Abstract
Pancreatic ductal adenocarcinoma (PDAC) presents a fatal clinical challenge characterized by a dismal 5-year overall survival rate, primarily due to the lack of early diagnosis and limited therapeutic efficacy. Immunotherapy, a proven success in multiple cancers, has yet to demonstrate significant benefits in PDAC. Recent studies have revealed the immunosuppressive characteristics of the PDAC tumor microenvironment (TME), including immune cells with suppressive properties, desmoplastic stroma, microbiome influences, and PDAC-specific signaling pathways. In this article, we review recent advances in understanding the immunosuppressive TME of PDAC, TME differences among various mouse models of pancreatic cancer, and the mechanisms underlying resistance to immunotherapeutic interventions. Furthermore, we discuss the potential of targeting cancer cell-intrinsic pathways and TME components to sensitize PDAC to immune therapies, providing insights into strategies and future perspectives to break through the barriers in improving pancreatic cancer treatment.
Similar content being viewed by others
Introduction
Pancreatic ductal adenocarcinoma (PDAC), accounting for 90% of pancreatic tumors, remains a formidable malignancy with a dismal 5-year survival rate of merely 12%1. More than 80% of patients are diagnosed at an advanced stage, either locally advanced or metastatic disease, rendering curative surgical intervention futile2,3. Although gemcitabine in combination with albumin-bound paclitaxel or modified FOLFIRINOX (5-fluorouracil, leucovorin, irinotecan, and oxaliplatin) has been established as the standard first-line chemotherapeutic protocol for metastatic cases2, the clinical median survival still falls short of 1 year4,5.
Cancer immunotherapeutic approaches, including immune checkpoint blockade (ICB), chimeric antigen receptor (CAR) T-cell therapies, and cancer vaccines, have achieved significant advancements in treating various cancers6,7,8, such as melanoma, lung cancer, renal cell carcinoma, and lymphoma9,10,11,12. However, their effectiveness in PDAC remains disappointing6. Clinical studies utilizing immune checkpoint inhibitors (ICIs), including anti-programmed death ligand-1 (anti-PD-L1) or anti-cytotoxic T-lymphocyte-associated protein-4 (anti-CTLA-4) mono-immunotherapy and combination therapy, have not been successful in treating pancreatic cancer13,14,15. In a recent phase 2 trial of metastatic PDAC, combining ICIs (durvalumab and tremelimumab) with chemotherapy (gemcitabine and nab-paclitaxel) did not improve survival compared with chemotherapy alone16.
The immunosuppressive tumor microenvironment (TME) in PDAC, a major factor contributing to immunotherapy resistance, includes tumor-infiltrating immune-suppressive cells, stromal cells, the microbiome, and the extracellular matrix (ECM). The immune infiltration in PDAC is characterized by an abundance of suppressive cells, a deficiency of anti-tumor immune cells, and immune dysfunction17,18,19. Exploring combination strategies involving immunotherapy and agents tailored to target these TME characteristics has emerged as a prominent area of research in pancreatic cancer.
In this article, we review recent advances in understanding the immunosuppressive TME of PDAC, describe TME differences among various animal models, discuss the mechanisms of immune resistance induced by TME and tumor cells, and summarize strategies aimed at improving the efficacy of immunotherapy in pancreatic cancer.
Highly immunosuppressive tumor microenvironment in PDAC
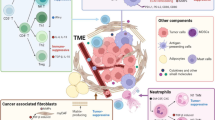
The TME of PDAC consists of various immune-suppressive cells, including immunosuppressive myeloid cells, M2 macrophages, N2 neutrophils, mast cells, Th2 cells, regulatory T cells, and regulatory B cells (Fig. 1 and Table 1). On the other hand, there is notable dysfunction and deficiency of anti-tumor immune cells, including CD8 + T cells, conventional dendritic cells, natural killer cells, M1 macrophages, N1 neutrophils, and Th1 cells (Fig. 1 and Table 2). The suppressive immune cells impede the cytotoxic T-cell-mediated tumor ablation effect, either directly or indirectly through inhibition of dendritic cells. In addition, the microbiome, stromal cells, and ECM modulate immune cell infiltration and function, contributing to the establishment of an immunosuppressive TME.
a Schematic representation of the interplay among tumor cells, tumor-infiltrating immune cells, and cancer-associated fibroblasts (CAFs) in the PDAC TME. Tumor cells and CAFs secrete chemokines and growth factors, such as granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), CXCL2/5, and CXCL12 to recruit suppressive immune cells to tumor tissues. Pro-tumor immune cells contribute to the exhaustion of effector T cells and the activation of CAFs. Activated CAFs, in turn, support tumor growth through desmoplasia and inflammatory cytokines such as IL-6; in addition, they may cooperate with mast cells to promote tumor cell proliferation and metastasis. Upregulation of immune checkpoint molecules (e.g., PD-L1 and TIGIT) on tumor cells and immune cells, as well as downregulation of MHC-I, contribute to T-cell dysfunction. b Pro-tumor cells include myeloid-derived suppressor cells (MDSCs), M2 macrophages, N2 neutrophils, regulatory T cells, regulatory B cells, mast cells, and Th2 cells. Anti-tumor immune cells include CD8 + T cells, dendritic cells (DC), M1 macrophages, natural killer (NK) cells, N1 neutrophils, and Th1 cells.
Immunosuppressive myeloid cells
Suppressive myeloid cells in the TME can be broadly categorized into myeloid-derived suppressor cells (MDSCs) comprising granulocytic MDSCs and monocytic MDSCs, tumor-associated macrophages (TAMs) derived from either the bone marrow or resident tissue macrophages6,20, tumor-associated neutrophils (TANs), and mast cells. These myeloid cells are recruited to the TME by various factors and attenuate anti-tumor T-cell responses in PDAC21,22.
MDSCs
MDSCs are a subset of anti-inflammatory, immunosuppressive cells, originating from immature myeloid cells under various pathological conditions such as chronic inflammation, cancer, and autoimmune disease23. In pancreatic cancer, MDSCs exert immunosuppressive functions and promote immune evasion through EGFR-MAPK-dependent upregulation of PD-L1 expression in tumor cells24. It has also been reported that MDSCs deplete nutrition through arginase-1 and the Xc- transporter, resulting in the downregulation of the T-cell receptor (TCR) and restriction of T-cell activation6,25. In addition, MDSCs can promote regulatory T (Treg) cell induction in a cell-cell-dependent manner26,27. In an autochthonous PDAC model, depletion of granulocytic MDSCs elevated CD8 + T-cell infiltration and increased tumor cell apoptosis28. Moreover, reducing MDSCs through loss or inhibition of CXCR2 mitigated tumor metastasis and conferred sensitivity to anti-PD-1 therapy, thus prolonging survival in mice with pancreatic cancer29. Notably, a recent preclinical study by DePinho and colleagues30 demonstrated that inhibition of chemokine receptors on MDSCs (by using a CXCR1/2 inhibitor) combined with modulation of T-cell immune checkpoints (by using a 41BB agonist and a LAG3 antagonist) could reprogram the highly suppressive tumor immune microenvironment of pancreatic cancer. This approach led to durable responses and survival benefits in a mouse model of PDAC, suggesting a potential clinical strategy.
TAMs
TAMs in the PDAC TME are characterized by an enrichment of pro-tumor M2-like phenotypes and a relatively low presence of anti-tumor M1-like phenotypes19. The immune suppression mediated by these pro-tumor TAMs stems from their ability to hamper the anti-tumor activity of CD8+ cytotoxic T lymphocytes by supporting PD-L1 expression in tumor cells and depleting nutrition in T cells24,27,31. Moreover, TAMs hinder adaptive immune responses through the dectin-1/galectin-9 axis32 and facilitate the production of immunosuppressive factors in tumor cells, such as CXCL1 and CXCL5, through elevated expression of apolipoprotein E (ApoE)33.
In PDAC models, reprogramming TAMs through the blockade of receptor-interacting serine/threonine protein kinase 1 (RIP1) leads to activation of cytotoxic T cells and differentiation of T helper (Th) cells into a mixed Th1/Th17 phenotype34. Previous studies have revealed the pro-tumor effects of Th2 cells and the anti-tumor effects of Th1 cells34,35. While the precise function of combined Th1/Th17 phenotypes remains to be defined in tumors, they appear to possess significant immunogenicity and are associated with the downregulation of FOXP3, a biomarker of Treg cells34. Interestingly, an exosome-based dual delivery biosystem, featuring electroporation-loaded galectin-9 siRNA and surface modification with an oxaliplatin prodrug, effectively reversed M2-like phenotypes of TAMs and enhanced anti-tumor immunity in mice36. In addition to their role in immune suppression, TAMs support cancer cells by secreting growth factors such as TGF-β37,38 and producing cytokines and chemokines that accelerate tumor metastasis directly or indirectly39,40,41.
A study comparing immune infiltrates in pancreatic cancer and melanoma identified VISTA (V-domain immunoglobulin suppressor of T-cell activation) as a potential immune checkpoint primarily expressed on CD68+ macrophages in PDAC42. Targeting VISTA-positive macrophages holds promise as a strategy to augment CD8 + T-cell responses and treat pancreatic cancer. Furthermore, a recent preclinical study demonstrated that dual antagonism of CCR2 and CCR5 (CCR2/5i), when combined with radiation therapy and an anti-PD-1 antibody, resulted in a reduction in tumor infiltration by Tregs, M2-like TAMs, and MDSCs43. Notably, this combination treatment increased intratumoral effector and memory T cells, supporting the clinical development of CCR2/5i in combination with radiation therapy and ICB for the treatment of PDAC.
TANs
Neutrophils are major players in innate immunity. A single-cell RNA-seq (scRNA-seq) analysis uncovered a terminally differentiated subpopulation of TANs exhibiting hyperactivated glycolysis and pro-tumor functions in PDAC, which is associated with unfavorable prognosis in patients44. Pro-tumor neutrophils secrete immunosuppressive cytokines and chemokines, thereby inhibiting the activity of cytotoxic CD8 + T cells45. Moreover, neutrophil extracellular traps (NETs) have been reported to contribute to immunotherapy resistance induced by TANs. One of the inducers of NETs, IL17, which is upregulated in PDAC, recruits neutrophils while excluding cytotoxic CD8 + T cells from tumors46. In preclinical mouse models, targeting TANs with lorlatinib has been shown to enhance the response to PD-1 blockade, highlighting the potential of modulating pro-tumor neutrophils in the TME as a treatment strategy for PDAC47.
