proteins or LC3 receptors18. Consequently, our findings suggest that H. pylori infection may cause aberrant mitochondrial division and fusion, poor mitochondrial dynamics, and the initiation of mitophagy.
H. pylori CagA induces mitochondrial dynamic imbalance in gastric epithelial cells. GES-1 and AGS cells infected with H. pylori for 48 h at an MOI of 60, respectively. (A) The expression of proteins related to mitochondrial dynamics of H. pylori-infected AGS cells were detected by western blotting; (B) The expression of proteins related to mitochondrial dynamics of H. pylori-infected GES-1 cells were detected by western blotting. The membrane strips were cut before hybridization with antibodies, which resulted in the inability to provide the original full-length blot images.The figure presents the average of three independent experiments. Data are presented as the means ± SD. Error bars represent the standard deviation. control, the uninfected cells; GZ7/ΔcagA, the GZ7/ΔcagA strains infected cells; GZ7/cagA, the GZ7/cagA strains infected cells; *p < 0.05, **p < 0.01; GZ7/cagA vs control, GZ7/cagA vs GZ7/ΔcagA.
Helicobacter pylori CagA mediates mitophagy in infected cells and mice
Mitochondrial damage usually occurs in response to microbial infection or cellular stress, with subsequent removal of damaged mitochondria by mitophagy to maintain mitochondrial stability19. There is increasing evidence that H. pylori or culture filtrate of H. pylori induces autophagy in cells20; however, whether CagA triggers mitophagy in H. pylori-infected human GES remains elusive. In this study, we co-cultured GES-1 and AGS cells with GZ7/cagA and GZ7/ΔcagA, respectively, for 48 h at an MOI of 60. The results showed that GZ7/cagA infection significantly increased the expression of mitophagy-related proteins PINK1, Parkin, P62, LC3, VDAC1, and TOM20 in the cells compared to the uninfected control cells and GZ7/ΔcagA-infected cells (see Fig. 3A). Confocal imaging revealed that LC3 punctate aggregation, increased co-localisation with mitochondria and aggregated autophagy-related proteins in mitochondria occurred following H. pylori infection. These effects were more pronounced in GZ7/cagA infected cells than in GZ7/ΔcagA infected cells (Fig. 3B). Furthermore, TEM was performed to determine whether mitophagy occurred in GZ7/cagA and GZ7/ΔcagA infected cells. The results showed that there was an accumulation of autophagosomes and autolysosomes in the GZ7/cagA and GZ7/ΔcagA-infected cells, with a more significant increase in the GZ7/cagA-infected cells (see Fig. 3C). These data suggest that CagA mediates mitophagy in H. pylori-infected cells. To further investigate the effects of mitophagy in mice during H. pylori infection, GZ7/cagA and GZ7/ΔcagA were orally administered to C57BL/6 mice for 3 months. Gastric tissues were collected to detect the expression of mitophagy-related proteins to verify the effects of H. pylori and its CagA on mitophagy. Compared to uninfected control mice and GZ7/ΔcagA-infected mice, the expression of mitophagy-related proteins P62, LC3, PINK1, and Parkin was significantly increased in GZ7/cagA-infected mice (see Fig. 4). These data indicate that H. pylori CagA can induce mitophagy in gastric tissue of infected mice, which is consistent with the in vitro experiments.
H. pylori CagA induces mitophagy in infected gastric epithelial cells. GES-1 and AGS cells infected with H. pylori for 48 h at an MOI of 60, respectively. (A) The expression of mitophagy- related proteins PINK1, PARKIN, P62, LC3, VDAC1and TOM20 were detected by western blotting. The membrane strips were cut before hybridization with antibodies, which resulted in the inability to provide the original full-length blot images.; (B) The immunofluorescence signal accumulation of LC3 co-localization with mitochondria were observed by confocal microscopy, Scale bar: 10 μm; (C) The accumulation of autophagosomes and autolysosomes was performed by TEM, Scale bar: 5 μm and 1 μm. The figure presents the average of three independent experiments. Data are presented as the means ± SD. Error bars represent the standard deviation. control, the uninfected cells; GZ7/ΔcagA, the GZ7/ΔcagA strains infected cells; GZ7/cagA, the GZ7/cagA strains infected cells; *p < 0.05, **p < 0.01; GZ7/cagA vs control, GZ7/cagA vs GZ7/ΔcagA.
