Abstract
Massive hemorrhage following tissue trauma has high mortality owing to the lack of timely intervention. However, research on utilizing hemostats for humans is limited; therefore, developing an efficient emergency hemostatic agent is imperative. We developed a hemostatic sponge using natural polysaccharide riclin, theoretically modified with 50% aldehyde content (AR50). The AR50 sponge, with quasi-honeycomb channels and appropriate aldehyde content, exhibits ultra-high blood absorption (59.4 g·g−1) and rapidly targets erythrocytes and platelets to form a stable barrier. It surpasses most commercial hemostats in porcine artery scission (reducing hemostasis time and blood loss by 53 s and 4.2 g), hepatic bleeding laceration (68 s and 2.6 g), and perforation models (140 s and 4.9 g). The AR50 sponge is easily removed post hemostasis, exhibits antibacterial properties by destroying bacterial cell walls, and is safely absorbed by day 5, making it an ideal emergency hemostatic agent for massive hemorrhages in humans.
Similar content being viewed by others
Introduction
Military conflicts, accidents, and clinical surgeries resulting in severe tissue trauma can lead to uncontrollable hemorrhaging and wound infection, pivotal factors that may contribute to mass fatalities1,2. Ideal hemostatic agents for emergencies, battlefield scenarios, and pre-hospital situations should possess characteristics such as rapid hemostasis, anti-infection properties, easy removal, promotion of healing, stability, affordability, and biocompatibility3,4. In clinical applications, achieving hemostasis poses a significant challenge owing to high blood flow3,5 and is currently addressed with the use of a high-expansion sponge4,5. Sponges, with porous structures and liquid absorption capacities, based on characteristics such as shape memory, light weight, portability, stability, and ease of operation, sponges offer advantages in treating moderate and severe arterial and visceral bleeding3,5.
Polysaccharides have gained attention as clinical frontier hemostatic materials owing to their outstanding biological activities, abundant sources, high biosafety levels, and easily modifiable structures3,6,7,8. Polysaccharides and their derivatives, including dextran (Bloxx®), cellulose (Surgicel®, BloodSTOP®), alginate (Algosteril®, KALTOSTAT®), chitosan (HemCon®, Celox®, Chito SAMTM 100), and starch (TraumaDEX®), are widely used in preparing commercial hemostats3,4,8. While most polysaccharide-based hemostatic agents exhibit excellent biological safety, they face challenges such as low hemostatic activity, a short shelf life, a high contamination risk, low coagulation and anti-infection capacities, limiting their application in treating massive bleeding in clinical settings4,9. Regrettably, limited research has been reported on effective polysaccharide hemostats for treating simulated massive human hemorrhage3,4,5,6,8.
Various strategies have been developed to comprehensively enhance polysaccharide hemostatic agents, including improving physical properties (shape, surface morphology, size, and formation process)3,4,5,10, chemical modifications (acylation, carboxymethylation, phosphorylation, sulfation and alkylation of polysaccharides)4,8,11, employing a bionic design with advanced functions4,11,12,13 and fabricating composite hemostats with polysaccharides and additional functional materials, conferring antibacterial functions and promoting wound absorption and tissue adhesion14,15,16. Despite these improvements, even the most effective hemostats present limitations such as the potential toxicity of the derivatives, slow wound absorption, challenges in removal, complex preparation, and low yields, rendering them unsuitable for treating massive emergency hemorrhaging, few studies have reported on the polysaccharide-based hemostasis of lethal large-animal hemorrhages to date3,4,5,9. Overcoming these challenges urgently requires the development of an ideal polysaccharide-based hemostatic agent that integrates active and passive hemostatic functions, rapid wound absorption, efficient antibacterial properties, high yield, portability, stability, and suitability for a wide range of application scenarios3,4,7,8.
Riclin, an extracellular polysaccharide derived from Agrobacterium sp. ZCC3656, exhibits outstanding bioactivity, biocompatibility, and high yield (production yield, 20 g·L−1)17,18,19. In this study, we designed an efficient aldehyde-modified riclin (AR) hemostatic sponge. The optimized sponge (AR50 sponge) could rapidly establish a stable hemostatic barrier on the wound, effectively controlling hemorrhaging of the porcine femoral artery and liver. Antibacterial activity, biodegradation, and biocompatibility of this novel polysaccharide hemostatic sponge were further examined. Collectively, the developed sponge holds promise as an ideal hemostatic agent for saving the lives of patients with tissue trauma and hemorrhaging in emergency, pre-hospital settings.
Results and discussion
Preparation and characterization of AR sponges
AR samples were prepared through NaIO4 oxidation at varying theoretical ratios20 of 25%, 50%, 75%, and 100%, denoted as AR25, AR50, AR75, and AR100, respectively. In the oxidation reaction, each ortho-hydroxyl unit generates two aldehyde groups by breaking the carbon-carbon bond. Briefly, a bond is formed between sodium periodate and the hydroxyl group in the first step of the reaction; the cycloaddition takes place in the next step; finally, the cyclic iodate ester decomposes and forms two aldehyde groups (Supplementary Fig. 1a). The five sugar rings in the molecular structure were opened to form a molecular chain containing 10 aldehyde groups at 100% oxidation (Fig. 1a). In addition, NaIO4 oxidized one residue of riclin: a double oxidation between C2 and C3 and between C3 and C4, respectively, in which C3 was released as HCOOH (Supplementary Fig. 2a), and the actual product included the formation of partial intra-/inter-molecular hemiacetal structure (Fig. 1b). The degrees of oxidation for AR25, AR50, AR75, and AR100 determined using hydroxylamine hydrochloride titration were 19.2%, 39.4%, 50.2%, and 68.4%, respectively (Supplementary Fig. 1b and Supplementary Table 1)21. Gel permeation chromatography and thermogravimetry-derivative thermogravimetry results indicated that the main chain of riclin was broken in an acidic environment or during oxidation; AR was opened to yield partial sugar rings and molecular chains compared with that of the riclin structure, resulting in a lower molecular weight (Supplementary Fig. 2c–h and Supplementary Table 1)22,23, these formed long and short molecular chains fold and ultimately create a cross-linked system of AR molecules (Fig. 1b).
a, b Principle of the aldehyde modification of riclin. c 1H-NMR (D2O) spectra. d FT-IR spectra. e Wide XPS spectra. f, g C1s XPS spectra of riclin and AR50. h UV-vis spectra. i Surface zeta potential (n = 6). Data are presented as mean ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001. “ns” denotes no significant difference.
Chemical characterization of AR
The aldehyde-modified structure of AR was further characterized using 1H-nuclear magnetic resonance (1H-NMR), Fourier-transform infrared spectroscopy (FT-IR), X-ray photoelectron spectroscopy (XPS), Ultraviolet–visible spectroscopy (UV-Vis), and zeta-potential measurements. 1H-NMR spectroscopy confirmed the addition of aldehyde (9.0–9.3 ppm) and hemiacetal groups (5.0–6.5 ppm) to the riclin molecular chain (Fig. 1c)24. The FT-IR spectra displayed a weak peak at 1720 cm−1, corresponding to stretching vibrations of the carbonyl group22, which increased with the degree of AR oxidation (Fig. 1d), confirming the formation of aldehyde moieties. The XPS spectra of riclin and AR displayed two similar peaks at 535 (C 1 s) and 286 eV (O 1 s) (Fig. 1e). The C 1 s spectrum of AR displayed an additional peak representing C = O (289 eV) (Fig. 1f, g); with an increase in the degree of oxidation, AR demonstrated a decrease in the carbon content and an increase in the oxygen content on the surface (Supplementary Table 2), corresponding to the formation of the aldehyde and acetal groups25. The UV-Vis absorption spectrum of AR revealed high absorption peaks at 228–230 nm (Fig. 1h), further confirming the characteristics of aldehyde groups22. Zeta-potential measurements revealed an elevated positive charge for AR compared with that of riclin (riclin: −38.5 ± 3.4 mV, AR25: −22.4 ± 3.4 mV, AR50: −18.2 ± 0.9 mV, AR 75: −18.0 ± 0.7 mV, AR100: −16.0 ± 1.0 mV), indicating that the more negatively charged hydroxyl groups were replaced by aldehyde groups with weaker charges (Fig. 1i)26. XRD (X-Ray Diffraction) results shown that AR appears with broad peaks between 5° and 30° (Supplementary Fig. 3a), revealing its amorphous structure. This is critical for porous structure formation and stability, which enhances flexibility, absorbency, and interaction with water, allowing greater deformation without cracking27. Collectively, these results confirmed the successful introduction of aldehyde group.