Aside from primary tolerance, the replenishment of TANs is a contributing factor to therapy resistance in pancreatic cancer. Targeting CCR2+ macrophages led to a compensatory influx of CXCR2+ TANs, a phenomenon associated with poor outcomes in PDAC patients; interestingly, dual inhibition of CCR2+ TAMs and CXCR2+ TANs significantly improved anti-tumor immunity and chemotherapeutic responses in orthotopic models of PDAC48. Similar to the plasticity of TAMs, recent studies have revealed the plasticity of TANs—the presence of both N1 and N2 phenotypes in the TME of PDAC patients. The N1/N2 ratios positively correlated with CD8 + T-cell infiltration, median overall survival (OS), and recurrence-free survival, and inversely correlated with the abundance of tumor-infiltrating Tregs49. In mouse models of PDAC, blockade of the TGF-β1 receptor promoted the polarization of neutrophils into an anti-tumor N1 phenotype, thus enhancing the response of tumors to the combination treatment with irreversible electroporation (which ablates tumors by inducing irreversible membrane destruction of cells) and anti-PD-150.
Mast cells
Mast cells, like neutrophils and macrophages, originate from myeloid progenitor cells. Compared with normal tissues, PDAC tissues exhibit a significant increase in infiltration by mast cells, which promote the proliferation and invasion of pancreatic cancer cells and contribute to chemotherapy resistance51,52,53,54. The exact role and mechanisms by which mast cells regulate tumor immunity in PDAC are not fully understood. Nevertheless, it is worth noting that in preclinical models of melanoma, combining anti-PD-1 therapy with the depletion of mast cells resulted in tumor regression55, suggesting that mast cells may suppress the immune response and limit the efficacy of immunotherapies.
Treg cells
Treg cells are a specialized subset of T cells that modulate effector T cells. The abundance of FOXP3+ Treg cells within pancreatic tumors increases during PDAC progression and correlates with poor survival56,57,58. The immunomodulatory effect of Treg cells has been studied in different models59,60. Bar-Sagi and colleagues61 reported that Treg cells inhibit the function of dendritic cells through direct contact, leading to the downregulation of dendritic cell-derived costimulatory ligands that are crucial for CD8 + T-cell activation in PDAC. This study also demonstrated that the elimination of Treg cells induced an effective anti-tumor immune response in mice bearing orthotopic pancreatic tumors derived from a KPC model (expressing mutant forms of Kras and Trp53)61. On the other hand, however, Pasca di Magliano and colleagues62 reported that the removal of Treg cells accelerated tumor progression by reprogramming cancer-associated fibroblasts in genetically engineered mouse models of PDAC. The conflicting results from orthotopic and autochthonous models indicate context-dependent crosstalk between Tregs and other cell types in pancreatic cancer TME.
B cells
Plasma cells, a subtype of terminally differentiated B cells, play an essential role in amplifying anti-tumor immune responses through antibody production. However, in PDAC patients and tumor-bearing mice, cancer can induce differentiation of naïve B cells into regulatory B cells (as opposed to plasma cells) through Bruton tyrosine kinase (BTK) signaling, which results in a reduction in tumor-infiltrating cytotoxic T cells, thereby contributing to immune evasion63,64. In addition, BTK induces the programming of T(H)2-type macrophages and diminishes CD8 + T-cell cytotoxicity by facilitating the communication between B cells and TAMs in PDAC65. Furthermore, extensive research has advanced the understanding of the mechanisms of immunosuppression induced by B cells and IL-3563,66,67,68,69. Targeting B cells through molecular blockade has shown promise in reducing tumor growth and disease progression, presenting a therapeutic strategy for treating PDAC in combination with immunotherapy63,64,65,68,69,70,71.
Stromal cells and extracellular matrix (ECM)
PDAC is characterized by extensive desmoplasia, where fibroblasts are the major cell type72. A subset of fibroblasts (myofibroblasts), along with tumor cells and macrophages, can produce a dense fibrotic matrix composed of ECM proteins, thereby influencing the progression of PDAC and its response to treatment73,74,75. The excessive desmoplastic stroma limits the penetration of tumors by drugs and cytotoxic CD8 + T cells76. Addressing this challenge, enzymatic degradation of hyaluronic acid (HA) has been shown to alleviate desmoplastic pressure. This approach not only expands the microvasculature but also contributes to a 2-fold increase in overall survival in mouse models when combined with chemotherapy77,78. In human PDAC tissues, elevated focal adhesion kinase (FAK) activity correlates with increased fibrosis and poor CD8 + T-cell infiltration79. Treatment with a FAK inhibitor led to a reduction in fibrosis and tumor-associated immunosuppressive cells, sensitizing the p48-Cre;LSL-KrasG12D/+;Trp53flox/+ mouse model of PDAC to T-cell immunotherapy and immune checkpoint inhibitors79. Furthermore, combining HA degradation with FAK inhibition promoted the survival of PDAC-bearing mice treated with an anti-PD-1 antibody80; notably, this combination treatment enhanced T-cell infiltration while concurrently reducing MDSCs.
Besides producing dense ECM, CAFs interact with immune cells and induce immune suppression through secreted factors such as IL-6, CXCL12, granulocyte-macrophage colony-stimulating factor (GM-CSF), and macrophage colony-stimulating factor (M-CSF)81,82. The removal of CAFs expressing fibroblast activation protein (FAP) has been shown to inhibit tumor growth and enhance the efficacy of anti-CTLA-4 and anti-PD-L1 antibodies in mouse models of PDAC83. On the other hand, however, depletion of alpha smooth muscle actin (αSMA)-positive CAFs hampers immune surveillance, leading to an increase in Treg cells and shorter survival in mice with PDAC84. Taken together with other studies73,74,85,86,87, these findings underscore the functional heterogeneity of CAFs. CAFs can be classified into inflammatory CAFs (iCAFs), αSMA + myofibroblasts (myCAFs), and antigen-presenting CAFs (apCAFs), with a small subset of CAFs being derived from pancreatic stellate cells74,87,88. While apCAFs support immune evasion by inducing expansion of Treg cells89, the pro-tumor role of iCAFs is associated with the cytokine IL-6, which suppresses anti-tumor immunity by eliciting metabolic stress and dendritic cell apoptosis90,91. Moreover, induction of iCAFs by IL-17A-producing CD8 + T cells promotes PDAC progression and is associated with a poor prognosis92. myCAFs are the major source of type I collagen in the PDAC stroma, and depletion of type I collagen leads to upregulation of Cxcl5 in tumor cells, which promotes the recruitment of MDSCs and dysfunction of CD8 + T cells, thereby accelerating pancreatic cancer progression and decreasing survival93. Intriguingly, whereas intact type I collagen triggers degradation of discoidin domain receptor 1 (DDR1) and impedes PDAC, matrix-metalloprotease-cleaved type I collagen promotes PDAC growth by activating the DDR1–NF-κB–NRF2 axis94.
Stroma-modulating drugs have shown the potential to enhance the efficacy of ICB therapy in preclinical models of PDAC. For instance, combination treatment with the sonic hedgehog inhibitor cyclopamine and the chemotherapeutic drug paclitaxel increased the infiltration of CD8 + T cells into tumors. A synergistic effect of this combination with anti-PD-1 therapy was observed in both orthotopic and genetically engineered mouse models of PDAC76. More recently, the combination of MEK inhibitor (MEKi) with STAT3 inhibitor (STAT3i) demonstrated promising results in mitigating the polarization of iCAFs and enriching CAFs with mesenchymal stem cell-like features. The resulting stroma remodeling facilitated the M2-to-M1 reprogramming of TAMs, improved the trafficking of CD8 + T cells, and impeded the infiltration of myeloid cells95. When the MEKi + STAT3i combination was added to anti-PD-1 treatment, prolonged survival was observed in PDAC-bearing mice compared with anti-PD-1 treatment alone. These findings suggest that targeting the stromal components holds promise as a strategy to overcome immunotherapy resistance in PDAC. The exploration of stroma-modulating drugs in combination with immunotherapies may pave the way for improved treatment outcomes.
Microbiome
Recent studies have implicated the microbiome in the pathogenesis and immune suppression in PDAC96,97. The gut microbiome can translocate into pancreatic cancer tissues, modulating the tumor microbiome and altering the immune landscape of TME97,98 (Fig. 2). Ablation of the gut microbiome by oral antibiotics has been shown to reverse the immunosuppressive TME. This reversal is characterized by an increase in anti-tumor interferon-γ-producing T cells, Th1 cells, and M1 macrophages, as well as a decrease in pro-tumor IL-17a- and IL-10-producing T cells and MDSCs, thereby enhancing the efficacy of ICB therapy97,99. Moreover, fungus-dependent IL-33 secretion by PDAC cells plays a role in recruiting and activating Th2 cells and innate lymphoid cells 2 (ILC2)100; these cells, in turn, contribute to immune suppression by secreting pro-tumorigenic cytokines. In addition, pathogenic fungi can promote PDAC progression via a mannose-binding lectin (MBL)–C3 axis96. It has also been reported that compared with healthy individuals, Proteobacteria, Synergistetes, and Euryarchaeota are enriched in PDAC patients, reprograming TAMs toward a pro-tumor M2-like phenotype through Toll-like receptor (TLR) signaling97. Intriguingly, Lactobacillus metabolization of dietary tryptophan fosters TAM polarization toward an immune-suppressive phenotype through the aryl hydrocarbon receptor, accelerating PDAC progression101.
a Intratumoral fungus-mediated IL-33 secretion by PDAC cells recruits and activates Th2 cells and innate lymphoid cells 2 (ILC2), which stimulate tumor growth by secreting pro-tumorigenic cytokines such as IL-4, IL-5, and IL-13. b Pathogenic fungi promote PDAC progression by driving the complement cascade via a mannose-binding lectin (MBL)–C3 axis. c Proteobacteria, Synergistetes, and Euryarchaeota are enriched in PDAC patients, reprogramming TAM toward a pro-tumor M2-like phenotype through Toll-like receptors (TLRs). (d) Lactobacillus-derived indole fosters TAM polarization toward an immune-suppressive phenotype through the aryl hydrocarbon receptor (AhR). Figure created with BioRender.com.
