Abstract
Perovskite solar cells have rapidly advanced, achieving over 26% power conversion efficiency on the laboratory scale. However, transitioning to large-scale production remains a challenge due to limitations in conventional fabrication methods like spin coating. Here, we introduce an optimized blade coating process for the scalable fabrication of large-area (15 cm × 15 cm) perovskite solar modules with a nickel oxide hole transport layer, performed in ambient air and utilizing a non-toxic solvent system. Self-assembled monolayers between the nickel oxide and perovskite layer improve the uniformity and morphology of the perovskite film. Perovskite solar modules with a 110 cm2 active area achieve a power conversion efficiency of 12.6%. Moreover, encapsulated modules retained 84% of their initial efficiency after 1,000 hours at 85 °C in air (ISOS-T-1). This study demonstrates progress in the large-scale production of perovskite solar cells that combine efficiency with long-term stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Global demand for energy continues to increase, requiring the development of renewable energies. Among the photovoltaic technologies, perovskite solar cells (PSCs) have proved very promising for their high power conversion efficiency (PCE), cost-effectiveness, diverse applications, and versatile fabrication methods1,2,3,4. Achieving PCE > 26% on the laboratory scale5,6, PSCs are set to revolutionize the solar industry. However, the adoption of industry-scale PSCs is hindered by significant challenges, particularly in terms of scalability, stability, and environmental impact7,8,9,10. Despite the impressive efficiency of small-scale PSCs, upscaling modules for industrial production remains challenging. Methods like spin coating contribute to high material waste and offer limited scalability7,8.
In addition, the need for sustainable processes is becoming increasingly urgent. To commercialize PSCs, fabrication in ambient environments is necessary. PSCs are often coated under an N2 environment, which is unsuitable for large-scale manufacturing processes. Several research groups have explored alternative large-area deposition techniques for PSC fabrication, including blade coating11,12, slot-die coating13,14, spray-coating15,16, screen printing17,18, and inkjet printing19. Among these deposition techniques, blade coating and slot coating, known as Meniscus coating technologies, have gained prominence. These methods are widely used due to their versatility, high efficiency, and scalability. Many efforts have been made to produce printable perovskite solar modules with large active areas using meniscus coating technologies20,21,22,23,24. Both methods are compatible with roll-to-roll (R2R) manufacturing processes, which makes them a step toward the commercial viability of PSCs25.
PSCs can be fabricated with two main architectures based on the order of charge transport materials: normal (n–i–p) and inverted (p–i–n). Inverted PSCs exhibit improved stability and reduced hysteresis behavior, making them more suitable for potential commercialization26. The commonly used hole transport layer (HTL) in inverted PSCs is poly [bis(4-phenyl)(2,4,6 trimethylphenyl) amine (PTAA), known for its high performance in printable PSCs27. However, the introduction of the inorganic hole transport layer, NiOx, resulted in not only high-performance PSCs but also enhanced long-term stability. In contrast to PTAA, NiOx is potentially low-cost due to its inorganic nature, is highly photostable, chemically stable, has excellent optical transmittance, and has a hydrophilic nature28,29.
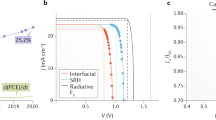
To produce compact nickel oxide in large-area perovskite solar cells (PSCs), various deposition methods are employed, classified into printable and non-printable methods. Printable methods include meniscus coating30,31 and spray coating32,33, while non-printable methods comprise evaporation techniques34,35 and chemical bath deposition36,37. Printable deposition techniques are essential for the practical application and commercial viability of perovskite solar cells18,38,39. They offer scalability and cost efficiency by minimizing material waste, speeding up deposition, and enabling fabrication in the ambient environment. However, while fabrication in ambient conditions using printable methods is feasible, it results in lower PCE compared to cells produced in controlled environments. Several groups demonstrated the remarkable long-term stability and high efficiency of NiOx HTL in small-scale devices, achieving up to 23.6% PCE32,33,35,40,41,42. Despite these advancements, there remains a substantial gap between the performance of small-scale and large-area printable NiOx-based PSCs, particularly with respect to the deposition in ambient air conditions. For instance, moving to ambient air-deposited PSCs, The efficiency dropped to 20.7% for small-scale (0.1 cm2)32 and 10.34% for modules with 3.7 cm2 active area43, as depicted in Fig. 1a.