Physical characterization of AR sponges
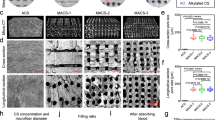
The liquid absorption ratio, shape memory, and compressive strength, which are vital mechanical characteristics of a sponge, strongly depend on the underlying physical structure and morphology5. After dialysis, the high-purity AR solution was freeze-thawed repeatedly, obtain a homogeneous AR solution with molecules arranged neatly according to size, and then freeze-dried to prepare sponge with strong cross-linking strength28,29. Scanning electron microscopy (SEM) images revealed the distinct porous quasi-honeycomb channel structures of the AR sponge and the normal distribution of interconnected channel apertures (Fig. 2a). With an increasing degree of aldehyde modification, the sizes of the channel apertures decreased and the honeycomb structure became more pronounced (Fig. 2b). Additionally, increasing the concentration from 1 to 3 wt.% substantially altered the sponge microstructure (Supplementary Fig. 4a). An excessively high aperture size led to channel collapse (1 wt.%), whereas a narrow aperture rendered the macrostructure excessively hard (3 wt.%) and increased the surface tension, hindering liquid adsorption or practical use (Supplementary Fig. 4a, Supplementary Fig. 5a and Supplementary Fig. 6a–c, Movie 11). However, the AR50 sponge (2 wt.%) exhibited a suitable and stable macrostructure (Supplementary Fig. 5 b–e, Movie 12), with high-porosity dense microchannels (92.52 ± 1.55 %, d Mean = 91.52 μm) (Fig. 2b and Supplementary Fig. 4b). The dry density gradually increased with a low amplitude with the rise in the degree of oxidation (Supplementary Fig. 4c), further confirming a decrease in the size of the channel apertures. The contact-angle measurements demonstrated that the AR50 sponge facilitates the rapid passage of liquids through its quasi-honeycomb channels, and displaying a substantially high liquid infiltration speed (Supplementary Fig. 4d). Consequently, 2wt.%-AR50 sponge exhibits robust shape memory and blood absorption characteristics, thereby may play a pivotal role in stabilizing compression and accelerating hemostasis10.
a Macroscopic photographs and SEM images of AR25/50/75/100 sponges (scale bar: 500 µm, 200 µm). b Aperture distribution of the AR sponges. c An 80% compression cycle of a water-soaked AR50 sponge. d Absorption ratios of NS, SBF, and blood for various samples (ricin, AR25/50/75/100 sponges, gauze, gelatin, Surgicel, Chito SAM, QCG; n = 5 per group). e, f NS/blood absorption kinetics of AR sponges (n = 3). g, h Schematic illustration and photographs of the porcine skin lap-shear test. i, j Curves of displacement with shear stress and adhesion strength of riclin, gelatin, and AR sponges. Data are presented as mean ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001. “ns” denotes no significant difference.
Mechanical properties of AR sponges
Shape memory properties of AR sponges
Hemostatic sponges for treating irregular tissue trauma should exhibit strong shape recovery and appropriate flexibility with maintained structural integrity5,10,14. Their use should result in the rapid cessation of bleeding in an external or internal high-pressure environment. Therefore, to evaluate the shape memory performances of the AR sponges after blood absorption, we conducted a cyclic compression-decompression test (Fig. 2c and Supplementary Movie 1). The AR50 (2wt.%) sponge displayed almost no loss of recovery at different cyclic compression intensities (40%, 60%, and 80%), with the ultimate compressive strength observed at 80% (19.3 kPa, 18.9 kPa, and 17.8 kPa for the first, second, and third cycle, respectively) (Supplementary Fig. 7a–d, h). However, after three cycles of 80% total compression, the AR25, AR75, and AR100 sponges exhibited significant loss of elasticity at the maximum compression strength30 (AR25: 32.3 kPa, 24.4 kPa, and 19.9 kPa; AR75: 15.8 kPa, 13.7 kPa, and 8.4 kPa; and AR100: 13.2 kPa, 9.8 kPa, and 8.4 kPa for the first, second, and third cycle, respectively), and all three sponges were damaged after 80% compression (Supplementary Fig. 7e–g, i). Additionally, the stress-strain curves revealed that as the aldehyde content of the AR sponge increased, the brittleness and softness increased gradually and the rigidity (hardness) decreased gradually, with the AR50 sponge exhibiting the most suitable elasticity and rigidity (Supplementary Fig. 7j, k)5,10,31. Liquid absorption analysis of the AR50 sponge under dry compression (compressive strength of 80%) indicated that the sponge rapidly absorbed NS or blood and then recovered, indicating its highly efficient shape memory (Supplementary Fig. 7i and Movie 2, 12, 16).
Liquid adsorption of the AR50 sponge
The principal hemostatic mechanism of the sponge involves mechanical compression triggered by water- or blood-induced shape recovery and volume expansion5,10. Therefore, efficient water or blood absorption with short-term stable fluid retention is crucial for a sponge with superior hemostatic efficacy4,32. Compared with various commercial hemostatic agents (gelatin sponge, Surgicel, Chito SAM, and QuikClot Combat Gauze [QCG]), the AR50 sponge exhibited the highest fluid absorption ratios for human blood (59.4 g·g−1), SBF (54.3 g·g−1), and NS (53.0 g·g−1) (Fig. 2d). Furthermore, we revealed that the AR50 sponge with a high, stable liquid absorption efficiency (NS, 10.46 ± 0.11 g·s−1; blood, 10.77 ± 0.07 g·s−1) (Fig. 2e, f and Supplementary Table 3). Consequently, the appropriate aldehyde-containing molecular chain imparts an ultra-high liquid absorption ratio to the AR50 sponge, potentially creating suitable apertures in its quasi-honeycomb channel structure for effective association with blood and water, resulting in macroscopic fluid retention5,10. Simultaneously, the AR50 molecular chains with appropriate hydrophobicity may form larger hydration layers after liquid contact, maintaining short-term stability and cross-linking with proteins in the blood to enhance adsorption strength toward blood and coagulation factors22. This demonstrated a highly efficient liquid preservation over a shorter time of AR50 sponge, surpassing the performance of other hemostatic agents5,10
Tissue adhesion of the AR50 sponge
In pre-hospital hemorrhage emergencies, continuous pressure application is inconvenient, rendering complete self-rescue challenging for the patient; therefore, the ideal hemostatic agent must possess suitable tissue adhesion properties1,3,33. Most existing polysaccharide-based hemostatic sponges lack sufficient cohesive strength for effective adhesion3,5,9. Therefore, to evaluate the tissue adhesion strength of the AR sponge, we performed lap-shear tests using porcine skin (Fig. 2g, h)33. At a bonding time of 10 min, the shear strengths of gelatin and riclin were <6 kPa, indicating weak adhesion. In contrast, AR exhibited enhanced adhesion to the skin compared with those of the other hemostatic agents (AR25, 7.32 ± 0.21 kPa; AR50, 9.01 ± 1.05 kPa; AR75, 15.05 ± 0.68 kPa; and AR100, 17.79 ± 0.55 kPa) (Fig. 2i, j). The robust tissue adhesion of the AR sponge may depend on the aldehyde groups of the molecular chains forming Schiff-base bonds with the amino groups of tissue proteins (Supplementary Fig. 2f)22. Notably, AR50 displayed suitable tissue adhesion properties for hemostasis and was easily removed, whereas AR75 and AR100 sponges exhibited excessively strong wet-tissue adhesion strengths, similar to that of general medical fibrin glue (12–20 kPa)3,33,34, indicating the potential application of the AR50 sponge in closing large wounds and healing small wounds35.
Collectively, based on its appropriate aldehyde-modified, quasi-honeycomb uniform channel structure, the AR50 sponge exhibits excellent flexibility, memory recovery, liquid absorption, proper adhesion and compressive strength, suggesting its potential suitability for hemostasis in irregularly shaped wounds and the ability to provide durable compression at the hemostatic site.
In vitro coagulation
Hemostasis involves three distinct mechanisms, namely erythrocyte accumulation, platelet activation, and fibrin structure formation, which collectively provide a physiological hemostatic barrier (thrombus)4,5. In this study, we explored the mechanisms by which the AR50 sponge affects the conformational changes of cells and substances in the blood, including erythrocytes, platelets, and plasma. This comprehensive and systematic evaluation method covers the field of polysaccharide hemostatic sponges3,4,5,6.