Collectively, these findings highlight the complex relationship between the microbiome and the immune response in PDAC, opening up potential avenues for therapeutic interventions. For instance, the delivery of gut microbiome-derived metabolite trimethylamine N-oxide (TMAO) has been shown to enhance anti-tumor immunity and restrain tumor growth in orthotopic models of PDAC. When combined with anti-PD-1 therapy, TMAO delivery led to a significant reduction in tumor burden and prolonged survival compared with treatment with TMAO or ICB alone102. In a study led by McAllister and colleagues98, analysis of the tumor microbiome in PDAC patients by using 16S rRNA gene sequencing revealed higher microbial diversity in patients with longer survival. Notably, human-to-mice fecal microbiota transplants from control, long-term survival, or short-term survival donors differentially modulated the tumor microbiome, tumor growth, and tumor immune infiltration98. Furthermore, a metagenomic analysis uncovered specific microbiome species associated with PDAC, including enrichment of Streptococcus and Veillonella spp and depletion of Faecalibacterium prausnitzii103. This study suggests that microorganisms could serve as potential sources of biomarkers for pancreatic cancer. It should be noted that recent re-analyses of previously reported pan-cancer microbial composition data have identified significant pitfalls, including contamination, false positive classifications, problematic handling of batch effects, and limitations in the machine learning approaches used104, which warrant corrections and future improvement.
Immune features of PDAC metastases
The liver and lung are the common sites of metastasis in patients with PDAC105,106. In general, patients with liver metastases have a poorer prognosis compared with those with lung metastases, indicating inherent disparities between these metastatic sites105,106. In mouse models of metastatic PDAC, significant differences were observed in the TME between the liver and the lung: whereas the liver exhibited immunosuppressive characteristics, the lung TME showed high levels of immune infiltration and activated immune signaling105. Using cytometry by time-of-flight, Jaffee, Fertig, and colleagues analyzed a mouse model of metastatic PDAC and found a reduction in dendritic cells, NK cells, cytotoxic T cells, and Th cells in the TME of liver metastases relative to those present in lung metastases105. Moreover, significant enrichment of PD-L1 and LAG3 was observed in the hepatic TME, along with higher levels of pro-tumorigenic chemokines such as CCL5, CCL22, and CXCL12 relative to the lung. In contrast, immune-activating chemokines, such as CXCL9 and CXCL10, were found to be enriched in lung metastases relative to liver metastases105.
A recent investigation of patient samples revealed significant immune heterogeneity in PDAC recurrences across various sites including the liver, lung, peritoneum, and local areas106. This study demonstrated low immunogenicity, stemness, and innate immune responses in patients with liver and/or peritoneal recurrences, contrasting with notable interferon-γ signaling and mixed adaptive and innate immune responses in PDACs with local and/or lung recurrences. In addition, accumulation of P2RX1-negative neutrophils was found in PDAC liver metastases, alongside various suppressive cells such as macrophages and fibroblasts, which promote metastatic progression in the liver107,108,109. Furthermore, JAK-STAT–dependent macrophage-fibroblast crosstalk was reported to facilitate liver metastatic outgrowth in PDAC. Notably, pharmacological inhibition of STAT3 or myofibroblastic metastasis-associated fibroblast (myMAF)-specific genetic depletion of STAT3 restored anti-tumor immunity and reduced liver metastases109.
TME differences among various mouse models of pancreatic cancer
Patient-derived xenograft (PDX) models, syngeneic mouse models, and genetically engineered mouse models (GEMMs) are widely used in pancreatic cancer research and preclinical drug testing110. It is important to recognize that these models exhibit distinct TMEs, necessitating careful selection based on the specific experimental objectives in preclinical studies. Moreover, there is a critical need to emphasize comparative validation across multiple models to ensure the robustness and reproducibility of findings.
Cell line-derived xenograft and PDX models of pancreatic cancer are widely used for gene function studies and evaluation of therapeutic approaches111. These models are categorized based on the implantation site into orthotopic and subcutaneous models. Subcutaneous models facilitate non-surgical implantation of tumor cells or tissue fragments, whereas orthotopic models, despite being more technically challenging to establish, better recapitulate tumor growth in the natural tissue microenvironment compared with subcutaneous models. It is important to note that PDX models require immunodeficient mice (typically nude mice, SCID mice, or NOD-SCID mice) to prevent immune rejection of human-derived xenografts. However, this immunodeficiency limits their utility for studying the immune cell composition of tumors or evaluating immunotherapy efficacy. Addressing this limitation, humanized mouse models have been developed for immunotherapy studies. For example, Chang and colleagues112 isolated CD34+ hematopoietic stem cells from human umbilical cord blood and injected them into 3- to 4-week-old NSG mice to establish a humanized model. Treatment with siRNA nanoparticles targeting PD-L1 upregulated interferon-γ-positive CD8 + T cells and inhibited pancreatic tumor growth in this model, underscoring the potential of humanized PDAC PDX models for advancing immunotherapy research.
Syngeneic mouse models of PDAC involve implanting immunologically compatible cancer cells or tissues into mice with an intact immune system, which distinguishes them from xenograft models. These models can also be established through subcutaneous or orthotopic implantation110. Orthotopic tumors actively interact with the tissue microenvironment, providing a more physiologically relevant model. However, due to the technical challenges associated with orthotopic models, subcutaneous models remain commonly used in preclinical drug trials. It should be noted that cell line- or model-dependent effects are often observed. For example, in a study investigating the potential of a CD47 monoclonal antibody to enhance the response of PDAC to ICIs, combination therapy targeting CD47 and PD-L1 showed synergistic inhibition of tumor growth in the Panc02 but not in the MPC-83 syngeneic mouse model113. This indicates that treatment responses may vary between models derived from different PDAC cell lines. scRNA-seq analysis revealed that anti-CD47 treatment reshaped the intratumoral lymphocyte and macrophage populations in Panc02 tumor-bearing mice, resulting in increased intratumoral CD8 + T cells, more active T-cell clusters, enhanced anti-tumor pro-inflammatory macrophages, and reduced anti-inflammatory macrophages113. These findings underscore the suitability of specific syngeneic models with intact immune systems for investigating the intratumoral immune microenvironment and conducting preclinical trials of immunotherapy.
GEMMs allow immunocompetent mice to spontaneously develop pancreatic cancer, eliminating the need for exogenous implantation methods. They have become indispensable for evaluating various therapeutic strategies111. Among these models, the LSL-KrasG12D/+;Trp53flox/flox;Pdx-1-Cre (KPC) model is a commonly used GEMM capable of generating spontaneous pancreatic tumors that closely mimic human PDAC, with features including prominent connective tissue hyperplasia, abnormal vascular distribution, and high metastatic potential. These tumors also exhibit extensive infiltration of immunosuppressive macrophages and low numbers of effector T cells110. Studies on KPC mice have demonstrated that CD40 activation induces tumor regression through a T-cell-independent mechanism. This finding aligns with histological analyses of human tumors treated with a combination of CD40 agonists and gemcitabine. However, these results differ from those observed in the implantable KPC model114,115, suggesting that GEMMs may be more similar to humans in terms of the TME and the mechanisms of immunotherapy response. This highlights the importance of model selection.
In conclusion, various PDAC mouse models exhibit significant differences in TME and response to immunotherapy. For preclinical experiments, selecting models with intact immune function, tumor pathological features, and microenvironments similar to those of humans is important for studying pancreatic carcinogenesis, metastasis, and immunotherapeutic strategies.
Pancreatic cancer cell-intrinsic factors contributing to immunotherapy resistance
In addition to the immunosuppressive TME of PDAC cells, tumor-intrinsic features also contribute to immune evasion (Fig. 3). KRAS mutations are present in more than 95% of PDAC116, among which the major mutations are G12D (40%), G12V (33%), and G12R (15%)117 (Fig. 3a). Downstream signaling pathways and metabolic networks in mutant KRAS (mKRAS)-driven tumors play a pivotal role in immune suppression and tumor progression116,117. For instance, mKRAS leads to upregulation of CXCL1, CXCL5, and GM-CSF through NF-κB, PI-3K, or MAPK pathways, fostering the proliferation, maturation, and recruitment of immunosuppressive myeloid cells33,118,119. Moreover, mKRAS boosts PD-L1 expression on tumor cells through p38 and MAPK pathways, thereby activating the PD-1/PD-L1 checkpoint and causing T-cell exhaustion24,120. In addition, mKRAS modulates the tumor cell metabolism, increasing glucose uptake, and aerobic glycolysis, as well as the production of reactive oxygen species (ROS)121,122. ROS, in turn, mediates mKRAS-induced PD-L1 expression through FGFR1 signaling122. It has also been shown that mKRAS recruits immunosuppressive IL-17-producing T cells35 and promotes the formation and maintenance of fibro-inflammatory stroma123.
a Mutated KRAS (mKRAS), which is permanently bound to GTP, constitutively activates downstream signaling pathways, resulting in PD-L1 overexpression and the recruitment of immunosuppressive cells to pancreatic tumors. b Aberrant activation of the WNT–β-catenin pathway, which can be caused by mutation of RNF43 or lncRNA-mediated inhibition of β-catenin degradation, reduces dendritic cell recruitment by downregulating CCL4 and upregulating PD-L1 expression. c Lipophilic glucocorticoids (GCs) diffuse through the cell membrane and bind to the glucocorticoid receptor (GR) in the cytoplasm of PDAC cells. This binding induces a change in the chaperone complex bound to GR, leading to its translocation into the nucleus. Once in the nucleus, GR activates PD-L1 expression and represses MHC-1 expression by binding to glucocorticoid response elements (GREs), ultimately leading to the reduction in the abundance and effector function of tumor-infiltrating CD8 + T cells.
Activation of WNT signaling is often observed in pancreatic cancer124,125 (Fig. 3b). In PDAC, WNT pathway activation is associated with the aberrant expression of WNT ligands. Moreover, a subset of PDAC tumors carry mutations in RNF43, which encodes ring finger 43, a ubiquitin ligase that inhibits WNT signaling by ubiquitinating FZD receptors and LRP5/6 co-receptors, leading to their internalization and lysosomal degradation126,127. The loss of RNF43 in a genetically engineered mouse model of PDAC accelerated tumor progression and upregulated regulatory T-cell immune checkpoint molecules, which could be a potential mechanism of immune evasion128. In addition, lncRNA-mediated inhibition of β-catenin degradation has been observed in pancreatic cancer cells129,130. Although current evidence indicates that WNT pathway activation is immunosuppressive in PDAC, the role of WNT–β-catenin signaling in regulating cancer immunosurveillance may be cancer-type-dependent131.
