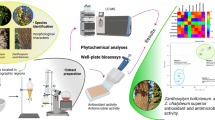
Abstract
Traditional herbalists have been relied on for many years by Algerians to cure a wide range of diseases. Regardless of their nutritional values, mushrooms have chemical properties that make them attractive, beneficial, and more likely to be studied by researchers, according to ethnobotanical literature on traditional phytotherapy. Among all the edible mushrooms, tubers are a type of fungus that are traditionally used in fine dining and have garnered attention recently because of their many therapeutic applications. This research delves into a meticulous analysis of bioactive constituents in Bunium bulbocastanum tubers, sourced from Mostaganem and Relizane regions, with a keen focus on polyphenols, flavonoids, and condensed tannins. The quantification of total phenolic content was executed through the Folin-Ciocalteu assay, while flavonoids were assessed via the aluminum chloride colorimetric method. In addition, condensed tannins were evaluated in this study. Antioxidant capacities were scrutinized employing the 2,2′-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay. Microbial inhibition studies were conducted against five benchmark bacterial strains, utilizing the agar disc diffusion technique. Furthermore, a comprehensive liquid chromatography–mass spectrometry (LC–MS) analysis was performed to identify and quantify bioactive compounds. The findings underscore that the Mostaganem extracts were particularly rich in polyphenols (11.65 mg GAE/g of extract) and tannins (1.30 mg CE/g of extract), while the Relizane extracts boasted significant flavonoid concentrations (9.421 mg QE/g of extract). Notably, 4-methylguaiacol (82.04 mg/L), caffeic acid dimethyl ether (27.76 mg/L), syringic acid (20.48 mg/L), and naringenin (16.05 mg/L) emerged as the predominant volatile compounds. Compositional investigation of the extracts by LC–MS confirmed the presence of various compounds that were linked to the bioactivities exhibited by B. bulbocastanum tubers. These findings demonstrate the effective antibacterial and antioxidant properties of B. bulbocastanum tubers, indicating their potential use in pharmaceutical and nutraceutical applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The utilization of secondary plant metabolites in primary health care is evident in both traditional medicine practices and the pharmaceutical industry. Traditionally, plant extracts or specific parts have been used to treat various ailments based on empirical knowledge passed down through generations. These practices involve using extracts or infusions made from whole plants or specific plant parts for their therapeutic properties1,2,3. In the pharmaceutical industry, plants have been served as a source of active principles that have been isolated, characterized, and used to develop pure pharmacological compounds. This transition from traditional preparations to purified active ingredients marks a significant advancement, allowing for more precise dosage, a better understanding of pharmacokinetics, and targeted therapy4,5,6. Traditional practices thus serve as valuable leads for identifying bioactive compounds with potential pharmaceutical applications7,8,9,10.
Medicinal plants have been an essential source for both therapeutic and preventive treatments in human medicine. The World Health Organization (WHO) estimates that approximately 21,000 plant species have the potential to be utilized as medicinal plants11,12. Different plant compounds offer varied therapeutic effects. For example, alkaloids can act as pain relievers or stimulants13, flavonoids are known for their antioxidant and anti-inflammatory properties14,15, terpenes may relieve respiratory ailments and enhance mood16,17, and saponins have roles in heart health and immune function18. Algeria’s flora is rich, comprising some 3000 plant species, with 15% being endemic, which underscores the region’s unique botanical richness19. This diversity serves as a crucial reservoir for traditional medicine systems that have relied on indigenous flora for centuries. The Bunium genus with around 50 species found across North Africa, Asia, and Europe, contains several species used in traditional medicine20. Specifically, Bunium bulbocastanum L., known locally as Talghouda or Nut, is recognized in rural Algerian regions for its therapeutic properties21.
Bunium bulbocastanum, a member of the Apiaceae family, has a long history in traditional medicine for its anti-inflammatory, antioxidant, and possible endocrine modulating benefits21,22. In the northern regions of Algeria, particularly in the districts of Relizane and Mostaganem, this plant is highly regarded in local healthcare practices for its potent antioxidant and anticancer properties, as well as its effectiveness in treating thyroid gland disorders23. Recent investigations reveal that the B. bulbocastanum tuber contains approximately 7% proteins, with lipid and ash contents of 3.34% and 3.96%, respectively24. The potential of B. bulbocastanum extends beyond its nutritional value. Amid rising antibiotic resistance and the side effects of synthetic drugs, this plant offers a viable alternative due to its bioactive substances, such as polyphenols, flavonoids, and condensed tannins, which are known for disease prevention and management properties. Nevertheless, little is known about the biological activities of this plant, and its phytochemicals have not been thoroughly studied. Thus, the objective of this study was to evaluate the antioxidant and antimicrobial properties and bioactive compounds of B. bulbocastanum from Sidi Ali (Mostaganem) and Ammi Moussa (Relizane). This research can merge traditional knowledge with scientific research, potentially yielding new therapeutic insights and supporting global biodiversity conservation and the sustainable use of medicinal plants.
Results and discussion
Total phenolic compounds
The total phenolic content of the extract obtained from B. bulbocastanum tubers was evaluated using spectrophotometric assays with the Folin-Ciocalteu reagent. The focus on total phenols is justified by its high antioxidant properties25 and their widespread use in herbal medicine26. Quantitative analysis of total phenols was determined from the linear regression equations y = 0.0119X + 0.0658 with a regression coefficient of R2 = 0.995 for the calibration curve, expressed in milligrams of gallic acid equivalent per gram of crude extract (mg GAE/g).
Table 1 indicates that the total phenolic content was 11.65 mg GAE/g for samples from Mostaganem and 7.35 mg GAE/g for those from Relizane with a statistically significant difference (p < 0.05). The difference in polyphenol content between the two regions was estimated at 36.88%. These values were significantly higher than those reported by Souri et al.27 for B. persicum, where the methanolic extract contained 2.14 mg/g dry weight in total phenolic content. Similarly, the phenol levels obtained by Chizzola et al.28 for the methanolic fraction of B. persicum were 0.57 mg/g.