Effects of the AR50 sponge on blood
Efficient blood aggregation is essential in hemostatic barrier formation1,3. Initially, we evaluated the coagulation strength and time of the AR50 sponge using whole-blood blotting kinetics and a whole-blood clotting test (WBCT). In whole-blood blotting kinetics, the blood clotting index (BCI) correlated negatively with blood clotting36. The AR50 sponge displayed the lowest BCI within 15 s (~ 20%) compared with those of clinically used hemostats (medical gauze, Celox, gelatin sponge, Surgicel) (Fig. 3a). The BCI of the AR50 sponge exhibited minimal fluctuation over 240 s, indicating the strongest clotting effect among the selected hemostatic agents (Supplementary Fig. 8). The results of the WBCT indicated that well-established commercial hemostats (medical gauze ~597 s, Celox ~347 s, gelatin sponge ~286 s, and Surgicel » 800 s) exhibited a hemostatic time >4 min, whereas the AR50 sponge displayed the lowest clotting time of ~50 s (Fig. 3b).
Comparison of a whole-blood clotting kinetics (n = 3) and b whole-blood clotting time on various samples (n = 6–13 per group: ricin, AR25/50/75/100 sponges, gauze, gelatin, Surgicel, Chito SAM, QCG). c SEM images depicting the morphological changes of whole blood in contact with riclin and the AR50 sponge for 15, 30, 60, 120, 180, and 240 s. The arrows represent activated platelets (Scale bar: 10 μm). d Traces of thromboelastography of the different whole-blood samples within 60 min. e, f Representative plasma clotting kinetics in 1 h and the slope of the linear range of the curves (n = 4) for riclin and AR sponges. g Erythrocyte adhesion rates of various hemostats (n = 6–7 per group). h Erythrocyte clotting kinetics within 20 min for different samples (n = 3 per group). i SEM observation of erythrocyte morphology on the AR50 sponge at various periods (1, 4, 8, 12, 16, and 20 min). (Scale bar: 10 μm). Data are presented as mean ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001. “ns” denotes no significant difference.
Further observation using SEM revealed partial platelet activation after only 15 s of contact between whole blood and the AR50 sponge. During 30–60 s, platelets were highly activated and moved to the contact surface, whereas erythrocytes aggregated and deformed to stack with the platelets. During 60–240 s, activated platelets and deformed erythrocytes became considerably stacked and adhered to the surface of the AR50 sponge in a layer-by-layer manner, whereas riclin exhibited almost no such characteristics (Fig. 3c).
Thromboelastography was performed to further evaluate coagulation37. Compared with the other groups, AR50 promoted coagulation, had a higher coagulation strength, and exhibited platelet hyperfunction (response time [R] = 5.6 ± 0.12 min, maximum amplitude [MA] = 85.9 ± 3.66 mm) (Fig. 3d and Supplementary Table 4). Furthermore, the curve angle (α) and the time from the endpoint (R) to 20 mm amplitude (k) of AR50 did not change significantly compared with those of the blank group. Additionally, the loss of the spongy structure of the AR50 powder still reduced R and enhanced the platelet function and MA. Conversely, the coagulation rate was unaffected (α/k), suggesting that the suitable quasi-honeycomb channels for AR50 were largely responsible for the accelerated coagulation. Notably, based on the chemical structure and physical channels, the AR50 sponge displayed the optimal coagulation effect among the tested commercial hemostats, suggesting that it may be a suitable clotting activator for accelerating coagulation.
Effects of AR sponges on plasma
Blood clotting is divided into extrinsic, intrinsic, and common pathways9. Prothrombin time (PT), activated partial thromboplastin (APTT), and thrombin time (TT) trials were performed based on platelet-poor plasma (PPP). The AR sponges exhibited a minimal effect on APTT and PT, whereas TT was substantially reduced in the AR50 and AR75 groups (Supplementary Fig. 9a–c). According to previous studies, increasing aldehyde content may inhibit coagulation factor activity and, consequently, plasma clotting22. However, the results of APTT/PT evaluations indicated that AR sponges had no notable effect on extrinsic and intrinsic coagulation pathways and did not affect human coagulation factor activity; the negative impact of the AR sponge series on coagulation factors was not expressed, indicating that the 3D physical structure of the AR sponge offsets this negative impact. Additionally, Thromboelastography shows that the coagulation rate of AR50 powder was unaffected (α/k), and the plasma clotting relies on fibrin synthesis38, therefore, it is hypothesized that exposure of plasma to the physical surface of the AR50 sponge triggers an external coagulation cascade that accelerates fibrin synthesis22,39.
Moreover, we observed decrease in TT indicated that the AR50 sponge structure could accelerate plasma fibrin synthesis and influence the common pathway. We then evaluated the plasma clotting kinetics of the AR sponges. AR50, AR75, and AR100 sponges promoted plasma clotting upon contact, and kinetic curves were stable within 10 min, especially for AR50, which reached stability at the fastest rate within 5 min (Fig. 3e). The decrease in turbidity indicated that the aldehyde-containing group reduced the total amount of fibrin synthesis. Moreover, AR50 displayed the highest slope, excluding that of the Ca2+ group (Fig. 3f), indicating that the AR50 sponge promoted low levels of fibrin synthesis22,34.
Furthermore, based on SEM images of PPP on the sponge surface, AR50 and AR75 sponges display a loose fibrin network—conversion of fibrinogen to fibrin monomer and accumulate to form a network (Supplementary Fig. 9d), the property of AR sponge structure to promote fibrin fiber formation is further demonstrated by these results40. Therefore, we speculated that AR50 sponge is based on a suitable 3D quasi-honeycomb channel, which highly adsorbs and aggregates proteins and coagulation factors in plasma22, exposure of plasma to the physical surface of AR50 sponge triggers an external coagulation cascade. The coagulation pathway is activated and rapidly amplified, among which the common coagulation pathway is most affected, ultimately accelerating the conversion of few fibrinogens in plasma into fibrin in the short term. Additionally, it shows that the high coagulation function of AR50 sponge mainly comes from its effect on blood cells, rather than the plasma.
Effects of the AR50 sponge on erythrocytes
Erythrocytes are major components of a thrombus, and an effective hemostat may induce substantial erythrocyte adherence and stable aggregation3,4,5,6,7,8,34. Additionally, concentrating erythrocytes in polyhedrocyte clusters could help form an impermeable seal, thereby effectively arresting bleeding41. Therefore, we evaluated the ability of the AR sponges to adhere to and contract erythrocytes. Among the different AR sponges, AR50 exhibited the highest erythrocyte adhesion rate and the most polyhedral erythrocytes (AR25, 84.82%; AR50, 97.81%; AR75, 90.68%; and AR100, 77.67%) (Supplementary Fig. 9e, f). Compared with the insufficient levels of erythrocyte adhesion observed with Celox (7.93%), the gelatin sponge (39.37%), and Surgicel (61.72%), the AR50 sponge exhibited the highest erythrocyte adhesion efficiency (96.74%) within 15 min (Fig. 3g). In terms of erythrocyte clotting kinetics, the AR50 sponge exhibited the lowest absorbance at 4 min, and the corresponding curve remained stable from 4–20 min, indicating rapid adhesion, concentration, and stabilization of erythrocyte clots (Fig. 3h). Furthermore, SEM revealed highly adherent and concentrated erythrocytes on the AR50 sponge surface (Fig. 3i and Supplementary Fig. 9e). Additionally, biconcave-shaped erythrocytes gradually contracted into polyhedra, tightly stacking to form larger polygonal cell clusters over time, with increased contraction and stacking in 16–20 min (Fig. 3i). The results of erythrocyte clotting after 12 and 24 h of immersion indicated that the AR50 sponge group displayed stable clots, and the surface erythrocytes remained highly stackable compared with the loose cells in the riclin group (Supplementary Fig. 9g). Therefore, the AR50 sponge induced the formation of tightly stacked polyhedral clusters, significantly enhancing the strength of surface erythrocyte clots41. Notably, during long-term soaking, the AR sponges exerted a minimal effect on pH compared with the change in pH values observed in the blank groups; furthermore, AR sponges exhibited safety and clot-stability characteristics (Supplementary Fig. 9h). However, the well-established commercial hemostat Surgicel caused erythrocyte lysis, inducing a low pH (~4.8) and forming a brown colloid with no apparent capacity to coagulate larger quantities of blood42. Collectively, these results suggest that the AR50 sponge rapidly aggregated, tightly adhered to, and concentrated erythrocytes considerably to form a tessellated array of polyhedra, ultimately creating a stable clot. Additionally, the polyhedral deformation of erythrocytes might help establish a tight barrier on the hemostatic surface of the sponge34,41.