In addition to KRAS mutation and RNF43 loss, downregulation of major histocompatibility complex class I (MHC-I) contributes to immune evasion and immunotherapy resistance due to impaired antigen presentation. Kimmelman and colleagues132 demonstrated that in pancreatic cancer cells, an autophagy-dependent mechanism, involving the autophagy cargo receptor NBR1, targets MHC-I molecules for lysosomal degradation. A subsequent study revealed that progranulin from tumor cells (not macrophages) correlates with poor overall survival in PDAC. Inhibition of progranulin effectively halted autophagy-dependent degradation of MHC-I and restored MHC-I expression in pancreatic cancer cells. Moreover, antibody-based progranulin blockade in a mouse model of PDAC impeded the initiation and progression of tumors133. Glucocorticoid receptor (GR) signaling was thought to suppress immunity by acting on immune cells134. In a recent study, our laboratory uncovered a new role of GR as a transcriptional activator of PD-L1 and a transcriptional repressor of MHC-I in pancreatic cancer cells135 (Fig. 3c). In preclinical models, either genetic depletion or pharmacological inhibition of GR promoted the infiltration and effector function of cytotoxic T cells, leading to enhanced immune surveillance and sensitization of pancreatic tumors to ICIs135. These findings highlight GR signaling in pancreatic cancer cells as a tumor-intrinsic mechanism of immune suppression and suggest that therapeutic intervention targeting GR holds promise for improving the responsiveness of pancreatic cancer to immunotherapy. Furthermore, it should be noted that p53, which is frequently altered in PDAC, has been shown to promote antigen processing and MHC-1 surface expression136,137. Both aspects of antigen presentation are downregulated in p53-null and p53-mutant cancer cells136,137.
Metabolic enzymes have crucial roles in multiple aspects of cancer, including tumorigenesis, progression, metastasis, and therapy resistance. Recently, Sherman and colleagues138 reported that pancreatic cancer cell-intrinsic glutamic-oxaloacetic transaminase 2 (GOT2), a key player in the malate-aspartate shuttle, remodels TME to suppress anticancer immunity138. Mechanistically, GOT2 promotes the transcriptional activity of nuclear receptor peroxisome proliferator-activated receptor delta (PPARδ) to restrict CD4+ and CD8 + T-cell infiltration of the TME, revealing a non-canonical function for this metabolic enzyme138. Moreover, Zhang and colleagues139 showed that deficiency in quinoid dihydropteridine reductase orchestrates a series of events culminating in the recruitment of MDSCs, which ultimately induce immunosuppression in PDAC. In addition, pancreatic cancer cells can impair NK cell activity by competitively depleting vitamin B6, thereby compromising anti-tumor immunity140. Interestingly, metabolism-focused CRISPR screens have identified genes linked to immune evasion in PDAC, including Tap1, Tapbp, and the autophagy gene Atg7141. Furthermore, tumor cell-intrinsic deficiency in the epigenetic regulator SETD2 has been shown to promote tumor progression through two mechanisms: 1) by enhancing mitochondrial oxidative phosphorylation through interactions with a subset of lipid-rich CAFs142, and 2) by boosting recruitment of immunosuppressive neutrophils through activation of the PI3K-AKT pathway143.
Reprogramming cancer microbiome has been linked to immune evasion, particularly in PDAC. The collagen I (Col1) homotrimer produced by pancreatic cancer cells fosters oncogenic signaling by binding to α3β1 integrin, resulting in the development of a tumor microbiome abundant in anaerobic Bacteroidales in hypoxic and immunosuppressive TME144. Deleting Col1 homotrimers in a mouse model of PDAC yielded significant benefits, including increased overall survival, enhanced T-cell infiltration, and improved responsiveness to anti-PD-1 immunotherapy144.
Therapeutic strategies and clinical trials of pancreatic cancer immunotherapies
Despite a number of breakthroughs in immunotherapies for multiple cancer types, their clinical utility in PDAC, whether administered as monotherapy or in combination with other therapies, has been insufficient. Nevertheless, extensive preclinical studies and clinical trials have provided valuable insights. In this section, we discuss three types of immunotherapeutic strategies for pancreatic cancer treatment (Table 3): (i) targeting myeloid cells, dendritic cells, or B cells to enhance T-cell trafficking and anti-tumor responses; (ii) reprogramming macrophages to be tumoricidal; and (iii) remodeling stroma cell and ECM.
Targeting myeloid cells
The reduction of immunosuppressive myeloid cells in the TME can be achieved by blocking the chemokine-receptor axis in these cells, resulting in diminished infiltration of myeloid cells. Targeting CD11b, CSF-1R, CCR2/5, and CXCR1/2 has emerged as therapeutic strategies.
CD11b is an integrin molecule expressed on myeloid cells, playing a role in chemotaxis and cellular functions. In preclinical studies, the administration of a small-molecule agonist of CD11b led to a reduction in suppressive myeloid cell infiltration and repolarization of TAMs toward an anti-tumor phenotype, thereby eliciting a T-cell response. This treatment also showed an enhancement in the therapeutic effects of an anti-PD-1 antibody145. However, a clinical trial (NCT04060342) investigating the combination of the CD11b agonist (GB1275) with gemcitabine, nab-paclitaxel, and pembrolizumab (an anti-PD-1 antibody) was terminated due to the lack of a clear benefit in PDAC patients when GB1275 was used either as a monotherapy or in combination with pembrolizumab146.
In an orthotopic model of PDAC, blocking CSF-1R not only reduced TAM infiltration but also reprogrammed TAMs to enhance T-cell activation, resulting in increased efficacy of anti-PD-1 and anti-CTLA-4147. Unfortunately, clinical trials combining an anti-CSF-1R antibody with immunotherapy for PDAC treatment have not yielded satisfactory results thus far. For example, a phase 1a/b single-arm study combining the anti-CSF-1R antibody cabiralizumab with PD-1 blockade (nivolumab) demonstrated discouraging outcomes with an objective response rate (ORR) of 6.0%, a median overall survival (OS) of 5.6 months, and a median progression-free survival (PFS) of 1.7 months (NCT02526017)148. Moreover, a phase 2 clinical study evaluating cabiralizumab in combination with nivolumab and chemotherapy did not improve PFS in patients with advanced PDAC (NCT03336216)6,149. Currently, several ongoing trials are testing the combination of small-molecule CSF-1R inhibitors with immunotherapy in pancreatic cancer. One such trial is a dose escalation phase 1 study evaluating the safety and efficacy of a small-molecule CSF-1R inhibitor (pexidartinib) combined with an anti-PD-L1 antibody (durvalumab) in patients with metastatic/advanced pancreatic or colorectal cancer (NCT02777710)150.
Chemokine receptors, CCR2/5 and CXCR2, play a crucial role in facilitating myeloid cell infiltration into the TME43,48. Blocking these pathways has emerged as a potential strategy to overcome immunosuppression. A phase 1b trial investigating the combination of the CCR2 inhibitor PF-04136309 with FOLFIRINOX not only demonstrated safety but also yielded an ORR of 49% and a disease control rate (DCR) of 97%, surpassing the 0% ORR and 80% DCR observed in the FOLFIRINOX alone group151. However, a phase 2 trial of PF-04136309 in combination with gemcitabine and nab-paclitaxel was terminated due to toxicity and a lack of superior efficacy compared with the gemcitabine plus nab-paclitaxel group152. Several early-phase clinical trials combining BMS-813160 (a CCR2/5 dual antagonist) with ICIs and chemotherapy or vaccines are underway (Table 3). Meanwhile, targeting CXCR2 with AZD5069 in combination with an anti-PD-L1 antibody in patients with metastatic PDAC resulted in disappointing results, with an ORR of 5.6%, OS of 2.8 months, and PFS of 1.6 months (NCT02583477)153. Currently, an early-phase clinical trial investigating a CXCR1/2 dual inhibitor (SX-682) plus an anti-PD-1 antibody (nivolumab) is recruiting patients with metastatic pancreatic cancer (NCT04477343)154.
Targeting dendritic cells
Pancreatic cancer often exhibits a paucity and dysfunction of dendritic cells, which underlies poor infiltration and effector function of T cells90,155,156. Restoring dendritic cells in PDAC might enhance anti-tumor immunity. CD40, a member of the tumor necrosis factor (TNF) receptor superfamily, holds the capacity to license dendritic cells for promoting anti-tumor T-cell activation157. Several formulations of agonistic CD40 antibodies have undergone testing in preclinical and clinical settings, demonstrating tolerability and feasibility. DeNardo and colleagues156 engineered a neoantigen-expressing mouse model of PDAC and showed that enhancing dendritic cell infiltration and activity using FLT3 ligand along with an agonistic CD40 antibody resulted in increased intratumoral CD8+ cells and prolonged survival when combined with radiation therapy. Currently, early-phase clinical trials are in progress, evaluating the combination of the agonistic CD40 antibody and FLT3 ligand with anti-PD-1, gemcitabine, and nab-paclitaxel for treating pancreatic cancer and other advanced cancers (e.g., NCT03329950)158.
Dendritic cell vaccination has emerged as a strategy to enhance anti-tumor immunity159. In a syngeneic Panc02 model of pancreatic cancer, combining dendritic cell-based vaccination with gemcitabine significantly improved the survival of tumor-bearing mice compared with vaccination or gemcitabine alone160. Moreover, the combination of an allogeneic tumor lysate-loaded dendritic cell vaccine with an agonistic CD40 antibody significantly increased survival in a mouse model of pancreatic cancer, which was accompanied by an increase in CD8 + T-cell infiltration161. A recent phase 1 clinical trial involving 10 patients with resected PDAC demonstrated the safety of an allogeneic tumor lysate-loaded autologous dendritic cell vaccine, with seven out of 10 patients showing no recurrence or progression at a median follow-up of 25 months162. An ongoing phase 1 clinical trial is evaluating the safety and efficacy of a tumor lysate-loaded dendritic cell vaccine in combination with an agonistic CD40 antibody in patients with metastatic PDAC following FOLFIRINOX chemotherapy (NCT05650918)163.
Targeting B cells
BTK-dependent crosstalk between B cells and TAMs has been identified as a driver of PDAC growth. In mice bearing PDAC, the use of a BTK inhibitor (ibrutinib) led to the restoration of anti-tumor immunity, inhibition of pancreatic tumor growth, and enhanced responsiveness to standard-of-care chemotherapy65. However, a phase 3 clinical trial combining ibrutinib with gemcitabine and nab-paclitaxel failed to show improvements in PFS and overall survival in PDAC patients compared with the placebo plus gemcitabine/nab-paclitaxel cohort164. Meanwhile, the combined treatment with acalabrutinib (a second-generation BTK inhibitor) and pembrolizumab produced modest clinical benefits, with an ORR of 7.9% and a DCR of 21.1%, compared with the 0% ORR and 14.3% DCR observed in the acalabrutinib monotherapy group165.