Herein, we proved the feasibility of upscaling printable NiOx-based perovskite solar modules in an ambient air environment. We established a procedure to print NiOx over 15 cm × 15 cm substrates without any spin coating step. The Modules were fabricated with an active area of 110 cm2 by doctor blading NiOx/MeO-2PACz/Perovskite stack in ambient conditions and thermal evaporation of the rest of the layers (Fig. 1b). The champion module achieved 12.6% PCE by optimizing the NiOx ink concentration and introducing the self-assembled monolayer (SAM). Figure 1a and Table S1 compare the PCE and active area of perovskite solar cells and modules with NiOx as HTL. The modules showed remarkable stability after being stored at 85 °C in the air for 1000 h. The fabricated perovskite solar modules outperformed previously reported printable large-area perovskite solar modules that are fabricated in ambient environments both in terms of efficiency and stability.
Results and discussion
NiOx film thickness and uniformity optimization
To deposit the NiOx film for large-area perovskite solar modules, we did the doctor blading of NiCl2·6H2O solution on ITO substrates ambient conditions. Then, the films were annealed at 300 °C to promote decomposition and oxidation, leveraging atmospheric oxygen to form the NiOx film44. The impact of varying precursor concentrations on the film thickness and uniformity were explored as critical parameters for efficient solar cell performance. To systematically investigate this, four different concentrations of nickel chloride hexahydrate precursor were deposited on glass/ITO substrates. Starting with a reference solution (0.15 M), we prepared three additional solutions with concentrations of 0.075 M (1:1 dilution), 0.050 M (1:2 dilution), and 0.037 M (1:3 dilution). For each concentration, we mapped the film thickness across a 140 × 140 mm centered area of the substrate using ellipsometry measurement, collecting data at 225 points. The obtained thickness maps as well as the distribution of average thickness across x and y axes are demonstrated in Fig. 2a–d.
The reference solution (Fig. 2a) indicates significant variation in film thickness across both the x and y axes due to non-uniform doctor blading. Increasing the solvent ratio displayed improved uniformity. The dilution results in lower ink viscosity and lower boiling point of the ink due to the presence of 2-methoxy ethanol. However, the dilution resulted in lower film thickness as well. The average measured thickness followed a downward trend with precursor dilution, obtaining 42.2, 40.0, and 36.2 nm for the films with 0.075 M (1:1), 0.05 M (1:2), and 0.037 M (1:3) concentrations, respectively. Figure S1 demonstrates the thickness histogram of films. In the case of 1:3 concentration, it is obvious from Fig. 2d that there is a reduction in film thickness and the lower edge of the substrate. This suggests the viscosity of the solution might be too low to keep a stable meniscus, resulting in a thinner film. Additionally, dilution causes the need to evaporate a larger volume of solvent which could cause uneven drying patterns.
X-ray photoelectron spectroscopy (XPS) and X-ray reflectometry (XRR) measurements were performed to evaluate the presence and contribution of the various oxidation states of NiOx over different parts of the substrate and the uniformity of the deposition over large areas. XPS spectra for the NiOx layers deposited at different nickel chloride hexahydrate precursor concentrations were measured (Fig. 3a–d). To show the possible effect of precursor concentration on the Ni oxidation states present on the film surface, in Fig. 3, the Ni 2p3/2 spectra in the relevant energy regions (850–860 eV) are shown. In this binding energy range, four different peaks can be identified corresponding to the different components of the nickel oxide films: Ni, NiO (Ni²⁺), NiOH (Ni²⁺), and Ni2O3 (Ni³⁺). The spectra were fitted using four Gaussian curves corresponding to Ni, NiO, NiOH, and Ni2O3 with calculated binding energies of approximately 852.0, 853.5, 855, and 856 eV, which agrees with values reported in the literature. (Surface passivation of sputtered NiOx using a SAM interface layer to enhance the performance of perovskite solar cells.)