Effects of the AR50 sponge on platelets
Platelet adhesion may lead to platelet aggregation and activation43,44; therefore, we evaluated the capacities of the AR sponges to adhere to platelets. SEM revealed that large platelets adhered to and irregularly tessellated on the AR50 sponge surface. Additionally, the AR50 sponge exhibited the highest platelet adhesion effect among the AR groups at 10 min (AR25, ~43.58%; AR50, ~62.88%; AR75, ~42.17%; and AR100, ~29.76%) (Fig. 4a, b). The platelet adhesion rates of the AR50 sponge reached 63.35% (15 min), 69.23% (30 min), and 94.33% (60 min), which surpassed those of the well-established commercial hemostats (<60%), indicating ultra-high-efficiency platelet adhesion (Fig. 4c).
a SEM images of AR sponges to platelets at 10 min. (Scale bar: 10 μm). b Platelet adhesion rate at 10 min (plasma removal) (n = 4). c Platelet adhesion rates of various hemostats (n = 3). d Effect of different hemostats on the fluorescence intensity (FI) of intracellular Ca2+ (n = 4–6). e SEM images showing platelet activation from the AR50 sponge at 10, 20, and 30 min (Scale bar: 10 μm). f Representative FACS graphs showing samples-mediated platelet activation (normal platelets, spongy riclin, and AR50 sponge-mediated platelets) (CD41a-PE and CD62p-APC). g Activation rate of platelets (CD41a/CD62p co-expression cells). Data are presented as mean ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. “ns” denotes no significant difference.
Previous studies regarding polysaccharide hemostatic sponges have mostly focused on physical structure adhesion or positive/negative surface charge stimulation to activate the platelets; however, few studies on platelet activation have been reported3,4,5. Therefore, we further evaluated the ability of the AR50 sponge to actively stimulate platelets. Platelet activation and deformation depend on intracellular Ca2+ release45; Changes in intracellular Ca2+ in platelets mainly rely on the release of intracellular Ca2+ pools and the Ca2+ pump on the cell membrane to transport extracellular Ca2+ into the cell46. Therefore, we initially measured the intracellular Ca2+ levels in treated platelets using a fluorescent probe (Fluo-4 AM). The Ca2+ fluorescence intensities indicated that AR significantly increased the Ca2+ concentration to a degree far superior to those of the other commercial hemostats (Fig. 4d). This suggests that AR50 sponge-induced platelet activation might guide intracellular Ca2+ release and cause irregular platelet deformation (Fig. 4d, e). However, the commercial hemostats could not induce an increase in the Ca2+ level within the platelets. Considering that AR50 sponge can wrap blood cells and exhibited a minimal effect coagulation factor (Supplementary Fig. 5a, b, g)22,47, we speculated that after AR50 sponge adheres to platelets, the mosaic structure formed not only inhibits the loss of Ca2+, but also promotes the release of intracellular Ca2+ pools in platelets48.
Furthermore, SEM revealed a gradual activation of platelets on the surface of the AR50 sponge (with large platelets extending irregularly), whereas we observed negligible changes in platelets detected in the riclin group (Fig. 4e and Supplementary Fig. 10)49, confirming that the AR50 sponge could actively promote platelet activation. Increased expression levels of P-selectin (CD62p) on the platelet surface are markers of platelet activation50, and platelets may be distinguished from other cells and impurities by the expression of the surface antigen CD41a51. Therefore, we tagged CD62p and CD41a on the surfaces of the platelets and further assessed the active stimulation by the AR50 sponge via fluorescence-activated cell sorting (FACS), with the blank and spongy riclin group used to exclude stimulation owing to the environment and structural contact, respectively. FACS results revealed that AR50 treatment resulted in CD41a/CD62p levels as high as 32.7% (blank 10.7%, spongy riclin 12.6%), with an excellent active platelet stimulation effect (Fig. 4f, g)52,53,54. Notably, the surface electronegativity of AR50 was lower than that of riclin, suggesting that the capacity of AR50 to actively stimulate platelets was attributed to its specific molecular structures and physical channels, especially the channel structure, rather than its electronegativity55. Collectively, these results suggest that the AR50 sponge provides rapid hemostasis via the strong ability for physical adhesion and platelet activation.
In vivo hemostasis
Considering the mechanical properties and coagulation effects of the sponges, we utilized the AR50 sponge in the hemostatic evaluation. Leveraging its efficient hemostatic performance in small animal hemorrhage models (Supplementary Figs. 11–14), we established lethal porcine and rabbit hepatic and arterial hemorrhage models to assess the potential of the AR50 sponge in treating massive hemorrhaging in humans. Commonly used medical hemostatic agents such as hemostatic gauze (cellulose-based), Chito SAM (chitosan-based cotton gauze), Celox (chitosan-based microspheres), Surgicel (oxidized regenerated cellulose-based gauze), gelatin sponge (derived from animal tissue collagen), and QCG (gauze loaded with kaolin) served as controls in this study owing to their recognized efficacy in hospital or pre-hospital scenarios4,10,56,57. Additionally, we examined the hemostatic barrier formed by the AR50 sponge in practical hemostasis and explored the link between the observed in vitro coagulation mechanism and the in vivo hemostatic mechanism of the AR50 sponge.
Hemostasis of porcine hepatic incision and perforation
In the context of pre-hospital visceral hemostasis, accurately filling visceral wounds with hemostatic agents poses more significant challenges than direct compression on the wound surface. Consequently, we strategically placed the AR50 sponge on the liver wound (Fig. 5a–c), capitalizing on its adhesive properties demonstrated in porcine skin studies (Fig. 2g–j). In the surgical treatment of a porcine hepatic laceration, the AR50 sponge facilitated hemostasis within 68 s, resulting in a substantial reduction in blood loss to 2.6 g. Notably, this represented an 86% decrease in hemostasis time and an 85% reduction in blood loss compared with that in the blank group (time/loss). These values were considerably lower than those observed when employing other hemostatic agents (Surgicel, 262 s and 7.9 g; Chito SAM, 284 s and 10.4 g; and medical gauze, 312 s and 15.4 g) (Fig. 5d, e; Supplementary Movies 3 and 4).
a Schematic illustration of the hemostatic process of the AR50 sponge in porcine hepatic laceration and perforation. b, c Photographs of the hemostasis effect in porcine hepatic laceration and perforation (stage 1, before hemostasis; stage 2, beginning of hemostasis; stage 3, completion of hemostasis; stage 4, removal hemostats). d, e Hemostasis time and blood loss in the porcine hepatic laceration model (n = 3–4 per group). f, g Hemostasis time and blood loss in the porcine hepatic perforation model (n = 3–5 per group). Data are presented as mean ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001. “ns” denotes no significant difference.
In a lethal porcine hepatic perforation model, the AR50 sponge effectively controlled hemostasis within 140 s. The corresponding reduction in hemostasis time and blood loss (4.9 g) amounted to 75% and 87%, respectively, when compared with those of the blank group. These values were significantly lower than those observed with Surgicel (242 s and 11.3 g), Chito SAM (332 s and 19.8 g), and medical gauze (436 s and 25.4 g) (Fig. 5f, g; Supplementary Movies 5 and 6). Notably, in the porcine liver hemorrhage model, minimal blood oozed from the surface of the AR50 sponge compared with that using other hemostats (Surgicel, Chito SAM, and medical gauze). Moreover, no significant blood flow was observed on the filter paper after sponge fixation (Fig. 5b, c, Supplementary Movies 3–6 and 13). Following sponge removal, we observed no fresh blood gushing from the liver wound (Supplementary Movies 4, 6 and 14), confirming the excellent hemostatic effect.
Inspection of hepatic incision sections revealed deep thromboses of tissue wounds, with the AR50 sponge group exhibiting more blood cell aggregates than those in the Surgicel, Chito SAM, Celox, gelatin sponge, gauze, and blank groups. This confirmed the potent blood cell clotting and concentration characteristics of the AR50 sponge (Supplementary Fig. 15), highlighting its potential to establish a high-strength hemostatic barrier.