Variations in phenolic content can be attributed to phenolic metabolism, environmental conditions, and the geographical distribution of the plant species. According to Khlifi et al.29, the phenolic composition of plant tissues varies considerably due to seasonal, genetic, and agronomic factors. High variability in phenolic content has been observed at different stages of maturation and under varying abiotic conditions of cultivation, such as temperature and precipitation30,31. Our findings align with those of Karouche et al.32, who reported significant variations in phenolic content based on geographical origin.
The higher polyphenol content in B. bulbocastanum samples from Mostaganem compared to those from Relizane highlights the influence of geographical location on the phenolic profile of the plant. Mostaganem’s specific environmental conditions, including soil type, climate, and agricultural practices, may contribute to the enhanced phenolic content. These findings are consistent with previous studies showing that environmental factors play a crucial role in the biosynthesis of phenolic compounds33,34. The robust antioxidant properties of phenols underline their importance in herbal medicine. The significantly higher phenolic content in the Mostaganem samples suggests a greater potential for antioxidant activity, which could enhance the therapeutic efficacy of B. bulbocastanum extracts from this region. This difference in polyphenol content could be leveraged in to develop region-specific extracts with optimized health benefits.
Comparing our results with those of other studies, the discrepancies in phenolic content may be attributed to differences in extraction methods, plant parts used, and preparation techniques35. For instance, variations in solvent type, extraction time, and temperature can significantly impact the yield of phenolic compounds. The use of methanol as a solvent, known for its efficiency in extracting a broad range of phytochemicals, likely contributed to the higher phenolic content observed in our study.
Total flavonoids content
The determination of total flavonoid levels as shown in Table 1 revealed that the extract from Relizane was significantly richer in flavonoids (9.42 mg QE/g) compared to the extract from Mostaganem (4.69 mg QE/g), representing a difference ratio of 50.15%. This result was statistically significant with p < 0.05. The flavonoids content was higher than those reported by Karouche et al.32. Our results were comparable to those found by Chizzola et al.28, who attributed these levels to environmental and climatic conditions, collection period, genetic factors, and experimental conditions. The significantly higher flavonoid content in the Relizane extract suggests that the environmental conditions in Relizane, such as temperature, sunlight exposure, and soil composition, are more conducive to flavonoid biosynthesis36. This observation was consistent with previous findings33,34, which highlighted the influence of climatic conditions on the production of secondary metabolites.
Determination of tannins
The condensed tannin content (Table 1) was significantly (p < 0.05) higher in the samples collected from Mostaganem (1.30 mg CE/g) compared to those from Relizane (0.69 mg CE/g), representing a difference ratio of 46.92%. These results indicate that the Mostaganem extract has the highest tannin content. However, this content was lower than that reported by Bansal et al.37 for the same species, which was 17.24 µg EAT/mg. This discrepancy may be attributed to various factors, including environmental conditions, climatic and agronomic factors, geographic region, genetic factors, processing, and storage [38–38.
The higher tannin content in the Mostaganem extract can be attributed to specific environmental conditions, such as higher humidity and varying soil compositions, which promote tannin biosynthesis. These findings are consistent with the research of Kumari et al.39 and Taghizadeh et al.40, who found that tannin levels are significantly affected by environmental and agronomic factors. The observed lower tannin content compared to Ogawa and Yazaki41, could be attributed to differences in extraction techniques, seasonal variations, or the age of the plant material at the time of extraction. Standardizing units and clearly defining terms, such as “CE” (catechin equivalent) and “EAT” (epicatechin equivalent) are crucial to prevent confusion and ensure clarity. This study underscores the need for a deeper understanding of how specific environmental factors and genetic variations influence the production of tannins in B. bulbocastanum.
Antioxidant activity
DPPH free radical scavenging
The DPPH free radical scavenging test (Table 1) revealed that the ground nut extract from Relizane exhibited a significantly higher DPPH scavenging capacity (p < 0.05) compared to the extract from Mostaganem. Specifically, the inhibition percentage for Relizane ground nut extract was 70.14%, while the Mostaganem ground nut extract showed an inhibition percentage of 40.90%. These results indicate that the antioxidant activity of the Relizane extract is superior to that of the Mostaganem extract. However, the inhibition percentages observed in this study were significantly lower than those reported by Karouche et al.32, who found an inhibition percentage of 75.41% at the same concentration for the methanolic extract from the tubers of B. bulbocastanum.
The lower DPPH scavenging activity observed in this study suggests that the antioxidant power of the B. bulbocastanum tuber extracts from both Relizane and Mostaganem is relatively low. The slight higher antioxidant activity reported by Karouche et al.32 could be attributed to differences in methodology and experimental conditions. The geographical and environmental conditions of Relizane, which may promote the accumulation of antioxidant compounds, appear to contribute to the relatively higher DPPH scavenging activity observed in its extracts compared to those from Mostaganem. This observation aligns with findings from other studies, such as Shahsavari et al.42, who reported significant antioxidant activity in essential oils from B. persicum, and Adelifar and Rezanejad33, who highlighted the influence of environmental conditions on antioxidant capacity in different organs of Bunium species.
The DPPH assay was used as general, simple and fast indication of antioxidant activity of B. bulbocastanum from the different locations. Although the DPPH assay is valuable for evaluating radical scavenging activity, it is subject to several limitations, such as non-physiological aspects, selectivity towards hydrogen-donating processes, susceptibility to experimental factors, and possible disruption by non-antioxidant substances. Additional experimental tests should be conducted to assess the antioxidant activity of this plant.
Antimicrobial activity
The antimicrobial potency of B. bulbocastanum crude extract from two different regions was evaluated in vitro using the AWDT well diffusion method on Mueller-Hinton agar, a widely recognized medium for antimicrobial testing43. The antimicrobial activity was assessed by measuring the diameter of the inhibition zone surrounding the wells containing the crude extract. This assay was conducted against five microorganisms from the laboratory collection, including three Gram-negative bacteria: P. aeruginosa ATCC 27853, E. coli ATCC 25922, and K. pneumoniae, one Gram-positive bacterium: S. aureus ATCC 33862, and one fungus: C. albicans ATCC 10231.
