Abstract
Scalable isogenic models of cancer-associated mutations are critical to studying dysregulated gene function. Nonsynonymous mutations of splicing factors, which typically affect one allele, are common in many cancers, but paradoxically confer growth disadvantage to cell lines, making their generation and expansion challenging. Here, we combine AAV-intron trap, CRISPR/Cas9, and inducible Cre-recombinase systems to achieve >90% efficiency to introduce the oncogenic K700E mutation in SF3B1, a splicing factor commonly mutated in multiple cancers. The intron-trap design of AAV vector limits editing to one allele. CRISPR/Cas9-induced double stranded DNA breaks direct homologous recombination to the desired genomic locus. Inducible Cre-recombinase allows for the expansion of cells prior to loxp excision and expression of the mutant allele. Importantly, AAV or CRISPR/Cas9 alone results in much lower editing efficiency and the edited cells do not expand due to toxicity of SF3B1-K700E. Our approach can be readily adapted to generate scalable isogenic systems where mutant oncogenes confer a growth disadvantage.
Similar content being viewed by others
Introduction
Isogenic cell line systems of non-synonymous mutations are essential to understanding the biochemistry and cellular consequences of disease mutations. The CRISPR/Cas9 system has revolutionized genomic editing for purposes of generating both in vitro and in vivo model systems1,2,3, but challenges of its adaptation to some scenarios remain. Such scenarios include generation of single-allele missense point mutations or when the resultant mutation confers a physiological growth disadvantage. Targeted double-stranded breaks (DSBs) created by clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) system are typically repaired by non-homologous end joining4,5 introducing gene knock-outs. By contrast, gene knock-ins of point mutations require a donor DNA template for homology-directed repair (HDR), a very low efficiency process often corrupted by additional target-site indels6, necessitating screening of multiple clones to isolate those with correct genome editing7,8. Additional difficulties arise when the introduced mutation is toxic to the cell resulting in loss of cell viability and/or loss of mutant allele on prolonged culture9, which significantly limits scalability of these edited cell systems. To exemplify this, we had tried to generate a SF3B1K700E mutation in K562 cell lines using a conventional CRISPR/Cas9 with linear ultramer template but were unable to successfully isolate edited clones despite screening several hundreds of them. It is likely that this was a consequence from the toxicity of the mutated protein.
Many disease-associated mutations, such as those involving splicing factors10, are heterozygous and paradoxically result in slower growth or cellular death in in vitro model systems while promoting clonal evolution in vivo9,11,12. This significantly limits our ability to keep them viable in culture and to investigate molecular mechanisms of disease, particularly in scenarios where a large number of cells are required. There is therefore an unmet need in the field to generate highly efficient in vitro isogenic model systems in which expression of the toxic mutation can be regulated temporally.
Adeno-associated virus (AAV)-based approaches have long been used for creation of gene sequence-specific knock-ins into endogenous alleles in tissue culture-based systems13. Recombinant AAV (rAAV) are replication-deficient genetic-payload vehicles generated by replacing the entire AAV genome except for the inverted terminal repeats (ITRs), which are essential for viral genome encapsidation and in vitro viral production14. Following cellular attachment and entry of encapsidated rAAV into cells, they exist mainly as extra-chromosomal episomes and rarely integrating into the genome15. Since the initial report of rAAV vectors as a promising gene-editing system in 199816, several groups have worked to improve the efficiency of this gene targeting approach by introducing gene-trap systems into targeting constructs17,18,19. Using a neomycin resistance (NeoR; G418)-based intron-trap gene-editing system, the Waldman laboratory has previously reported on the use of rAAV gene editing to introduce epitope tags18 as well as oncogenic point mutations20 into endogenous alleles of genes in human cell lines. However, the targeting efficiency of gene editing with this approach is typically modest, in the range of 1–40% among G418-selected clones18,20. The antibiotic selection of these resistant clones may be attributed to low frequency, random integration of the rAAV genomes (and not necessarily targeted to desired genomic coordinates), despite consisting of one Kilobyte (Kbp) long homology arms21.
Previous studies have combined rAAV- and targeted nuclease-based (such as CRISPR/Cas9) approaches to improve upon the efficiency of either approach when deployed alone22,23,24. In these studies, the rAAV vector provides a long single-stranded (ss) DNA template for HDR following double-stranded DNA breaks introduced by CRISPR/Cas9. Initial efforts that combined CRISPR/Cas9 components and HDR template in the same vector were limited by the maximum length that rAAV vectors could accommodate (~4.5 Kb between ITR). Bak et al.25 first demonstrated the feasibility of delivering CRISPR components as ribonucleoproteins (RNPs) thereby freeing up the space in the rAAV to fill with the repair template. Subsequently, it was reported that combining RNP and ss linear rAAV donor delivery increased the efficiency of gene editing by up to tenfold over RNP and double-stranded conventional plasmid donor systems26. Additional adaptations of the rAAV-CRISPR gene-editing methodology with varied in vivo and in vitro applications have since been reported24,25,27,28,29,30. Strategies requiring targeted transgene insertions specifically at the DNA break site may however be undesirable for some applications requiring scarless gene editing; integrating AAV targeting constructs typically contain a selection marker, which leaves a scar upon loxp excision in the final cell lines13.
