Abstract
Inherited retinal dystrophies/degenerations (IRDs) are the leading cause of visual impairment and incurable familial blindness in the Western world. Given the clinical and genetic heterogeneity, establishing a molecular diagnosis is especially relevant. The aim of this study was to perform the first nationwide survey to understand the prevalence and current management of IRDs in Portugal. A response was obtained from 26 healthcare providers (HCP) (76.5% response rate). Only 4 respondents reported not managing IRD patients. Most HCPs (68.1%) reported managing up to 100 patients, while three currently manage between 501 and 1000 patients. Based on the Portuguese population, an estimated IRD prevalence of 0.031%, i.e., about 1 in 3000 individuals, was calculated. In most HCPs (86.3%), most patients are adults, and non-syndromic retinitis pigmentosa is the most frequent diagnosis. Only 4 HCPs currently use the national, web-based IRD registry (IRD-PT). However, all but one respondent expressed interest in participating in such a registry. Genetic testing is available in 54.5%, with 58.3% HCPs reporting solved rates between 61–80%, but 4 to 9 months to get a genetic test result in 83.4% of cases. Based on this survey, the prevalence of biallelic RPE65-associated disease in Portugal is 0.00031%, i.e., approximately 1:300,000 individuals. Data from this study provide vital background information on national differences in the diagnosis and management of IRD patients. Nationwide implementation of the IRD-PT registry should be encouraged and supported to provide population-based reference data and to identify patients eligible for current and future therapies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Inherited retinal dystrophies/degenerations (IRDs) comprise a heterogeneous group of rare ocular diseases characterized by progressive retinal dysfunction and/or degeneration that result in significant visual impairment1,2. With an estimated global prevalence of 1:2000–3000, and affecting more than 2 million people worldwide3,4, IRDs are the leading cause of incurable familial blindness in the Western world5.
Due to the phenotypic and genotypic heterogeneity of IRDs, establishing a final clinical diagnosis can be challenging. Consequently, patients often experience a diagnostic odyssey, resulting in delayed diagnosis and poor access to specialized care6. To date, more than 280 IRD-causing genes have been identified and catalogued by RetNet (Retinal Information Network, https://sph.uth.edu/retnet/). As a result, effective and individualized approaches to the clinical management of these patients depend on a comprehensive means of providing genetic testing. In Europe, genetic testing has been recommended by the European Reference Network for Rare Eye Diseases (ERN-EYE) for all individuals with suspected/probable IRDs6. In addition to providing a molecular diagnosis, genetic testing is essential for accurate genetic counseling, identification of candidates for new therapies, or enrollment in gene therapy-based clinical trials5.
Despite technological advances and national health system reimbursement, the implementation of genetic testing for individual IRD patients has been slow in Portugal, ultimately impacting genetic counseling and access to specialized care. Moreover, data on the overall prevalence and genetic landscape of IRDs in the country is currently scarce7,8. This knowledge is critical as the genetic profiles of IRDs have been shown to vary considerably between regions and ethnic groups, highlighting the importance of obtaining population-based reference data9,10,11.
Bearing this in mind, the Ophthalmic Genetics Group of the Portuguese Society of Ophthalmology conducted an online survey targeting 34 public healthcare providers (HCPs) to assess the current management of IRD patients across Portugal. The main goal was to estimate the overall prevalence and understand the current management of Portuguese patients with IRDs, hoping to identify possible gaps and avenues for improvement across the country.
Methods
Study design and questionnaire
The IRD-PT survey (Supplementary Table S1) was an electronic questionnaire developed by two IRD specialists (J.P.M. and E.S.) in a research collaboration between the Portuguese Society of Ophthalmology and Novartis Farma, Portugal.
The electronic questionnaire comprised 31 main questions divided into four sections: 1) IRDs demographics, 2) genetic testing and counseling, 3) IRD patient management, and 4) RPE65-associated disease. Some of the main questions followed conditional branching. The questionnaire was designed to be predominantly single-choice (closed-ended), but it also included a few multiple-choice and open-ended questions. Some questions presented options representing a range of values, meaning that only estimates were requested. After its electronic development, the questionnaire was sent by e-mail to the head of the Ophthalmology Department of 34 HCPs from all five continental Nomenclature of Territorial Units for Statistics (NUTS) II regions, Madeira and Azores by the Portuguese Society of Ophthalmology. Only one reply per HCP was allowed. The identification of the HCP and the name, subspecialty, and e-mail of the respondent were requested. All responses collected between 21 September and 19 October 2022 were considered for the analysis. Strategies to maximize the response rate were follow-up contact, personalized e-mails, and giving an ultimate deadline. The present study complied with the ethical standards of the Human Research Ethics Committee (HREC) of CHUC/Faculty of Medicine, University of Coimbra (Reference Number: CE 125/2019), and with the tenets of the 1964 Helsinki declaration for biomedical research and its later amendments or comparable ethical standards.