The antimicrobial properties of the Relizane extract (Table 2) demonstrated significant efficacy against E. coli ATCC 25922, C. albicans ATCC 10231, and S. aureus ATCC 33862, with lower activity observed against P. aeruginosa ATCC 27853 and K. pneumoniae ATCC E47. The inhibition zone diameters ranged from 7.66 to 20 mm, with the highest activity recorded against S. aureus ATCC 33862 (20 mm). C. albicans ATCC 10231 exhibited an inhibition diameter of 14.66 mm, closely followed by E. coli ATCC 25922 (14.33 mm). In contrast, K. pneumoniae ATCC E47 showed a modest an inhibition diameter of 9.00 mm, while P. aeruginosa ATCC 27853 displayed the smallest inhibition zone at 7.66 mm.
The Mostaganem extract demonstrated antimicrobial activity against all tested bacteria. The highest sensitivity was observed in S. aureus, with an inhibition zone of 21 mm, followed by C. albicans (10.00 mm) and E. coli (9.66 mm). The smallest inhibition zones were observed for P. aeruginosa (7.66 mm) and K. pneumoniae (8.33 mm). Both the Relizane and Mostaganem extracts exhibited antibacterial effects across all tested strains, aligning with previous findings by Bousetla et al.44, who reported significant antibacterial activity in the hydro-methanolic extract of B. incrassatum. These results are further supported by the systematic review by Adoui et al.21, which emphasized the substantial antimicrobial properties of the Bunium genus. Furthermore, Majidi et al.45 reviewed the ethnopharmacology, phytochemistry, and biological activities of B. persicum, reinforcing the antimicrobial potential observed in our study. Additionally, Bansal et al.37 provided a comprehensive review of B. persicum, emphasizing its medicinal value and antimicrobial activity, which further supports our findings. Moreover, Bakhti et al.46 demonstrated the antimicrobial activity of zinc oxide nanoparticles synthesized using B. persicum plant extract, indicating the plant’s potential to enhance antimicrobial properties through various applications. The previous study reported chemical composition of essential oils from different Bunium species, reinforcing the importance of phytochemical constituents in the antimicrobial efficacy of these extracts47. The variability in inhibition diameters highlights the potential of B. bulbocastanum as a source of antimicrobial agent. These results contribute to the growing body of evidence supporting the use of plant-based extracts in antimicrobial treatments, emphasizing the need for further research to isolate and characterize the active compounds responsible for these effects.
Bioactive metabolites of Bunium bulbocastanum
The UHPLC-ESI-MS/MS analysis was utilized in both negative and positive ion modes to identify the metabolites present in this fingerprint profile. All of the chemicals compounds detected in Relizane and Mostaganem samples are listed in Tables 3 and 4. By careful examination of MS-MS of samples spectral data, retention time, and comparability with available literature data, the metabolites were tentatively identified. Furthermore, the dereplication provided by library database of MzMine and UV spectra of the detected metabolites provided valuable insights, allowing for the classification of metabolite groups by comparing their absorption wavelengths with those reported in the literature. This comprehensive LC–MS/MS method enabled the detailed profiling of up to 43 phytochemicals in B. bulbocastanum. The identified compounds included various alkaloids, flavonoids, terpenoids, and saponins, highlighting the plant’s significant medicinal and nutritional properties. Notably, compounds such as quercetin, kaempferol, and rutin were detected in high concentrations, indicating their potential roles in the plant’s antioxidant and anti-inflammatory activities. The optimized LC–MS/MS method stands out for its precision, sensitivity, and reliability in phytochemical analysis. By providing detailed chromatographic and mass spectrometric conditions, along with thorough validation data, this study offers a robust framework for the comprehensive identification and quantification of phytochemicals in B. bulbocastanum.
In Mostaganem’s samples (Table 3), Compound 1 displayed a pseudo-molecular ion at m/z 147 in negative mode. which was tentatively identified as trans-cinnamic acid (m/z 147.0524) with the chemical formula C9H8O2,, based on research published by Redeuil et al.48. The fragmentation pattern of this compound revealed two prominent base peaks at m/z 119 and m/z 103. The fragments at m/z 119 [M-H-CO] resulted from the loss of the (CO) moiety from the parent ion; while, the fragment at m/z 103 [M-H- CO2] was formed by the loss of the (CO2) moiety. Compound 2 was observed at m/z 150.0681 in the positive mode, and it has been proposed as hydrocinnamic acid (C15H10O6). The key fragments ion that produced by this compound appeared at m/z 149 [M-H], consistent with findings from previous studies49. Compound 3 suggested as nordihydroguaiaretic acid (C18H22O4), exhibiting a pseudo- molecular ion at m/z 301.2 in the negative mode [M-H], followed by the loss of [M-H-C7H6O2] to form a fragment ion at m/z 122. These findings are in agreement with those reported by Ražná et al.50.
The negative electrospray mass spectrum of compound 4 displayed a [M − H] ion at m/z 227 in the negative mode. According to Steckel and Schlosser51, two major fragment ions at 143 and 185 were produced by the m/z 227 spectrum. The loss of 42 (C2H2O) from the ion at m/z 227 generated the fragment ion at m/z 185. Following the loss of 42(C2H2O), the m/z 185 further fragmented to m/z 143. Compound 4 has been tentatively assigned as resveratrol (C14H22O4). Compound 5 exhibited a pseudo- molecular ion at m/z 179 [M − H], Considering its molecular weight, retention time, and fragments ion produced in the negative mode, this compound was successfully identified as 3-methoxyhydrocinnamic acid (C10H10O3). The intense fragment ion of 3-methoxyhydrocinnamic acid at m/z 120 resulted from the loss of (CO), followed by the loss of OCH3 moieties. The production of the final fragment ion at m/z 104 appeared after the loss of CO2 and OCH3 from the parent ion. Compound 6 was identified as p-coumaric acid (C9H8O3), with a pseudo-molecular ion at m/z 163 [M-H] in negative mode. The compound generated a fragmentation ion at m/z 119 [M-H-CO2], which is a characteristic fragment ion for the coumaric acids derivatives, as reported by Wang et al.14. Compound 7 was tentatively assigned as m-coumaric acid, which is an isomer of compound 6. The MS/MS spectrum of this compound in the negative mode has shown the same principal fragment ion at m/z 119 [M + H- C2O]14.