Reprogramming macrophages
Beyond its role in activating conventional dendritic cells, CD40 activation has been found to reprogram macrophages into tumoricidal TAMs in PDAC-bearing mice115. However, in a randomized phase 2 trial involving 105 patients with PDAC, sotigalimab, an agonistic CD40 antibody, did not demonstrate survival benefits when combined with chemotherapy (gemcitabine/nab-paclitaxel) and an anti-PD-1 antibody (nivolumab)166. Currently, a phase 1 clinical trial is recruiting patients with metastatic PDAC to assess the response to gemcitabine/nab-paclitaxel in combination with the anti-CTLA-4 antibody ipilimumab plus nivolumab, hydroxychloroquine, or NG350A (a CD40 agonist) (NCT04787991)167. In addition to CD40 agonism, the cellular inhibitor of apoptosis proteins 1 and 2 (cIAP1/2) antagonist has demonstrated the induction of T-cell-dependent reprogramming of TAMs to be tumoricidal, reducing tumor burdens by enhancing phagocytosis in orthotopic models of PDAC168. A phase 1 trial evaluating the combination of the cIAP1/2 antagonist ASTX660 with the anti-PD-1 antibody pembrolizumab is recruiting patients with pancreatic cancer or other solid tumors (NCT05082259)169.
CD47 expressed on tumor cells acts as a “don’t eat me” signal by engaging signal-regulating protein alpha (SIRPα) expressed on macrophages, thereby blocking phagocytosis. This phagocytic checkpoint has gained attention as an attractive target for immunotherapy170. Given the substantial T-cell dysfunction and exhaustion observed in PDAC and other “cold” tumor types, macrophage-based therapeutic strategies have emerged to address resistance to T-cell-based immunotherapy21. A phase1/2 clinical trial is currently recruiting patients to evaluate the safety and efficacy of PT886, a bispecific antibody targeting both CD47 and claudin 18.2—a tumor antigen that is overexpressed in PDAC (NCT05482893)171.
Targeting stroma
Following encouraging preclinical studies, clinical trials focused on targeting PDAC stroma to enhance therapy responses have emerged. In preclinical models, the degradation of hyaluronan by PEGPH20 demonstrated a significant survival benefit when combined with gemcitabine77,78. In PDAC patients not selected for tumor hyaluronan status, the addition of PEGPH20 to FOLFIRINOX led to increased toxicity and a reduced median OS (7.7 months compared with 14.4 months in the FOLFIRINOX alone group)172. On the other hand, in a randomized phase 3 clinical trial involving patients with hyaluronan-high metastatic PDAC, the combination of PEGPH20 with gemcitabine/nab-paclitaxel improved the ORR but did not increase OS or PFS when compared with the placebo plus gemcitabine/nab-paclitaxel arm173. Currently, a phase 2 trial is evaluating the combination treatment with PEGPH20 and pembrolizumab in patients with hyaluronan-high metastatic PDAC (NCT03634332)174.
In a genetically engineered mouse model of PDAC, the administration of an FAK inhibitor led to a reduction of fibrosis and increased sensitivity to immunotherapy (anti-PD-1 plus anti-CTLA-4)79. A phase 1 study demonstrated that the combination of defactinib (a small-molecule inhibitor of FAK) with PD-1 blockade plus gemcitabine resulted in stable disease in 11 out of 20 patients with metastatic PDAC175; this outcome was accompanied by an increase in CD8 + T cells and a decrease in Tregs, macrophages, and stromal cells. Currently, a phase 2 trial is underway to evaluate the efficacy of pembrolizumab plus defactinib following chemotherapy as a neoadjuvant and adjuvant treatment in patients with resectable PDAC (NCT03727880)176.
CXCL12, produced by FAP+ CAFs, inhibits T-cell infiltration in pancreatic cancer. In a preclinical model of PDAC, pharmacological inhibition of CXCR4, the cognate receptor of CXCL12, enhanced the anti-tumor efficacy of PD-L1 blockade83. A phase 1 study investigated the safety and tolerability of the CXCR4 antagonist LY2510924 in combination with the anti-PD-L1 antibody durvalumab, revealing modest clinical benefits, with three out of eight patients achieving stable disease177. Of note, a recent phase 2 trial assessing the combination of BL-8040 (a CXCR4 antagonist), pembrolizumab, and Onivyde (topoisomerase I inhibitor) demonstrated encouraging results, including an ORR of 32%, a DCR of 77%, and duration of response (DOR) of 7.8 months in patients with metastatic chemotherapy-refractory PDAC178.
The JAK-STAT pathway activates pancreatic stellate cells (PSCs) and induces inflammatory CAFs, contributing to immunosuppression in PDAC81,87,179. In a phase 2 study of ruxolitinib, a small-molecule JAK1/JAK2 inhibitor, in combination with the chemotherapeutic agent capecitabine, there was no observed benefit in OS generally, but a significant increase in OS was noted in patients with systemic inflammation compared with the placebo plus capecitabine group180. Unfortunately, two subsequent randomized phase 3 studies combining ruxolitinib and capecitabine for treating advanced/metastatic pancreatic cancer showed no improvement in OS or PFS181. Currently, a phase 1 clinical trial combining ruxolitinib, retifanlimab (an anti-PD-1 antibody), and trametinib (a MEK inhibitor) is recruiting patients with metastatic PDAC (NCT05440942)182.
Discussion
Both pancreatic cancer cell-intrinsic signaling pathways and the TME play significant roles in immune suppression, contributing to the inherent resistance of pancreatic cancer to immunotherapy. Various therapeutic strategies, when combined with immunotherapy, have shown encouraging results in preclinical studies. However, the translation of these findings into clinical benefits has been challenging thus far. It is crucial to elucidate the mechanisms by which human PDAC evades immune surveillance and to devise approaches that effectively overcome immunotherapy resistance in patients. It should be noted that unrestricted cell death or tissue damage induced by chemotherapy might lead to an immunosuppressive TME183. In contrast, radiation therapy was reported to enhance the response to immunotherapy in patients with microsatellite-stable pancreatic cancer184.
Improving pancreatic cancer immunotherapy involves exploring various strategies. Some key strategies and future perspectives include: (1) combination therapies: combining different immunotherapeutic agents, including inhibitors of established and newly discovered immune checkpoints, with targeted therapies or other immunomodulators, may enhance the overall anti-tumor response. (2) Stroma-targeted approaches: targeting the dense stroma in pancreatic cancer to reduce fibrosis holds promise for improving drug delivery and enhancing immune cell infiltration. (3) Vaccine development: developing personalized cancer vaccines based on individual tumor profiles has the potential to stimulate a specific and robust immune response against cancer cells. (4) Bispecific antibodies: designing antibodies that can simultaneously target multiple antigens is likely to enhance their specificity and efficacy in engaging immune cells against cancer. (5) Adoptive cell therapies: CAR T-cell therapies, which use genetically engineered T cells directed to specific cancer-associated antigens to elicit cytotoxic activity, represent a promising therapeutic modality, although significant challenges exist for CAR T-cells to infiltrate the immunosuppressive TME of pancreatic tumors. (6) Biomarker identification: identifying reliable biomarkers to predict the response to immunotherapy will allow for better patient stratification and treatment selection. (7) Clinical trial innovation: designing innovative clinical trials to test emerging therapies and combinations can ensure a rapid translation of promising preclinical findings into clinical benefits.
These strategies are active areas of research and development, with the potential to significantly impact the future of pancreatic cancer immunotherapy. In addition, investigating strategies for early detection of pancreatic cancer will enable timely intervention, potentially improving the success of immunotherapeutic approaches.
References
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023).
Park, W., Chawla, A. & O’Reilly, E. M. Pancreatic cancer: a review. JAMA 326, 851–862, (2021).
Mizrahi, J. D., Surana, R., Valle, J. W. & Shroff, R. T. Pancreatic cancer. Lancet 395, 2008–2020 (2020).
Von Hoff, D. D. et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 369, 1691–1703 (2013).
Conroy, T. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364, 1817–1825 (2011).
Ullman, N. A., Burchard, P. R., Dunne, R. F. & Linehan, D. C. Immunologic strategies in pancreatic cancer: making cold tumors hot. J. Clin. Oncol. 40, 2789–2805 (2022).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Lu, J. & Jiang, G. The journey of CAR-T therapy in hematological malignancies. Mol. Cancer 21, 194 (2022).
Larkin, J. et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 381, 1535–1546 (2019).
Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med. 378, 2078–2092 (2018).
Motzer, R. J. et al. Nivolumab plus Ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 378, 1277–1290 (2018).
Ansell, S. M. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 372, 311–319 (2015).
Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012).
Royal, R. E. et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother. 33, 828–833 (2010).
O’Reilly, E. M. et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 5, 1431–1438, (2019).
Renouf, D. J. et al. The CCTG PA.7 phase II trial of gemcitabine and nab-paclitaxel with or without durvalumab and tremelimumab as initial therapy in metastatic pancreatic ductal adenocarcinoma. Nat. Commun. 13, 5020 (2022).
Steele, N. G. et al. Multimodal mapping of the tumor and peripheral blood immune landscape in human pancreatic cancer. Nat. Cancer 1, 1097–1112 (2020).
Pollini, T. et al. The tumour immune microenvironment and microbiome of pancreatic intraductal papillary mucinous neoplasms. Lancet Gastroenterol. Hepatol. 7, 1141–1150 (2022).
Muller, M. et al. The immune landscape of human pancreatic ductal carcinoma: key players, clinical implications, and challenges. Cancers 14, https://doi.org/10.3390/cancers14040995 (2022).
Balachandran, V. P., Beatty, G. L. & Dougan, S. K. Broadening the impact of immunotherapy to pancreatic cancer: challenges and opportunities. Gastroenterology 156, 2056–2072 (2019).
Hosein, A. N., Dougan, S. K., Aguirre, A. J. & Maitra, A. Translational advances in pancreatic ductal adenocarcinoma therapy. Nat. Cancer 3, 272–286 (2022).