As shown in Fig. 3a, the spectrum shows a dominant NiO peak with smaller contributions from Ni, NiOH, and Ni2O3. The high intensity of the NiO peak suggests a highly oxidized film, which is optimal for applications requiring stable Ni²⁺ oxidation states, such as hole transport layers in perovskite solar cells. For the 1:1 Concentration (Fig. 3b), the NiO peak remains prominent, but there is a slight increase in the Ni and Ni2O3 contributions. This indicates that even with dilution, the film retains a significant amount of Ni²⁺, hole transport. However, the increased presence of Ni and Ni2O3 suggests reduced oxidation efficiency or a more complex chemical environment. There is a notable increase in the Ni2O3 component for 1:2 Concentration, as shown in Fig. 3c, suggesting a higher oxidation state (Ni³⁺). This could result from a more thorough oxidation process, possibly due to thinner films allowing better oxygen access during annealing. The increase in Ni2O3 might enhance charge separation in solar cell applications45,46. Upon further dilution of nickel chloride (1:3 concentration), Fig. 3d shows a further shift toward higher oxidation states, with greater intensity in the Ni2O3 peak relative to NiO. The variability and shift in peak intensity could point to non-uniformity in film thickness and oxidation across the film. Hence, as the concentration of the precursor decreases, there is a trend towards higher oxidation states and increased complexity in the nickel oxide chemistry. The contribution from NiO has a decreasing tendency with decreasing concentration of the precursor, as can be seen in Fig. S2. This trend correlates with the observed decrease in uniformity and thickness of the films, as discussed. The fitting parameters and the deduced Ni oxidation states are summarized in Table 1.
Furthermore, XRR measurements probed the electron density perpendicular to the surface on four different points across 150 × 150 mm NiOx deposited substrates. Fresnel XRR profiles were collected upon several portions of each deposited plate since no Kiessig fringes were visible due to poor electron density contrast between NiOx and ITO, as expected. Since the glass/ITO substrate is the same for all tested sections of the various samples, the derived electron densities—which are directly proportional to the observed critical angles—correlate with different scattering volumes, such as the film thickness observed across various parts of the large area NiOx films47. The dispersion of the critical angle, reflecting variations in scattering length densities, directly reveals the uniformity of deposition: low dispersion suggests uniformity, while high dispersion indicates a less homogeneous deposition. The electron density values show a trend of increasing with decreasing precursor concentration. This suggests that the films become denser with higher levels of dilution. The increase in electron density from the reference to the 1:3 concentration indicates that thinner films, while potentially less uniform in thickness (as noted in your ellipsometry data), may have a denser packing of atoms. This could result from a higher degree of oxidation or compaction during the annealing process. Higher electron densities in dilute samples (1:3 concentration) suggest that these films, despite their reduced thickness, may possess enhanced functional properties, such as improved barrier characteristics or different optical properties, which could improve performance in certain layers of perovskite solar cells. It is evident from Fig. 4 that the reference sample and the 1:2 concentrated NiOx deposition are the most homogenous, while 1:3 concentration results in a less uniform film. The Fresnel Reflectivity allowed for an interface/surface roughness estimation of about 4.5 (5) nm for the reference sample and the 1:2 concentration sample. This value is consistent across all patterns. Samples obtained from the 1:1 and 1:3 concentration plate show a higher roughness dispersion, with values ranging from about 2.5 (5) nm to about 4.5 (5) nm for the 1:1 XRR profiles and from about 4.5 (5) nm to about 7.0 (5) nm for the 1:3 XRR profiles. Such scattered roughness values are reported in Table S4. These observations are in good agreement with the thickness profile maps reported in Fig. 2 and the electron density values reported in Table S3. Similar species and trends were observed in the oxygen spectra.
Considering the trade-off between film uniformity and thickness, oxidation states, and electron density values, the optimum concentration of 0.05 M (1:2) was selected.
Interface engineering and perovskite film morphology
Continuing the exploration into the optimization of perovskite solar modules, the focus was directed towards the deposition of the perovskite layer. The perovskite deposition was built upon our previous work, which introduced a two-step blade coating deposition for perovskite solar modules in ambient air for flexible n–i–p devices, notably avoiding toxic solvents23. We developed a double-cation Cs0.15FA0.85PbI3−xBrx-based perovskite through optimized blade coating parameters and additive engineering to enhance film morphology and phase purity23. Briefly, a two-step deposition of the perovskite is performed by the deposition of PbI2-(FAI)0.3-(CsI)0.15 in dimethyl sulfoxide (DMSO) followed by the deposition of FAI/FABr in isopropyl alcohol. We proposed four main drying methods for the first step ink, namely, ambient, partial, optimal, and excessive drying23. Building on that foundation, this study examined how similar green solvent-based blade coating techniques could be applied to rigid substrates. We used the same approach to create uniform, high-quality perovskite films on larger 15 cm × 15 cm substrates. This advancement sets the stage for developing a universal green perovskite formulation applicable to different device configurations and substrate materials.