Compound 8, which was detected in the negative mode, displayed a pseudo-molecular ion at m/z 197 [M-H] and was identified as syringic acid (C9H9O5). The MS/MS spectrum of this compound revealed characteristic fragment ions at m/z 182 [M + H- CH3], and 153 [M + H- CO2], consistent with previously reported syringic acid fragmentation patterns14,52. Compound 9 was identified as 4-ethulphenol (C8H10O), as described by del Mar Contreras and Castro52. In positive mode, it produced a product ion at m/z 123 [M + H]. Meanwhile, compound 9 showed more fragments with ions at m/z 109 [M + H-CH3] and m/z 95 [M + H-CH3–O]. Compound 10 was tentatively identified as salicylic acid (C7H6O3). The MS/MS spectrum of this compound in the negative mode displayed a principal fragment ion at m/z 137 [M-H]. The compound gave two extra fragments ions at m/z 108 [M-H- CO] and m/z 93 [M-H-CO2]52.
In the negative mode, compound 11 displayed a pseudo-molecular ion at m/z 223 [M-H]. The primary fragment ion of this compound was detected at m/z 208 [M-H-CH3]. allowing for the identification of the molecule as sinapic acid (C11H12O5) based on the methodology used in this study and comparison with MS and MS/MS data from the literature as reported by del Mar Contreras and Castro52. Similarly, compound 12, which was identified as ferulic acid (C10H10O4) since it has a pseudo-molecular ion at m/z 193 [M-H] in the negative mode. The loss of the CH3 moiety resulted in the formation of a fragment ion at m/z 178 [M-H-CH3], which was previously identified as the major fragment ion of ferulic acid. Among the distinctive fragment ions were two more that were seen at m/z 149 [M-H-CH3–CO] and m/z 134 [M-H-CH3–CO–CH3]. These fragments were previously discussed by del Mar Contreras and Castro52.
The MS2 base peak fragmentation ions of compound 13 were observed at m/z 169 [M-H], which was tentatively identified as gallic acid. The fragment ion at m/z 125 was detected as well by the neutral loss of CO2, which is characteristic MS fragments of a gallic acid. Compound 14, with a pseudo-molecular ion at m/z 125 in the negative mode and predicted molecular formula (C6H6O3) was proposed to be phloroglucinol53. Compound 15 was identified as 3,5-dimethoxyphenol (C8H10O3), showing a pseudo-molecular ion at m/z 155 [M + H] in positive mode. It produced a fragment ion at m/z 110 [M − H−CO2], indicative of the loss of CO2 and hydrogen, alongside a ion fragment at m/z 69, suggesting the presence of a benzene ring. Compound 16 that has been assigned as homovanillic acid (C9H10O4) was found at m/z 181 [M − H] in negative mode. It generated a fragment ion at m/z 137 [M − H−CO2] with 100% relative intensity due to loss CO2 moiety. Add to the list compound 17 which was detected at m/z 169 [M + H] in positive mode. Since this compound regenerates a principal fragment ion at m/z 119 [M + H-CO–CH3] due to loss CO2, suggesting it was acetylphloroglucinol (C8H8O4). Additionally, compound 18 detected at m/z 179 [M − H], was identified as caffeic acid (C9H8O4), compound 19 at m/z 152 [M-H] which was 4-acetocatechol (C7H8O2), and compound 20 at m/z 161 [M − H] which was α-methylcinnamic acid (C10H10O2), were formed an intensive fragments ion at m/z 135, m/z 109, and m/z 117 respectively. All these produced fragments were formed by the loos of CO2 moiety53. In this sample, compound 21 was identified as tyrosol (C8H10O2), detected at m/z 137 [M − H] with a fragment ion at m/z 110 [M-H- C2H2] due to loss C2H2. Compound 22 showed a base peak m/z 237 [M − H] and fragment at m/z 103 [M − H-3(OC2H3). This compound was successfully identified as 3,4,5- trimethoxycinnammic acid (C12H14O5). Additionally, compound 23 at m/z 95 [M + H], and compound 24 at m/z 151 [M − H] were detected with their main and only fragment ions leading to their identification as phenol (C6H6O), and 2- acetylresorcinol (C8H8O3), respectively. In contrast, several compounds were found only in sample from Relizane, namely 4-hydroxybenzoic acid, dihydrocaffeic acid, ferulic acid, hydroferulic acid, sinapic acid, m-coumaric acid, vanillic acid, coniferyl alcohol.
Materials and methods
Earth nut tubers sampling
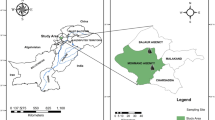
Sampling of B. bulbocastanum L. tubers was conducted in June 2023 from Sidi Ali (Mostaganem) and Ammi Moussa (Relizane), located in Northwest Algeria. Taxonomic identification and classification were performed by late Dr Bahi Kheira and Prof Dr Bouzouina Mohamed, who are botanists from the Department of Agronomy, Faculty of Nature and Life Sciences, UMAB University, following standard taxonomic protocols to ensure precise identification. The voucher specimen (LABUB23062018) was deposited at the Herbarium of the Faculty. Fresh and mature tubers, characterized by uniform size (1.5–2.5 cm diameter), color, and spherical shape, were selected. Maturity was determined by the absence of green coloration and full tuber development. The samples were cleaned with water and dried with tissue to remove dirt and debris22,24. Visual inspection was done to ensure homogeneity in color, size, and spherical geometry.
