Abstract
The Mott insulator-to-metal transition (IMT) driven by electron correlations has been among the main research topics in materials science over the past decades. The complex interplay between electronic and lattice degrees of freedom leads to various transition scenarios. Of particular interest may be the case of a transition involving the formation of complex phases comprising regions that differ significantly in their physical properties within the same material. Here, we present the results that advance the understanding of the IMT phenomenon, offering the documentation of a pure site-selective mechanism that is not complicated by any structural and spin transformation. Combining XRD, resistivity, Mössbauer and Raman spectroscopy measurements, we provide evidence for a pure pressure-induced Mott transition in α-LiFe5O8, characterized by site-selective delocalization of electrons, leading to the formation, above ~65 GPa, of a site-selective Mott phase consisting of metallic and insulating sublattices. We note that the electron delocalization in the partially disordered octahedral sublattice cannot be understood purely in terms of a Mott transition, the Anderson-Mott transition picture seems more adequate.
Similar content being viewed by others
Introduction
The insulator-metal transition (IMT) driven by electronic correlations is one of the most fundamental concepts in condensed matter. The complex interplay between electronic correlations and the spin, orbital, and lattice degrees of freedom leads to a wealth of possible scenarios of the IMT and the appearance of complex phases, which could be attractive for technological applications. The connective Mott transition, is of particular importance because it is essential to understanding the properties of strongly correlated transition-metal compounds being especially relevant to understanding high-temperature superconductivity, as well as heavy-fermion behavior1,2,3,4,5,6,7,8. A definitive electronic phenomenon in such compounds, induced by pressure, composition, or other means, is the breakdown of the d- or f-electron localization, causing the Mott IMT, which usually goes along with a collapse of the magnetic interactions1,2,3,9,10 or a high- to low-spin (HS-LS) transition11,12,13,14.
Of particular interest is the case of the Mott IMT in materials with a crystal structure containing transition-metal cations in different coordination polyhedra, where the interplay of electronic correlations and crystal structure may result in rather complex behavior of the electronic and magnetic states. Recent theoretical calculations of rare-earth nickelates15,16 suggest the formation of a Mott phase that remains electrically insulating while exhibiting the formation of site-selective local moments and Ni–d, O–p singlet states. Furthermore, high-pressure (HP) studies of Fe2O3 suggest that this material undergoes a site-selective Mott transition, accompanied by a site-dependent collapse of magnetic moments17 (which goes along with site-dependent delocalization (metallization) of the 3d electrons). However, in Fe2O3 the electron delocalization corroborating with the spin transition coincides also with a structural phase transition, all these complicating an analysis of the role of structural and electronic components in the transition. Undoubtedly, additional studies of the phenomenon of the site-selective Mott transition are essential to further understanding of its features.
Ferrite oxides and particularly ferric spinels seem to be suitable objects for the study of the site-selective Mott IMT phenomenon since their structures are usually characterized by two distinct coordination sites suggesting that in these systems a delocalization/metallization of the 3d electrons should not occur simultaneously and propagate through different crystallographic sites at different degrees of compression17,18. We note that ferrite spinels are some of the most studied materials, not only due to their rich physical properties, but also diverse applications in the field of magnetic recording media, ferrofluid, gas sensing, soft or hard magnetic materials, microwave absorption, etc.19,20,21. Most of the above-mentioned materials order magnetically above room temperature demonstrating a variety of magnetic ordering types, and their properties can be altered by doping20,21, or application of pressure22,23.
In the particular case of ferric oxides, the Mott and spin transitions are usually first-order transitions observed at the 40–60 GPa pressure range10,13,24,25. However, a signature of a partial sluggish second-order HS-LS transition has been found recently for some ferrite post-spinels26 and in disordered α-LiFeO2, characterized by a random distribution of Li and Fe ions27. Sluggish partial site-specific spin crossover appears to be the preferred electronic response of ferrous spinels at sufficiently high density28,29,30. Noteworthy, at high pressures, all these materials are characterized by the random environment of Fe ions, and specific features of the Mott IMT in case of such disordered systems are of particular interest31,32.
Recent high-pressure studies of AFe2O4 (A= Zn, Mg, Co and Fe) ferrite spinels revealed a structural transition in between 25–30 GPa to a post-spinel phase22,23,26,33,34,35,36,37,38,39 characterized by the Cmcm structure containing 6 and 8-fold coordination Fe3+ polyhedra26. With this, we note that lithium ferrite LiFe5O8 is the only member of the spinel ferrite family, which can be prepared in ordered and disordered phases40, α-LiFe5O8 and β-LiFe5O8, respectively41,42. This feature makes LiFe5O8 especially attractive in terms of studying not only the pressure-induced site-selective Mott transition but also the influence of a structural disorder on this phenomenon.
In the present work, we reveal a pure site-selective Mott IMT in α-LiFe5O8 not complicated by any structural and spin transformation, and resulting in the formation of a site-selective Mott phase consisting of metallic/nonmagnetic and insulating sublattices. Furthermore, we find that electron delocalization in LiFe5O8 octahedral sublattice is a sluggish process due to a significant inhomogeneity of the iron local environment. Our results suggest that site-selective Mott transitions involving the formation of sublattices, which differ in their physical properties, within the same material, may constitute a rather general phenomenon in correlated-electron materials with a complex crystal structure.