Statistical analysis
A descriptive analysis was conducted for all variables. Discrete variables were summarized using absolute and relative frequencies. Continuous variables were summarized by measures of central tendency and dispersion, including number (n), mean, standard deviation (SD), median (P50), interquartile range (P25 and P75), minimum (Min), and maximum (Max).
Comparisons of continuous variables between three and two independent subgroups were performed using the Kruskal–Wallis test and the Mann–Whitney test, respectively. Comparisons of categorical variables between groups were performed using the Fisher exact test.
Questionnaires with missing values were not excluded. However, each analysis was restricted to respondents with no missing values for that question, so the total number of respondents differed between questions.
All hypotheses were tested using two-tailed tests at a significance level of the ɑ = 0.05.
Statistical analysis was performed using R® version 4.1.2 software.
Results
Participants
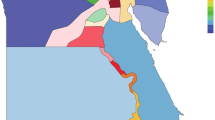
The IRD-PT electronic survey was sent to a total of 34 public HCPs, with a response rate of 76.5%, representing a final number of 26 participating centers (please refer to Supplementary Table S2 for a complete list). The geographic distribution and subspecialty of the respondents are shown in Table 1. The Northern, Central, and Lisbon and Tagus Valley (LTV) regions were the ones receiving the highest number of responses, with 9 (34.6%), 6 (23.1%), and 7 (26.9%) participating HCPs, respectively (Fig. 1A). In addition, there were two replies from Alentejo (7.7%), one from the Algarve (3.8%), and one from Madeira Island (3.8%). Most respondents were medical and/or surgical retina specialists (n = 16, 61.5%), followed by IRD specialists (n = 4, 15.4%), general ophthalmologists (n = 3, 11.5%), pediatric ophthalmologists (n = 1, 3.8%), or other—one neuro-ophthalmologist (3.8%) and one ocular inflammation specialist (3.8%).
Geographic distribution of survey respondents and genetic testing facilities across Portugal. Locations of (A) survey respondents (red for centers that replied to the survey and yellow indicating centers that did not respond) and (B) genetic testing facilities (black pins), across various regions in Portugal.
Of the 26 survey participants, only 4 (15.4%) reported not managing IRD patients, either because patients are sent to a referral hospital or because they do not have enough patients with this diagnosis. These participants did not answer any further questions in the questionnaire.
IRD demographics
Of the centers managing IRD patients (n = 22), the majority (n = 15, 68.1%) reported managing between 11 and 100 patients, while 2 centers (9.1%) indicated managing between 101 and 200 patients (Fig. 2). Two centers from the Central NUTS II region reported the lowest number of managed patients (≤ 10). In contrast, centers from Coimbra and Porto reported the highest numbers of IRD patients (between 501 and 1000). Based on the Portuguese population (10,343,066 people12 and considering the survey's broad national coverage, the overall prevalence of IRDs in Portugal is estimated to be 0.031%, i.e., about 1 in 3000 individuals.
An analysis of the distribution of IRD patients across the country shows that the North is the region that manages the highest number of patients (40.9%), followed by LTV (32.6%) and the Central region (24.3%). On the other hand, Alentejo and Madeira handle less than 2% of total Portuguese IRD patients combined.
When assessing the approximate ratio of adult to pediatric patients followed by each participating HCP, the majority of replies (n = 19; 86.3%) indicated managing between 70–100% of adults vs. 0–30% of pediatric patients. Two centers reported an equal proportion of adult and pediatric patients, while one center in the Northern region of Portugal reported managing 80% pediatric vs. 20% adult IRD patients (Fig. 2B).
Non-syndromic retinitis pigmentosa was reported as the most frequent IRD diagnosis (81.8%). However, two centers (9.1%) reported syndromic retinitis pigmentosa as the most frequent diagnosis. In the remaining HCPs, Stargardt disease (n = 1, 4.5%) and Best disease (n = 1, 4.5%) were the most frequent diagnoses.