Drying procedure
The drying process commenced with storing the samples at 4 °C to mitigate physiological and chemical changes. Convective hot air drying was employed using a cabinet food dehydrator set at 105 °C for 24 h, based on previous studies indicating optimal conditions for preserving bioactive compounds in similar tubers58. Prior to drying, samples were equilibrated to room temperature. This method followed the analytical protocols outlined by Chemists and Horwitz59, ensuring a consistent drying process that minimized compounds loss while preserving bioactive substance integrity. Post drying, the tubers were ground into a fine powder to increase surface area for subsequent extraction procedures. Methodological Consistency and Standardization Methodological choices, including the use of a cabinet food dehydrator and fine grinding, were justified through relevant literature citations and empirical results from preliminary experiments. Adhering to AOAC guidelines provided a standardized and reproducible methodological framework. Controls and duplicates were incorporated in the sampling and drying processes to ensure methodological reliability and statistical robustness.
Total phenolic compounds
The total phenolic content of B. bulbocastanum tubers was performed using the method of Singleton and Rossi60, as adapted by Miliauskas et al.61. A 1 mL aliquot of the extract (1 mg/mL) was combined with 5 mL of Folin-Ciocalteu reagent (2 M), diluted tenfold with distilled water. After a 5 min incubation at room temperature, 4 mL of sodium carbonate solution (75 g/L) was added. Following 1 h of incubation at room temperature, the absorbance was measured at 765 nm using a Jenway 6715 spectrophotometer against a blank. A standard calibration curve was prepared using gallic acid, with concentrations ranging from 0 to 100 µg/mL. The total phenolic content was expressed as milligrams of gallic acid equivalents per gram of crude extract (mg GAE/g).
Total flavonoid content
Total flavonoid content was determined using the method described by Chang et al.62. A 0.75 mL volume of 2% aluminum chloride (AlCl₃) in methanol was mixed with an equal volume of the extract. The mixture was incubated in the dark at room temperature for 10 min. The absorbance was then measured at 430 nm using a spectrophotometer. A standard calibration curve was prepared using quercetin, with concentrations ranging from 0 to 100 µg/mL. The flavonoid content was expressed as milligrams of quercetin equivalents per gram of dry matter (mg QE/g).
Condensed tannin content
Condensed tannin content was quantified using the method of Broadhurst and Jones63, with modifications by Heimler et al.64. A 400 µL aliquot of the extract was added to 3 mL of a 4% vanillin methanolic solution, followed by the addition of 1.5 mL of concentrated hydrochloric acid. The reaction was allowed to proceed for 15 min at room temperature. The absorbance was measured at 550 nm using a spectrophotometer. A standard calibration curve was prepared using catechin, with concentrations ranging from 100 to 1000 µg/mL. The condensed tannin content was expressed as milligrams of catechin equivalents per gram of crude extract (mg CE/g).
DPPH radical scavenging test
The antioxidant potency of the methanolic extracts of B. bulbocastanum tubers was evaluated using the DPPH radical scavenging assay, as described by Zakaria et al.65. Fifty microliters of 5000 ppm of methanolic extract were mixed with 5 mL of DPPH methanolic solution (0.004%). The mixture was incubated at a controlled room temperature of 25 °C for 30 min. The absorbance was measured at 517 nm using a spectrophotometer, considering the characteristic absorption peak of the DPPH radical at this wavelength. The blank sample consisted of 5 mL of DPPH methanolic solution without any extract. The percentage of DPPH radical inhibition was calculated using the following formula:
where Ablank is the absorbance of the blank and Asample is the absorbance of the sample containing the extract.
Antimicrobial activities
Reactivation of pathogenic strains
The pathogenic strains Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 33862, Pseudomonas aeruginosa ATCC 27853, Candida albicans ATCC 10231, and Klebsiella pneumoniae ATCC E47 IV.2.2 were reactivated in Brain Heart Infusion Broth (BHIB) and incubated at 37 °C for 24 h to ensure purity and viability. For each antagonism test, the pathogenic strains were inoculated into BHIB broth and incubated at 37 °C for 24 h to obtain a fresh culture. The optical density of the culture was adjusted to a range of 0.08–0.1 at 600 nm, corresponding to 108 CFU/mL, following the method described by Kishor et al.66.
Antimicrobial well diffusion test (AWDT)
The antimicrobial activity of the extracts was evaluated using the well diffusion method described by Barefoot and Klaenhammer43. Fifteen milliliters of Muller Hinton agar, inoculated with 100 µL of a 24-h culture (108 CFU/mL) of the test organism, were poured into a Petri dish. After the agar solidified, wells of 6 mm diameter were created using a sterile pipette tip. Each well was filled with 50 µL of the crude extract. The plates were then incubated at 37 °C for 24 h. Following incubation, antimicrobial activity was assessed by measuring the diameter of the inhibition zones around the wells. Positive controls (antibiotics known to be effective against the test organisms) and negative controls (solvent without plant extract) were included to validate the results. All tests were performed in triplicate to ensure reproducibility.
LC–MS/MS identification of phytochemicals in Bunium Bulbocastanum
In the analysis of B. bulbocastanum phytochemicals, a precise LC–MS/MS method was employed. Initially, a methanol extract of the plant was prepared, diluted to a concentration of 1000 mg/L, and filtered using a 0.2 μm syringe filter. The analytical system comprised a UHPLC Nexera connected to an 8040 triple quadrupole mass spectrometer, incorporating a degasser, dual pumps, a column oven, and an autosampler. Chromatographic separation was performed on a C18 column (150 mm x 2.1 mm, 1.7 μm particle size). The mobile phase consisted of water with 5 mM ammonium formate and 0.1% formic acid (solvent A) and methanol (solvent B). A detailed gradient program was used as follows: starting at 5% B, increasing to 95% B over 30 min, holding at 95% B for 5 min, and then returning to 5% B over 5 min, with a total run time of 40 min. The flow rate was set at 0.3 mL/min, and the column temperature was maintained at 40 °C to ensure optimal separation efficiency.
Mass spectrometry analysis employed Electrospray Ionization (ESI) in both positive and negative modes. Specific operational parameters included an ion source temperature of 400 °C, a capillary voltage of 4.5 kV, and a collision energy optimized for each phytochemical. The analysis was conducted in Multiple Reaction Monitoring (MRM) mode to ensure the specificity and sensitivity of the detections. Characteristic MRM transitions were selected for each phytochemical, allowing for accurate quantification. The method’s robustness was validated through rigorous testing for linearity, accuracy, precision, and detection limits. Linearity was confirmed with correlation coefficients (R²) greater than 0.99 for all analytes. Accuracy and precision were evaluated through intra-and inter day assays, with relative standard deviations (RSD) below 5%. Detection limits were established for each compound, ensuring reliable performance even at low concentrations.