Hemostasis of the femoral artery
Owing to high pressure on the artery vessel walls, stopping bleeding after injury using only the coagulation function of the body is challenging1,3. Therefore, we simulated massive arterial hemorrhages in rabbits and pigs by incising the femoral arteries (Fig. 6a, b). After inducing a massive hemorrhage in the rabbit femoral artery, the AR50 sponge significantly reduced the hemostasis time and blood loss by approximately 82% and 87%, respectively, compared with those of the gauze control, stabilizing at 54 s and 2.6 g, respectively. These values were superior to those observed when using the other hemostats (Surgicel, 119 s and 6.6 g; gelatin sponge with extra gauze, 184 s and 14.7 g; and Celox, 216 s and 14.8 g) (Fig. 6c, e, f and Supplementary Movies 7 and 8). In a more clinically relevant setup of a lethal porcine femoral arterial laceration model, the AR50 sponge effectively controlled hemostasis within 140 s. The corresponding reduction in hemostasis time and blood loss (4.2 g) amounted to 75% and 87%, respectively, compared with that in the blank group. These values were significantly lower than those observed with Surgicel (242 s and 11.3 g), QCG (171 s and 19.5 g), and Chito SAM (126 s and 21.3 g) (Fig. 6d, g, h and Supplementary Movies 9 and 10). Similarly, no blood exudation or flow was observed at the wound site treated using the AR50 sponge (Fig. 6d and Supplementary Movies 7–10), displaying a top-notch hemostatic effect.
a, b Schematic illustration of the hemostatic process of the AR50 sponge in pig/rabbit femoral artery scission. c, d Photographs of the hemostasis effect in pig/rabbit femoral artery scission (stage 1, before hemostasis; stage 2, beginning of hemostasis; stage 3, completion of hemostasis; stage 4, removal of hemostats). e, f Hemostasis time and blood loss in the model of rabbit femoral artery scission (n = 6–7 per group). g, h Hemostasis time and blood loss in the model of porcine femoral artery scission (n = 5–6 per group). Data are presented as mean ± S.D. *P < 0.05, **P < 0.01, ***P < 0.001. “ns” denotes no significant difference.
After successfully achieving hemostasis in the porcine femoral arteries, we further explored the in vivo hemostatic properties and potential mechanisms using SEM. We meticulously examined three sections of the hemostatic surface of the AR50 sponge, specifically focusing on the center of the contact surface (A), the inside surface (B), and the side of the surface (C). On surfaces A2–A5, erythrocytes and platelets exhibited irregular patterns, with large, activated platelets aggregating with polyhedral erythrocytes to form tightly packed cell clusters. Few fibrin filaments were observed (Supplementary Fig. 16a). Since fibrin plays a limited role in the hemostasis process, the contraction stress produced by activated platelets alone may not be fully effective34, suggesting that the quasi-honeycomb channel structure of the AR50 sponge could replace the pulling and supporting effect of fibrin to a certain extent. The sponge-induced aggregation of erythrocytes promotes deformation, potentially enhancing mutual adhesion. Additionally, activated platelets increase their viscosity44,45, enhancing adhesion with nearby platelets and erythrocytes. Consequently, massive platelets were recruited onto the erythrocyte layer, forming robust cell clusters that contributed to establishing a stable barrier with the AR50 sponge on the hemostatic interface. These findings aligned with the effects of the sponge on human blood cells (Figs. 3c, i and 4e), indicating that during hemostasis, the AR50 sponge specifically targets erythrocytes and platelets, with minimal impact on fibrin synthesis, yet still demonstrating superior hemostatic capability relative to commercial hemostats. Moreover, these irregular platelets and erythrocyte stacks were predominantly observed in the center of the contact surface (A1–A5), whereas the sides and interior exhibited a considerably large area of typical biconcave disk-shaped erythrocyte adhesion (Supplementary Fig. 16a–c). This noteworthy observation suggests that the AR50 sponge is tailored to the hemostatic surface, recruiting and inducing polyhedral erythrocytes and activated platelets to create a barrier, unequivocally enhancing its hemostatic efficiency34,41. Based on the procoagulant mechanism and superior hemostatic effect of the AR50 sponge, we propose the following unique hemostatic mechanism consisting of three phases: (I) a large number of erythrocytes and platelets are rapidly recruited and targeted to accumulate on the hemostatic surface (Fig. 3a, c and Supplementary Fig. 16); (II) at the contact interface, numerous platelets quickly activate and erythrocytes deform into polyhedral shapes, synergistically forming a robust, stable cell cluster (Figs. 3c, i and 4a, e; Supplementary Fig. 16); and (III) the quasi-honeycomb channel structure of the sponge itself could partially replace the supporting and pulling effect of fibrin, which combines with the cell clusters composed of erythrocytes and platelets to create a stable hemostatic barrier (i.e., a blood clot) to complete hemostasis in sizable bleeding wounds in large animals (Supplementary Figs. 15 and 16). Notably, although a small amount of fibrin synthesis on the hemostatic surface stabilizes the hemostatic barrier, the rapid hemostatic process involving the AR50 sponge used in treating massive hemorrhage does not rely on fibrin.
Additionally, the potential for secondary rebleeding injuries, stemming from the robust adhesion levels and the challenging removal of residual hemostats, warrants consideration57,58. Therefore, we evaluated whether the hemostatic agents could be easily disassembled after hemostatic stabilization. Following hemostasis completion, the AR50 sponge could be effortlessly removed from the wound, mitigating the risk of debridement (Supplementary Movies 4, 6, 8, and 10). Notably, hemostats that achieve hemostasis at low doses typically exhibit efficient hemostasis59.In this study, the AR50 sponge was administered at the lowest dosage among all tested hemostats, highlighting its excellent hemostatic effect. Furthermore, all animals survived after the application of AR50 sponges to halt bleeding. These compelling results suggest that the AR50 sponge is an ideal hemostatic agent for treating traumatic massive hemorrhaging, demonstrating an effective life-saving effect in crises.
Antibacterial properties and mechanisms
Severe bacterial infections are among the primary causes of death from traumatic hemorrhage; therefore, an effective hemostatic agent should exhibit antibacterial characteristics4,9. However, most previously reported polysaccharide-based hemostats exhibit poor antibacterial activities3,4,5,7,8. Consequently, we established in vitro models of Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa infection to evaluate the antibacterial capacity of the AR sponge and explore its mechanism of action. The AR50 sponge displayed evident bacterial inhibition rings (E. coli, 5.1 mm; S. aureus, 4.2 mm; P. aeruginosa, 2 mm) and significantly inhibited the rates of bacterial proliferation (Fig. 7a; Supplementary Fig. 17a, b, d; Supplementary Fig. 18a–c). Furthermore, the activities of the AR50 sponge-treated bacteria were substantially reduced, and the AR50 sponge displayed superior antibacterial activity compared with those of the other commercial hemostats (Fig. 7b; Supplementary Fig. 17c, e; Supplementary Fig. 18d). We used two nucleic acid dyes, SYTO 9 (green fluorescence) and propidium iodide (PI; red fluorescence), to stain the treated bacteria to accurately observe the bactericidal effects of the AR50 sponge. After AR50 sponge treatment for 2 h, the bacteria displayed strong PI signals (Fig. 7c and Supplementary Fig. 18e), suggesting that the AR50 sponge exhibited the highest sterilizing effect among the tested hemostats.
a, b Effects of riclin and AR sponges against E. coli and S. aureus tested in vitro based on the formation of the inhibition ring and the diluted spread plate method. c SYTO9/PI fluorescence staining images displaying the live/dead status of E. coli and S. aureus after 2 h of treatment (figures were merged by Image J). SYTO9 (green fluorescence) is a membrane-permeable marker, and PI (red fluorescence) labels bacteria with damaged membranes (scale bar, 200 µm). d, e SEM and transmission electron microscopy (TEM) images illustrating the morphologies and structures of E. coli and S. aureus treated with the AR50 sponge (SEM image scale bar: 500 nm; TEM image scale bars: 500 nm, 200 nm).
As AR50, a macromolecule, may have difficulty crossing intracellular membranes60, it likely utilizes a broad-spectrum antibacterial mechanism against bacterial surface structures. We used E. coli and P. aeruginosa as a representative gram-negative bacterium and S. aureus as a representative gram-positive bacterium to explore the antibacterial mechanism. SEM observations revealed morphological changes in the treated bacteria. In contrast to the smooth surfaces of the untreated bacteria, the AR50-treated E. coli bacterial skeletons were sunken and broken, with a shriveled, wrinkled, and twisted appearance. The biofilm of S. aureus shrank and cracked, with content leakage, after AR50 treatment (Fig. 7d and Supplementary Fig. 19a).