General practitioners were reported as the most common referral source of IRD patients to the HCPs (32.5%), followed by referrals from other subspecialties within the center (30.1%), patient self-referral (19.0%), and pediatricians (10.8%) (Fig. 3). Of note, participants indicated that, on average, only 2.5% of IRD patients were referred by Medical Geneticists. Analysis of referral routes showed that patients referred by geneticists were almost exclusively assigned to central hospitals rather than smaller and less resourced regional hospitals (9% vs. 0.6% of patient referrals, p = 0.003).
Percentage of patient referrals to the participating center. Respondents were asked to estimate the percentage of patients referred by each of the six routes presented. Box plots are presented with the first quartile (P25) in grey and the third quartile (P75) in white. The black line separating P25 and P75 represents the median. The dashed line shows the minimum and maximum values obtained.
Less than half the centers (n = 9, 40.9%) use a database for IRD patients. Of those, 44.4% (n = 4) have their patients enrolled in the national, web-based IRD registry – the IRD-PT registry13 –and 55.5% (n = 5) in local databases (44.4% in Excel files and 11.1% in hospital databases). The majority of participating centers (66.6%) have between 20 and 500 patients in their databases. Coimbra and Central Lisbon hospitals have databases with 500 to 1000 IRD patients. All but one respondent (95.5%) agreed that having a single national registry specifically for IRD patients is important, and expressed interest in participating in such a registry.
Genetic testing and counselling
A total of 54.5% (n = 12) of centers managing IRD patients currently offer genetic testing (Fig. 1B and Fig. 4A). Among these, 5 centers (41.7%) have performed genetic testing to nearly half (41–60%) of their IRD patients (Fig. 4B). For most centers, a molecular characterization of the disease could be established in 61–80% of patients (Fig. 4C).
Estimated percentage of IRD patients genetically tested and those molecularly characterized. (A) Frequency (%) and number (n) of participating centers that offer genetic testing and estimated percentage of IRD patients in those centers that has been (B) genetically tested and (C) molecularly characterized, ie solved.
Lack of a medical genetics department was the most common reason (90%) for not genetically testing IRD patients. Others cited the lack of an IRD specialist, limited financial resources, or bureaucracy as bottlenecks for not offering genetic testing to IRD patients. However, all centers currently not performing genetic testing expressed their willingness to consider referring patients to specialized centers for genetic testing. Most centers in the North and LTV regions perform genetic testing (66.7% and 71.4%, respectively), while only 1 in 4 centers from the Central region of Portugal offers genetic testing. Centers from Alentejo and Madeira regions do not currently perform genetic testing. In 75% of cases, genetic testing is requested by ophthalmologists, while in 50%, medical geneticists oversee genetic testing. Notably, in some centers, genetic testing is solely requested by ophthalmologists (41.7%), while in others, both ophthalmologists and medical geneticists order these tests (33.3%). This depends on the availability of a genetics department and/or case complexity (genetic testing in syndromic IRDs is mostly requested by a geneticist and not by an ophthalmologist). The most frequently requested tests are next-generation sequencing (NGS) panels (83.3%), followed by whole exome sequencing (WES) and Sanger sequencing (16.7% each; Table 2).
Fifty-eight percent use a national private laboratory, 25% use a national academic or research laboratory, and the remaining either use private and national laboratories or do not know (Table 3). Of the 12 centers that genetically test their IRD patients, 11 (91.7%) offer genetic counseling. Of these, 63.6% offer in-house genetic counseling, and 36.4% refer patients to an external medical geneticist. The only center that reported not offering genetic counseling cited the lack of a medical genetics department in the hospital as the reason for this. In most cases (83.4%), a waiting time of 4 to 9 months is needed to get a genetic test result.
Despite this, one center (8.3%) claimed to get results between 1 and 3 months, and another (8.3%) reported having results only after 10 months. The average time to obtain a genetic test result by country region is shown in Fig. 5.