Targeting gene alterations that do not confer a selectable growth advantage and subsequent recovery of knock-in cells requires a high-efficiency gene-editing strategy. In this regard, advances in rAAV vector gene targeting, through the creation of promoter trap targeting vectors carrying gene entrapment cassettes termed synthetic exon promoter trap (SEPT)31, have greatly simplified and improved upon the rAAV gene-editing efficacy. The pAAV-SEPT-Acceptor plasmid-based (Addgene # 25648) HDR template used in our model contains a SEPT cassette, which begins with a short intron sequence followed by an IgG variable region-derived splice acceptor, a IRES that permits the translation of the NeoR open reading frame from RNA transcripts initiated upstream, and a polyadenylation site32,33. The intron-trap cassette is flanked by tandem LoxP sites oriented so that expression of the Cre-recombinase results in excision of all sequences within them. Despite such high efficiency of rAAV-HDR, a significant problem with the approach is that of non-specific integration of the repair template in the transcribed regions of the genome that will generate Neo-resistant clones that do not contain the desired modification. We postulated that if the HDR template is directed to the correct genomic locus, the proportion of correct clones can be vastly improved. To this purpose, we combined the rAAV approach with CRISPR/Cas9 to facilitate high-precision genome editing. This approach is an extension of our previously developed inducible AAV-based intron-trap system. We demonstrate that combining rAAV and CRISPR/Cas9 is feasible and leads to much higher efficiency than attainable by rAAV alone. Additionally, we use inducible expression of Cre-recombinase to temporally control expression of mutant allele, thereby, overcoming problems of toxicity of the mutant protein.
Results
Genome editing with rAAV alone for single base pair changes results in low efficiency editing without incorporating the desired mutation
The lysine to glutamic acid substitution at position 700 (K700E) is the most common non-synonymous mutation described in multiple clonal processes such as myelodysplastic syndromes, chronic lymphocytic leukemia, uveal melanoma, lung cancer and breast cancer34. Like other splicing factor mutations, SF3B1 mutations are heterozygous and non-synonymous suggesting a gain-of-function change. It is therefore critical to study these mutations in isogenic model systems, and not in overexpression models where the proportion of wild-type to mutant protein is unlikely to be equal.
The rAAV model with intron trap, as we have previously described, is ideal for this purpose given that it can specifically select clones edited at a single allele. To introduce the K700E mutation in exon 15 of human SF3B1 gene locus, we sequentially cloned the two homology arms into the polylinkers that had been built into the pAAV-SEPT-Acceptor plasmid (Fig. 1: steps 1 and 2). The additional screening primer was also introduced by site-directed mutagenesis (SDM) to accurately identify single-allele insertions (Fig. 1: step 3). To introduce the K700E mutation in exon 15 of the human SF3B1 genomic locus, we introduced a G mutation (for AAA to GAA codon change) through SDM in the right homology arm (RHA) (Fig. 1: step 4). Cloning of the arms into rAAV acceptor and the SDM reaction to introduce the screening primer and desired K700E mutation were extremely efficient and took ∼10 days to perform.
Step 1: left (LHA) and right (RHA) homology arms were created by PCR from human genomic DNA template using PCR primers tailed with restriction enzyme sites (REs; RE 1, 2 for LHA and RE 3, 4 for RHA) for subsequently cloning into the pAAV acceptor. Step 2: The left and right homologous arms were digested and sequentially cloned into the pAAV-SEPT-Acceptor plasmid, which has been built with a Neomycin (NeoR) gene-trap cassette. Step 3: The extra 20 bp from upstream exon (primer 2 sequence) was introduced in the beginning of the RHA of the pAAV-SEPT-Acceptor by site-directed mutagenesis (SDM). Step 4: The desired substitution (K700E mutation) was introduced in the right arm of the pAAV-SEPT-Acceptor by SDM (shown in magenta).
An important consideration must be made in designing the homology arms flanking the NeoR gene-trap cassette: if the target intron is too short, the cassette may be integrated too close to the 3’ splicing acceptor site at the intron/exon junction, potentially disrupting 3’ branchpoint recognition leading to a failure of splicing out of the gene-trap-containing intron upon Cre-activation. To avoid this possibility, the longer of the two introns flanking the exon of interest (in which to introduce the desired modification) should be preferred for introduction of the gene-trap cassette. In this case, intron 13 was chosen over intron 14 due to its larger size.
Two recipient cell lines were chosen, both harboring wild-type SF3B1 (transformed human K562 erythroleukemia cells and doxycycline-immortalized human umbilical cord-derived erythroid progenitor-2 (HUDEP-2) cells). K562 cells were transfected with the rAAV virus and selected for G-418 resistance at 48 h for 10–12 days at which point resistant cells (evident as doublets) had emerged. Single-cell clones were selected with limiting dilution and crude DNA preparation (purified from cultures using standard methods) was analyzed by polymerase chain reaction (PCR) using the “In–Out” screening strategy outlined in the “Methods” section (Fig. 2a, b). In the SF3B1 example, using the first screening primer located in the targeted genomic locus outside the RHA and the second primer located in the middle of the left homology arm (LHA), this PCR screening strategy would generate 1.5 Kbp in the case of unedited wild type, 1.5 Kbp, and 1.1 bp with monoallelic HR and 1.1 bp with biallelic HR. This screening strategy did not fail in our experience and we found it to be a cheap and reliable method that demands very low cell numbers.
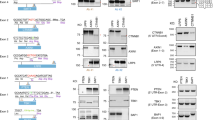
a Schematic of “In–Out” PCR screening strategy of clones after single-allele recombination before Cre activation (screening primer pair denoted as black and blue arrows) and after Cre activation (denoted as red arrows) is shown. b PCR analysis of genomic DNA isolated from unedited and edited K562 cells that underwent recombination (using screening primer pair from the middle panel of a). c PCR analysis of genomic DNA from edited K562 cells post Cre activation (using screening primer pair from the lower panel of a). d Confirmation of successful single-allele K700E mutation by Sanger sequencing of genomic DNA.