Statistical analysis
All assays were conducted in triplicate (n = 3), and the results were expressed as mean values with their corresponding standard deviations (mean ± SD). The data were analyzed using one way analysis of variance (ANOVA) to evaluate the differences between the samples. A p value of less than 0.05 was considered statistically significant. The ANOVA was followed by post-hoc tests, including Tukey’s Honest Significant Difference (HSD) test, to determine specific differences between sample means67. This rigorous statistical approach ensures the robustness and reliability of the results, providing a comprehensive evaluation of the phytochemical content and biological activities of different B. bulbocastanum samples.
Conclusions
This comprehensive study highlights the significant therapeutic potential of the tubers of B. bulbocastanum, a species indigenous to the Algerian regions of Mostaganem and Relizane. Through rigorous quantification and analysis, it’s evidenced that these tubers are a rich source of bioactive compounds, particularly polyphenols, flavonoids, and condensed tannins, which contribute to their potential antioxidant and antibacterial properties. The observed variations in bioactive constituents between the extracts from Mostaganem and Relizane underscores the impact of environmental, climatic, and geographical factors on phytochemical profiles. These differences emphasize the importance of considering local conditions in the cultivation and therapeutic utilization of B. bulbocastanum, as these factors evidently impact the biosynthesis of key bioactive compounds. Furthermore, the identification of potent compounds, such as 4-methylguaiacol, caffeic acid dimethyl ether, syringic acid, and naringenin through LC–MS/MS analysis accentuates the tuber’s medicinal viability. These compounds, known for their health promoting effects, present a compelling case for the integration of B. bulbocastanum into pharmaceutical endeavors, potentially offering remedies for contemporary health challenges. Moreover, the pronounced antibacterial activity against pathogenic strains reaffirms the tuber’s role in traditional medicine, particularly as a viable alternative in the era of antibiotic resistance. There was a scarce of information about the phytochemical and biological analyses of B. bulbocastanum. This is the first report comparing the effect of different location on phytochemical and biological properties of this plant. This research thereby not only bridges the gap between traditional practices and scientific inquiry but also propels B. bulbocastanum to the forefront of discussions on natural bioactive sources for health enhancement. This detailed phytochemical profiling underscores the plant’s medicinal and nutritional significance, paving the way for further pharmacological investigations.
Data availability
The data involved in this study is available in the manuscript. Any other data can be available upon request from the corresponding author.
References
Alamgir, A. N. M. Therapeutic Use of Medicinal Plants and Their Extracts: Volume 1 (Springer, 2017).
Giannenas, I., Sidiropoulou, E., Bonos, E., Christaki, E. & Florou-Paneri, P. The history of herbs, medicinal and aromatic plants, and their extracts: Past, current situation and future perspectives. In Feed Additives 1–18 (Elsevier, 2020).
Nwozo, O. S., Effiong, E. M., Aja, P. M. & Awuchi, C. G. Antioxidant, phytochemical, and therapeutic properties of medicinal plants: A review. Int. J. Food Prop.26(1), 359–388 (2023).
Zhao, X. et al. Lycium barbarum L. leaves ameliorate type 2 diabetes in rats by modulating metabolic profiles and gut microbiota composition. Biomed. Pharmacother.121, 109559. https://doi.org/10.1016/j.biopha.2019.109559 (2019).
Srivastava, A. K., Kashyap, P. L., Santoyo, G. & Newcombe, G. Plant microbiome: Interactions, mechanisms of action, and applications. Front. Microbiol.12, 706049 (2021).
Daley, D. K. & Badal, S. Plant crude drugs. In Pharmacognosy 75–89 (Elsevier, 2024).
Mustafa, G., Arif, R., Atta, A., Sharif, S. & Jamil, A. Bioactive compounds from medicinal plants and their importance in drug discovery in Pakistan. Matrix Sci. Pharma1(1), 17–26 (2017).
Anand, U., Jacobo-Herrera, N., Altemimi, A. & Lakhssassi, N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites9(11), 258 (2019).
Sun, W. & Shahrajabian, M. H. Therapeutic potential of phenolic compounds in medicinal plants: Natural health products for human health. Molecules28(4), 1845 (2023).
Ahmed, M. H., Karkush, S. I., Ali, S. A. & Mohammed, A. A. Phytochemicals: A new arsenal in drug discovery. Int. J. Med. Sci. Dent. Health10(01), 29–44 (2024).
Gupta, N., Shukla, S. & Bharatia, R. A review on some potential traditional phytomedicine with antidiabetic properties. Int. J. Pharma Prof. Res.15(1), 97–104 (2024).
Parfait, B. T. S. & Lawrence, M. T. J. Pharmacognosy, phytotherapy and modern medicine. Int. J. Infect. Dis. Epidemol.23(4), 1 (2023).
Farkas, D. Kratom Alkaloid Mitragynine: Therapeutic Role and Potential Utility Against Chemotherapy-Induced Peripheral Neuropathy (Temple University Libraries, 2023).
Wang, Z. et al. In vitro antioxidant analysis of flavonoids extracted from Artemisia argyi stem and their anti-inflammatory activity in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem.407, 135198 (2023).
Melrose, J. The potential of flavonoids and flavonoid metabolites in the treatment of neurodegenerative pathology in disorders of cognitive decline. Antioxidants. 12 (3), 663 (2023).
Agatonovic-Kustrin, S., Kustrin, E., Gegechkori, V. & Morton, D. W. Anxiolytic terpenoids and aromatherapy for anxiety and depression. Rev. New. Drug Targets Age-Relat. Disord. 283–296 (2020).
Powder-George, Y. L. Terpenoids. In Pharmacognosy 253–294 (Elsevier, 2024).