Management of IRD patients
Most participating centers managing IRD patients reported annual follow-up visits (72.8%). Twenty-three percent indicated monitoring their patients every six months, while one center reported managing the follow-up time according to the patient's profile. Regarding the type of examinations considered relevant for establishing the clinical diagnosis and follow-up of IRD patients (Table 4), all respondents agreed to use best corrected visual acuity test (BCVA), complemented by spectral domain optical coherence tomography (SD-OCT; 90.9%), electrophysiology testing (81.8%), fundus autofluorescence and automated perimetry (each 77.3%), OCT-angiography (22.7%), and fluorescein angiography (18.2%). Indocyanine green angiography, microperimetry, kinetic perimetry, or other tests were reported by 4.5% of the centers.
Retinal dystrophies associated with RPE65 gene
Twenty-three percent of HCPs managing IRD patients (n = 5) reported following patients with RPE65-associated retinal degeneration (RPE65-RD). Of these, most (n = 3, 60%) indicated to have between 1 and 5 patients with biallelic RPE65 gene mutations. One center declared to have between 6 and 10 biallelic RPE65-RD patients, and another between 11 and 20. Based on these findings, the prevalence of RPE65-RD patients carrying biallelic mutations in Portugal was estimated to be 0.00031%, which translates to approximately 1 in 300,000 Portuguese individuals.
Among the centers following RPE65-RD patients, 80% (n = 4) considered to have 1 to 5 patients eligible for treatment (Table 5). Of note, of all centers following RPE65-RD patients, 2 (40%) reported that approximately 60% of their patients had already received treatment with voretigene neparvovec in at least one eye, 2 (40%) claimed to have 12.5% of their RPE65-RD patients treated and 1 (20%) stated that none of their RPE65 patients received treatment.
Discussion
This is the first Portuguese nationwide survey on the management of IRD patients. Information from this study generates important knowledge and adds value for HCPs and policymakers to reflect on current management practices, identify bottlenecks across the country and take action to overcome them. Although considered rare diseases, IRDs are among the most common causes of severe visual impairment and blindness in children and young adults in developed countries14, posing a significant burden on economies, as recently illustrated by a report from the Republic of Ireland and the United Kingdom15. The lack of equitable access to genetic testing, genetic counselling and IRD referral centers (both within and between countries) accentuates the inequalities in IRD patient management16,17. As a result, the development and commissioning of clinical services, provision of therapies, and planning/implementation of clinical studies are profoundly hampered. Unlike other European countries, genetic testing in the public setting in Portugal is fully reimbursed by the Portuguese National Health System18. This encompasses all available genetic testing modalities. In cases where the patient is managed in a private HCP, genetic testing costs are usually out-of-pocket, as most health insurance companies operating in Portugal do not cover genetic testing. This survey has targeted only public HCP, where we believe most IRD patients are managed. Of all HCPs contacted, a significant number replied (76.5%), ensuring a broad national coverage. Most HCPs reported managing between 11 and 100 IRD patients, while three national expert centers in the North (Porto), Centre (Coimbra), and South (Lisbon) each handle between 500 to 1,000 IRD patients. These are well distributed across the country, providing access to advanced medical facilities and IRD specialists. In contrast, regions such as Alentejo and some areas in the inland Central region report managing significantly fewer IRD patients, likely due to variations in healthcare resources and limited access to specialized services, highlighting potential access limitations to specialized care in rural areas. We believe the results of this survey may help improve patient referral pathways and develop targeted aid strategies to ensure that all IRD patients are granted full clinical and socioeconomic support. Based on the projected number of patients followed by each participating center, the estimated prevalence of IRD patients in Portugal was found to be around 1:3000, similar to the reported global prevalence of these diseases19. Of note, Centro Hospitalar Universitário de Coimbra (CHUC) manages nearly a quarter of the national IRD population and is the only Portuguese HCP integrating the ERN-EYE.
Most centers reported managing a higher proportion of adult than pediatric patients, which is somewhat expected as IRDs are more frequently diagnosed in adulthood14. However, this may also reflect a delayed diagnosis due to the so-called diagnostic odyssey that IRD patients usually face and the lack of direct referral pathways to expert centers. Nevertheless, centers that do not manage IRD patients indicated referring them to specialized centers. The survey results show that general practitioners are the most common referral source of IRD patients in Portugal. This may be partly explained by how the Portuguese National Health Service is organized and managed, but it also highlights the need to establish direct referral routes in the country. Importantly, the survey revealed that a significant portion of referrals (30.1%) comes from other subspecialties within the centers, indicating that a variety of medical professionals, beyond ophthalmologists, are involved in recognizing and managing these complex cases. This interdisciplinary approach not only facilitates early and accurate diagnosis but also ensures comprehensive management of IRD patients, involving specialists who can address the broader spectrum of clinical needs. Referral pathways are paramount to ensuring that IRD patients are followed in expert centers, i.e., centers that can provide the best possible care from diagnosis to treatment (low vision aids, clinical trials, or newly approved therapies). In addition, these centers typically have genetics departments that offer genetic testing and counseling. In fact, data from this survey show that genetic counseling is most often provided by an in-house geneticist (63.6%), but when this is not available, it may be outsourced.