Of the 25 clones analyzed, only four were found to have the correct targeted integration (Fig. 3a). Furthermore, Sanger sequencing of their genomic DNA revealed that none of the four clones carried the A → G mutation (Fig. 3c). This suggested that, while the rAAV vector was utilized as a template for HDR, the mutation was not incorporated likely due to the large distance (~300 bp) between the obligatory NeoR cassette and the mutation (Fig. 3e). We concluded that using rAAV alone as a strategy to introduce single-allele point mutations is limited by a long distance between the selection cassette and the mutation site, since the homologous recombination likely occurs proximal to and terminates before reaching the mutated site on the AAV template.
a PCR-based identification of on-target events in SF3B1 alleles using rAAV-based homologous recombination alone strategy. Four of the 25 (16%) G418-resistant clones were PCR positive for on-target integration of the expression cassette (marked with asterisks); C control wild type. b Depicted is the Sanger sequencing of the gDNA target sequence of one of the representative PCR-positive clones generated by rAAV-alone-based HR. The clone did not undergo the desired K700E modification indicating failure of HR between the target DNA and rAAV in region of the desired modification (∼300 bp from the gene-trap cassette integration). c Illustration of the HR event between rAAV vector and genome locus. In the absence of a targeted DSB, HR begins in the intron at the site of obligatory NeoR intron-trap insertion (depicted in yellow) spreading out with decreasing efficiency before falling off due to significant homology between the rAAV HAs and the host genome. d PCR-based identification of on-target events in SF3B1 alleles using rAAV + CRISPR/Cas9-based homologous recombination strategy. Thirty-one of the 33 (94%) G418-resistant clones demonstrated on-target integration of the expression cassette (marked with asterisks). e Depicted is the Sanger sequencing of the gDNA target sequence of one of the representative PCR-positive clones generated by rAAV-CRISPR/Cas9-based HR, showing successful single-allele editing of the SF3B1 K700 locus. f The double-stranded break introduced by Cas9 facilitates locus-specific HR of the nascent DNA strand with the rAAV donor template containing the desired mutation.
Combining CRISPR/Cas9 with rAAV vastly improves efficiency of introducing single-allele mutations at the correct genomic locus
We postulated that lack of incorporation of the desired mutation, despite successful editing at the correct locus, could be overcome by directing the HDR to this site through a DSB in its immediate vicinity. We therefore designed single-guide RNA (sgRNA) close to the K700 locus and generated Cas9/sgRNA ribonuclear proteins (as described in “Methods”). After confirming efficiency of the sgRNA to introduce DSBs through T7EI cleavage assay35 (Fig. S1), RNPs were transfected into K562 cells (and HUDEP2 cells by nucleofection) followed by transduction of rAAV 1 h later. Cells were then allowed to recover and selected for Neo resistance followed by single-cell cloning (as described for rAAV-only methodology; Fig. S2). Single-cell clones were then screened by PCR for single-allele insertion at the correct locus (Fig. 3b). Twenty-three of the 25 clones were noted to have correct insertion (targeted integration efficiency of 92%). Furthermore, all these clones were noted to incorporate the desired A → G mutation (Fig. 3d–e). The DSB introduced by Cas9 facilitates locus-specific HR of the nascent DNA strand with the rAAV donor (Fig. 3f). It has been previously reported that editing is most efficient when targeted site is within 15 bp (ideally <10) of the Cas9-induced DSB, with efficiencies rapidly dropping beyond 20 nucleotides from the DSB site36.
Clones thus identified were expanded, transfected with Cre-recombinase plasmid, and single clones were selected (based on correct Cre-mediated recombination as per PCR strategy, Fig. 2c). RNA from these “post-Cre recombination” cells were analyzed for K700E mutation (through sequencing of cDNA amplicon), which showed comparable expression of wild-type and mutant transcripts (Fig. 4a). As shown in western blot (Fig. 4b), heterozygous SF3B1 cells, post-induction, had double the SF3B1 protein expression as compared to uninduced K562 isogenic cells due to expression of the mutant allele along with the unmodified allele. We further confirmed the functional expression of the mutant allele by analyzing the cDNA for the presence of aberrant 3’ splice sites (cryptic 3’SS), the characteristic splicing aberration brought about by the SF3B1 mutation37,38. SF3B1 mutations result in widespread use of cryptic 3’SS a few base pairs upstream of the canonical sites in previously reported genes such as UBR4, ABCB7, SEPT6, ENOSF1, and DYNLL1; Fig. 4c).
a Sanger sequencing of cDNA from K562 isogenic cell line sample (5 days post-induction) confirming mutant SF3B1 mRNA expression. b Western blot-based confirmation of expression of the mutant SF3B1 allele. Depicted is the western blot with SF3B1 antibodies on lysates from parental (SF3B1wt/null) clone and cells 10 days post-induction (SF3B1wt/K700E). The induction of mutant SF3B1 allele leading to expression of mutant SF3B1 protein (same size as the wild-type SF3B1 protein) doubles the SF3B1 protein expression as compared to the pre-induced sample. c Validation of cryptic 3’ splice site usage in five selected genes frequently mis-spliced in SF3B1 mutant myelodysplastic syndrome. PCR products of the five genes (UBR4, ABCB7, SEPT6, ENOSF1, DYNL11) showing aberrant cryptic 3’SS usage in the K562 SF3B1 mutant (cryptic transcripts indicated by black arrow heads).