Results
X-ray diffraction
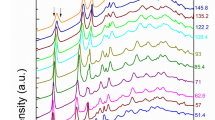
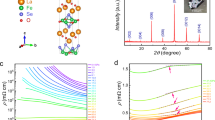
XRD patterns obtained up to about 83 GPa are shown in Fig. 1a, and Supplementary Fig. 1 and 2. Low pressure (LP) patterns up to 36 GPa can be identified as the cubic phase with space group P4332 with Fe3+ in tetrahedral (8c) and octahedral (12d) sites, whereas Li+ ions occupy the (4b) octahedral positions (Supplementary Fig. 3a)40. At 33.6 GPa, new diffraction peaks start appearing. However, the LP cubic phase still dominates up to about 50 GPa and coexists with a new high-pressure (HP) phase nearly up to 57 GPa, after which no signature of the LP phase is found. Rietveld refinement was used in order to obtain lattice parameters for the LP phase according to space group P4332 as seen in Supplementary Fig. 4i. The LP phase V(P) data can be fit well with a second-order Birch-Murnaghan (BM2) equation of state (EOS)43 (Fig. 1c, and Supplementary Table 1). (Supplementary Materials). The patterns of the HP phase could be fitted (Supplementary Fig. 4(ii)) well with the CaTi2O4-type (Cmcm) structure. This structure is characterized by two cationic sites: octahedral 8f and a bicapped trigonal prism 4c Wyckoff sites, characterized by 6 and 8-fold coordination polyhedra, respectively (Supplementary Fig. 3b). The structural transition to the HP phase is accompanied by ~ 2.1% volume reduction. Another noticeable change in the V(P) data, namely a steeper decrease of the unit-cell volume of the HP phase with pressure increase occurs at about 70 GPa (Fig. 1(c)). Such V(P) behavior suggests an appreciable electronic transformation taking place within the HP phase. Correspondingly the V(P) data for the HP phase could not be fitted with a “standard” equation of state. Meanwhile, one can use the data at a pressure range of 51<P< 69 GPa to define an EOS for the HP phase before the steeper decrease of V (Supplementary Table 1). An extrapolation of the obtained EOS to higher pressures allows us to estimate a volume reduction corresponding with the proposed electronic transition (Fig. 1c). This estimation gives ΔV/V70GPa ≈ 0.9%.
a X-ray powder diffraction patterns of α-LiFe5O8 at T=298 K at various pressures (λ = 0.3344 Å). Diffraction peaks of the Cmcm high-pressure phase, which first appears at ~ 34 GPa (marked with asterisks) is clearly seen at 61 GPa. Miller indices in blue color correspond to the diffraction peaks of the Cmcm phase, whereas those in yellow represent peaks of the P4332 phase. b Zoomed portion of the plot clearly showing structural phase transition. Positions of main diffraction peaks of the Pt pressure marker are shown with dashed lines. c Unit-cell volume of the various structural phases of α-LiFe5O8 as a function of pressure. Dash-dotted line is a fit for the cubic P4332 phase using the BM2 EOS. The dotted line represents a fit to the orthorhombic Cmcm phase at the pressure range 51–69 GPa. Note a steeper decrease of unit-cell volume with pressure increase above 69 GPa. The volume and pressure error bars are within the symbol sizes.
Raman spectroscopy
Room temperature Raman spectra of α-LiFe5O8 were collected up to nearly 78 GPa. The spectra measured upon compression are shown in Fig. 2a. Up to ~41 GPa all the peaks can be identified as the Raman modes calculated for α-LiFe5O8 sample in the P4332 space group44. The appearance of new Raman peaks and the disappearance of previous ones can be seen above 41 GPa (Fig. 2a), suggesting a first-order structural transition at this pressure range. Another distinct change in the spectra can be seen above 60 GPa, above which some of the modes significantly decrease in intensity and an additional mode appears around 480 cm−1. The results of the Raman measurements are summarized in Fig. 2c by plotting the most intense peak positions as a function of wavenumber. Two distinct changes can be seen at ~41 and 61 GPa as discussed above. Upon decompression, a distinct change in the Raman modes is observed at ~55GPa (Figs. 2b, d) with the recovery of the peaks, which remain down to ambient pressure. These peaks do not match with the LP structure but are consistent with the modes observed at the range 41–61 GPa upon compression. This suggests an irreversible structural phase transition in LiFe5O8, similar to other spinel ferrites26.
Mössbauer spectroscopy
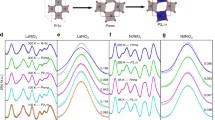
α-LiFe5O8 is ferrimagnetic below ~ 900 K40. At ambient conditions, the Mössbauer spectrum of LiFe5O8 consists of two sextets (Fig. 3a) corresponding to two LP Fe3+ structural sites, tetrahedral (T) and octahedral (O) (Supplementary Fig. 3a). The two components exhibit magnetic hyperfine fields Hhf = 49.7(2) and 51.6(2) T, typical for a ferric-oxide high-spin 6A1g state in tetrahedral and octahedral environments, respectively. The area ratio for these two components is restricted to 40:60 to remain consistent with the structural data discussed earlier. Between ambient pressure and 44 GPa the observed spectra barely change, with the only observable variation being a slight decrease in isomer shift and Hhf (Fig. 3d).