Figure 5a shows the SEM images of the perovskite layer on ITO/NiOx substrates. The non-optimized NiOx layer (0.15 M) resulted in a perovskite film with defects, including visible bars and pinholes, indicative of uneven deposition. In contrast, the perovskite film deposited on the optimized NiOx (0.05 M precursor solution), displayed remarkable reduction in such defects, with smaller particles and fewer pinholes. Despite these improvements, the occurrence of pinholes persisted, indicating a major challenge attributed to the adhesion issues between the NiOx film and perovskite precursor inks, particularly for large-area perovskite deposition35. Conventional surface treatments, such as UV-ozone and plasma, were found to negatively affect the NiOx film, exacerbating interface issues such as the formation of excess PbI2, which can act as a hole extraction barrier and reduce open circuit voltage of the device48,49. In addition to interface defects and traps, the low conductivity of NiO can be detrimental to the performance of PCSs28. To improve interface adhesion, open-circuit voltage, and higher conductivity, we introduced a self-assembled monolayer (SAM) of MeO-2PACz at the HTL/perovskite interface.
The SEM image of the perovskite film on optimized NiOx + SAM shown in Fig. 5a, confirms the effectiveness of this approach. The presence of SAM led to highly uniform perovskite films and the elimination of pinholes. The uniformity of the perovskite film is further evidenced by the ellipsometry thickness map (Fig. 5b), which indicates the average perovskite film thickness of 570 nm. Nevertheless, a gradient is observed with the film starting at 700 nm thickness as well as a slight decrease in the film thickness at the end of the coating. This gradient is due to the nature of the sheet-to-sheet doctor blading process since there is an acceleration at the beginning and the end of coating due to blade movement, and it is recommended to consider a margin for module active area due to this reason. The uniformity of perovskite film is essential for the performance of solar modules. The implementation of the SAM layer mitigated the adhesion challenges, which are crucial for the advancement toward industrial-compatible, stable perovskite solar modules.
Modules and long-term stability
The perovskite solar module’s structure was completed by evaporation of C60/BCP as ETL, performing the P2 laser scribing followed by evaporation of Cu electrode and performing of P3 laser scribing. The photovoltaic parameters of the module are shown in Fig. 6a. The module showed an efficiency of 12.6%, ISC of 98.13 mA, a Fill Factor of 63.49% and a VOC of 22.3 V (22 cells in series). The near-unity hysteresis index of 1.02 indicates the consistency in performance between forward and reverse measurement scan directions, underscoring the reliable operation of the module. The photographic image of the module (Fig. 6b) and a detailed schematic of the module layout (Fig. 6c) provide a visual and structural context for our work. Long-term stability, which is a critical benchmark for perovskite solar module’s performance, was assessed through the ISOS-T-150 thermal test at 85 °C in ambient air conditions. Modules maintained 84% of their initial efficiency after 1000-h testing time without the application of any encapsulation, as shown in Fig. 6d. This stability record is remarkable among NiOx-based encapsulated devices as other studies stored encapsulated devices at room temperature or at 85 °C under N2 environment33,37.
Conclusion
This research represented a significant step forward in addressing the scalability challenges of perovskite solar cells for commercial application. By adopting doctor blading, we successfully fabricated large-area PSC modules using NiOx as the HTL. Our findings show that not only can NiOx be effectively printed on large substrates, but it also significantly enhances the performance and stability of the modules. Using previous research experience, we developed a multi-functional and non-toxic perovskite formulation suitable for rigid and flexible substrates as well as p–i–n and n–i–p configurations. This achievement highlights the potential for large-area production of PSCs without compromising environmental sustainability or efficiency. The champion module, with an active area of 110 cm², achieved a power conversion efficiency of 12.6% and maintained 84% of its initial efficiency after 1000 h of thermal stress testing at 85 °C in air. These results underscore the potential of NiOx in PSCs and open new avenues for the large-scale, cost-effective production of perovskite solar modules. Future research should focus on further optimizing the fabrication process and exploring the commercial viability of these technologies.