Vonderheide, R. H. & Bear, A. S. Tumor-derived myeloid cell chemoattractants and T cell exclusion in pancreatic cancer. Front. Immunol. 11, 605619 (2020).
Li, K. et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 6, 362 (2021).
Zhang, Y. et al. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut 66, 124–136 (2017).
Srivastava, M. K., Sinha, P., Clements, V. K., Rodriguez, P. & Ostrand-Rosenberg, S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 70, 68–77 (2010).
Siret, C. et al. Deciphering the crosstalk between myeloid-derived suppressor cells and regulatory T cells in pancreatic ductal adenocarcinoma. Front. Immunol. 10, 3070 (2019).
Gabrilovich, D. I., Ostrand-Rosenberg, S. & Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012).
Stromnes, I. M. et al. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut 63, 1769–1781 (2014).
Steele, C. W. et al. CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell 29, 832–845 (2016).
Gulhati, P. et al. Targeting T cell checkpoints 41BB and LAG3 and myeloid cell CXCR1/CXCR2 results in antitumor immunity and durable response in pancreatic cancer. Nat. Cancer 4, 62–80 (2023).
Xia, Q. et al. Tumor-associated macrophages promote PD-L1 expression in tumor cells by regulating PKM2 nuclear translocation in pancreatic ductal adenocarcinoma. Oncogene 41, 865–877 (2022).
Daley, D. et al. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat. Med. 23, 556–567 (2017).
Kemp, S. B. et al. Apolipoprotein E promotes immune suppression in pancreatic cancer through NF-κB-mediated production of CXCL1. Cancer Res. 81, 4305–4318 (2021).
Wang, W. et al. RIP1 kinase drives macrophage-mediated adaptive immune tolerance in pancreatic cancer. Cancer Cell 34, 757–774.e757 (2018).
McAllister, F. et al. Oncogenic Kras activates a hematopoietic-to-epithelial IL-17 signaling axis in preinvasive pancreatic neoplasia. Cancer Cell 25, 621–637 (2014).
Zhou, W. et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials 268, 120546 (2021).
Hou, P. et al. Tumor microenvironment remodeling enables bypass of oncogenic KRAS dependency in pancreatic cancer. Cancer Discov. 10, 1058–1077 (2020).
Zhang, B. et al. Macrophage-expressed CD51 promotes cancer stem cell properties via the TGF-β1/smad2/3 axis in pancreatic cancer. Cancer Lett. 459, 204–215 (2019).
Sun, X. et al. Inflammatory cell-derived CXCL3 promotes pancreatic cancer metastasis through a novel myofibroblast-hijacked cancer escape mechanism. Gut 71, 129–147 (2022).
Alonso-Nocelo, M. et al. Macrophages direct cancer cells through a LOXL2-mediated metastatic cascade in pancreatic ductal adenocarcinoma. Gut https://doi.org/10.1136/gutjnl-2021-325564 (2022).
Lee, B. Y. et al. Heterocellular OSM-OSMR signalling reprograms fibroblasts to promote pancreatic cancer growth and metastasis. Nat. Commun. 12, 7336 (2021).
Blando, J. et al. Comparison of immune infiltrates in melanoma and pancreatic cancer highlights VISTA as a potential target in pancreatic cancer. Proc. Natl Acad. Sci. USA 116, 1692–1697 (2019).
Wang, J. et al. CCR2/CCR5 inhibitor permits the radiation-induced effector T cell infiltration in pancreatic adenocarcinoma. J. Exp. Med. 219, e20211631 (2022).
Wang, L. et al. Single-cell RNA-seq analysis reveals BHLHE40-driven pro-tumour neutrophils with hyperactivated glycolysis in pancreatic tumour microenvironment. Gut https://doi.org/10.1136/gutjnl-2021-326070 (2022).
Hester, R., Mazur, P. K. & McAllister, F. Immunotherapy in pancreatic adenocarcinoma: beyond “copy/paste. Clin. Cancer Res. 27, 6287–6297 (2021).
Zhang, Y. et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J. Exp. Med. 217, e20190354 (2020).
Nielsen, S. R. et al. Suppression of tumor-associated neutrophils by lorlatinib attenuates pancreatic cancer growth and improves treatment with immune checkpoint blockade. Nat. Commun. 12, 3414 (2021).
Nywening, T. M. et al. Targeting both tumour-associated CXCR2+ neutrophils and CCR2+ macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 67, 1112–1123 (2018).
Chen, Q. et al. Prognostic value of tumor-associated N1/N2 neutrophil plasticity in patients following radical resection of pancreas ductal adenocarcinoma. J. Immunother. Cancer 10, e005798 (2022).
Peng, H. et al. Local release of TGF-β inhibitor modulates tumor-associated neutrophils and enhances pancreatic cancer response to combined irreversible electroporation and immunotherapy. Adv. Sci. 9, e2105240 (2022).
Ma, Y., Hwang, R. F., Logsdon, C. D. & Ullrich, S. E. Dynamic mast cell–stromal cell interactions promote growth of pancreatic cancer. Cancer Res. 73, 3927–3937 (2013).
Chang, D. Z. et al. Mast cells in tumor microenvironment promotes the in vivo growth of pancreatic ductal adenocarcinoma. Clin. Cancer Res. 17, 7015–7023 (2011).
Strouch, M. J. et al. Crosstalk between mast cells and pancreatic cancer cells contributes to pancreatic tumor progression. Clin. Cancer Res. 16, 2257–2265 (2010).
Shi, S., Ye, L., Yu, X., Jin, K. & Wu, W. Focus on mast cells in the tumor microenvironment: Current knowledge and future directions. Biochim. Biophys. Acta Rev. Cancer 1878, 188845 (2023).
Somasundaram, R. et al. Tumor-infiltrating mast cells are associated with resistance to anti-PD-1 therapy. Nat. Commun. 12, 346 (2021).
Stromnes, I. M., Hulbert, A., Pierce, R. H., Greenberg, P. D. & Hingorani, S. R. T-cell localization, activation, and clonal expansion in human pancreatic ductal adenocarcinoma. Cancer Immunol. Res. 5, 978–991 (2017).
Tang, Y. et al. An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS One 9, e91551 (2014).
Hiraoka, N., Onozato, K., Kosuge, T. & Hirohashi, S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin. Cancer Res. 12, 5423–5434 (2006).
Sakaguchi, S. et al. Regulatory T cells and human disease. Annu. Rev. Immunol. 38, 541–566 (2020).
Zou, W. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 6, 295–307 (2006).
Jang, J.-E. et al. Crosstalk between regulatory T Cells and tumor-associated dendritic cells negates anti-tumor immunity in pancreatic cancer. Cell Rep. 20, 558–571 (2017).
Zhang, Y. et al. Regulatory T-cell depletion alters the tumor microenvironment and accelerates pancreatic carcinogenesis. Cancer Discov. 10, 422–439 (2020).
Mirlekar, B. et al. Balance between immunoregulatory B cells and plasma cells drives pancreatic tumor immunity. Cell Rep. Med. 3, 100744 (2022).
Das, S. & Bar-Sagi, D. BTK signaling drives CD1dhiCD5+ regulatory B-cell differentiation to promote pancreatic carcinogenesis. Oncogene 38, 3316–3324 (2019).
Gunderson, A. J. et al. Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discov. 6, 270–285 (2016).
Li, S. et al. STING-induced regulatory B cells compromise NK function in cancer immunity. Nature 610, 373–380 (2022).
Takahashi, R. et al. Interleukin-1β-induced pancreatitis promotes pancreatic ductal adenocarcinoma via B lymphocyte-mediated immune suppression. Gut 70, 330–341 (2021).
Michaud, D., Mirlekar, B., Steward, C., Bishop, G. & Pylayeva-Gupta, Y. B cell receptor signaling and protein kinase D2 support regulatory B cell function in pancreatic cancer. Front. Immunol. 12, 745873 (2021).
Mirlekar, B. et al. B cell-derived IL35 drives STAT3-dependent CD8+ T-cell exclusion in pancreatic cancer. Cancer Immunol. Res. 8, 292–308 (2020).
Lee, K. E. et al. Hif1a deletion reveals pro-neoplastic function of B cells in pancreatic neoplasia. Cancer Discov. 6, 256–269 (2016).
Zhao, Y. et al. Regulatory B cells induced by pancreatic cancer cell-derived interleukin-18 promote immune tolerance via the PD-1/PD-L1 pathway. Oncotarget 9, 14803–14814 (2018).
Chen, Y.-I. et al. Homophilic ATP1A1 binding induces activin A secretion to promote EMT of tumor cells and myofibroblast activation. Nat. Commun. 13, 2945 (2022).
Öhlund, D. et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 214, 579–596 (2017).
Hosein, A. N., Brekken, R. A. & Maitra, A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 17, 487–505 (2020).
LaRue, M. M. et al. Metabolic reprogramming of tumor-associated macrophages by collagen turnover promotes fibrosis in pancreatic cancer. Proc. Natl Acad. Sci. USA 119, e2119168119 (2022).
Zhao, J. et al. Stromal modulation reverses primary resistance to immune checkpoint blockade in pancreatic cancer. ACS Nano 12, 9881–9893 (2018).
Jacobetz, M. A. et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62, 112–120 (2013).
Provenzano, P. P. et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429 (2012).
Jiang, H. et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat. Med. 22, 851–860 (2016).
Blair, A. B. et al. Dual stromal targeting sensitizes pancreatic adenocarcinoma for anti-programmed cell death protein 1 therapy. Gastroenterology 163, 1267–1280.e1267 (2022).
Mace, T. A., Bloomston, M. & Lesinski, G. B. Pancreatic cancer-associated stellate cells: a viable target for reducing immunosuppression in the tumor microenvironment. Oncoimmunology 2, e24891 (2013).
Liu, H., Shi, Y. & Qian, F. Opportunities and delusions regarding drug delivery targeting pancreatic cancer-associated fibroblasts. Adv. Drug Deliv. Rev. 172, 37–51 (2021).
Feig, C. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA 110, 20212–20217 (2013).
McAndrews, K. M. et al. Identification of functional heterogeneity of carcinoma-associated fibroblasts with distinct IL6-mediated therapy resistance in pancreatic cancer. Cancer Discov. 12, 1580–1597 (2022).
Özdemir, B. C. et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014).
Elyada, E. et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 9, 1102–1123 (2019).
Biffi, G. et al. IL1-induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discov. 9, 282–301 (2019).