Selected Fe Mössbauer spectra of α-LiFe5O8 at various pressures and temperatures; at room temperature a, c and low temperatures b, upon compression a, b and decompression (c). Empty circles represent experimental data points whereas the black solid line through the data points represents the overall fit to the data from the sum of sub-components shown. Gray and pink shaded sub-components refer to the P4332 phase tetrahedral and octahedral sites, respectively, whereas olive dark-yellow and red sub-components represent the Cmcm phase HP1, HP2, and HP3 components, respectively. Pressure dependence of the isomer shift (IS), hyperfine field (HF) and abundances or area percentage extracted from best fits to the Mössbauer spectra of α-LiFe5O8 for (d) compression and (e) decompression cycles at RT. In (d) black squares and pink diamonds refer to parameters for the octahedral and tetrahedral sites of the P4332 phase, respectively, whereas dark yellow triangles, olive asterisks and red spheres in both (d) and (e) represent HP1, HP2 and HP3 components for the Cmcm phase, respectively. We note that in the case of SMS measurements (panel b) there is an appreciable delay in the HP1 to HP3 transformation compared to in-house measurements (panel a): even at 90 GPa the abundance of the HP1 component remains rather high, ~ 20%. This may be attributed to the different pressure transmitting media used and how the degree of non-hydrostaticity (strain and stress) affects the electronic transition58.
Above 52 GPa two new sextets appear (Figs. 3a, d) with smaller hyperfine field values of 41.6(9) and 35.7(1.7) T, suggesting Fe3+ HS states characterized by a magnetic ordering temperature (TN) close to room temperature. These two new components, designated as HP1 and HP2, are characterized by isomer shift values of 0.23(4) and 0.36(3) mm/s (Fig. 3d). The larger isomer shift value obtained for the HP2 component suggests an 8-fold coordinated nearest-neighbor environment characterized by larger Fe–O distances (isomer shift has a negative linear dependence on the effective s-electron density at the nucleus ρs(0)45). Furthermore, the isomer shift values for the HP1 and LP(O) components are reasonably close supporting similar nearest-neighbor environments; namely, an octahedral configuration. Magnetic sextets from the LP phase coexist with the two new sextets up to at least 59 GPa (Figs. 3a, d). At 65 GPa, no traces of the LP phase have been found in the Mössbauer spectra. Alongside the magnetic sextets a non-magnetic component, designated as HP3, is observed in the central part of the spectra, characterized by a reduced isomer shift value of 0.19(2) mm/s. The abundance of the new component increases with pressure, replacing the HP1 component (Figs. 3a, d) and its isomer shift value shows an appreciable decrease above 65 GPa. Meanwhile, the abundance of the HP2 HS component stabilizes above 60 GPa at ~ 40% and does not change with pressure.
The HP3 component, observed above 65 GPa, can originate from a non-magnetic state or a low-spin state. To clarify the nature of this component we performed low–temperature Synchrotron MS measurements46 at temperatures down to 2.4 K(Fig. 3b). (We note that when fitting the area ratio for the P4332 phase we obtain the expected 60:40 in agreement with the structural data, but when all parameters are free, the uncertainty is large due to the overlap of the two components. Thus, we fixed the ratio at 60:40 to avoid larger uncertainty.). These measurements show no sign of broadening or splitting of the absorption spectrum and no increase of quadrupole splitting for HP3 component with temperature decrease down to 2.4 K, which means no features of a magnetic interaction. The collapse of magnetic interactions, observed above ~ 65 GPa, coinciding with the sharp decrease of the isomer shift (which means an increase of the effective s-electron density at the nucleus ρs(0)45) point to a collapse of electron localization rather than a transition to a low-spin state (see9,10,45).
Upon decompression, we do not observe a recovery of the original spectra typical for the LP phase. However, below 50 GPa the features of the spectra change significantly, compared to the spectra observed above 70 GPa (Figs. 3c, e). Particularly, the feature of the non-magnetic doublet changes with pressure decrease: it broadens considerably, decreases in magnitude and the corresponding isomer shift value increases by ~ 0.2 mm/s, almost coinciding with the isomer shift value of the HP1 component. In addition, Hhf values of the magnetic HP2 and HP1 components increase to about 49 and 43 T, respectively, suggesting an increase of the TN values. One can propose a partial collapse of the magnetic order of the HP1 component, showing a central peak representing the fast spin-spin relaxation typical of the T ~ TN regime. The observed features of MS spectra upon decompression are undoubtedly a signature of an irreversible phase transition.