Mitophagy-related proteins expression were increased in gastric tissues of H. pylori infected mice. C57BL/6 mice were inoculated orally with the GZ7/cagA and GZ7/ΔcagA for 3 months, respectively. (A) H. pylori colonization status was detected by immunohistochemical staining; (B) Gastric tissues were collected and the expression of P62, LC3, PINK1 and PARKIN were detected by immunochemistry, Scale bar: 100 μm. The figure presents the average of three independent experiments. Data are presented as the means ± SD. Error bars represent the standard deviation. control, the uninfected cells; GZ7/ΔcagA, the GZ7/ΔcagA strains infected cells; GZ7/cagA, the GZ7/cagA strains infected cells; *p < 0.05, **p < 0.01; GZ7/cagA vs control, GZ7/cagA vs GZ7/ΔcagA.
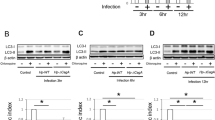
Autophagic flux blocked during H. pylori infection
The autophagosome, like the phagosome, culminates in fusion with the lysosome to form a degradative autolysosome, and the entire process of autophagy is called autophagic flux21. Autophagosome-lysosome fusion is required for autophagic flux, and inhibition of this process impairs autophagic degradation22. Therefore, we used mRFP-GFP-LC3 as a tandem reporter to investigate whether H. pylori infection affects autophagic flux. We transfected AGS and GES-1 cells with mRFP-GFP-LC3 for 24 h and further infected them with GZ7/cagA and GZ7/ΔcagA (MOI, 60:1), respectively, for another 48 h. To assess autophagic flux, confocal microscopy was employed to observe the co-localization of autophagosomes and lysosomes. As shown in Fig. 5, GZ7/cagA and GZ7/ΔcagA infection enhanced the formation of both green and red dots; co-localization caused an increase in the number of yellow puncta, whereas GZ7/cagA-infected cells showed a marked increase in yellow puncta. Our results showed that H. pylori infection increased autophagosome formation without lysosome fusion, indicating a barrier to autophagic flux. Blocked autophagic flux leads to the accumulation of dysfunctional mitochondria, which may contribute to H. pylori-induced tumorigenesis.
The autophagic flux was blocked due to H. pylori infection. GES-1 and AGS cells infected with H. pylori for 48 h at an MOI of 60, respectively.,then, the co-localization of autophagosomes and lysosomes were observed by confocal microscopy. (A) AGS cells infected with GZ7/cagA and GZ7/Δ cagA strains; (B) GES-1 cells infected with GZ7/cagA and GZ7/Δ cagA strains; Scale bar: 10 μm. The figure presents the average of three independent experiments. Data are presented as the means ± SD. Error bars represent the standard deviation. control, the uninfected cells; GZ7/ΔcagA, the GZ7/ΔcagA strains infected cells; GZ7/cagA, the GZ7/cagA strains infected cells; *p < 0.05, **p < 0.01; GZ7/cagA vs control, GZ7/cagA vs GZ7/ΔcagA.