Inducible expression of Cre-recombinase overcomes toxicity of mutant SF3B1
While we were able to reliably generate single-cell mutant clones using rAAV/CRISPR editing, we were not able to maintain them in culture beyond 2–3 weeks. Cells slowed in their growth, increased in size, and eventually became apoptotic. Our results were similar to data from previous studies which have noted toxicity of mutant splicing factors that resulted in silencing of transgene, loss of mutant allele, or cell death9. This is clearly a major obstacle in biochemical experiments that require large number of cells. We posited that, if mutant factors are expressed only after the clone is expanded to a sufficiently large number, this limitation could be overcome. Therefore, we opted to transduce the PCR-positive clones with inducible FLAG-tagged Cre-recombinase expressing lentiviral vectors (as described in the “Methods” section) to generate stable inducible Cre-recombinase-based isogenic cells. We found this strategy to successfully generate stably integrated, inducible Cre-recombinase cell lines. Cre-recombinase expression in the clones, upon doxycycline administration, was confirmed by western blot using anti-FLAG antibody (Fig. S3). Regulated induction of the Cre-expressing clones to activate Cre expression would remove the floxed NeoR cassette and allow expression of the targeted mutant allele (Fig. 2a, c, d). Upon doxycycline induction, G → A substitution was observed beginning at 2 days and noted to be equal to A allele by about day 5 (Fig. S4). We speculate that this is because, although the LoxP cassette is floxed promptly upon Cre-recombinase expression, the epigenetic memory of silencing marks on the “null” allele is not reversed until at least a round of cellular replication.
These inducible isogenic cells are currently being used for biochemical studies that investigate mechanisms of SF3B1K700E. We have used the strategy to generate similar SF3B1-mutant cells in a second hematopoietic cell line (HUDEP-2) and to generate isogenic cell lines involving oncogenic mutation of another splicing factor (S34F mutation in U2AF1) (Fig. S5). To further demonstrate the generality of our approach, we have also generated stable HeLa adherent cell lines with endogenous FLAG-epitope-tagged SF3B1 gene (depicted in Fig. 5). Unlike in the case with mutations in SF3B1, pure FLAG-tagged-SF3B1 isogenic cell lines can be generated and expanded after transient Cre expression without requiring an inducible Cre-recombinase system.
a Schematic of genomic SF3B1 locus and rAAV vector is shown. AAV vector consists of left homology arm (LHA) with FLAG-tag and extra 20 bp from downstream exons added to the end for PCR screening, neomycin cassette with IRES, poly(A) termination signal, and a right homology arm (RHA). LoxP sites (shown by black triangles) were placed as indicated to excise out the neomycin cassette after recombination was confirmed. Initially a null allele is formed due to termination of transcript at the p(A) signal following the NeoR cassette. Cre recombination leads to elimination of the cassette flanked by two LoxP sequences and the FLAG-tagged allele is expressed again, with a FLAG tag at N-terminus. b PCR analysis of genomic DNA isolated from unedited and edited K562 cells that underwent recombination. c PCR analysis of genomic DNA from edited K562 cells pre- and post-Cre activation. d Confirmation of successful FLAG-tagged SF3B1 by western blot with FLAG antibody.
Discussion
Isogenic model systems are critical to defining complex molecular mechanisms of clonal diseases such as cancer, where mutations are often limited to one allele. Overexpression models where mutated genes with neomorphic functions are expressed several folds of their wild-type counterparts are clearly of limited utility. In some instances, like oncogenic mutations of splicing factors, paradoxical growth suppression or toxicity is noted in vitro. These scenarios have created roadblocks in the generation of physiological models and need specialized genome-editing approaches capable of creating scalable isogenic cell lines at high efficiency.
Herein we establish a novel combinatory approach of CRISPR/Cas9 with rAAV gene targeting and demonstrate its utility in creating isogenic human cell lines.
Single strand DNA templates are associated with improved knock-in efficiency and fewer off-target insertions compared to double-stranded DNA donors39,40,41. Since our initial attempts to generate isogenic cell lines using conventional CRISPR/Cas9 and short ssDNA templates (~200 bp synthesized oligonucleotides) were unsuccessful, we did not systematically compare knock-in efficiencies of this approach to the one we describe here (i.e., rAAV with CRISPR/Cas9). Given the toxicity of mutant SFs, it is likely that, even when generated through extensive screening, these cell lines will likely lose the mutant allele or die out upon extended culture.
With regard to linear ssDNA oligomers versus rAAV donor templates, it must be noted that the rAAV templates have extremely high knock-in efficiencies due to the virus’ natural ability to stimulate HR. As ssDNA viruses, rAAVs are naturally suited to be HDR templates. Additionally, the pAAV-SEPT-Acceptor plasmid uses a promoterless splice acceptor-IRES-NeoR gene cassette to select correctly integrated clones, which further enhances the HR efficiency. Combining CRISPR/Cas9 and rAAV in this fashion, we obtained >90% clones to be directed to the accurate genomic locus as compared to ~15% with rAAV alone, representing a significant improvement compared to other reports of rAAV-based gene editing18,20,25. To our knowledge, this is the first demonstration where CRISPR was used to specifically target the mutation site and a single rAAV donor served as the HDR template for both the desired modification as well as the gene trap. Our approach differs from the other CRISPR-AAV systems as it relies on HDR-mediated transgene cassette insertion in the adjacent intron but not at the Cas9 targeted DSB site.
While we attained recombination efficiencies of about 15% in the case of SF3B1 editing, it is notable that none of these clones incorporated the K700E mutation. We speculate that this is likely due to the distance of ~300 bp between the mutation site and intron-trap cassette. Without a DSB to direct the HDR, the HR process likely terminates proximal to the K700 locus (Fig. 3c, f). Introducing a targeted DSB with CRISPR/Cas9 avoids this problem by directing HDR to the vicinity of the K700E locus. Hence, in addition to achieving >90% homologous recombination efficiency, all the clones screened were positive for the G → A at the K700E locus.
While combining rAAV and CRISPR/Cas9 resulted in high efficiency of gene editing, toxicity of SF3B1-K700E resulted in death of these clones during their expansion. We overcame this by expressing Cre-recombinase from an doxycycline-inducible system. Thus, cells can be expanded prior to Cre recombination and induced to express Cre-recombinase. Editing efficiency upon doxycycline induction is nearly 100% after a few days, confirming utility of the strategy for biochemistry studies that require several million cells. To demonstrate the generalizability of our approach, we have also optimized the strategy in HUDEP-2, a non-transformed cell line that is difficult to edit compared to transformed cell lines such as K562.