Electrical resistance
Room-temperature resistance for both compression and decompression cycles are shown in Fig. 4 as a function of pressure. A steep decrease in the resistance is observed at the 65–70 GPa pressure range, concurring with the growing of the non-magnetic doublet in Mössbauer spectra. The variation of lnR as a function of inverse temperature at various pressures are shown in the inset of Fig. 4. Up to 63 GPa, the resistance increases with decreasing temperature, indicating insulating-like behavior. Above 65 GPa, the resistance shows metallic behavior. The activation energy Ea(P) obtained from the slope of linear fits to these plots is shown in the upper inset of Fig. 4: a continuous decrease in the Ea with increasing pressure results in a gap closure at ~ 68 GPa.
a Pressure dependence of the resistance upon compression and decompression at 300 K in the main panel. Dashed green vertical line in the panel (a) delineates pressures where the new Cmcm phase starts to dominate according to XRD experiments (see text and preceding Fig. 1). Dashed orange line delineates the pressure where the insulator-to-metal transition takes place. The error in the pressure determination also should be taken into account. b shows linearized temperature-dependent data at various pressures assuming Arrhenius activated hopping transport (below 32 GPa such measurements were unrealizable using our experimental setup because of the high resistance value). The activation energy Ea is obtained from the slope of linear fits to these plots to obtain the charge gap for intrinsic conduction Eg = 2Ea. (c) shows the pressure dependence of Ea, which drops to zero at ~ 65 GPa.
Upon decompression, down to 60 GPa the R(P) behavior is similar to that of the compression cycle, indicating a pure electronic nature of the transition observed at the 60–70 GPa range. Upon further pressure decrease the R(P) behavior changes significantly. The resistance value at ambient pressure is above two orders of magnitude lower compared to the compression cycle, despite the decrease in sample thickness. This agrees well with the signs of an irreversible structural phase transition, in accordance with Raman and MS data.
Discussions
Summarizing the obtained XRD, Raman spectroscopy and MS data, we can conclude that LiFe5O8, similar to a few other ferric spinels MFe2O4 (M=Fe, Mg, Zn)22,23,26,33,34,35,36,37,38, undergoes a first order structural phase transition upon pressure increase; however, at the pressure range 33–57 GPa compared to 25–40 GPa typical for other ferric spinels. This phase transition is manifested by the observed changes in XRD patterns, Raman spectra and dramatic changes of the hyperfine interaction parameters. As in many other ferrites, the high-pressure phase of LiFe5O8 comprises two distinct Fe3+ sites characterized by different Hhf and IS values. Analysis of the powder XRD data allows assuming for the HP phase the Cmcm structure typical for ferric26 and some ferrous post-spinels28,29,30. In this structure, two Fe3+ sites, distinct according to MS studies, correspond well with two cationic sites: namely, the octahedral and a bicapped trigonal prism, characterized by 6 and 8-fold coordination polyhedra, respectively. The abundances of Fe3+ occupying octahedrons and bicapped trigonal prisms stand at 60:40% (Fig. 3c), respectively, which suggests that the rest of the octahedral sites are occupied by Li+1 ions.
Similar to the above-mentioned ferric spinels, immediately following the phase transition Fe3+ in both sites is in the HS state. With further pressure increase, a steeper decrease of unit-cell volume of the HP phase is seen at ~ 70 GPa (Fig. 1d). This behavior of the unit-cell volume corroborates with an appreciable change in the features of the MS spectra: namely, a new non-magnetic quadrupole-split component appears above ~ 59 GPa, with abundance increasing rapidly with pressure (Fig. 3c). The new HP3 component is characterized above 65 GPa by a significantly reduced IS value, which corresponds to an increase of the effective s-electron density at the nucleus that may result from either (i) a spin crossover, or (ii) a correlation breakdown (a Mott transition) in which magnetic interactions vanish10,45. In the present case, the lack of any signs of magnetic correlations on Mössbauer timescales ( ≈ 10−7 s) down to 2.4 K prompted us to designate the HP3 component as a non-magnetic resulting from an electron correlation breakdown. A corroborating IMT deduced from R(P, T) measurements, confirms this conclusion. Since MS observations refer to the HP1 component as undergoing an electronic transition, we conclude that the Mott transition originates only on the octahedral sites, while the electronic state of Fe3+ ions on the trigonal prisms does not change at least up to ~ 90 GPa.