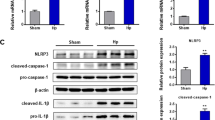
Inhibiting mitophagy resulted in increased NLRP3 inflammasome activation and apoptosis and decreased cell viability in H. pylori-infected cells
As shown in our previous study, H. pylori CagA can activate the NLRP3 inflammasome and promote the invasion and migration of gastric cancer cells8. Recent evidence suggests that mitophagy is associated with the immune response and can suppress NLRP3 inflammasome overactivation and subsequent immune response23. Disruption of mitophagy can lead to the progressive accumulation of damaged mitochondria, which activates apoptosis24. However, the role of H. pylori-induced mitophagy in NLRP3 inflammasome activation, apoptosis, and cell survival remains elusive. Therefore, we pre-treated AGS and GES-1 cells with mitophagy inhibitor (BafA1, 10 μM) for 24 h and further infected them with GZ7/cagA and GZ7/ΔcagA, respectively, at an MOI of 60. Western blotting, flow cytometry, and the CCK-8 assay were used to detect NLRP3 inflammasome-related protein expression, cell apoptosis, and cell viability in H. pylori-infected cells. The results showed that the expression of mitophagy-related proteins was decreased, whereas the expression of NLRP3 inflammasome-related proteins was increased in BafA1-treated cells (see Fig. 6A,B). Compared to H. pylori-infected cells alone, BafA1 pretreatment significantly increased the expression levels of NLRP3, caspase-1 and IL-18, suggesting that inhibition of mitophagy may cause overactivation of the NLRP3 inflammasome in H. pylori-infected cells (see Fig. 6C). It was observed that BafA1 pre-treatment significantly increased the apoptosis rate (see Fig. 7A) and decreased the viability of H. pylori-infected cells (see Fig. 7B) when compared to H. pylori-infected cells alone. Based on these data, GZ7/cagA-infected cells outperformed GZ7/ΔcagA-infected cells in terms of overt outcomes. Our results indicate that CagA mediates H. pylori to induce mitophagy, which attenuates NLRP3 inflammasome activation to evade host immune surveillance and improves the survival and viability of infected cells. Mitophagy is believed to play a critical role in the immune escape and survival of H. pylori-infected cells, and inhibition of mitophagy may be one of the novel strategies for the prevention and treatment of H. pylori-induced gastric cancer.
Inhibition of Mitophagy can increase NLRP3 inflammasome activation of H. pylori infected cells. AGS and GES-1 cells were pretreated with mitophagy inhibitor (BafA1, 10 μM) for 24 h, respectively, and then infected with GZ7/cagA and GZ7/Δ cagA at an MOI of 60 for 48 h, respectively. (A) Western blotting analysis of the effect of BafA1treatment on the expression of mitophagy-related protein P62 and PARKIN in AGS and GES-1 cells. (B) Western blotting analysis of the effect of BafA1 treatment on the expression of NLRP3 inflammasome protein NLRP3, Caspase-1 and IL-18 in AGS and GES-1 cells. (C) Western blotting analysis of the effect of BafA1 pretreatment on the expression of NLRP3 inflammasome protein NLRP3, Caspase-1 and IL-18 in AGS and GES-1 cells with H. pylori infection. The figure presents the average of three independent experiments. Data are presented as the means ± SD. Error bars represent the standard deviation. control, the uninfected cells; GZ7/ΔcagA, the GZ7/ΔcagA strains infected cells; GZ7/cagA, the GZ7/cagA strains infected cells; *p < 0.05, **p < 0.01; GZ7/cagA vs control, GZ7/cagA vs BafA1 + GZ7/cagA, BafA1 + GZ7/cagA vs BafA1 + GZ7/ΔcagA.
Inhibition of Mitophagy can increase apoptosis, and decrease the cell viability of H. pylori infected cells. AGS and GES-1 cells were pretreated with mitophagy inhibitor (BafA1, 10 μM) for 24 h, respectively, and then infected with GZ7/cagA and GZ7/Δ cagA at an MOI of 60. (A) The apoptosis rate of H. pylori-infected cells were detected by flow cytometry after H. pylori infection 48 h. (B) The cell viability of H. pylori-infected cells were detected by CCK8 assay after H. pylori infection 6 h, 24 h and 48 h. The figure presents the average of three independent experiments. Data are presented as the means ± SD. Error bars represent the standard deviation. control, the uninfected cells; GZ7/ΔcagA, the GZ7/ΔcagA strains infected cells; GZ7/cagA, the GZ7/cagA strains infected cells; *p < 0.05, **p < 0.01; GZ7/cagA vs control, GZ7/cagA vs BafA1 + GZ7/cagA, BafA1 + GZ7/cagA vs BafA1 + GZ7/ΔcagA.