While the intron-trap design can be adapted for most genes, the following considerations must be kept in mind. First, a robust PCR-based screening strategy must be in place before the editing of a particular gene. This may be problematic for GC-rich or low-complexity genomic sequences and may require optimization of primer design or cycling conditions. Second, the cis regulators of splicing (such as branch point, 3’ and 5’ splice sites) may be perturbed by transgene cassette insertion, especially when introns are small. Therefore, the boundaries of LHA and RHA must be carefully defined such that these sequences are not disturbed resulting in splicing failure. Third, the approach requires G418 selection of infected clones, making it unsuitable for in vivo applications and primary cell line editing. Fourth, unlike CRISPR–Cas9 approaches which can be multiplexed, the combinatory approach is not ideal for applications requiring simultaneous modifications of multiple genes in a cell or multiple loci within a single gene. Finally, it is important to keep in mind that transgene insertions may not only physically disrupt genes at their site of insertion42 and influence gene function but also potentially have effects on function of neighboring genes43,44. Therefore, it is recommended to generate multiple cell lines of the same transgene and compare them for any unexpected phenotypes that may be due to the insertion site. It must be mentioned here that the strategy of using a drug resistance cassette flanked by LoxP sites within introns mitigates effects on the expression of neighboring genes by allowing for the removal of the NeoR minigene upon Cre-recombinase activation.
In addition to its utility to generate inducible single-allele mutants, the rAAV intron-trap CRISPR/Cas9 approach lends itself to other applications, such as creation of heterozygous endogenous epitope tags and single-allele knockouts. In summary, we demonstrate the feasibility combining rAAV and CRISPR for targeted gene editing that significantly improves upon conventional approaches for generation of isogenic cell lines, both in terms of precision and efficiency. Using an inducible Cre-recombinase also overcomes the issue of toxicity of the resulting mutant protein.
Methods
rAAV donor plasmid production
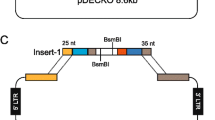
All PCR primers used are listed in Table S1. To efficiently screen accurately recombined cells, a robust screening strategy is essential. An “In–Out” PCR screening strategy was employed as we have previously reported18 (Fig. 2a). Briefly, the first screening primer is located in the targeted genomic locus outside one of the homology arms (RHA in this case) and the second primer is located within the other arm to amplify an ~1.5 Kbp fragment. A copy of the second primer is inserted into the 5’ end of the RHA (adjacent to the NeoR trap cassette).
Homology arms for the human SF3B1 gene were generated by PCR using human genomic DNA as template. LHA and RHA were approximately 1 Kbp in size. As illustrated in Fig. 1, LHA and RHA spanned a genomic region from chr2:197401496-197403593. LHA and RHA were cloned to the AgeI/SacI and ClaI/SalI sites of the pAAV-SEPT-Acceptor plasmid at the multiple cloning sites I and II, respectively. SDM for the insertion of the screening primer and generation of a point mutation in the RHA of the SF3B1 vector was sequentially performed using the Q5 SDM Kit (NEB Biolabs) with primers designed to create the screening primer and K700 locus (K700K → K700E) nucleotide substitution, respectively. The integrity of the plasmid was confirmed by restriction analysis and sequence verified by Sanger sequencing.
rAAV virus production
The rAAV vectors were produced using the AAV-Helper-free System protocol (Agilent Technologies) with modifications, as previously described18. Briefly, AAV-293 cells (HEK-293 cells transformed by adenovirus type 5 DNA)45 were plated at 3 × 106 cells per 100-mm tissue culture plate in 10 ml of Dulbecco’s Modified Eagle Medium (DMEM) growth medium (Gibco) supplemented with 10% (v/v) fetal bovine serum (FBS) (Thermofisher) and 1% penicillin–streptomycin (Gibco) in a humidified 5% CO2 at 37 °C. At 48 h after seeding, the cells were co-transfected with 10 µg each of pAAV-SEPT, pAAV-RC (rAAV2 serotype; Stratagene), and pAAV-Helper (Stratagene) plasmids using a standard calcium phosphate-based protocol, as previously described46. At 72 h post transfections, cells were harvested and the cell suspensions in the DMEM growth medium were subjected to lysis through four rounds of freeze/thaw by alternating the tubes between a dry ice-ethanol bath and the 37 °C water bath. The lysates were centrifuged at 10,000 × g for 10 min at room temperature (RT). The lysis supernatant was transferred to a fresh tube and viral stocks were stored at −80 °C.
Titration of the rAAV-2 viral stock was carried out as previously published47. Briefly, to determine the viral genomic (VG) physical titer per ml, we treated 10 µl of purified virus with DNAse1 (NEB M0303S) following purification of viral DNA using the DNAeasy Blood and Tissue Kit (Qiagen 69506) as per the manufacturer’s protocol. A standard curve was obtained using the pAAV-SEPT purified plasmid by using serial 10× dilutions of 108–104 copies. Quantitative PCR (qPCR) was performed on BioRad CFX96 using KAPA qPCR mix. The DNAseI-treated VG titers were determined to be 6 × 107 copies/ml and 7.3 × 108 copies/ml for rAAV-SF3B1K700E and rAAV-U2AF1S34F (discussed later) viral supernatants, respectively (see Table S3). Estimation of rAAV VG titers is generally recommended to ensure reproducibility of transduction efficiency. Given the relatively high transduction rates associated with rAAV248, we chose to infect all our study cell lines (K562, HUDEP-2, HeLa) with a constant multiplicity of infection (MOI) of ~500.