The existence of a site-selective Mott phase has recently been discussed in the rare-earth nickelates15,16,47. However, the latter remains electrically insulating, while exhibiting formation of site-selective local moments. Furthermore, recent studies of Fe2O3 provide an evidence for a site-selective Mott phase, in which the 3d electrons on only half of the Fe sites (octahedral) are metallic, while the rest remain insulating17. However, in Fe2O3 the electronic transition coincides with the structural transition complicating an analysis of the role of structural and electronic components in the phase transition. In contrast to Fe2O3, in the present case we observe a pure electronic site-selective Mott transition not complicated by any structural transformations, which takes place in the Cmcm phase above 65 GPa. This transition is accompanied only by a minor volume decrease of ~ 0.9% suggesting a decrease of the FeO6 octahedral volume below 2%. Such octahedral volume change at the transition is significantly less than that observed in Fe2O3 ( ~ 14 %), which signifies a different mechanism of the electronic transition in both cases. In Fe2O3 the octahedral volume change upon metallization is similar to that observed in CaFe2O4, FeOOH and FeBO348,49,50 (ΔV/Voct ~ 12–12.4%) during the Fe3+ HS-LS transition, suggesting in accord with theoretical calculations17 a closure of the Mott-Hubbard gap associated with a spin crossover12,13,14. (We note that differences in the Mott transition onset pressure identified by the four techniques is ascribed to the different pressure transmitting media and degrees of hydrostaticity in the DAC (see supplementary materials). Undoubtedly, such a mechanism is not relevant to the present case. In the present case it seems that a more suitable scenario is the transformation of electron states from localized to itinerant without an accompanying change of the spin state. In such a case, the corresponding volume change could be relatively small10. Therefore, one can expect in LiFe5O8 a closure of the gap on the octahedral sites driven by a classical bandwidth broadening mechanism1,2 and resulting in the formation of a site-selective Mott phase consisting of metallic and insulating sublattices.
The question remains, why the behavior of LiFe5O8 is different from many other ferric oxides, characterized by the HS-LS transition of the Fe3+ in the octahedral environment at the 40–60 GPa pressure range10,13,24,25,26. This feature could be related to a significant octahedral distortion due to the presence of Li+1, whose ionic radius exceeds that of Fe3+ by ~ 18%51. It could be proposed that an additional ~ 16% decrease of Fe ionic radii due to the transition to the LS state and a corresponding further increase of the octahedral distortion does not facilitate this electronic transition. Meanwhile, in the case of another lithium ferrite, LiFeO2, a very sluggish second-order Fe3+ HS-LS transition is observed, starting at ~ 50 GPa and not completed even at ~ 100 GPa (however, in contrast to LiFe5O8, LiFeO2 remains insulating at least up to 115 GPa).
An important feature distinguishing these lithium ferrites is that rocksalt LiFeO2 and the Cmcm phase of LiFe5O8 are microscopic disorder systems due to the presence of Li+1 ions in the octahedral sublattice. It is noteworthy that according to Mott’s argument1,2, in the case of doping a transition to a metal takes place at some critical concentration nc. However, in fact, the doping process not only introduces excess electrons but at the same time creates disorder since the dopant atoms are randomly distributed in the host lattice. As a result, the observed transition is continuous and cannot be understood purely in terms of a Mott transition. Thus an Anderson-Mott transition picture seems more adequate31. In our case a transition to a metal can be achieved by inducing a d band broadening at some critical pressure Pc. However, the pure Mott transition picture is inadequate also in this case since one has to deal simultaneously with disorder and interactions between the electrons. Correspondingly one would expect the transition, in part, to follow the Anderson model52,53,54, and a continuous feature of the transition. Namely, a random distribution of Fe3+ and Li1+ cations in octahedral sites should result in significant inhomogeneity of the iron local environment, a possible clusters formation and therefore smearing the value of the critical pressure required for electron delocalization (or spin crossover in the case of LiFeO2). Indeed, in contrast to many other ferric compounds10 the Mott transition in LiFe5O8 is characterized by a rather gradual transformation. The onset of metallization is observed at ~ 65 GPa. However, according to SMS data (Fig. 3b), He pressure medium) about a third of octahedral Fe3+ ions (HP1 component) are still not involved in the transition and their fraction remains quite large up to ~ 90 GPa, the highest pressure measured. In the case of N2 pressure medium the transition is sharper (Fig. 3a) suggesting that a transition diffuseness strongly depends on the hydrostatic conditions shaping the sample microstructure. Hence, another noticeable feature of the Mott transition in LiFe5O8 is the interplay between electron-electron interactions and disorder, which results in the sluggish second-order-type metallization on the octahedral sites associated with a nonhomogeneous environment of Fe3+ ions. This special case of the site-selective Mott transition is of great interest and undoubtedly deserves further in-depth study.
In Fig. 5 we present the pressure-temperature phase diagram of LiFe5O8 based on our XRD, MS, resistivity and Raman spectroscopy results. We note that LiFe5O8 turns metallic at Pc ~ 65 GPa but with a minimum in R(T) plots above Pc, which shifts to lower temperatures under pressure. The change of the R(T) slope sign at lower temperature could be related with a weak electron localization originating from the presence of disorder potentials in the octahedral sublattice. According to MS data this disorder results also in a smearing of the transition pressure range and an incompleteness of the electron delocalization process up to the highest pressures measured. Upon decompression, according to the Raman and MS data, the Cmcm structure remains unchanged. However, we observe a reversal in electronic properties with no sign of hysteresis down to ~ 60 GPa confirming a pure electronic nature of the observed site-selective electronic transition, not complicated by any coinciding structural transformations.
A part of the R(T) plot is expanded in the inset to emphasize the metallic behavior above ~ 65 GPa and the change of R(T) slope sign with temperature decrease. We note that a minimum in R(T) plots shifts to lower temperatures under pressure and above the minimum resistance shows (R-R0) ~ Tn behavior. Solid lines are fits using this expression where n value decreases gradually from n ~ 1.19 to 0.93 at the 68–78 GPa pressure range demonstrating a non-Fermi-liquid behavior.