Discussion
Our study confirmed that H. pylori CagA induces mitochondrial oxidative damage, mitochondrial dysfunction, dynamic imbalance, mitophagy, and autophagy flux blockage in infected cells. Mitophagy activation attenuates H. pylori infection-induced NLRP3 inflammasome activation and increases survival and viability of infected cells. This suggests that mitophagy activated by H. pylori CagA may be an adaptive response of cells to evade host immune surveillance against H. pylori infection. In addition, blockade of autophagic flux leads to the accumulation of dysfunctional mitochondria, which may contribute to tumourigenesis induced by H. pylori infection.
Mitochondria continue the normal physiological activities of the cell as an essential generator of energy to maintain cell homeostasis only when their morphology and function are normal. Virulence factors secreted by bacteria can interact with mitochondria as a part of a key strategy to hijack host cell physiology and promote infection25. H. pylori is a pathogen that affects the human gastric mucosa and is a major contributor to the development of gastric cancer. It has also been reported to damage mucosal epithelial cells and target mitochondria, decreasing the activity of oxidative phosphorylation and electron transport chain activity and causing mitochondrial dysfunction and apoptosis26. To date, the vacuolating cytotoxin A (VacA) is the only H. pylori protein reported to target mitochondria and decrease mitochondrial membrane potential, reducing ATP production and cytochrome c release27. However, the role of CagA in causing mitochondrial function injury remains elusive, although it is a major virulence factor of H. pylori. In this study, we infected AGS and GES-1 cells with GZ7/cagA and GZ7/ΔcagA strains, respectively, followed by the detection of mitochondrial membrane potential, ATP production, cell apoptosis, and mitochondrial fusion division. The results showed that H. pylori infection decreased mitochondrial membrane potential and ATP production levels, promoted cell apoptosis, decreased the expression of mitochondrial fusion-related proteins MFN1 and Mfn2, and increased the expression of mitochondrial fission-related proteins DRP1 and FIS1. The results of the GZ7/cagA infected group were more significant in these processes. It was also confirmed that H. pylori can induce mitochondrial dysfunction, mitochondrial dynamic imbalance, and apoptosis of infected cells, in all of which CagA plays an important role.
Damaged mitochondria can induce mitophagy as a defence mechanism to eliminate themselves and excess ROS, maintain mitochondrial homeostasis, and promote cell survival in stressful environments28. Mitophagy is widely regarded as a major regulatory mechanism of mitochondrial quality control processes, which is involved in the regulation of host immune responses29. Previous research has suggested that H. pylori can induce defective autophagy or inhibit autophagy, allowing H. pylori to proliferate30. The VacA produced by H. pylori specifically targets mitochondria, disrupting mitochondrial membrane potential, and causing mitochondrial damage and mitophagy31. Furthermore, research suggests that CagA can inhibit autophagy via the mesenchymal-epithelial transition factor-phosphatidylinositol 3-kinase/protein kinase B-mechanistic target of the rapamycin signalling pathway32. However, CagA-induced mitochondrial damage and its effect on mitophagy remain elusive. In our study, we observed slight bleeding and inflammatory cell infiltration in the submucosa of the gastric tissue from GZ7/cagA-infected C57BL/6 mice in vivo, indicating an inflammatory response in the gastric mucosa of H. pylori-infected mice. We also found that the expression of mitophagy-related proteins P62, LC3, PINK1, and Parkin was significantly increased in the gastric mucosa of GZ7/cagA-infected mice. Consistent with that in H. pylori-infected mice in vivo, the expression of mitophagy-related proteins PINK1, Parkin, P62, LC3, VDAC1, and TOM20 increased significantly in H. pylori-infected cells in vitro. Immunofluorescence labelling revealed that H. pylori-infected cells exhibited punctate LC3 aggregation and increased colocalisation with mitochondria. In addition, TEM revealed an accumulation of autophagosomes and autolysosomes in H. pylori-infected cells. Furthermore, the autophagic flux observed by confocal microscopy revealed that H. pylori infection increased the level of mitophagy in infected cells, although the fusion of autophagosomes and lysosomes was limited. The results of GZ7/cagA infected cells were more significant in these processes. Although autophagy can limit exogenous infections, it has been reported that bacterial pathogens have evolved mechanisms to interfere with autophagy initiation or autophagy flux for their benefit33. Our results showed that H. pylori CagA promotes mitophagy induction but inhibits a specific stage of autophagosome maturation, thereby blocking autophagy flux. Considering that the accumulation of dysfunctional mitochondria is involved in tumourigenesis, this may be one of the pathogenic mechanisms by which H. pylori and its CagA cause gastric cancer.