Cas9 protein expression
The Cas9 nuclease from Streptococcus pyogenes (SpyCas9) was expressed in Escherichia coli (NEB #M0386) and purified49. The protein was stored at −20 °C in a preservation buffer containing 10 mM Tris-HCl, 300 mM NaCl, 1 mM DTT, 0.1 mM EDTA, and 50% glycerol.
sgRNA in vitro transcription
The sgRNAs were designed using the GPP sgRNA Designer online webtool (https://portals.broadinstitute.org/gpp/public/analysis-tools/sgrna-design). The DNA template encoding the sgRNA containing the targeting sequence in the region of interest was assembled using synthetic oligonucleotides, as previously described50. The sgRNAs were generated by in vitro transcription of DNA template using the HiScribe T7 Quick High-Yield RNA Synthesis Kit (NEB #E2050). The sgRNA sequences are provided in Table S2.
Cell culture
Human K562 erythroleukemia cells and HUDEP-2 cells were used for SF3B1 editing. The K562 cells were established from human chronic myelogenous leukemia cells as described previously51, tested free of mycoplasma contamination, and short-tandem repeat validated by ATCC. Cells were maintained in a medium containing 90% RPMI 1640 (Gibco) supplemented with 10% FBS (Thermofisher) and 1% penicillin–streptomycin (Gibco). The HUDEP-2 cells were established from human umbilical blood as described previously52 and provided as a kind gift from Dr. Nakamura53. Cells were maintained in a HUDEP-2 expansion medium54.
Cas9 RNP and rAAV delivery
K562 cells were seeded in 12-well plates at a density of 2.5 × 105 cells per well. After 24 h, 6 nM of Cas9 nuclease (0.3 µl of 20 µM stock) was mixed with 12 nM of guide RNA (0.3 of 40 µM stock), in a 1:2 molar ratio, diluted with 100 µl of Opti-MEM Reduced-Serum Medium (Gibco), followed by addition of TransIT-X2® (2 µl) to generate TransIT-X2®:RNP complexes (Mirus Bio). The TransIT-X2®:RNP complex mixture was then added to the cells. After 1h incubation at 37 °C, cells were spinfected with the rAAV viral supernatant (volume determined by MOI) at 1800 RPM for 45 min at RT. Cells were maintained in serum-containing medium in a humidified 5% CO2 at 37 °C.
The HUDEP-2 cells were seeded at a density of 2.5 × 105 cells, per well. After 24 h, cells were harvested, centrifuged at 300 × g for 5 min, washed once with phosphate-buffered saline, and resuspended in 20 µl of electroporation Solution (Mirus Ingenio) in a 0.2 cm cuvette. In a separate tube, 4 µl of Cas9 nuclease was mixed with 5 µl of guide RNA in a 1:2 molar ratio (1500 nM gRNA:750 nM Cas9; final concentration in cuvette) and incubated for 5 min at RT. The RNP complex mixture was subsequently added to the cell mixture and cells were nucleofected using the Amaxa® 96-well Shuttle system (Lonza) and the program U-008. Immediately after nucleofection, 200 μl of serum-containing medium was added to the nucleofector cuvettes, and cells were transferred onto 24-well plates and incubated in HUDEP-2 expansion medium at 37 °C. After 1-h incubation at 37 °C, cells were spinfected with the rAAV vector (volume determined by MOI) at 1800 RPM for 45 min at RT. Cells were maintained in serum-containing medium in a humidified 5% CO2 at 37 °C.
Identification of the mutant clones
Two days after spinfection, G418 (Sigma) at a final concentration of 0.2 mg/ml was added to the cell culture medium. The cell culture media with G418 was replenished every 3 days. Addition of antibiotics was terminated after 2 weeks at which time resistant clones emerged. To isolate the live cells, Ficoll gradient separation with Histopaque-1077® (Sigma) was used. Single-cell clones were then isolated using limited dilution into 96-well plates in the presence of G418 at 0.2 mg/ml. Individual G418R clones were expanded and then checked for homologous recombination of the targeting rAAV vector using PCR primer pairs specific for the targeted allele using a PCR screening strategy described in the “rAAV donor plasmid production” section.
Generation of inducible SpCas9/CRISPR cell line systems
To generate the inducible Cre-recombinase-based cell lines, K562 (inducible for Cre with doxycycline) and HUDEP-2 cells (inducible for Cre with tamoxifen [doxycycline is already a component of the HUDEP-2 expansion medium]) were transduced with lentiviral vectors encoding Cre-2A-Puro (Tet-pLKO-Puro [Addgene # 21915] for K562; MSCV-CreERT2-Puro [Addgene # 22776] for HUDEP-2) followed by selection in a serum-containing media with 1 μg/ml of puromycin (Sigma) and 0.2 mg/ml of G418. Individual clones were expanded by limiting dilution and the Cre-recombined clones were identified through PCR of genomic DNA using PCR primer pairs specific for the targeted allele. Genomic editing was confirmed at the K700 locus through Sanger sequencing of PCR amplicons, which demonstrated a mixed pattern of AAA (lysine) and GAA (glutamic acid).
Finally, a fraction of the cell population from each of the clones were washed, resuspended in culture medium containing puromycin 1 µg/ml but without G418 (since G418 sensitivity is restored with floxing of G418-resistance LoxP cassette upon Cre-recombinase induction), and induced with 1 µg/ml doxycycline (Sigma) or 1 mM 4-OH-tamoxifen (Cayman chemicals) in ethanol for K562 and HUDEP2, respectively. Confirmation of mRNA expression of the mutant SF3B1 allele was made based on Sanger sequencing of cDNA prepared from RNA extracted from cell lysates, 2–7 days after induction. RNA was extracted using the RNeasy Mini Kit (Qiagen). cDNA synthesis was performed using standard PCR-based techniques. The primer sequences used in this and the previous section are provided in Table S1. For a more easy-to-follow protocol version format, the reader may refer to https://www.protocols.io/edit/high-efficiency-gene-editing-using-adeno-associate-bv99n996.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The datasets generated and/or analyzed during the current study not included in this published article (and its supplementary information files) are available from the corresponding author on reasonable request.