Kaur, R. et al. Industrial and environmental applications of plant-derived saponins: An overview and future prospective. J. Plant. Growth Regul. 1–15 (2024).
Boutlelis, D. A. Etude phytochimique et activité antimicrobienne, antioxydante, antihépatotoxique du Marrube blanc ouMarrubium vulgare L. (UniversitéBadji Mokhtar de Annaba, Départeme nt de Biologie, 2014).
Giancarlo, S., Rosa, L. M., Nadjafi, F. & Francesco, M. Hypoglycaemic activity of two spices extracts: Rhus coriaria L. and Bunium persicum Boiss. Nat. Prod. Res.20(9), 882–886 (2006).
Adoui, N. et al. Ethnomedicinal uses, phytochemistry and biological activities of Talghouda (Bunium fontanesii Batt. and related synonyms): A review. J. EcoAgriTourism18(1) (2022).
Attoui, N., Berroukeche, F. & Toul, F. Effect of the roots of Algerian Bunium incrassatum on biological, biochemical and histological parameters of mature female rats. Plant. Arch.21(1) (2021).
Chentouh, S. et al. Effets des extraits organiques de Bunium incrassatum sur quelques paramètres hématologiques chez les lapines de Population la race locale (2018).
Warda, B. A., Djilali, B., Said, D., Ahmed, B. & Zineb, B. Physicochemical and rheological properties of ‘Bunium bulbocastanum’ earth-nut flour. Agric. Sci. Dig.43(6), 829–833 (2023).
Haleng, J., Pincemail, J., Defraigne, J. O., Charlier, C. & Chapelle, J. P. Le stress oxydant. Rev. Med. Liege62(10) (2007).
Hennebelle, T., Sahpaz, S. & Bailleul, F. Polyphénols végétaux, sources, utilisations et potentiel dans la lutte contre le stress oxydatif. Phytothérapie2, 3–6 (2004).
Souri, E., Amin, G., Farsam, H. & Barazandeh, T. M. Screening of antioxidant activity and phenolic content of 24 medicinal plant extracts (2008).
Chizzola, R., Saeidnejad, A. H., Azizi, M., Oroojalian, F. & Mardani, H. Bunium persicum: Variability in essential oil and antioxidants activity of fruits from different Iranian wild populations. Genet. Resour. Crop Evol.61, 1621–1631 (2014).
Khlifi, D. et al. Composition and anti-oxidant, anti-cancer and anti-inflammatory activities of Artemisia herba-alba, Ruta chalpensis L. and Peganum harmala L.. Food Chem. Toxicol.55, 202–208 (2013).
Aganga, A. A. & Mosase, K. W. Tannin content, nutritive value and dry matter digestibility of Lonchocarpus capassa, Zizyphus mucronata, Sclerocarya birrea, Kirkia acuminata and Rhus lancea seeds. Anim. Feed Sci. Technol.91(1–2), 107–113 (2001).
Pedneault, K. et al. Variations in concentration of active compounds in four hydroponically-and field-grown medicinal plant species. In IV International ISHS Symposium on Artificial Lighting, Vol. 580, 255–262 (2000).
Karouche, S., Benbott, A., Henouda, S., Malki, S. & Boudchicha, I. Evaluation of phenolic content and biological activities of Bunium mauritanicum tuberss. J. Fundam. Appl. Sci.12(2), 916–930 (2020).
Adelifar, N. & Rezanejad, F. A comparative study of essential oil constituents, total phenolics and antioxidant capacity of the different organs of four species of the genus bunium. Flavour. Fragr. J.36 (3), 384–394 (2021).
Toul, F., Djendar, A., Seladji, M. & Berroukeche, F. Algerian Bunium incrassatum seeds: Effects of extraction solvent polarity on phenolic profile and antioxidant activity. J. Turk. Chem. Soc. Sect. Chem.9(2), 415–422 (2022).
Zengin, G. et al. Chemical profiling and pharmaco-toxicological activity of Origanum sipyleum extracts: Exploring for novel sources for potential therapeutic agents. J. Food Biochem.43(11), e13003 (2019).
Atmani, D. et al. Antioxidant capacity and phenol content of selected Algerian medicinal plants. Food Chem.112(2), 303–309 (2009).
Bansal, S., Malhotra, E. V. & Joshi, P. Bioactive compounds and biological activities of black cumin (Elwendia persica (Boiss.) Pimenov & Kljuykov). In Bioactive Compounds in the Storage Organs of Plants 1–18 (Springer, 2023).
Manach, C., Scalbert, A., Morand, C., Rémésy, C. & Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr.79(5), 727–747 (2004).
Kumari, B., Tiwari, B. K., Hossain, M. B., Rai, D. K. & Brunton, N. P. Ultrasound-assisted extraction of polyphenols from potato peels: Profiling and kinetic modelling. Int. J. Food Sci. Technol.52(6), 1432–1439 (2017).
Taghizadeh, S. F., Davarynejad, G., Asili, J., Nemati, S. H. & Karimi, G. Assessment of phenolic profile and antioxidant power of five pistachio (Pistacia vera) cultivars collected from four geographical regions of Iran. Avicenna J. Phytomed.8(1), 33 (2018).
Ogawa, S. & Yazaki, Y. Tannins from Acacia mearnsii De Wild. Bark: Tannin determination and biological activities. Molecules23(4), 837 (2018).
Shahsavari, N., Barzegar, M., Sahari, M. A. & Naghdibadi, H. Antioxidant activity and chemical characterization of essential oil of Bunium persicum. Plant. Foods Hum. Nutr.63, 183–188 (2008).
Barefoot, S. F. & Klaenhammer, T. R. Detection and activity of lactacin B, a bacteriocin produced by Lactobacillus acidophilus. Appl. Environ. Microbiol.45(6), 1808–1815 (1983).
Bousetla, A., Zellagui, A., Derouiche, K. & Rhouati, S. Chemical constituents of the roots of Algerian Bunium incrassatum and evaluation of its antimicrobial activity. Arab. J. Chem.8(3), 313–316 (2015).