Fifty-four percent of the respondent HCPs offer genetic testing to their IRD patients, which is still lagging behind a recent report from the European Vision Institute Clinical Research Network (EVICR.net), where 84% of the respondents claimed to offer genetic testing to their IRD patients17. However, it should be noted that most EVICR.net centers are University Hospitals from across Europe, actively involved in clinical and basic research. Thus, the percentage of European centers offering genetic testing to IRD patients is likely biased. Additionally, the response rate to that survey was approximately 50%, well below that of the present nationwide survey. Had we only considered answers from the five largest hospital centers in Portugal (ULS de Coimbra [Coimbra], ULS de Santo António [Porto], ULS de São José [Lisbon], ULS de Santa Maria [Lisbon], and ULS de São João [Porto]), we would have had a 100% response rate and 100% centers genotyping IRD patients. On the other hand, the estimated number of IRD patients genetically tested by each HCP (40–60%) and those where a molecular characterization of the disease was possible (60–80%) is in line with that of our European counterparts17. As expected, non-syndromic retinitis pigmentosa was reported as the most prevalent IRD diagnosis, as it is considered the most common IRD, with a worldwide prevalence of approximately 1:4000 individuals19. Most respondents declared to wait around 6 months between requesting a genetic test and receiving the final molecular genetic report. Note that no question was asked to specify the time to obtain a result by type of test, which may have impacted the answers given. Notwithstanding, these figures are somewhat disappointing and show room for improvement, particularly when there are European HCPs where test results are available within 2 to 4 weeks17. Interestingly, there was no significant variation in the test response speed between the country's regions.
The majority of centers managing IRD patients reported to perform annual follow-ups of IRD patients. In addition, most respondents use deep phenotyping in the diagnosis and management of their IRD patients, including both functional and structural testing, as established by international guidelines20.
Approximately one-quarter of the participating centers manage RPE65-RD patients, 50% carrying American College of Medical Genetics and Genomics (ACMG) class IV or V biallelic variants. Based on the present survey, the estimated prevalence of RPE65-RD patients carrying biallelic mutations in Portugal is 0.00031%, i.e., approximately 1:300,000 Portuguese individuals. However, these data should be interpreted with caution, as it is based on an inference made from an estimated number of patients reported by the participating HCPs. According to the respondents, 80% of their biallelic RPE65-RD patients would be eligible for treatment with voretigene neparvovec. Yet, at the time of this survey, only one center (ULS de Coimbra) in Portugal had provided the treatment to a total of 10 patients, resulting in 20 treated eyes. In December 2022, a second Portuguese center (ULS de São José) treated its first RPE65-RD patient.
In a rapidly evolving field as IRDs, there is an urgent need to improve the quality of care and to keep pace with treatment guidelines. This study shows that four major bottlenecks exist in the country: (1) lack of established referral pathways for IRD patients; (2) less than desirable adoption of the national IRD registry (IRD-PT registry; retina.com.pt); (3) a significant number of centers (45.5%) that still do not offer genetic testing to their patients; and (4) long waiting times for genetic testing results. Several respondents still use Excel files as databases for their IRD patients, despite the existence of a national web-based registry specifically developed for IRD patients: the IRD-PT registry7,13. Despite previous initiatives21, there is still room for greater dissemination of this platform among those who manage IRD patients. To facilitate the nationwide adoption of genetic testing for IRD patients, the development of national genetic testing guidelines for IRDs would be important. To enhance the efficiency of genetic test results, national health policies should set benchmarks for acceptable turnaround times and increase funding to expand genetic testing facilities. This funding should be directed towards modernizing laboratory equipment and enhancing the technical skills of staff for sophisticated genetic testing procedures. Fostering collaboration between hospitals, private laboratories, and academic centers is also essential. Such partnerships can facilitate resource sharing — from specialized equipment to expert knowledge — thus enhancing the overall capacity and reducing bottlenecks in the genetic testing pipeline. Importantly, as a result of this survey, many of these measures are already being implemented. Additionally, an expert committee composed of geneticists and ophthalmologists has recently come together to formulate guidelines for genetic testing in IRDs in Portugal. The outcome will soon be published for the international community.