References
Lino, C. A., Harper, J. C., Carney, J. P. & Timlin, J. A. Delivering CRISPR: a review of the challenges and approaches. Drug Deliv. 25, 1234–1257 (2018).
Jacinto, F. V., Link, W. & Ferreira, B. I. CRISPR/Cas9-mediated genome editing: from basic research to translational medicine. J. Cell. Mol. Med. 24, 3766–3778 (2020).
Kato, T. & Takada, S. In vivo and in vitro disease modeling with CRISPR/Cas9. Brief. Funct. Genomics 16, 13–24 (2017).
Guo, T. et al. Harnessing accurate non-homologous end joining for efficient precise deletion in CRISPR/Cas9-mediated genome editing. Genome Biol. 19, 170 (2018).
Yang, F. et al. Functional non-homologous end joining patterns triggered by CRISPR/Cas9 in human cells. J. Genet. Genomics https://doi.org/10.1016/j.jgg.2018.02.009 (2018).
Inui, M. et al. Rapid generation of mouse models with defined point mutations by the CRISPR/Cas9 system. Sci. Rep. 4, 5396 (2014).
Lin, J. & Musunuru, K. Genome engineering tools for building cellular models of disease. FEBS J. 283, 3222–3231 (2016).
Ding, Q. et al. Enhanced efficiency of human pluripotent stem cell genome editing through replacing TALENs with CRISPRs. Cell Stem Cell 12, 393–394 (2013).
Pollyea, D. A. et al. Myelodysplastic syndrome-associated spliceosome gene mutations enhance innate immune signaling. Haematologica 104, e388–e392 (2019).
Sperling, A. S., Gibson, C. J. & Ebert, B. L. The genetics of myelodysplastic syndrome: from clonal haematopoiesis to secondary leukaemia. Nat. Rev. Cancer 17, 5–19 (2017).
Bapat, A. et al. Myeloid disease mutations of splicing factor SRSF2 cause G2-M arrest and skewed differentiation of human hematopoietic stem and progenitor cells. Stem Cells 36, 1663–1675 (2018).
Chen, L. et al. The augmented R-loop is a unifying mechanism for myelodysplastic syndromes induced by high-risk splicing factor mutations. Mol. Cell 69, 412.e6–425.e6 (2018).
Howes, R. & Schofield, C. Genome engineering using adeno-associated virus (AAV). Methods Mol. Biol. 1239, 75–103 (2015).
Balakrishnan, B. & Jayandharan, G. R. Basic biology of adeno-associated virus (AAV) vectors used in gene therapy. Curr. Gene Ther. 14, 86–100 (2014).
Dhungel, B. P., Bailey, C. G. & Rasko, J. E. J. Journey to the center of the cell: tracing the path of AAV transduction. Trends Mol. Med. 27, 172–184 (2021).
Russell, D. W. & Hirata, R. K. Human gene targeting by viral vectors. Nat. Genet. 18, 325–330 (1998).
Karnan, S. et al. Improved methods of AAV-mediated gene targeting for human cell lines using ribosome-skipping 2A peptide. Nucleic Acids Res. 44, e54 (2016).
Kim, J. S., Bonifant, C., Bunz, F., Lane, W. S. & Waldman, T. Epitope tagging of endogenous genes in diverse human cell lines. Nucleic Acids Res. 36, e127 (2008).
Kohli, M., Rago, C., Lengauer, C., Kinzler, K. W. & Vogelstein, B. Facile methods for generating human somatic cell gene knockouts using recombinant adeno-associated viruses. Nucleic Acids Res. 32, e3 (2004).
Kim, J. S. et al. Intact cohesion, anaphase, and chromosome segregation in human cells harboring tumor-derived mutations in STAG2. PLoS Genet. 12, e1005865 (2016).
Vasileva, A., Linden, R. M. & Jessberger, R. Homologous recombination is required for AAV-mediated gene targeting. Nucleic Acids Res. 34, 3345–3360 (2006).
Epstein, B. E. & Schaffer, D. V. Combining engineered nucleases with adeno-associated viral vectors for therapeutic gene editing. Adv. Exp. Med. Biol. 1016, 29–42 (2017).
Moser, R. J. & Hirsch, M. L. AAV vectorization of DSB-mediated gene editing technologies. Curr. Gene Ther. 16, 207–219 (2016).
Reber, S. et al. CRISPR-Trap: a clean approach for the generation of gene knockouts and gene replacements in human cells. Mol. Biol. Cell 29, 75–83 (2018).
Bak, R. O. & Porteus, M. H. CRISPR-mediated integration of large gene cassettes using AAV donor vectors. Cell Rep. 20, 750–756 (2017).
Gaj, T. et al. Targeted gene knock-in by homology-directed genome editing using Cas9 ribonucleoprotein and AAV donor delivery. Nucleic Acids Res. 45, e98 (2017).
Kumar, N. et al. The development of an AAV-based CRISPR SaCas9 genome editing system that can be delivered to neurons in vivo and regulated via doxycycline and Cre-recombinase. Front. Mol. Neurosci. 11, 413 (2018).
Bak, R. O., Dever, D. P. & Porteus, M. H. CRISPR/Cas9 genome editing in human hematopoietic stem cells. Nat. Protoc. 13, 358–376 (2018).