Helms, E. J. et al. Mesenchymal lineage heterogeneity underlies nonredundant functions of pancreatic cancer-associated fibroblasts. Cancer Discov. 12, 484–501 (2022).
Huang, H. et al. Mesothelial cell-derived antigen-presenting cancer-associated fibroblasts induce expansion of regulatory T cells in pancreatic cancer. Cancer Cell 40, 656–673.e657 (2022).
Lin, J. H. et al. Type 1 conventional dendritic cells are systemically dysregulated early in pancreatic carcinogenesis. J. Exp. Med. 217, e20190673 (2020).
Flint, T. R. et al. Tumor-induced IL-6 reprograms host metabolism to suppress anti-tumor immunity. Cell Metab. 24, 672–684 (2016).
Picard, F. S. R. et al. IL-17A-producing CD8(+) T cells promote PDAC via induction of inflammatory cancer-associated fibroblasts. Gut 72, 1510–1522 (2023).
Chen, Y. et al. Type I collagen deletion in αSMA+ myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell 39, 548–565.e546 (2021).
Su, H. et al. Collagenolysis-dependent DDR1 signalling dictates pancreatic cancer outcome. Nature 610, 366–372 (2022).
Datta, J. et al. Combined MEK and STAT3 inhibition uncovers stromal plasticity by enriching for cancer-associated fibroblasts with mesenchymal stem cell-like features to overcome immunotherapy resistance in pancreatic cancer. Gastroenterology 163, 1593–1612 (2022).
Aykut, B. et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 574, 264–267 (2019).
Pushalkar, S. et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 8, 403–416 (2018).
Riquelme, E. et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 178, 795–806.e712 (2019).
Sethi, V. et al. Gut microbiota promotes tumor growth in mice by modulating immune response. Gastroenterology 155, 33–37.e36 (2018).
Alam, A. et al. Fungal mycobiome drives IL-33 secretion and type 2 immunity in pancreatic cancer. Cancer Cell 40, 153–167.e111 (2022).
Hezaveh, K. et al. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity 55, 324–340.e328 (2022).
Mirji, G. et al. The microbiome-derived metabolite TMAO drives immune activation and boosts responses to immune checkpoint blockade in pancreatic cancer. Sci. Immunol. 7, eabn0704 (2022).
Nagata, N. et al. Metagenomic identification of microbial signatures predicting pancreatic cancer from a multinational study. Gastroenterology 163, 222–238 (2022).
Gihawi, A. et al. Major data analysis errors invalidate cancer microbiome findings. mBio 14, e0160723 (2023).
Ho, W. J. et al. Multi-omic profiling of lung and liver tumor microenvironments of metastatic pancreatic cancer reveals site-specific immune regulatory pathways. Genome Biol. 22, 154 (2021).
Karamitopoulou, E. et al. Spatially restricted tumour-associated and host-associated immune drivers correlate with the recurrence sites of pancreatic cancer. Gut 72, 1523–1533 (2023).
Wang, X. et al. Identification of a subset of immunosuppressive P2RX1-negative neutrophils in pancreatic cancer liver metastasis. Nat. Commun. 12, 174 (2021).
Astuti, Y. et al. Efferocytosis reprograms the tumor microenvironment to promote pancreatic cancer liver metastasis. Nat. Cancer 5, 774–790 (2024).
Raymant, M. et al. Macrophage-fibroblast JAK/STAT dependent crosstalk promotes liver metastatic outgrowth in pancreatic cancer. Nat. Commun. 15, 3593 (2024).
Pham, T. N. D. et al. Preclinical models of pancreatic ductal adenocarcinoma and their utility in immunotherapy studies. Cancers 13, https://doi.org/10.3390/cancers13030440 (2021).
Mallya, K., Gautam, S. K., Aithal, A., Batra, S. K. & Jain, M. Modeling pancreatic cancer in mice for experimental therapeutics. Biochim. Biophys. Acta Rev. Cancer 1876, 188554 (2021).
Jung, J. Y. et al. siRNA nanoparticle targeting PD-L1 activates tumor immunity and abrogates pancreatic cancer growth in humanized preclinical model. Cells 10, https://doi.org/10.3390/cells10102734 (2021).
Pan, Y. et al. Single-cell RNA sequencing reveals compartmental remodeling of tumor-infiltrating immune cells induced by anti-CD47 targeting in pancreatic cancer. J. Hematol. Oncol. 12, 124 (2019).
Vonderheide, R. H. et al. CD40 immunotherapy for pancreatic cancer. Cancer Immunol. Immunother. 62, 949–954 (2013).
Beatty, G. L. et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331, 1612–1616 (2011).
Kerk, S. A., Papagiannakopoulos, T., Shah, Y. M. & Lyssiotis, C. A. Metabolic networks in mutant KRAS-driven tumours: tissue specificities and the microenvironment. Nat. Rev. Cancer 21, 510–525 (2021).
Buscail, L., Bournet, B. & Cordelier, P. Role of oncogenic KRAS in the diagnosis, prognosis and treatment of pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 17, 153–168 (2020).
Chao, T., Furth, E. E. & Vonderheide, R. H. CXCR2-dependent accumulation of tumor-associated neutrophils regulates t-cell immunity in pancreatic ductal adenocarcinoma. Cancer Immunol. Res. 4, 968–982 (2016).
Pylayeva-Gupta, Y., Lee, K. E., Hajdu, C. H., Miller, G. & Bar-Sagi, D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 21, 836–847 (2012).
Coelho, M. A. et al. Oncogenic RAS signaling promotes tumor immunoresistance by stabilizing PD-L1 mRNA. Immunity 47, 1083–1099.e1086 (2017).
Hu, Y. et al. K-ras(G12V) transformation leads to mitochondrial dysfunction and a metabolic switch from oxidative phosphorylation to glycolysis. Cell Res. 22, 399–412 (2012).
Glorieux, C. et al. Regulation of PD-L1 expression in K-ras-driven cancers through ROS-mediated FGFR1 signaling. Redox Biol. 38, 101780 (2021).
Collins, M. A. et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J. Clin. Investig. 122, 639–653 (2012).
Jones, S. et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321, 1801–1806 (2008).
Zeng, G. et al. Aberrant Wnt/beta-catenin signaling in pancreatic adenocarcinoma. Neoplasia 8, 279–289 (2006).
Aguilera, K. Y. & Dawson, D. W. WNT ligand dependencies in pancreatic cancer. Front. Cell Dev. Biol. 9, 671022 (2021).
Jiang, X. et al. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc. Natl Acad. Sci. USA 110, 12649–12654 (2013).
Hosein, A. N. et al. Loss of Rnf43 accelerates Kras-mediated neoplasia and remodels the tumor immune microenvironment in pancreatic adenocarcinoma. Gastroenterology 162, 1303–1318.e1318 (2022).
Zhou, M. et al. LncRNA FAM83H-AS1 promotes the malignant progression of pancreatic ductal adenocarcinoma by stabilizing FAM83H mRNA to protect β-catenin from degradation. J. Exp. Clin. Cancer Res. 41, 288 (2022).
Chen, Y. et al. Oncogenic collagen I homotrimers from cancer cells bind to α3β1 integrin and impact tumor microbiome and immunity to promote pancreatic cancer. Cancer Cell 40, 818–834.e819 (2022).
Galluzzi, L., Spranger, S., Fuchs, E. & López-Soto, A. WNT signaling in cancer immunosurveillance. Trends Cell Biol. 29, 44–65 (2019).
Yamamoto, K. et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature 581, 100–105 (2020).
Cheung, P. F. et al. Progranulin mediates immune evasion of pancreatic ductal adenocarcinoma through regulation of MHCI expression. Nat. Commun. 13, 156 (2022).
Cain, D. W. & Cidlowski, J. A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 17, 233–247 (2017).
Deng, Y. et al. Glucocorticoid receptor regulates PD-L1 and MHC-I in pancreatic cancer cells to promote immune evasion and immunotherapy resistance. Nat. Commun. 12, 7041 (2021).
Wang, B., Niu, D., Lai, L. & Ren, E. C. p53 increases MHC class I expression by upregulating the endoplasmic reticulum aminopeptidase ERAP1. Nat. Commun. 4, 2359 (2013).
Zhu, K. et al. p53 induces TAP1 and enhances the transport of MHC class I peptides. Oncogene 18, 7740–7747 (1999).
Abrego, J. et al. A cancer cell-intrinsic GOT2-PPARδ axis suppresses antitumor immunity. Cancer Discov. 12, 2414–2433 (2022).
Liu, J. et al. QDPR deficiency drives immune suppression in pancreatic cancer. Cell Metab. 36, 984–999.e988 (2024).
He, C. et al. Vitamin B6 competition in the tumor microenvironment hampers antitumor functions of NK cells. Cancer Discov. 14, 176–193 (2024).
Zhu, X. G. et al. Functional genomics in vivo reveal metabolic dependencies of pancreatic cancer cells. Cell Metab. 33, 211–221.e216 (2021).
Niu, N. et al. Tumor cell-intrinsic epigenetic dysregulation shapes cancer-associated fibroblasts heterogeneity to metabolically support pancreatic cancer. Cancer Cell 42, 869–884.e869 (2024).
Niu, N. et al. Tumor cell-intrinsic SETD2 deficiency reprograms neutrophils to foster immune escape in pancreatic tumorigenesis. Adv. Sci. 10, e2202937 (2023).
Mills, B. N. et al. Modulation of the human pancreatic ductal adenocarcinoma immune microenvironment by stereotactic body radiotherapy. Clin. Cancer Res. 28, 150–162 (2022).
Panni, R. Z. et al. Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies. Sci. Transl. Med. 11, eaau9240 (2019).
GB006 Inc. (a wholly owned subsidiary of Gossamer Bio, Inc.) & Merck Sharp & Dohme LLC. A Phase 1/2, First-in-Human, Open-label, Dose Escalation Study of GB1275 Monotherapy and in Combination With an Anti-PD-1 Antibody in Patients With Specified Advanced Solid Tumors or in Combination With Standard of Care in Patients With Metastatic Pancreatic Adenocarcinoma, Followed by Basket Expansion of GB1275 With Standard of Care or in Combination With an Anti-PD-1 Antibody in Patients With Specified Metastatic Solid Tumors. Report No. NCT04060342 (clinicaltrials.gov, 2022).
Zhu, Y. et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 74, 5057–5069 (2014).
Five Prime Therapeutics, I. A Phase 1a/1b Study of Cabiralizumab in Combination With Nivolumab in Patients With Selected Advanced Cancers. Report No. NCT02526017, (clinicaltrials.gov, 2022).