The disruption of bacterial biofilms by AR50 was further observed using transmission electron microscopy. AR50 treatment of E. coli resulted in the disruption of the cell membrane and wall, with uneven ripples, along with lysis of the surface and large internal vacuoles, resulting in continuous leakage of the contents. The cell wall and membrane of S. aureus were likewise noticeably disrupted after treatment, with severe leakage of the cytoplasm and large internal vacuoles evident (Fig. 7e and Supplementary Fig. 19b). Notably, we observed stronger antibacterial effects with increasing aldehyde content in the AR sponge, suggesting that its antibacterial properties could be attributed to the aldehyde groups in its molecular structure61. Specifically, the antibacterial activity could be primarily attributed to the reactions between the aldehyde groups and the bacterial cell wall or cleavage of the peptide bonds of peptidoglycan or other proteins62.
Additionally, the AR50 sponge exhibited a reduced killing effect and less bacterial biofilm damage to P. aeruginosa than to E. coli and S. aureus (Supplementary Fig. 18e, f), suggesting that the lower cell wall permeability owing to stronger cell outer membranes (closed conformer) in P. aeruginosa inhibits the killing effect of AR5063. Conversely, the outer membrane of E. coli may facilitate the passage of AR50, contributing to a better antibacterial effect observed macroscopically61. The AR-induced severely affected bacterial cell permeability and disrupted bacterial mechanics (Fig. 7c–e and Supplementary Fig. 18e, f) suggested that AR50 could readily break through the outer membrane of E. coli but has more difficulty in penetrating the outer membrane of P. aeruginosa, primarily damaging the peptidoglycan of the bacterial cell wall to exert its bactericidal effect64,65. This also explains the stronger killing effect of AR50 against E. coli compared with that against S. aureus and P. aeruginosa because E. coli displays a thinner cell wall than S. aureus and P. aeruginosa has a notably lower cell wall permeability than does E. coli (Fig. 7 and Supplementary Fig. 18)66.
More significantly, AR is a polysaccharide-based polymer with an increased number of ring-opened chain structures with an increased degree of oxidation, indicating that these chain structures within AR may enhance its damaging effect on the bacterial cell wall67,68,69. Notably, the AR50 sponge exhibited stronger antibacterial properties than its solution counterpart (Supplementary Fig. 17f, g), suggesting that the excellent antibacterial capacity of this sponge could be attributed to its unique physical structure; notably, the quasi-honeycomb physical structure with channels provides a large and stable contact area to exert a long-lasting antibacterial effect. The antibacterial activity of the AR50 sponge is, therefore, attributed to the combination of physical and chemical structures that effectively kill bacteria. The AR50 sponge exhibits satisfactory broad-spectrum antibacterial activity, which is far superior to that of other commercial hemostats, suggesting that it should effectively prevent bacterial infections in the treatment of massive hemorrhaging of tissue in a pre-hospital setting. Notably, the antibacterial effect displayed by AR sponge targeting the peptidoglycan layer in the cell wall is almost among the top-notch among polysaccharide hemostatic sponges.
Rapid degradation of the AR50 sponge in vitro and in vivo
Previously reported degradable polysaccharide-based hemostats (based on cellulose, chitosan, starch, and their derivative raw materials) exhibited an extended degradation time in vitro and slow absorption in vivo3,4,7,8; thus, developing rapidly absorbable polysaccharide-based efficient hemostatic agents remains a major challenge8. We evaluated the degradation and absorption capacities of the AR sponge using in vitro and in vivo methods. First, we examined the degradation of the gelatin and AR sponges in SBF at room temperature (22–25 °C) and observed that the AR sponge was completely degraded within 24 h (riclin > 24 h; AR25, ~21 h; AR50, ~16 h; AR75, ~4 h; AR100 ~ 2 h) (Fig. 8a and Supplementary Fig. 20a). Conversely, the gelatin sponge was almost unchanged, indicating that the AR sponge exhibits excellent capacity for in vitro degradation.
a Degradation time of cylindrical spongy riclin and AR sponges in SBF at 25 °C (50 mg or 50 mL) (n = 5–6 per group). b Relative biodegraded absorbance of various samples after 24 h incubation in Dulbecco’s phosphate-buffered saline (PBS) at 37 °C (25 mg or 10 mL) (n = 5 per group). c Degradation time of the AR50 sponge in SBF at different pH values at 25 °C and 37 °C (pH = 5–10, 50 mg or 50 mL) (n = 3 per group). d Degradation time–mass curve of the subcutaneously implanted AR sponge (days 0, 2, 5, 10, 15, 20, and 25) (n = 6 per group). e After 25, 10, 15, and 20 days, hematoxylin and eosin (H&E) staining of the surrounding skin tissue shows the infiltration of inflammatory cells. The arrows represent inflammatory cell infiltration (Scale bar: 100 µm). f Hemolysis photographs after treatment of various samples at 2.5, 5, and 7.5 mg·mL–1. g Hemolysis rate of each group (n = 3 per group). h Cell proliferation and reactive oxygen species (ROS) response after 24 h of culture of the AR50 sponge with NIH3T3 cells (100, 200, 400, 800 µg·mL–1). (Scale bar: 100 µm). Data are presented as mean ± S.D. *P < 0.05, ***P < 0.001, ****P < 0.0001. “ns” denotes no significant difference.
Moreover, we evaluated the degradation ability of the hemostats in Dulbecco’s phosphate-buffered saline. The AR sponge displayed the highest degradation efficiency, with complete degradation observed for 15–60 min (Fig. 8b and Supplementary Fig. 20b). Notably, increasing the aldehyde content accelerated the swelling and dispersion of the sponge (Supplementary Fig. 20a). The enhanced hydrophobicity and distinct honeycomb channels could contribute to the potent exudation characteristics of the AR sponge with a high aldehyde content. Simultaneously, the decrease in molecular weight and changes in the intramolecular structure weakened the inter- and intramolecular forces (Supplementary Fig. 2c–h and Supplementary Table 1), which macroscopically resulted in rapid in vitro degradation.
To further evaluate the applicability of the AR sponge in vivo and in vitro, we simulated the degradation of AR50 sponges in various pH environments at 25 °C and 37 °C. The degradation time of the AR sponge, which was extended under acidic conditions, substantially decreased with increasing pH and temperature (pH 5 = 1051 and 386 min, pH 6 = 585 and 323 min, pH 7 = 237 and 103 min, pH 8 = 137 and 87 min, pH 9 = 61 and 54 min, and pH 10 = 64 and 53 min at 22–25 °C and 37 °C, respectively; Fig. 8c). These differences could be attributed to β-elimination and acid hydrolysis70, rendering AR50, produced via ring-opening with aldehyde-based chains, more sensitive to alkalinity.
The temperature-sensitive and pH-responsive degradation mechanism suggested the absorbability of the AR50 sponge in various wounds. Therefore, to further evaluate the degradation ability of the AR50 sponge in vivo, we implanted the sponges on the backs of rats and observed their residual masses and degradation levels from days 0 to 25. Approximately 72.3% of the AR50 sponge mass was absorbed on day 2; degradation was virtually completed between days 10 and 15, and wound healing on day 5 did not differ from that of the surgical group (Fig. 8d and Supplementary Fig. 21a, b). Therefore, the AR50 sponge could be metabolized in the short term without affecting wound healing, which is consistent with the results of the in vitro rapid degradation evaluation.
Biocompatibility of the AR50 sponge
Biocompatibility is a prerequisite for the utilization of a biomaterial in hemostatic applications71. Polysaccharides typically exhibit a broad spectrum of biocompatibilities as hemostats; however, derivatives with an excess of aldehyde contents are typically cytotoxic72. To ascertain the biocompatibility of the AR50 sponge, we initially assessed its toxicity during in vivo degradation. Pathological section staining indicated the absence of the day-5 inflammatory reaction in the skin surrounding the implanted sponge (Fig. 8e). The liver, spleen, heart, lungs, and kidneys exhibited no discernible differences compared with those of the control groups at various intervals (Supplementary Fig. 21c), thus confirming the excellent biocompatibility of the AR50 sponge. Moreover, the absence of skin irritation in the rabbits suggested that the AR sponge was devoid of potential allergens (Supplementary Fig. 21d, e). Furthermore, the hemolysis rate of the AR50 sponge in the hemolysis assay was <0.5%, falling below the 5% toxicity standard (Fig. 8f, g), in line with previous observations, signifying the optimal hemocompatibility of the AR50 sponge. Notably, the cell proliferation and ROS responses indicated that AR50 was virtually non-toxic (Fig. 8h and Supplementary Fig. 21f, g). In addition, the excellent degradation ability of AR50 sponge in vivo and in vitro did not affect its long-term storage and clinical application (Supplementary Fig. 22a, b and Movies 15, 16). Collectively, these results suggest that the AR sponge is biocompatible and exhibits promising potential for clinical translation as an advanced hemostatic agent.