Mitophagy is important in limiting inflammasome activation to prevent to prevent apoptosis and is used by viruses and bacteria to evade host immune clearance34. Mitophagy, a critical component in maintaining mitochondrial homeostasis, occurs via the elimination of damaged mitochondria, thereby, preventing hyperinflammation triggered by NLRP3 inflammasome activation and destruction of intracellular pathogens35. Similarly, the NLRP3 inflammasome signaling pathway can regulate autophagy and help maintain a balance between the necessary host defence inflammatory response and the prevention of excessive and harmful inflammation13. Recent studies have shown that some pathogens can directly or indirectly induce mitophagy and regulate the process of mitophagy via various strategies, thereby, weakening the innate immune response and allowing pathogens to promote persistent infection, which impacts the development of infectious diseases. Pseudomonas aeruginosa can disrupt mitochondrial homeostasis in host cells, thereby activating mitophagy and diminishing host defence against the toxic responses induced by its virulence factor pyoverdin36. Mycobacterium bovis (M. bovis) infection induces mitophagy in murine macrophages, and induction of mitophagy improves M. bovis growth, whereas inhibition of mitophagy improves growth restriction37. AGS and GES-1 cells were infected with GZ7/cagA and GZ7/Δ cagA strains combined with a mitophagy inhibitor (BafA1). The results showed that inhibition of mitophagy by BafA1 can cause the over-activation of the NLRP3 inflammasome in H. pylori-infected cells, suggesting that mitophagy induced by H. pylori CagA can attenuate the NLRP3 inflammasome activation and thus inhibit the antibacterial innate immune response, which may be one of the causes of H. pylori persistence.
Mitophagy is important in cancer cell homeostasis and tumour progression, acting as a pro- or anti-cancer factor, and its failure demotes oncogenic stress and tumorigenesis38,39,40. The normal level of mitophagy is the body's defence and protective mechanism against various environmental stresses; however, excessive abnormal mitophagy can cause an abnormal mitochondrial network and energy metabolism disorder, leading to cancer initiation and progression41. Although H. pylori is an extracellular pathogen, it can also survive intracellularly in GES, causing chronic infection and even the development of gastric cancer. The potential of mitophagy as a mechanism and therapeutic strategy in this process remains elusive. In our study, we confirmed that inhibition of mitophagy can increase the rate of apoptosis and decrease the cell viability of H. pylori-infected cells, suggesting that CagA mediates H. pylori to induce mitophagy, which attenuates the activation of the NLRP3 inflammasome to escape host immune surveillance and enhance the survival and viability of infected cells from a contrasting perspective. This ultimately leads to chronic H. pylori infection and promotes gastric cancer development. Furthermore, mitophagy inhibition may be considered as one of the new strategies for the prevention and treatment of H. pylori-caused gastric cancer.
In conclusion, our results showed that H. pylori induces mitochondrial oxidative damage, mitochondrial dysfunction, dynamic imbalance, mitophagy, and autophagy flux blockade mainly via its CagA. Mitophagy induction regulates the activation of the NLRP3 inflammasome, which may be one of the strategies of H. pylori to evade the immune response and increase the survival and viability of infected cells, potentially enhancing the intracellular survival of the pathogen and causing persistent infections. Blocking autophagy flux can lead to the accumulation of dysfunctional mitochondria, which may play a role in tumourigenesis caused by H. pylori infection. Our findings improve our understanding of the pathogenesis of H. pylori-induced gastric cancer, and mitophagy inhibition may be one of the novel strategies for the prevention and treatment of H. pylori-induced gastric cancer.
Data availability
The dataset supporting the conclusions of this article is included within the article.
References
Zamani, M. et al. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment Pharmacol. Ther. 47(7), 868–876 (2018).