Conclusions
We provide experimental evidence for a pressure-induced site-selective Mott IMT in LiFe5O8 characterized by site-selective delocalization of electrons above 65 GPa. Within the Cmcm crystal structure, characterized by two distinct coordination sites (VI and VIII), we observe 40% of ferric ions occupying bicapped trigonal prism sites and above 65 GPa ions on the octahedral sites having delocalized electrons. The transition is characterized by delocalization/metallization of the 3d electrons only on the Fe sites in the octahedral sublattice, with a site-dependent collapse of magnetic interactions. The delocalization of electrons on the octahedral sites coincides with a minor volume decrease suggesting a classical Mott IMT driven by a band-width broadening mechanism. Due to a significant inhomogeneity of the iron local environment in the octahedral sublattice, containing also Li ions, the electron delocalization is a sluggish process, which is not completed yet at about 90 GPa: instead of a certain critical pressure of the IMT one observes a broad critical pressures distribution. Hence, in the case of LiFe5O8 at high pressures the material is characterized by two sublattices, which differ significantly not only by the Pc value but also by the character of the IM transition. Thus, our results extend the classification of Mott insulator-to-metal transition, offering the documentation of a pure site-selective mechanism, not complicated by any structural and spin transformation, in this case–a second-order type. They suggest that the concept of a site-selective Mott transition may be applicable to correlated-electron materials: in particular, in materials with a complex crystal structure and intricate short-range ordering features.
Methods
A polycrystalline sample of α-LiFe5O8 was synthesized at Tel Aviv University (TAU) (enriched to 25% with 57Fe isotope when needed for MS experiments). Custom TAU diamond anvil cells (DACs)55 were used to induce high pressure, with He, Ne or N2 as a pressure-transmitting medium. The pressure was determined using the ruby R1 fluorescence line as a pressure marker56, diamond Raman spectra57, and the Ne and Pt unit-cell volumes in the case of x-ray diffraction studies. Powder XRD experiments were performed at the 13-ID-D beamline of the Advanced Photon Source (APS), Argonne National Laboratory (Argonne, IL) and at the beamline of the Synchrotron Soleil (Paris). Diffraction patterns up to ~ 30 GPa were analyzed by Rietveld refinement and above ~ 30 GPa by using the Whole Profile Fitting (Pawley) method (see Supplementary Materials www.xxxxx.com). 57Fe Mössbauer studies were performed using a 10 mCi 57Co (Rh) point source. Low-temperature measurements at 76 and 90 GPa were performed using energy-domain synchrotron Mössbauer spectroscopy carried out at the beamline ID18 at ESRF (Grenoble). Electrical resistance measurements were performed as a function of pressure and temperature using a standard four-probe method. High-pressure Raman spectra of LiFe5O8 were collected at room temperature using a Horiba (iHr-550) spectrometer with a 487.8 nm excitation source. Further technical details about the methods used can be found in the Supporting Information www.xxxxx.com.
Data availability
The data sets supporting the findings of the present study are available from the corresponding author upon reasonable request.
References
Mott, N. Metal-insulator transitions (CRC Press, 2004).
Mott, N. F. Metal-insulator transition. Rev. Mod. Phys. 40, 677 (1968).
Imada, M., Fujimori, A. & Tokura, Y. Metal-insulator transitions. Rev. Mod. Phys. 70, 1039 (1998).
Si, Q. & Abrahams, E. Strong correlations and magnetic frustration in the high Tc iron pnictides. Phys. Rev. Lett. 101, 076401 (2008).
de’Medici, L., Giovannetti, G. & Capone, M. Selective mott physics as a key to iron superconductors. Phys. Rev. Lett. 112, 177001 (2014).
Gebhard, F. Metal—insulator transitions (Springer, 1997).
Edwards, P. & Rao, C. N. R.The Metal-Nonmetal Transition Revisited (CRC Press, 2018).
Khomskii, D. I. et al. Transition metal compounds (Cambridge University Press, 2014).
Arielly, R. et al. Intriguing sequence of GaFeO3 structures and electronic states to 70 GPa. Phys. Rev. B 84, 094109 (2011).
Rozenberg, G. Kh., Xu, W. & Pasternak, M. P. The mott insulators at extreme conditions; structural consequences of pressure-induced electronic transitions. Z. Kristallogr. Cryst. Mater. 229, 210–222 (2014).
Saxena, S. K. et al. Advances In Physical Geochemistry, vol. 2 (Springer Science & Business Media, 2012).
Kuneš, J., Lukoyanov, A. V., Anisimov, V. I., Scalettar, R. T. & Pickett, W. E. Collapse of magnetic moment drives the mott transition in MnO. Nat. Mater. 7, 198–202 (2008).
Lyubutin, I., Ovchinnikov, S., Gavriliuk, A. & Struzhkin, V. Spin-crossover-induced mott transition and the other scenarios of metallization in 3d n metal compounds. Phys. Rev. B 79, 085125 (2009).