In summary, we present a novel, cost-effective, easily fabricated, handled, and manipulated hemostatic AR50 sponge that is swiftly absorbable, safe, robust, antibacterial, and highly efficient for treating tissue trauma and massive hemorrhage in humans within a pre-hospital setting (Fig. 9). This monomeric polysaccharide-based hemostatic sponge possesses both active and passive hemostatic functions, addressing the limitations of many currently available hemostatic agents. Leveraging its distinctive aldehyde-modified molecular structure and quasi-honeycomb spatial channels, the AR50 sponge exhibits exceptional mechanical properties, including efficient liquid absorption, top-tier shape recovery, and appropriate tissue adhesion. The AR50 sponge demonstrates outstanding hemostatic efficacy through the rapid and targeted adherence and aggregation of numerous erythrocytes and platelets to the bleeding interface. It possesses the ability to activate platelets and concentrate erythrocytes into a polyhedron, forming tightly packed cell clusters. The quasi-honeycomb structure of the sponge contributes to support, creating a robustly stable hemostatic barrier in the short term without relying on fibrin. In massive hemorrhage models of animals such as pigs and rabbits, the AR50 sponge exhibited superior hemostatic effects. Notably, the AR50 sponge displayed efficient broad-spectrum antibacterial functionality by destroying the peptidoglycan of the bacterial cell wall. It is rapidly and safely absorbed in vivo, characteristics that most existing hemostats lack. In addition, compared with current research on polysaccharide hemostatic sponge10,22,25,30,31,32,59, the performance of AR50 sponge is among the best in the evaluation system of hemostatic sponges3,4,5, it not only further improves the hemostatic ability, but also has outstanding achievements in antibacterial and in vitro and in vivo degradation.
Given these advantages, we posit that the AR50 sponge could serve as an ideal hemostatic agent for saving injured soldiers on the battlefield, civilians in accidents, and other acute traumatic massive hemorrhage scenarios, holding promise in terms of production and application. Preclinical and clinical investigations are currently being conducted to evaluate the hemostatic effectiveness and biosafety of the AR50 sponge in humans.
Methods
Materials
Surgicel® 1953 was sourced from Johnson & Johnson Medical, Celox® from Medtrade Products Ltd. (Electra House), and Chito SAM™ 100 and QuikClot ® were obtained from Z-Medica Corporation (Wallingford, CT, USA). Absorbent medical gelatin sponge and medical sterile hemostatic gauze were purchased from Artivion (USA). All other reagents were acquired from Merck KGaA. The lactate dehydrogenase (LDH) kit, ROS assay kit, and Actin-Tracker Green were obtained from Beyotime Biotech Inc. Live/DeadTM BacLightTM Bacterial Viability Kits (L7012), CD41a monoclonal antibody (HIP8), and PE eBioscience™ (12-0419-42) were purchased from Thermo Fisher Scientific. Fluo-4 AM and APC mouse anti-human CD62P (561920) were purchased from Becton, Dickinson and Company.
Animals
Healthy male pigs (18–20 kg) and New Zealand white rabbits (~3 kg) were purchased from a livestock market in Linyi, Yantai (Shandong, China). Male Sprague–Dawley rats were supplied by Sai Bo company (Hefei, Anhui, China), and male C57BL/6 J mice (7 weeks, 24 ± 2 g) were supplied by the Center for Comparative Experimentation of Yangzhou University, China. All animals were housed in the animal facilities at Bengbu medical university, and all experimental protocols involving animals were conducted in strict accordance with the US Public Health Service’s Policy on Animal Research Advisory Committee Guidelines (Animal Research Advisory Committee (ARAC) Guidelines | OACU (nih.gov)) and received approval from the Animal Care Ethics Committee of Bengbu Medical College (BBMCEC2020018). The animals were humanely euthanized with a sodium pentobarbital overdose after completing the experiment.
Preparation of AR sponges
Riclin was obtained according to previously established methodologies73. A riclin solution (2 wt.%) was mixed with NaIO4 at molar ratios of 1:1.25, 1:2.5, 1:3.75, and 1:5. The mixture was stirred for 2 h and dialyzed for 24 h (molecular weight range 500–1000 Da). The solution was then freeze-dried after cyclic freeze-thawing to obtain theoretical aldehyde-modified AR sponges with an aldehyde content of 25%, 50%, 75%, and 100%.
Chemical characterization
The chemical structure of AR was characterized using various techniques: 1H NMR (Bruker Avance III HD 400 MHz, Germany), FT-IR (Nicolet iS50, USA), UV-Vis (Evolution 220, USA), XPS (Thermo Scientific K-Alpha, USA), DLS (Malvern Zetasizer Nano ZS90, UK), XRD (Rigaku SmartLab SE, Japan) and GPC (PL-GPC220, USA) assays. The hydroxylamine hydrochloride titration method was used for the determination of the actual aldehyde content as previously described21.
Macro and micro physical characterization
The macro- and microstructures of the AR sponge were characterized using a digital camera (Nikon Z50) and a Zeiss Gemini 300 scanning electron microscope (Zeiss Gemini 300, Germany), respectively. The aperture distribution of the AR sponges was quantitatively assessed using Nano Measure (Version 1.2).
Mechanical test
Using a universal tensile machine (INSTRON 5982, USA), the cylindrical AR sponge (diameter = 20 mm, height = 10 mm) was mechanically compressed. During the dry compression assessment, the AR sponge was compressed to an 80% strain threshold at a consistent velocity of 5 mm per minute. For the cyclic compression-decompression assessment, a hydrous cylindrical AR sponge was vertically compressed at a velocity of 5 mm min−1 to strains of 40%, 60%, and 80%, and each compression cycle was performed thrice10,30,31.
Adhesion test
The tissue adhesion strength of the AR sponge was assessed using the lap-shear method on porcine skin with the fat layer completely removed. The AR sponge (3 mg, 25 mm × 30 mm) was placed on overlapping wet porcine skin samples and pressed with a 200 g weight for 10 min. The adhesion strength was measured at the point of separation using a universal tensile machine (CMT6103, USA) set at a consistent extension rate of 5 mm min−1 33,74.
Liquid absorption
To assess liquid absorption recovery, the dry AR sponge was compressed to approximately 80% of its maximum strain before immersion in NS or blood. This recovery process was meticulously documented using photographic methods. The liquid absorption ratio (LAR) was evaluated by initially weighing the dry AR sponge (W0, g); immersing it in blood, SBF, or NS for 60 s; and then reweighing it (W, g) following the removal of excess liquid using filter paper. LAR calculations for AR sponges in NS and blood were conducted at various intervals within a 60-s timeframe based on the following equation:
The speed of liquid absorption was determined by calculating the slope of the initial 5 s of the NS or blood absorptive capacity versus time curve10,32.
Blood samples for in vitro experiments
All of the fresh blood used in coagulation experiments was obtained from healthy human volunteers, in accordance with the Regulations of the People’s Republic of China on the Administration of Blood Products, Nuremberg Code, and Ethical Principles of Human Experimentation. Informed written consents were obtained from volunteers. Approval was granted by the Ethics Committee of Bengbu Medical College (BBMCEC2020025).
In vitro calcification clotting time
Ten milligrams of AR sponge were added to a test tube, followed by immediate mixing of 1 mL of blood (ACD-anticoagulant) and 80 µL of CaCl2 (0.25 M). The mixture was then shaken gently for 5 s. The test tube was tilted to the horizontal plane at an angle of 60° every 2–3 s until the liquid level stopped changing and the blood clotting time was determined.