Park, J. Y., Forman, D., Waskito, L. A., Yamaoka, Y. & Crabtree, J. E. Epidemiology of Helicobacter pylori and CagA-positive infections and global variations in gastric cancer. Toxins 10(4), 163–170 (2018).
Bakhti, S. Z., Latifi-Navid, S. & Safaralizadeh, R. Helicobacter pylori-related risk predictors of gastric cancer: The latest models, challenges, and future prospects. Cancer Med. 9(13), 4808–4822 (2020).
Xu, J. S., Wang, B. & Xie, C. Advances in the association of Helicobacter pylori strain specificity with gastric cancer. Chin. General Pract. 21(18), 2258–2262 (2018).
Chmiela, M., Karwowska, Z., Gonciarz, W., Allushi, B. & Stączek, P. Host pathogen interactions in Helicobacter pylori related gastric cancer. World J. Gastroenterol. 429(24), 933–940 (2017).
Jang, S., Hansen, L. M., Su, H., Solnick, J. V. & Cha, J. H. Host immune response mediates changes in cagA copy number and virulence potential of Helicobacter pylori. Gut Microbes 14(1), 2044721 (2022).
Baj, J. et al. Helicobacter pylori virulence factors-mechanisms of bacterial pathogenicity in the gastric microenvironment. Cells. 10(1), 27 (2020).
Zhang, X. et al. pylori CagA activates the NLRP3 inflammasome to promote gastric cancer cell migration and invasion. Inflamm. Res. 71(1), 141–155 (2022).
Broz, P. & Dixit, V. M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 16, 407–420 (2016).
Heid, M. E. et al. Mitochondrial reactive oxygen species induces NLRP3-dependent lysosomal damage and inflammasome activation. J. Immunol. 191(10), 5230–5238 (2013).
Yang, S. & Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell Biochem. 467(1–2), 1–12 (2020).
Yuk, J. M., Silwal, P. & Jo, E. K. Inflammasome and mitophagy connection in health and disease. Int. J. Mol. Sci. 21(13), 4714 (2020).
Biasizzo, M. & Kopitar-Jerala, N. Interplay between NLRP3 inflammasome and autophagy. Front. Immunol. 9(11), 591803 (2020).
Niya, L. X. L. et al. Sequences difference of CagA between East Asian strain and Western strain of Helicobacter pylori and its impact on the proliferation and apoptosis. Microbiol. China 45(04), 848–855 (2018).
Simon, H. U., Haj-Yehia, A. & Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 5(5), 415–418 (2000).
Otera, H. & Mihara, K. Mitochondrial dynamics: Functional link with apoptosis. Int J Cell Biol. 2012, 821676 (2012).
Anzell, A. R., Maizy, R., Przyklenk, K. & Sanderson, T. H. Mitochondrial quality control and disease: Insights into ischemia-reperfusion injury. Mol Neurobiol. 55(3), 2547–2564 (2018).
Jing, C., Lin, W. & Miao, L. Progress in regulation of mitochondrial dynamics and mitochondrial autophagy. Acta Physiol. Sin. 72(4), 475–487 (2020).
Cho, D. H., Kim, J. K. & Jo, E. K. Mitophagy and innate immunity in infection. Mol. Cells 43(1), 10–22 (2020).
Eslami, M., Yousefi, B., Kokhaei, P., Arabkari, V. & Ghasemian, A. Current information on the association of helicobacter pylori with autophagy and gastric cancer. J. Cell Physiol. 234, 14800–14811 (2019).
Ge, P. et al. tuberculosis PknG manipulates host autophagy flux to promote pathogen intracellular survival. Autophagy. 18(3), 576–594 (2022).
Mizushima, N., Yoshimori, T. & Levine, B. Methods in mammalian autophagy research. Cell. 140, 313–326 (2010).
Onishi, M., Yamano, K., Sato, M., Matsuda, N. & Okamoto, K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 40(3), e104705 (2021).
Li, S. et al. The role of mitophagy in regulating cell death. Oxid. Med. Cell Longev. 18(2021), 6617256 (2021).