Greenberg, E. et al. Mott transition in CaFe2O4 at around 50 GPa. Phys. Rev. B 88, 214109 (2013).
Park, H., Millis, A. J. & Marianetti, C. A. Site-selective mott transition in rare-earth-element nickelates. Phys. Rev. Lett. 109, 156402 (2012).
Subedi, A., Peil, O. E. & Georges, A. Low-energy description of the metal-insulator transition in the rare-earth nickelates. Phys. Rev. B 91, 075128 (2015).
Greenberg, E. et al. Pressure-induced site-selective mott insulator-metal transition in Fe2O3. Phys. Rev. X 8, 031059 (2018).
Layek, S. et al. Verwey-type charge ordering and site-selective mott transition in Fe4O5 under pressure. J. Am. Chem. Soc. 144, 10259 (2022).
Goldman, A. Modern Ferrite Technology (Springer Science & Business Media, 2006).
Brabers, V. Progress in spinel ferrite research. Handbook of Magnetic Materials 8, 189–324 (1995).
Van Groenou, A. B., Bongers, P. & Stuyts, A. Magnetism, microstructure and crystal chemistry of spinel ferrites. Materials Science and Engineering 3, 317–392 (1969).
Levy, D., Pavese, A. & Hanfland, M. Phase transition of synthetic zinc ferrite spinel (ZnFe2O4) at high pressure, from synchrotron x-ray powder diffraction. Phys. Chem. Miner. 27, 638–644 (2000).
Andrault, D. & Bolfan-Casanova, N. High-pressure phase transformations in the MgFe2O4 and Fe2O3–MgSiO3 systems. Phys. Chem. Miner. 28, 211–217 (2001).
Friedrich, A. et al. Pressure-induced spin collapse of octahedrally coordinated Fe3+ in Ca3Fe2[SiO4] from experiment and theory. Phys. Rev. B 90, 094105 (2014).
Lyubutin, I. S. & Gavriliuk, A. G. Research on phase transformations in 3d-metal oxides at high and ultrahigh pressure: State of the art. Phys.-Uspekhi 52, 989 (2009).
Greenberg, E. et al. High-pressure magnetic, electronic, and structural properties of MFe2O4 (M= Mg, Zn, Fe) ferric spinels. Phys. Rev. B 95, 195150 (2017).
Layek, S. et al. Pressure-induced spin crossover in disordered α-LiFeO2. Phys. Rev. B 94, 125129 (2016).
Xu, W. et al. FeCr2O4 spinel to near megabar pressures: Orbital moment collapse and site-inversion facilitated spin crossover. Phys. Rev. B 95, 045110 (2017).
Xu, W. et al. Site-specific spin crossover in Fe2TiO4 post-spinel under high pressure up to nearly a megabar. Phys. Rev. B 96, 045108 (2017).
Xu, W. et al. Interplay between structural and magnetic-electronic responses of FeAl2O4 to a megabar: Site inversion and spin crossover. Phys. Rev. B 97, 085120 (2018).
Belitz, D. & Kirkpatrick, T. The Anderson-Mott transition. Rev. Mod. Phys. 66, 261 (1994).
Li, T. et al. Continuous mott transition in semiconductor moiré superlattices. Nature 597, 350–354 (2021).
Finger, L. W., Hazen, R. M. & Hofmeister, A. M. High-pressure crystal chemistry of spinel (MgAl2O4) and magnetite (Fe3O4): comparisons with silicate spinels. Phys. Chem. Miner. 13, 215–220 (1986).
Fei, Y., Frost, D. J., Mao, H.-K., Prewitt, C. T. & Haeusermann, D. In situ structure determination of the high-pressure phase of Fe3O4. Am. Mineral. 84, 203–206 (1999).
Haavik, C., Stølen, S., Fjellvag, H., Hanfland, M. & Hausermann, D. Equation of state of magnetite and its high-pressure modification: thermodynamics of the Fe-O system at high pressure. Am. Mineral. 85, 514–523 (2000).
Dubrovinsky, L. et al. The structure of the metallic high-pressure Fe3O4 polymorph: experimental and theoretical study. J. Phys. Condens. Matter 15, 7697 (2003).
Greenberg, E. et al. On the compressibility of ferrite spinels: a high-pressure x-ray diffraction study of MFe2O4 (M= Mg, Co, Zn). High Pressure Research 29, 764–779 (2009).
Xu, W., Machavariani, G. Y., Rozenberg, G. Kh. & Pasternak, M. Mössbauer and resistivity studies of the magnetic and electronic properties of the high-pressure phase of Fe3O4. Phys. Rev. B 70, 174106 (2004).
Yamanaka, T., Uchida, A. & Nakamoto, Y. Structural transition of post-spinel phases CaMn2O4, CaFe2O4, and CaTi2O4 under high pressures up to 80 GPa. Am. Mineral. 93, 1874–1881 (2008).
Verma, S. & Joy, P. Magnetic properties of superparamagnetic lithium ferrite nanoparticles. J. Appl. Phys. 98, 124312 (2005).