Li, A. et al. A self-deleting AAV-CRISPR system for in vivo genome editing. Mol. Ther. Methods Clin. Dev. 12, 111–122 (2019).
Martin, R. M. et al. Selection-free, high frequency genome editing by homologous recombination of human pluripotent stem cells using Cas9 RNP and AAV6. Cell Stem Cell 24, 821.e5–828.e5 (2019).
Topaloglu, O., Hurley, P. J., Yildirim, O., Civin, C. I. & Bunz, F. Improved methods for the generation of human gene knockout and knockin cell lines. Nucleic Acids Res. 33, e158 (2005).
Hellen, C. U. & Sarnow, P. Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev. 15, 1593–1612 (2001).
Huang, M. T. & Gorman, C. M. Intervening sequences increase efficiency of RNA 3' processing and accumulation of cytoplasmic RNA. Nucleic Acids Res. 18, 937–947 (1990).
Seiler, M. et al. Somatic mutational landscape of splicing factor genes and their functional consequences across 33 cancer types. Cell Rep. 23, 282.e4–296.e4 (2018).
Sentmanat, M. F., Peters, S. T., Florian, C. P., Connelly, J. P. & Pruett-Miller, S. M. A survey of validation strategies for CRISPR-Cas9 editing. Sci. Rep. 8, 888 (2018).
Paquet, D. et al. Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature 533, 125–129 (2016).
Kesarwani, A. K. et al. Cancer-associated SF3B1 mutants recognize otherwise inaccessible cryptic 3' splice sites within RNA secondary structures. Oncogene 36, 1123–1133 (2017).
Darman, R. B. et al. Cancer-associated SF3B1 hotspot mutations induce cryptic 3' splice site selection through use of a different branch point. Cell Rep. 13, 1033–1045 (2015).
Renaud, J. B. et al. Improved genome editing efficiency and flexibility using modified oligonucleotides with TALEN and CRISPR-Cas9 nucleases. Cell Rep. 14, 2263–2272 (2016).
Miura, H., Quadros, R. M., Gurumurthy, C. B. & Ohtsuka, M. Easi-CRISPR for creating knock-in and conditional knockout mouse models using long ssDNA donors. Nat. Protoc. 13, 195–215 (2018).
Zhou, X. Empowering chimeric antigen receptor T-cell therapy with CRISPR. Biotechniques 68, 169–171 (2020).
Delpy, L., Decourt, C., Le Bert, M. & Cogne, M. B cell development arrest upon insertion of a neo gene between JH and Emu: promoter competition results in transcriptional silencing of germline JH and complete VDJ rearrangements. J. Immunol. 169, 6875–6882 (2002).
Olson, E. N., Arnold, H. H., Rigby, P. W. & Wold, B. J. Know your neighbors: three phenotypes in null mutants of the myogenic bHLH gene MRF4. Cell 85, 1–4 (1996).
Yoon, J. K., Olson, E. N., Arnold, H. H. & Wold, B. J. Different MRF4 knockout alleles differentially disrupt Myf-5 expression: cis-regulatory interactions at the MRF4/Myf-5 locus. Dev. Biol. 188, 349–362 (1997).
Graham, F. L., Smiley, J., Russell, W. C. & Nairn, R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36, 59–74 (1977).
Wigler, M. et al. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc. Natl Acad. Sci. USA 76, 1373–1376 (1979).
Zheng, W. et al. Development and validation of quantitative real-time pcr for the detection of residual CHO host cell DNA and optimization of sample pretreatment method in biopharmaceutical products. Biol. Proced. Online 21, 17 (2019).
Chen, C., Akerstrom, V., Baus, J., Lan, M. S. & Breslin, M. B. Comparative analysis of the transduction efficiency of five adeno associated virus serotypes and VSV-G pseudotype lentiviral vector in lung cancer cells. Virol. J. 10, 86 (2013).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821 (2012).
Lin, S., Staahl, B. T., Alla, R. K. & Doudna, J. A. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. Elife 3, e04766 (2014).
Lozzio, C. B. & Lozzio, B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood 45, 321–334 (1975).
Daniels, D. E. et al. Comparing the two leading erythroid lines BEL-A and HUDEP-2. Haematologica 105, e389–e394 (2020).
Kurita, R. et al. Establishment of immortalized human erythroid progenitor cell lines able to produce enucleated red blood cells. PLoS ONE 8, e59890 (2013).
Vinjamur, D. S. & Bauer, D. E. Growing and genetically manipulating human umbilical cord blood-derived erythroid progenitor (HUDEP) cell lines. Methods Mol. Biol. 1698, 275–284 (2018).
Acknowledgements
We would like to thank the Yale Center for Genome Analysis (YCGA) and Yale Keck Center for DNA sequencing. The work was supported in part by National Institutes of Health (NIH) grants HL133406 (to M.M.P. and K.M.N.) and T32 CA233414 (to P.C.B.), a pilot grant from the NIDDK Core Centers for Excellence in Hematology (to P.C.B)., a U54 pilot grant from YCCEH (A.K.G). and a pilot grant from the Frederick A DeLuca Foundation (to M.M.P.).
Author information
Authors and Affiliations
Contributions
P.C.B. and A.K.G. designed and performed experiments and wrote the manuscript. J.-S.K., T.W., and K.M.N. provided experimental guidance and edited the manuscript. M.M.P. was involved in designing the strategy and writing and editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary handling editor: Eve Rogers. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boddu, P.C., Gupta, A.K., Kim, JS. et al. Generation of scalable cancer models by combining AAV-intron-trap, CRISPR/Cas9, and inducible Cre-recombinase. Commun Biol 4, 1184 (2021). https://doi.org/10.1038/s42003-021-02690-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s42003-021-02690-1
- Springer Nature Limited