Fielden, L. F., Kang, Y., Newton, H. J. & Stojanovski, D. Targeting mitochondria: How intravacuolar bacterial pathogens manipulate mitochondria. Cell Tissue Res. 367, 141–154 (2017).
Machado, A. M. et al. Helicobacter pylori infection affects mitochondrial function and DNA repair, thus, mediating genetic instability in gastric cells. Mech. Ageing Dev. 134, 460–466 (2013).
Chauhan, N., Tay, A. C. Y., Marshall, B. J. & Jain, U. Helicobacter pylori VacA, a distinct toxin exerts diverse functionalities in numerous cells: An overview. Helicobacter. 24, e12544 (2018).
Pickles, S., Vigié, P. & Youle, R. J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr Biol. 28(4), R170–R185 (2018).
Ashrafi, G. & Schwarz, T. L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 20(1), 31–42 (2013).
Yang, Y., Shu, X. & Xie, C. An overview of autophagy in Helicobacter pylori infection and related gastric cancer. Front. Cell Infect. Microbiol. 8(12), 847716 (2022).
Wang, L. et al. Vacuolating cytotoxin a triggers mitophagy in Helicobacter pylori-infected human gastric epithelium cells. Front. Oncol. 14(12), 881829 (2022).
Li, N. et al. Helicobacter pylori CagA protein negatively regulates autophagy and promotes inflammatory response via c-Met-PI3K/Akt-mTOR signaling pathway. Front. Cell Infect. Microbiol. 21(7), 417 (2017).
Li, J. et al. Brucella BtpB manipulates apoptosis and autophagic flux in RAW2647 cells. Int. J. Mol. Sci. 23(22), 14439 (2022).
Zhang, Y. et al. Listeria hijacks host mitophagy through a novel mitophagy receptor to evade killing. NatImmunol 20(4), 433–446 (2019).
Kim, M.-J., Yoon, J.-H. & Ryu, J.-H. Mitophagy: A balance regulator of NLRP3 inflammasome activation. BMB Rep. 49(10), 529–535 (2016).
Kirienko, N. V., Ausubel, F. M. & Ruvkun, G. Mitophagy confers resistance to siderophore-mediated killing by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 112(6), 1821–1826 (2015).
Song, Y. et al. Mycobacterium bovis induces mitophagy to suppress host xenophagy for its intracellular survival. Autophagy. 18(6), 1401–1415 (2022).
Chourasia, A. H., Boland, M. L. & Macleod, K. F. Mitophagy and cancer. Cancer Metab. 3, 4 (2015).
Drake, L. E., Springer, M. Z., Poole, L. P., Kim, C. J. & Macleod, K. F. Expanding perspectives on the significance of mitophagy in cancer. Semin. Cancer Biol. 47, 110–124 (2017).
Chang, J. Y., Yi, H. S., Kim, H. W. & Shong, M. Dysregulation of mitophagy in carcinogenesis and tumor progression. Biochim. Biophys. Acta Bioenerg. 1858(8), 633–640 (2017).
Song, C., Pan, S., Zhang, J., Li, N. & Geng, Q. Mitophagy: A novel perspective for insighting into cancer and cancer treatment. Cell Prolif. 55(12), e13327 (2022).
Acknowledgements
The authors are grateful to the editor, the associate editor, and the reviewer. We thank bmcscience (https://bmcscience.com/) for editing this manuscript
Funding
The work was supported by the National Natural Science Foundation of China (82260405, 31960028, 32160166), The Guizhou Provincial Science and Technology Projects (QianKeHe JC [2020]1Z010, ZK[2022]041, [2020]1Y333), The project of Guizhou Medical University (21NSFC03).
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal experiments were approved by the Animal Care Welfare Committee of Guizhou Medical University (Guiyang, China; Approval No. 1900801).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, D., Wu, L., Liu, X. et al. Helicobacter pylori CagA mediated mitophagy to attenuate the NLRP3 inflammasome activation and enhance the survival of infected cells. Sci Rep 14, 21648 (2024). https://doi.org/10.1038/s41598-024-72534-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72534-5
- Springer Nature Limited