Methods
NiOx synthesis and deposition
The ink is prepared by dissolving 35.5 mg/ml (0.15 M) of NiCl2·6H2O in 2-methoxyethanol, followed by the addition of 20 µl of 70% HNO3 per ml of solution. The solution is then heated to 75 °C for 2 h using a hotplate. This prepared solution remains stable for over a month. For the doctor blading technique, we set the blade height at 100 µm and the plate speed at 5 mm/s, applying 300 µl of the solution for each deposition. After this, the film undergoes a sintering process at 300 °C for one hour to facilitate the conversion and compaction of the nickel oxide formation.
SAM deposition
2-(3,6-Dimethoxy-9H-carbazol-9-yl) ethyl]phosphonic Acid (MeO-2PACz) from TCI (Purity > 98.0%) was dissolved in anhydrous ethanol to achieve a concentration of 0.001 M, equivalent to 0.33 mg/ml. The solution was then stirred continuously at room temperature overnight. Prior to the deposition, the MeO-2PACz solution underwent sonication in an ultrasonic bath at 40 °C for 10 minutes. The solution exhibited a shelf-life exceeding 1 month, with no observed drop in efficiencies. The doctor blading of MeO-2PACz was conducted at a concentration of 0.1 mg/ml in ethanol, following the methodology described in the study by Ernst et al.22.
Module fabrication
All the doctor-bladed layers were fabricated in ambient air (relative humidity of 20-40% at room temperature). The perovskite film is deposited via double-step doctor blading similar to previous work23. Briefly, PbI2:(FAI)0.3:(CsI)0.15 in DMSO containing 0.25 mg/ml l-alpha- phosphatidylcholine as an additive is prepared. P1, P2, and P3 processes for modules were performed with a UV ns laser (Spectra physics—Andover, MA, USA). The ink is doctor-bladed with a gap of 100 µm, coating speed of 2 mm/s, and hotplate temperature of 60 °C. During the deposition, an air knife with a flow rate of 120 l/min helped the drying process of the ink. Then, a solution composed of 30 mg/ml FAI and 7.5 mg/ml FABr in 2-propanol is doctor-bladed on top with the same gap, hotplate temperature, blading speed, and air-knife parameters. The perovskite was under an IR lamp (Helios Quartz) for 1 min at 60 °C followed by annealing for 1 h at 130 °C. C60/BCP (20/7 nm) were evaporated on top of the perovskite film, followed by P2 laser processing. Finally, 100 nm of Cu electrode is evaporated, and the P3 laser processing is performed to complete the module fabrication. Modules were encapsulated for stability tests by covering the active area with 1 mm of clear glass and by placing a UV-curable resin (ThreeBond) as an edge sealant.
Modules layout optimization
The optimization of 15 × 15 cm² substrate parameters like cell width, laser process (P1–P2–P3) thickness, and the dimensions of inactive areas were found to be comparable. Consequently, a unified geometric approach was adopted. The chosen module geometry, as depicted in Fig. 4C, demonstrates 22 cells in series for 15 × 15 cm² substrates. Each cell has a width of 5 mm, and the module’s dead area (which encompasses the widths of scribes P1–P2–P3 and the margins between p1 and p2 and between p2 and p3 to prevent overlap in laser processes) is set at 500 µm. The active area length of the module was determined to be 11 cm, accounting for a 2 cm allowance on each side for potential accumulation and deposition discharge during solution deposition using slot die and/or blade coating methods. With these dimensions, the aperture ratio, representing the geometrical fill factor, is calculated to be 91%. The calculated aperture area of the module is 121 cm2.