This study is not exempt from limitations. Several factors complicated the analysis of our data. First, questions often presented a range of values. This meant that estimates and medians had to be used to calculate statistically meaningful data. Second, some questions included space for free text to give the possibility to add aspects that might have been missed in the survey, but quite often participants gave repetitive answers in these fields or evaded the core of the question. Third, as Portugal is a small country, some patients are currently followed in more than one HCP, thus creating possible duplications. Fourth, the survey targeted only public hospitals and did not capture data from IRD patients managed outside these centers, particularly those who may be exclusively managed in the private sector. Consequently, the estimated prevalence of IRD presented in this study may be underestimated.
In summary, the present study highlights the significance of obtaining data on IRD patient management, particularly in relation to genetic testing. Replication of this nationwide survey at the international level could be of considerable benefit for several reasons. First, it would provide a more comprehensive understanding of the landscape and difficulties pertaining to IRD genetic testing across different regions and populations, including those from distinct ethnic or cultural backgrounds. Second, it could contribute to raise awareness on the importance of IRD genetic testing and promote collaboration among researchers, HCPs, and patient advocacy groups across different countries. Finally, it could stimulate the development of standard testing protocols and enhance the precision and accessibility of IRD genetic testing for patients worldwide.
Conclusions
This is the first survey on the management of IRD patients in Portugal. Data from this study provides vital background information on national differences in the diagnosis and management of IRD patients and identifies gaps for future intervention. The nationwide adoption of the IRD-PT registry should be encouraged and supported to obtain more precise prevalence data and help in the continuous monitoring and identification of patients eligible for current and future therapies.
Data availability
The dataset generated and analyzed during the current study is available in the Open Science Framework, IRD-PT Survey Database.
Abbreviations
- ACMG:
-
American college of medical genetics and genomics
- BCVA:
-
Best corrected visual acuity
- CHUC:
-
Centro hospitalar universitário coimbra
- ERN-EYE:
-
European reference network dedicated to rare eye diseases
- EVICR.net:
-
Network of European ophthalmological clinical research sites
- HCP:
-
Healthcare provider
- IRD:
-
Inherited retinal dystrophies/degenerations
- LTV:
-
Lisbon and tagus valley
- MLPA:
-
Multiplex ligation-dependent probe amplification
- NGS:
-
Next-generation sequencing
- NUTS:
-
Nomenclature of territorial units for statistics
- OCT:
-
Optical coherence tomography
- RetNet:
-
Retinal information network
- RPE65:
-
Retinal Epihelium-specific 65 kDa Protein Gene (retinoid isomerohydrolase)
- RPE65-RD:
-
RPE65-associated retinal degeneration
- SD:
-
Standard deviation
- SD-OCT:
-
Spectral-domain optical coherence tomography
- WES:
-
Whole exome sequencing
References
Griffith, J. 3rd. et al. Inherited Retinal Dystrophy in Southeastern United States: Characterization of South Carolina Patients and Comparative Literature Review. Genes (Basel) https://doi.org/10.3390/genes13081490 (2022).
Olivares-Gonzalez, L., Velasco, S., Campillo, I. & Rodrigo, R. Retinal Inflammation, Cell Death and Inherited Retinal Dystrophies. Int. J. Mol. Sci. 22, 2096 (2021).
Lam, B. L. et al. Genetic testing and diagnosis of inherited retinal diseases. Orphanet J. Rare Dis. 16(1), 514 (2021).
Broadgate, S., Yu, J., Downes, S. M. & Halford, S. Unravelling the genetics of inherited retinal dystrophies: Past, present and future. Prog. Retin. Eye Res. 59, 53–96 (2017).
Lee, K. & Garg, S. Navigating the current landscape of clinical genetic testing for inherited retinal dystrophies. Genet. Med. 17(4), 245–252 (2015).
Black, G. C. et al. The need for widely available genomic testing in rare eye diseases: An ERN-EYE position statement. Orphanet J. Rare Dis. 16(1), 142 (2021).