Majidi, Z., Bina, F., Kahkeshani, N. & Rahimi, R. Bunium persicum: A review of ethnopharmacology, phytochemistry, and biological activities. Tradit Integr. Med. (2020).
Bakhti, M., Baghbani-Arani, F., Shishesaz, P. & Mahdavi-Ourtakand, M. Antimicrobial activity of zinc oxide nanoparticles synthesized using Bunium persicum plant extract. Qom Univ. Med. Sci. J.16(5), 366–377 (2022).
Jaimand, K. et al. Chemical composition essential oils of Bunium kuhitangi Nevski and Bunium microcarpum (Boiss) Freyn & Bornm. J. Med. Plants By-Prod.10(2), 179–182 (2021).
Redeuil, K. et al. Quantification of flavan-3-ols and phenolic acids in milk-based food products by reversed-phase liquid chromatography–tandem mass spectrometry. J. Chromatogr. A1216(47), 8362–8370 (2009).
Sharafati Chaleshtori, R. & Fallah, M. Antibacterial effect of Bunium persicum, Eucalyptus globules, and Allium ampeloprasum extracts on STEC and MRSA in commercial barley soup. Adv. Herb. Med.5(2), 9–20 (2019).
Ražná, K., Khasanova, N., Ivanišová, E., Qahramon, D. & Habán, M. Antioxidant properties of cumin (Bunium persicum Boiss.) extract and its protective role against ultrasound-induced oxidative stress tested by microRNA based markers. Potravinarstvo12(1) (2018).
Steckel, A. & Schlosser, G. An organic chemist’s guide to electrospray mass spectrometric structure elucidation. Molecules24(3), 611 (2019).
del Contreras, M. & Castro, E. Extraction Strategies to Recover Bioactive Compounds, Incorporation into Food and Health Benefits (MDPI, 2020).
Kim, H. et al. Determination of phloroglucinol in human plasma by high-performance liquid chromatography–mass spectrometry. J. Chromatogr. B792(2), 307–312 (2003).
Ali, A., Bashmil, Y. M., Cottrell, J. J., Suleria, H. A. R. & Dunshea, F. R. LC–MS/MS-QTOF screening and identification of phenolic compounds from Australian grown herbs and their antioxidant potential. Antioxidants10(11), 1770 (2021).
Sinosaki, N. et al. Structural study of phenolic acids by triple quadrupole mass spectrometry with electrospray ionization in negative mode and H/D isotopic exchange. J. Braz Chem. Soc.31, 402–408 (2020).
Martínez-Huélamo, M. et al. Sensitive and rapid UHPLC–MS/MS for the analysis of tomato phenolics in human biological samples. Molecules20(11), 20409–20425 (2015).
Karonen, M. & Pihlava, J. M. Identification of oxindoleacetic acid conjugates in quinoa (Chenopodium quinoa Willd.) seeds by high-resolution UHPLC–MS/MS. Molecules27(17), 5629 (2022).
Abano, E. E. Microwave assisted convective air drying of sugarloaf pineapples (ananas comosus). Appl. Eng. Agric.37(5), 763–774 (2021).
Chemists, A. A. Official Methods of Analysis, Vol I 15th ed AOAC, Arlington, VA (1990).
Singleton, V. L. & Rossi, J. A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic.16(3), 144–158 (1965).
Miliauskas, G., Venskutonis, P. R. & Van Beek, T. A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem.85(2), 231–237 (2004).
Chang, C. C., Yang, M. H., Wen, H. M. & Chern, J. C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. food drug Anal.10(3) (2002).
Broadhurst, R. B. & Jones, W. T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric.29(9), 788–794 (1978).
Heimler, D., Vignolini, P., Dini, M. G., Vincieri, F. F. & Romani, A. Antiradical activity and polyphenol composition of local Brassicaceae edible varieties. Food Chem.99(3), 464–469 (2006).
Zakaria, Z., Aziz, R., Lachimanan, Y. L., Sreenivasan, S. & Rathinam, X. Antioxidant activity of Coleus blumei, Orthosiphon stamineus, Ocimum basilicum and Mentha arvensis from Lamiaceae family. Int. J. Nat. Eng. Sci.2(1), 93–95 (2008).
Kishor, P. B. K. et al. Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: Its implications in plant growth and abiotic stress tolerance. Curr. Sci. 424–438 (2005).
Lohr, S. L. SAS® Software Companion for Sampling: Design and Analysis (Chapman and Hall/CRC, 2021).
Acknowledgements
This study was supported by Researchers Supporting Project Number (RSP2023R111), King Saud University, Riyadh, Saudi Arabia.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to accomplishing this study, Conceptualization, A.-W.B., D.B., Z.B., W.S.M.Q., A.M., A.B., H.A. and A.M.; Data curation, S.D.; Formal analysis, A.-W.B., D.B., S.D., Z.B., K.H., W.S.M.Q., E.A.-O., A.M., A.B., A.M. and N.B.; Investigation, A.-W.B., D.B., S.D., K.H., W.S.M.Q., E.A.-O., A.M., A.B., H.A. and A.M.; Methodology, A.-W.B., D.B., S.D., Z.B., K.H., E.A.-O., A.M., A.B., H.A. and N.B.; Software, D.B., S.D., Z.B., W.S.M.Q., E.A.-O., A.M., A.B., H.A., A.M. and N.B.; Supervision, H.A. and A.M.; Validation, D.B., S.D., K.H., E.A.-O., A.M., A.B., H.A. and A.M.; Visualization, A.-W.B., D.B., Z.B., K.H., W.S.M.Q., E.A.-O., A.M., A.B., H.A. and A.M.; Writing—original draft, A.-W.B.; Writing—review and editing, A.-W.B., D.B., S.D., Z.B., K.H., W.S.M.Q., H.A., A.M. and N.B.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bouhalla, AW., Benabdelmoumene, D., Dahmouni, S. et al. Comparative LC–MS-based metabolite profiling, antioxidant, and antibacterial properties of Bunium bulbocastanum tubers from two regions in Algeria. Sci Rep 14, 21719 (2024). https://doi.org/10.1038/s41598-024-72758-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-024-72758-5
- Springer Nature Limited