Characterizations
Solar modules were measured under Air Mas 1.5 Global (AM1.5 G) conditions using a class A sun simulator (ABET Sun 2000). The thicknesses of the NiOx layers were measured by spectroscopic ellipsometry. Measurements were performed in a Semilab SE2000 ellipsometer at a 70° angle for a wavelength range 245–967 nm. The optical properties of NiOx were modeled by a Tauc-Lorentz and a Drude function. Scanning electron microscopy images were taken with TESCAN MIRA. X-ray reflectometry (XRR) measurements were performed by means of a Panalytical Empyrean apparatus (Cu-anode: K-Alpha1 [Å] = 1.54060; K-Alpha2 [Å] = 1.54443) equipped with a parallel plate collimator on the reflected beam path. 2 theta configuration was used as an incident optical pathway focusing the impinging beam with fixed divergent slits (1/32°−1/16°). Detection was accomplished by means of a solid-state hybrid Pix’cel 3D detector, working in 0D detection mode. Acquisitions were made in the 0.610 < °2θ < 1.500 angular range, using a continuous scan with a step size °2θ = 0.0020 and an acquisition time of 1 s for each step. XPS spectra were recorded in a vacuum generator VG-450 ultrahigh-vacuum (UHV) chamber equipped with an Al Kα radiation source with an estimated resolution of 0.1 eV. C1s spectra of adventitious carbon were used as a reference to discard any possible charging effects. The spectra for oxides were fitted using Gaussian curves.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Liu, S., Biju, V. P., Qi, Y., Chen, W. & Liu, Z. Recent progress in the development of high-efficiency inverted perovskite solar cells. NPG Asia Mater. 15, 27 (2023).
Stefanelli, M., Vesce, L. & Di Carlo, A. Upscaling of carbon-based perovskite solar module. Nanomaterials (Basel) 13, 313 (2023).
Park, N.-G. & Zhu, K. Scalable fabrication and coating methods for perovskite solar cells and solar modules. Nat. Rev. Mater. 5, 333–350 (2020).
Bati, A. S. R. et al. Next-generation applications for integrated perovskite solar cells. Commun. Mater. 4, 2 (2023).
Liang, Z. et al. Out-of-plane cations homogenise perovskite composition for solar cells. Nature https://doi.org/10.1038/s41586-023-06784-0 (2023).
Park, J. et al. Controlled growth of perovskite layers with volatile alkylammonium chlorides. Nature 616, 724–730 (2023).
Yang, J., Lim, E. L., Tan, L. & Wei, Z. Ink engineering in blade‐coating large‐area perovskite solar cells. Adv. Energy Mater. 12, 2270122 (2022).
Ma, Y. & Zhao, Q. A strategic review on processing routes towards scalable fabrication of perovskite solar cells. J. Energy Chem. 64, 538–560 (2022).
Podapangi, S. K. et al. Green solvents, materials, and lead-free semiconductors for sustainable fabrication of perovskite solar cells. RSC Adv. 13, 18165–18206 (2023).
Chowdhury, T. A. et al. Stability of perovskite solar cells: issues and prospects. RSC Adv. 13, 1787–1810 (2023).
Chen, S., Xiao, X., Gu, H. & Huang, J. Iodine reduction for reproducible and high-performance perovskite solar cells and modules. Sci. Adv. 7, 1–7 (2021).
Castriotta, L. A. et al. Air-processed infrared-annealed printed methylammonium-free perovskite solar cells and modules incorporating potassium-doped graphene oxide as an interlayer. ACS Appl. Mater. Interfaces 13, 11741–11754 (2021).
Li, J. et al. Ink design enabling slot‐die coated perovskite solar cells with >22% power conversion efficiency, micro‐modules, and 1 year of outdoor performance evaluation. Adv. Energy Mater. 13 2203898 (2023).
Beynon, D. et al. All-printed roll-to-roll Perovskite photovoltaics enabled by solution-processed carbon electrode. Adv. Mater. 35, e2208561 (2023).
Bishop, J. E., Read, C. D., Smith, J. A., Routledge, T. J. & Lidzey, D. G. Fully spray-coated triple-cation perovskite solar cells. Sci. Rep. 10, 1–8 (2020).
Chou, L.-H., Chan, J. M. W. & Liu, C.-L. Progress in spray coated perovskite films for solar cell applications. Sol. RRL 6, 2101035 (2022).
Chen, C. et al. Perovskite solar cells based on screen-printed thin films. Nature 612, 266–271 (2022).
Chen, C. et al. Screen-printing technology for scale manufacturing of perovskite solar cells. Adv. Sci. 10, e2303992 (2023).