Marques, J. P., Pires, J., Costa, J., Murta, J. & Silva, R. Inherited Retinal Degenerations in Portugal: Addressing the Unmet Needs. Acta Médica Portuguesa. 34(5), 332–334 (2021).
Peter, V. G. et al. The first genetic landscape of inherited retinal dystrophies in Portuguese patients identifies recurrent homozygous mutations as a frequent cause of pathogenesis. PNAS Nexus. https://doi.org/10.1093/pnasnexus/pgad043 (2023).
Weisschuh, N. et al. Genetic architecture of inherited retinal degeneration in Germany: A large cohort study from a single diagnostic center over a 9-year period. Hum. Mutat. 41(9), 1514–1527 (2020).
Sen, P. et al. Prevalence of retinitis pigmentosa in South Indian population aged above 40 years. Ophthalmic Epidemiol. 15(4), 279–281 (2008).
Sia, D. I. et al. A survey of visual impairment and blindness in children attending four schools for the blind in Cambodia. Ophthalmic Epidemiol. 17(4), 225–233 (2010).
Estatística INd. Censos 2021 2022 [Available from: https://tabulador.ine.pt/indicador/?id=0011609
Marques, J. P. et al. Design, development and deployment of a web-based interoperable registry for inherited retinal dystrophies in Portugal: the IRD-PT. Orphanet. J. Rare Dis. 15(1), 304 (2020).
Heath Jeffery, R. C. et al. Inherited retinal diseases are the most common cause of blindness in the working-age population in Australia. Ophthalmic Genet. 42(4), 431–439 (2021).
Galvin, O. et al. The Impact of Inherited Retinal Diseases in the Republic of Ireland (ROI) and the United Kingdom (UK) from a Cost-of-Illness Perspective. Clin. Ophthalmol. 14, 707–719 (2020).
Lorenz B, Tavares J, van den Born LI, Marques JP, Scholl HPN, Group EVn. Current Management of Inherited Retinal Degeneration Patients in Europe: Results of a Multinational Survey by the European Vision Institute Clinical Research Network. Ophthalmic Res. 64(4):622–38. (2021).
Lorenz, B. et al. Current management of Inherited Retinal Degenerations (IRD) patients in Europe. Results of a 2 years follow-up multinational survey by the European Vision Institute Clinical Research Network EVICRnet. Ophthalmic Res https://doi.org/10.1159/000529777 (2023).
Calzetti, G. et al. Genetic Testing of Patients with Inherited Retinal Diseases in the European Countries: An International Survey by the European Vision Institute. Ophthalmic. Res. 67(1), 448–457 (2024).
Verbakel, S. K. et al. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 66, 157–186 (2018).
Menghini, M., Cehajic-Kapetanovic, J. & MacLaren, R. E. Monitoring progression of retinitis pigmentosa: Current recommendations and recent advances. Expert Opin. Orphan. Drugs. 8(2–3), 67–78 (2020).
Marques, J. P. et al. Challenges, facilitators and barriers to the adoption and use of a web-based national IRD registry: Lessons learned from the IRD-PT registry. Orphanet. J. Rare Dis. 17(1), 323 (2022).
Acknowledgements
This article was prepared with the assistance of Carla Gomes (medical writing) and Adriana Belo (statistical analysis) from W4Research.
Funding
This study was funded by Novartis Farma, Portugal. Novartis provided non-binding comments/input to the IRD Survey Expert Committee into all elements of the scientific collaboration agreement.
Author information
Authors and Affiliations
Contributions
Study conception and design, data analysis, interpretation of results and draft preparation: JPM and ES; JPM, NF, NM, AM, SVP, SES, JC, ARC, PN, LD, DM, JP, CM, FR, PA, AC, DC, IC, MR, MM, SB, FI, FGR, JPCS, MFM and ES contributed to data collection. All authors substantially revised the manuscript and approved its final version. All authors have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This survey was conducted in accordance with the World Medical Association Declaration of Helsinki. As no personal data were collected, the use of a written and informed consent form was not applicable.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Marques, J.P., Ferreira, N., Moreno, N. et al. Current management of inherited retinal degenerations in Portugal (IRD-PT survey). Sci Rep 14, 21473 (2024). https://doi.org/10.1038/s41598-024-72589-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72589-4
- Springer Nature Limited