Braun, P. A superstructure in spinels. Nature 170, 1123–1123 (1952).
Tomas, A., Laruelle, P., Dormann, J. & Nogues, M. Affinement de la structure des formes ordonnée et désordonnée de l’octaoxopentaferrate de lithium, LiFe5O8. Acta Crystallogr. C 39, 1615–1617 (1983).
Anderson, O. L. et al. Equations of state of solids for geophysics and ceramic science. 31 (Oxford University Press on Demand, 1995).
Iliev, M. et al. Lattice dynamics of the α and β phases of LiFe5O8. Phys. Rev. B 83, 174111 (2011).
Gütlich, P., Bill, E. & Trautwein, A. X. Mössbauer spectroscopy and transition metal chemistry: fundamentals and applications (Springer Berlin, Heidelberg, 2010).
Potapkin, V. et al. The 57Fe synchrotron Mössbauer source at the ESRF. J. Synchrotron Radiat. 19, 559–569 (2012).
Middey, S. et al. Disentangled cooperative orderings in artificial rare-earth nickelates. Phys. Rev. Lett. 120, 156801 (2018).
Merlini, M. et al. Fe3+ spin transition in CaFe2O4 at high pressure. Am. Mineral. 95, 200–203 (2010).
Xu, W. et al. Pressure-induced hydrogen bond symmetrization in iron oxyhydroxide. Phys. Rev. Lett. 111, 175501 (2013).
Xu, W. et al. Pressure-induced high-spin/low-spin disproportionated state in the mott insulator FeBO3. Sci. Rep. 12, 9647 (2022).
Shannon, R. D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallographica Section A 32, 751–767 (1976).
Anderson, P. W. Absence of diffusion in certain random lattices. Phys. Rev. 109, 1492 (1958).
Lee, P. A. & Ramakrishnan, T. Disordered electronic systems. Rev. Mod. Phys. 57, 287 (1985).
Brandes, T. & Kettemann, S. Anderson Localization And Its Ramifications: Disorder, Phase Coherence, And Electron Correlations (Springer Berlin, Heidelberg, 2010).
Yu. Machavariani, G., Pasternak, M. P., Hearne, G. R. & Rozenberg, G. Kh. A multipurpose miniature piston-cylinder diamond-anvil cell for pressures beyond 100 GPa. Rev. Sci. Instrum. 69, 1423–1425 (1998).
Dewaele, A., Torrent, M., Loubeyre, P. & Mezouar, M. Compression curves of transition metals in the mbar range: Experiments and projector augmented-wave calculations. Phys. Rev. B 78, 104102 (2008).
Akahama, Y. & Kawamura, H. Pressure calibration of diamond anvil raman gauge to 310 GPa. J. Appl. Phys. 100, 043516 (2006).
Glazyrin, K., Miyajima, N., Smith, J. S. & Lee, K. K. Compression of a multiphase mantle assemblage: effects of undesirable stress and stress annealing on the iron spin state crossover in ferropericlase. J. Geophys. Res. Solid Earth 121, 3377–3392 (2016).
Acknowledgements
This research was supported by the Israeli Science Foundation (Grants No. 1189/14, No. 1552/18 and No. 1748/20). S. L. sincerely acknowledges Planning and Budget Commission from Higher Education Ministry of Israel for providing PBC post-doctoral fellowship. We thank Prof. J. Lashley for his interest in this research and for fruitful discussions. Authors are thankful to Mark Shulman for his help in the initial stages of a few experiments. We are grateful to J. P. Itié and the beamline team at the Synchrotron Soleil for their assistance in using beamline PSICHÉ. A few Mössbauer spectrum at low temperature were collected at the ID-18 beamline of the European Synchrotron Radiation Facility, Grenoble, France. We are grateful to D. G. Merkel for his assistance in using beamline ID-18. Portions of this work were performed at GeoSoilEnviroCARS (The University of Chicago, Sector 13), Advanced Photon Source (APS), Argonne National Laboratory. GeoSoilEnviroCARS is supported by the National Science Foundation - Earth Sciences (EAR - 1634415). This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. S. L. sincerely acknowledge SEED (Grant No.: UPES/R&D-SOE/20062022/02) and SEED-INFRA (Grants No.: UPES/R&D-SEED-INFRA/24082023/04 and UPES/R&D-SoAE/08042024/20) research funding from UPES, Dehradun, India. S.S.S. would like to acknowledge support (Grants No.: G117669) from Department of Science Innovation and Technology, UK.
Author information
Authors and Affiliations
Contributions
S.L., E.G., D.L., V.P., and G.K.R. conducted the experiments; S.L., E.G., and D.L. performed the data analysis; G.K.R., S.L., and S.S.S. conceived and supervised the project and wrote the paper. All the authors contributed to the writing of the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Peer review
Peer review information
Communications Materials thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Aldo Isidori.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Layek, S., Greenberg, E., Levy, D. et al. Correlated electron physics near a site-selective pressure-induced Mott transition in α-LiFe5O8. Commun Mater 5, 155 (2024). https://doi.org/10.1038/s43246-024-00560-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43246-024-00560-x
- Springer Nature Limited