Schackmar, F. et al. Perovskite solar cells with all‐inkjet‐printed absorber and charge transport layers. Adv. Mater. Technol. 6, 2000271 (2021).
Wu, Z. et al. Natural amino acid enables scalable fabrication of high-performance flexible perovskite solar cells and modules with areas over 300 cm2. Small Methods 6, e2200669 (2022).
Bu, T. et al. Modulating crystal growth of formamidinium–caesium perovskites for over 200 cm2 photovoltaic sub-modules. Nat. Energy 7, 528–536 (2022).
Ernst, M., Herterich, J.-P., Margenfeld, C., Kohlstädt, M. & Würfel, U. Multilayer blade‐coating fabrication of methylammonium‐free perovskite photovoltaic modules with 66 cm2 active area. Sol. RRL 6, 2100535 (2022).
Jafarzadeh, F. et al. All-blade-coated flexible perovskite solar cells & modules processed in air from a sustainable dimethyl sulfoxide (DMSO)-based solvent system. Sustain. Energy Fuels 7, 2219–2228 (2023).
Vesce, L. et al. Perovskite solar cell technology scaling‐up: Eco‐efficient and industrially compatible sub‐module manufacturing by fully ambient air slot‐die/blade meniscus coating. Prog. Photovolt. https://doi.org/10.1002/pip.3741 (2023).
Ma, Y., Lu, Z., Su, X., Zou, G. & Zhao, Q. Recent progress toward commercialization of flexible perovskite solar cells: from materials and structures to mechanical stabilities. Adv. Energy Sustain. Res. 4, 2200133 (2023).
Zhu, H. et al. Long-term operating stability in perovskite photovoltaics. Nat. Rev. Mater. 8, 569–586 (2023).
Wang, Y. et al. PTAA as efficient hole transport materials in perovskite solar cells: a review. Sol. RRL 6, 2200234 (2022).
Zhang, X. et al. Blade-coated inverted perovskite solar cells in an ambient environment. Sol. Energy Mater. Sol. Cells 246, 111894 (2022).
Parida, B. et al. Recent developments in upscalable printing techniques for perovskite solar cells. Adv. Sci. 9, e2200308 (2022).
Le, T. S. et al. All‐slot‐die‐coated inverted perovskite solar cells in ambient conditions with chlorine additives. Sol. RRL 6, 2100807 (2022).
Li, C.-F. et al. High-performance perovskite solar cells and modules fabricated by slot-die coating with nontoxic solvents. Nanomaterials (Basel) 13, 1760 (2023).
Zhang, X. et al. Blade coating inverted perovskite solar cells with vacuum‐assisted nucleation based on bottom quasi‐2D passivation. Sol. RRL 7, 2200900 (2023).
Li, C. et al. Efficient inverted perovskite solar cells with a fill factor over 86% via surface modification of the nickel oxide hole contact. Adv. Funct. Mater. 33, 2214774 (2023).
Tutundzic, M. et al. Toward efficient and fully scalable sputtered NiOx‐based inverted perovskite solar modules via co‐ordinated modification strategies. Sol. RRL https://doi.org/10.1002/solr.202300862 (2023).
Du, M. et al. Surface redox engineering of vacuum-deposited NiOx for top-performance perovskite solar cells and modules. Joule 6, 1931–1943 (2022).
Li, S. et al. Low-temperature chemical bath deposition of conformal and compact NiOX for scalable and efficient perovskite solar modules. Small 19, e2301110 (2023).
Liao, Y. et al. Seed‐assisted Cu‐doped chemical bath deposition for preparing high‐quality NiOx hole‐transport layers in perovskite solar cells. Sol. RRL 7, 2300364 (2023).
Zarabinia, N. et al. Simple and effective deposition method for solar cell perovskite films using a sheet of paper. iScience 25, 103712 (2022).
Wang, Y. et al. Printing strategies for scaling-up perovskite solar cells. Natl Sci. Rev. 8, nwab075 (2021).
Eggers, H. et al. Inkjet‐printed micrometer‐thick perovskite solar cells with large columnar grains. Adv. Energy Mater. 10, 1903184 (2020).
Solar, P. et al. Stabilized and operational PbI 2 precursor ink for large-scale perovskite solar cells via two-step blade-coating. J. Phys. Chem. C 124, 8129–8139 (2020).