Squibb, B.-M. A Phase 2 Study of Cabiralizumab (BMS-986227, FPA008) Administered in Combination With Nivolumab (BMS-936558) With and Without Chemotherapy in Patients With Advanced Pancreatic Cancer. Report No. study/NCT03336216, (clinicaltrials.gov, 2022).
Berard, C. L. A Dose Escalation Phase I Study With an Extension Part Evaluating the Safety and Activity of an Anti-PDL1 Antibody (DURVALUMAB) Combined With a Small Molecule CSF-1R Tyrosine Kinase Inhibitor (PEXIDARTINIB) in Patients With Metastatic/Advanced Pancreatic or Colorectal Cancers. Report No. NCT02777710, (clinicaltrials.gov, 2021).
Nywening, T. M. et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 17, 651–662 (2016).
Noel, M. et al. Phase 1b study of a small molecule antagonist of human chemokine (C-C motif) receptor 2 (PF-04136309) in combination with nab-paclitaxel/gemcitabine in first-line treatment of metastatic pancreatic ductal adenocarcinoma. Invest. N. Drugs 38, 800–811 (2020).
AstraZeneca. A Phase Ib and II Open-Label, Multi-Center Study of MEDI4736 Evaluated in Different Combinations in Patients With Metastatic Pancreatic Ductal Adenocarcinoma. Report No. NCT02583477 (clinicaltrials.gov, 2019).
Mulkerin, D. An Open-label Phase 1 Study to Evaluate the Safety and Tolerability of SX-682 in Combination With Nivolumab as a Maintenance Therapy in Patients With Metastatic Pancreatic Ductal Adenocarcinoma. Report No. NCT04477343 (clinicaltrials.gov, 2022).
Meyer, M. A. et al. Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance. Nat. Commun. 9, 1250 (2018).
Hegde, S. et al. Dendritic cell paucity leads to dysfunctional immune surveillance in pancreatic cancer. Cancer Cell 37, 289–307.e289 (2020).
Vonderheide, R. H. CD40 agonist antibodies in cancer immunotherapy. Annu. Rev. Med. 71, 47–58 (2020).
Therapeutics, C. A Phase 1 Study of CDX-1140 as Monotherapy or in Combination in Patients With Advanced Malignancies. Report No. NCT03329950, (clinicaltrials.gov, 2022).
Harari, A., Graciotti, M., Bassani-Sternberg, M. & Kandalaft, L. E. Antitumour dendritic cell vaccination in a priming and boosting approach. Nat. Rev. Drug Discov. 19, 635–652 (2020).
Bauer, C. et al. Dendritic cell-based vaccination combined with gemcitabine increases survival in a murine pancreatic carcinoma model. Gut 56, 1275–1282 (2007).
Lau, S. P. et al. Dendritic cell vaccination and CD40-agonist combination therapy licenses T cell-dependent antitumor immunity in a pancreatic carcinoma murine model. J. Immunother. Cancer 8, https://doi.org/10.1136/jitc-2020-000772 (2020).
Lau, S. P. et al. Autologous dendritic cells pulsed with allogeneic tumour cell lysate induce tumour-reactive T-cell responses in patients with pancreatic cancer: a phase I study. Eur. J. Cancer 169, 20–31 (2022).
Aerts, J. Dendritic Cells Loaded With Allogeneic Tumor Lysate (MesoPher) in Combination With a CD40 Agonist (Mitazalimab) in Metastatic Pancreatic Disease. Report No. study/NCT05650918 (clinicaltrials.gov, 2022). https://clinicaltrials.gov/study/NCT05650918?term=NCT05650918&rank=1#collaborators-andinvestigators.
Tempero, M. et al. Ibrutinib in combination with nab-paclitaxel and gemcitabine for first-line treatment of patients with metastatic pancreatic adenocarcinoma: phase III RESOLVE study. Ann. Oncol. 32, 600–608 (2021).
Overman, M. et al. Randomized phase II study of the Bruton tyrosine kinase inhibitor acalabrutinib, alone or with pembrolizumab in patients with advanced pancreatic cancer. J. Immunother. Cancer 8, https://doi.org/10.1136/jitc-2020-000587 (2020).
Padrón, L. J. et al. Sotigalimab and/or nivolumab with chemotherapy in first-line metastatic pancreatic cancer: clinical and immunologic analyses from the randomized phase 2 PRINCE trial. Nat. Med. 28, 1167–1177 (2022).
Immunotherapy, P. I. f. C. A Multicenter, Open-label, ExploRatory Platform Trial to EValuate ImmunOtherapy Combinations With Chemotherapy for the Treatment of Patients With PreviousLy UnTreated Metastatic Pancreatic AdenOcarciNoma (REVOLUTION). Report No. NCT04787991 (clinicaltrials.gov, 2023).
Roehle, K. et al. cIAP1/2 antagonism eliminates MHC class I-negative tumors through T cell-dependent reprogramming of mononuclear phagocytes. Sci. Transl. Med. 13, eabf5058 (2021).
Institute of Cancer Research, U. K. ASTEROID: A Phase I Trial of ASTX660 in Combination With Pembrolizumab in Patients With Solid Tumours: Utilizing Triple IAP Blockade as a Strategy to Maximize Immunogenic Cell Death and the Generation of an Efficient Adaptive Immune Response. Report No. NCT05082259 (clinicaltrials.gov, 2022).
Feng, M. et al. Phagocytosis checkpoints as new targets for cancer immunotherapy. Nat. Rev. Cancer 19, 568–586 (2019).
Therapeutics, P. A Phase I, First-in-Human, Open-Label, Dose Escalation and Expansion Study of PT886 in Adult Patients With Advanced Gastric, Gastroesophageal Junction and Pancreatic Adenocarcinomas. Report No. NCT05482893 (clinicaltrials.gov, 2022).
Ramanathan, R. K. et al. Phase IB/II randomized study of FOLFIRINOX plus pegylated recombinant human hyaluronidase versus FOLFIRINOX alone in patients with metastatic pancreatic adenocarcinoma: SWOG S1313. J. Clin. Oncol. 37, 1062–1069 (2019).
Van Cutsem, E. et al. Randomized phase III trial of pegvorhyaluronidase alfa with nab-paclitaxel plus gemcitabine for patients with hyaluronan-high metastatic pancreatic adenocarcinoma. J. Clin. Oncol. 38, 3185–3194 (2020).
Team, P. C. R. Phase II Study of PEGPH20 and Pembrolizumab (MK-3475) for Patients With Previously Treated Hyaluronan High (HA-High) Metastatic Pancreatic Ductal Adenocarcinoma. Report No. NCT03634332, (clinicaltrials.gov, 2019).
Wang-Gillam, A. et al. Abstract CT118: phase I study of defactinib combined with pembrolizumab and gemcitabine in patients with advanced cancer: Experiences of pancreatic ductal adenocarcinoma (PDAC) patients. Cancer Res. 80, CT118 (2020).
Hopkins, S. K. C. C. C. A. J. A Randomized Phase II Study of Pembrolizumab With or Without Defactinib, a Focal Adhesion Kinase Inhibitor Following Chemotherapy as a Neoadjuvant and Adjuvant Treatment for Resectable Pancreatic Ductal Adenocarcinoma (PDAC). Report No. NCT03727880 (clinicaltrials.gov, 2022).
O’Hara, M. H. et al. Safety and pharmacokinetics of CXCR4 peptide antagonist, LY2510924, in combination with durvalumab in advanced refractory solid tumors. J. Pancreat. Cancer 6, 21–31 (2020).
Bockorny, B. et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat. Med. 26, 878–885 (2020).
Komar, H. M. et al. Inhibition of Jak/STAT signaling reduces the activation of pancreatic stellate cells in vitro and limits caerulein-induced chronic pancreatitis in vivo. Sci. Rep. 7, 1787 (2017).
Hurwitz, H. I. et al. Randomized, double-blind, phase II study of ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. J. Clin. Oncol. 33, 4039–4047 (2015).
Hurwitz, H. et al. Ruxolitinib + capecitabine in advanced/metastatic pancreatic cancer after disease progression/intolerance to first-line therapy: JANUS 1 and 2 randomized phase III studies. Invest. N. Drugs 36, 683–695 (2018).
Hosein, P., Incyte Corporation, Novartis Pharmaceuticals & University of Miami Sylvester Comprehensive Cancer Center. A Phase 1 Trial of Combined MEK, STAT3 and PD-1 Inhibition in Metastatic Pancreatic Ductal Adenocarcinoma. Report No. NCT05440942 (clinicaltrials.gov, 2023).
Chen, X., Zeh, H. J., Kang, R., Kroemer, G. & Tang, D. Cell death in pancreatic cancer: from pathogenesis to therapy. Nat. Rev. Gastroenterol. Hepatol. 18, 804–823 (2021).
Parikh, A. R. et al. Radiation therapy enhances immunotherapy response in microsatellite stable colorectal and pancreatic adenocarcinoma in a phase II trial. Nat. Cancer 2, 1124–1135 (2021).
Acknowledgements
We are grateful to members of the Yao Lab and the Ma Lab for the discussion. We thank Mr. Haoli Ma from Wuhan University Zhongnan Hospital for his support. W.B. is supported by Fundamental Research Funds for the Central Universities (2662021SYQD001). F.Y. is supported by the Hubei Provincial Natural Science Foundation (2022CFA108), the Major Project of Hubei Hongshan Laboratory (2022hszd028 and 2021hszd012), HZAU-AGIS Cooperation Fund (SZYJY2023008) and the Youth Research Project of Health Commission of Hubei Province (ZY2023Q037). L.M. is supported by US National Institutes of Health (NIH) grants R01CA166051 and R01CA269140, an American Cancer Society grant (award number: DBG-22-161-01-MM), and the Nylene Eckles Distinguished Professorship of MD Anderson Cancer Center.
Author information
Authors and Affiliations
Contributions
Y.J. wrote the draft. D.X., Y.J., and M.L. made the figures and tables. Y.S., F.Y., and L.M. provided intellectual input. Y.S., W.B., F.Y., and L.M. edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests. Figure 2 was created with BioRender.com.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ju, Y., Xu, D., Liao, Mm. et al. Barriers and opportunities in pancreatic cancer immunotherapy. npj Precis. Onc. 8, 199 (2024). https://doi.org/10.1038/s41698-024-00681-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41698-024-00681-z
- Springer Nature Limited