Li, N. et al. Zwitterion-stabilizing scalable bladed α-phase Cs0.1FA0.9PbI3 films for efficient inverted planar perovskite solar cells. ACS Sustain. Chem. Eng. 8, 7020–7030 (2020).
Huang, S.-H. et al. Toward all slot‐die fabricated high efficiency large area perovskite solar cell using rapid near infrared heating in ambient air. Adv. Energy Mater. 10, 2001567 (2020).
Di Girolamo, D. et al. Ion migration‐induced amorphization and phase segregation as a degradation mechanism in planar perovskite solar cells. Adv. Energy Mater. 10, 2000310 (2020).
Fakharuddin, A. et al. Robust inorganic hole transport materials for organic and perovskite solar cells: insights into materials electronic properties and device performance. Sol. RRL 5, 2000555 (2021).
Ochoa-Martinez, E. et al. Nanocrystalline flash annealed nickel oxide for large area perovskite solar cells. Adv. Sci. 10, e2302549 (2023).
Benediktovich, A., Feranchuk, I. & Ulyanenkov, A. Theoretical Concepts of X-Ray Nanoscale Analysis (Springer, Berlin, Heidelberg, 2014).
Nishihara, Y., Chikamatsu, M., Kazaoui, S., Miyadera, T. & Yoshida, Y. Influence of O2 plasma treatment on NiOx layer in perovskite solar cells. Jpn. J. Appl. Phys. (2008) 57, 04FS07 (2018).
Boyd, C. C. et al. Overcoming redox reactions at perovskite-nickel oxide interfaces to boost voltages in perovskite solar cells. Joule 4, 1759–1775 (2020).
Khenkin, M. V. et al. Consensus statement for stability assessment and reporting for perovskite photovoltaics based on ISOS procedures. Nat. Energy 5, 35–49 (2020).
Acknowledgements
L.A.C. and A.D.C. acknowledge funding from the Italian Ministry of Environment and Energy Security in the framework of the Operating Agreement with ENEA for Research on the Electric System (PTR-RdS 2022-2024). A.D.C. acknowledges the support of the Ministry of University of Research via the PON FSE-REACT EU project DM 1062 of 10/08/2021. This publication has received funding from the European Union’s Horizon 2020 research and innovation program under Grant Agreement No. 101007084 CITYSOLAR (F.M. and F.J.), Grant Agreement no. 763989 APOLO (F.J. and F.B.) and Grant Agreement no. 101006715 VIPERLAB (F.D.G.). This project has received funding from the European Union’s Framework Program for Research and Innovation Horizon Europe (2021–2027) under the Marie Sktodowska-Curie Grant Agreement No. 101068387 EFESO. This publication reflects only the authors’ views, and the European Union is not liable for any use that may be made of the information contained therein.
Author information
Authors and Affiliations
Contributions
L.A.C. conceived the idea. L.A.C. and F.J. fabricated and characterized the PSMs, both coordinating the experimental activities. E.C. and F.D.G. prepared the NiOx precursor solution and gave guidance for a proper deposition with blade coating. P.S. characterized NiOx film by ellipsometer and elaborated data on that. A.G. and B.P. performed XRR analysis, D.B.R., M.L., and A.C. characterized NiOx film by XPS. F.M., F.B., and A.D.C. supervised the activities by giving suggestions for improvement. F.J. wrote the first draft and revised the manuscript with contributions from all the authors. L.A.C. supervised, planned the experimental activities and finalized the draft. All authors participated in the preparation of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
F.D.G. and P.S. are co-founders of Solertix and senior researchers of Saule Technologies, respectively, two companies that are working on commercializing perovskite solar technologies. E.C. is a senior researcher at Halocell Europe, a company developing PV modules. The remaining authors declare no competing interests.
Peer review
Peer review information
Communications Materials thanks Shangshang Chen, Yu-Ching Huang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: John Plummer. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jafarzadeh, F., Castriotta, L.A., Calabrò, E. et al. Stable and sustainable perovskite solar modules by optimizing blade coating nickel oxide deposition over 15 × 15 cm2 area. Commun Mater 5, 186 (2024). https://doi.org/10.1038/s43246-024-00576-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43246-024-00576-3
- Springer Nature Limited