Whole-blood clotting kinetics
A 25 mg sponge sample was positioned in the center of the petri dish and treated with a mixture of 250 µL of blood (ACD-anticoagulant) and 25 µL of CaCl2 (0.2 M) at 37 °C. This was followed by the addition of 20 mL of deionized water to ensure complete submersion at various periods (0, 15, 30, 60, 120, 180, and 240 s). Unbound blood cells were lysed by setting the shaker at 60 rpm for 10 min. The absorbance of the lysed cell solution (optical density [OD] experiment) was determined at 540 nm using a microplate reader (BioTek Epoch), with deionized water and the whole-blood mixture serving as the control (OD control). The BCI was then calculated according to the following equation36:
To further observe the process of the blood cells changing on the sponge, 20 µL of blood was applied to 2 mg of the AR50 sponge and washed thrice with PBS at specified time points. The sample was then fixed in 2.5% glutaraldehyde for 15 min, followed by a sequential dehydration process with alcohol solutions of increasing strengths (30%, 50%, 70%, 90%, 100%) for 5 min each time. After natural drying, the blood contact interface was observed using SEM, with spongy riclin serving as the control.
Thromboelastography (TEG) analysis
TEG was determined using the Thrombelastography Analyzer (CFMSLEPU-8800)24,37. Owing to the specificity of the TEG test, no kaolin activator was added, and the powdered sample was pre-made. Subsequently, 340 µL of blood (ACD-anticoagulant) was homogeneously mixed with 5 mg of the sample in the TEG test cup at 37 °C, followed by the addition of 20 µL of 0.25 M CaCl2. Finally, the sample cups were loaded for measurement.
PPP analysis
Plasma clotting kinetics were determined, as previously reported22,34. Specifically, in a 96-well plate, 60 µL AR (2 wt.%) was evenly mixed with 60 µL of PPP, followed by adding and mixing 60 µL of 0.25 M CaCl2. The absorbance of the plasma mixture was recorded at 405 nm every minute for 1 h, and a dynamic curve was drawn. The plasma procoagulant efficiency of each group was compared according to the trends of their kinetic curves and the slopes of their linear range.
Erythrocyte adhesion and morphology
The blood (ACD-anticoagulant) was centrifuged at 200 × g for 10 min at room temperature, the plasma was replaced with an equal volume of PBS, and subsequently, the erythrocytes were obtained; approximately 45 µL of these cells were added to 5 mg of the sponge and incubated in a 24-well plate at 37 °C for 15 min. Subsequently, the samples were washed thrice with PBS to effectively remove any non-adherent erythrocytes. The adhered erythrocytes were then lysed using 5 mL of deionized water, facilitating the quantification of hemoglobin through absorbance measurement at 540 nm. SEM was employed to examine the adhesion morphology of the erythrocytes on the sponge surface. This analysis was complemented by a pre-treatment protocol, consistent with the whole-blood test parameters.
Erythrocyte clotting kinetics and morphology
To further explore the strength of sponge erythrocyte adsorption, we slightly modified the protocol for whole-blood clotting kinetics. Briefly, 150 µL erythrocytes were dropped onto the sample at various periods (1, 4, 8, 12, 16, and 20 min), followed by the addition of 5 mL of deionized water and then transferred to a shaker at 60 rpm for 5 min. The absorbance of the hemoglobin solution was then recorded at 540 nm. SEM was used to observe changes in erythrocytes at the sponge contact interface pre-treatment, as described earlier. After the kinetic experiments were completed, the clotted mixture was immersed in 3 mL sterilized water for 24 h.
Platelet adhesion
Analysis of platelet adhesion was performed using an LDH assay. Initially, the blood treated with ACD-anticoagulant was centrifuged at 250 × g for 5 min to obtain platelet-rich plasma (PRP). The PRP underwent further centrifugation at 1000 × g for 5 min to separate the plasma, which was then replaced with an equivalent volume of PBS to acquire the platelet (PLT) suspension. The PLT concentration was adjusted to 1 × 108 mL−1 with PBS. Subsequently, 20 µL of the PLT suspension was applied to 3 mg of the sponge and incubated at 37 °C for 15, 30, and 60 min separately, followed by three washes with PBS. The platelets that adhered to the sponge were lysed in 1 mL of 1% Triton X-100 for 60 min. Finally, the platelet adhesion rate was measured using an LDH kit. Moreover, SEM observation of the platelet adhesion morphology on the sponge surface was conducted following the aforementioned pre-treatment protocol.
Ca2+ fluorescence intensity
Fifty microliters of the PLT suspension were incubated with 10 µL Fluo-4 AM, a calcium ion fluorescent probe, at room temperature for 15 min in the dark. Subsequently, 3 mg of sponge was incubated with the PLT suspension at room temperature for 15 min, followed by the addition of 200 µL of 1% Triton X-100 to lyse the platelets for 60 min. The mixture underwent centrifugation at 12,000 × g for 5 min and the supernatant was collected. Fluorescence intensity was measured with a spectral scanning multimode reader (Thermo Scientific Varioskan Flash, USA) at excitation and emission wavelengths of 494 and 516 nm, respectively22.
Flow cytometry analysis
The PLTs isolated from blood samples within 15 min of the collection were prepared at a concentration of 2 × 107 mL−1 after the removal of plasma. Subsequently, 500 µL of the PLT suspension was added to saturate 3 mg of spongy riclin and AR sponge. The incubation was conducted at room temperature for 25 min, and then the sponge was removed. After the PLTs were centrifuged at 1200 × g for 5 min, the supernatant was discarded to collect the platelets. The activated platelets were labeled using a CD41a/CD62p mixture and incubated in the dark at room temperature for 5 min. Finally, a flow cytometer (Cytoflex, China) was used to analyze the fluorescence expression distribution of the samples53,55.
Hemostasis of porcine hepatic laceration models
Hemostatic efficacy in hepatic lacerations was assessed in male pigs, rabbits, and rats. Following thorough cleaning and shaving, male pigs were intraperitoneally anesthetized using sodium pentobarbital (3 wt.%, 1 mL kg−1). A scalpel (No. 23 blade) was then used to create a cross incision approximately 3 cm in diameter and 1 cm deep in the largest lobe of the liver. Immediate application of an AR sponge (0.3 g) to the bleeding site was followed by compression for 5–10 s. The hemostasis time was determined by observing the cessation of blood oozing on the hemostatic agent and the halt of blood flow and dripping onto the filter paper10,56,57,75. The sponge was removed after 3–5 min of bleeding, and the blood loss was recorded. Except for the untreated control group, Surgicel (0.3 g), Chito SAM® (0.5 g), and medical gauze (1 g) were used for treatment. The hemostatic situation was recorded using a camera. The injury site after bleeding was preserved in 4% paraformaldehyde (PBS, pH = 7.4), and tissue sections were stained with Masson dye for observation.
Hemostasis of porcine hepatic perforation models
Male pigs were fully anesthetized, as described above, and the large lobe of the liver was exposed. Subsequently, a 2 cm-diameter round perforated wound was introduced on the large lobe, which was immediately covered with an AR sponge (0.3 g), Surgicel (0.3 g), Chito SAM (0.5 g), and medical gauze (1 g) and pressed for 5–10 s. The hemostasis time and blood loss were determined as described earlier.
Hemostasis of porcine artery scission models
Following complete anesthesia, the deep femoral arteries of male pigs were surgically exposed and incised, allowing natural bleeding to occur for approximately 10 seconds. Subsequent to the bleeding phase, the wound site was cleansed with gauze and promptly subjected to manual compression using an AR sponge (1 g). The hemostasis time was recorded when no further bleeding was observed during this manual pressure. Upon complete hemostasis, the sponge was removed and the amount of blood loss was documented. For comparative efficacy analysis, additional hemostatic agents, including medical gauze (8 g), QCG (2 g), Chito SAM (2 g), and Surgicel (1 g), were applied for treatment in a similar manner. The entire hemostatic procedure for each agent was visually documented using photographic equipment. Additionally, for specific samples within the sponge treatment group, the sponge was retrieved after 1 min of treatment (following a manual 1 min sponge compression) and its contact area was examined using SEM.
Hemostasis of rabbit hepatic laceration model
Male rabbits were completely anesthetized with sodium pentobarbital (3 wt.%, 1 ml·kg-1) by ear marginal vein injection. Next, the largest lobe of the liver was exposed and made a cross incision (2 cm diameter and 1 cm depth). Then, the injury site was covered with Gauze (1 g), Gelatin sponge (0.3 g), Celox (0.5 g), Surgicel (0.3 g) and AR sponge (0.2 g), respectively, the untreated group as a control. Both hemostasis time and blood loss were recorded. The hemostatic process was recorded by camera. After hemostasis, the cross-liver injury site was preserved into 4% paraformaldehyde and performed pathological observation.
Hemostasis of rat hepatic laceration model
Male SD rats were completely anesthetized with sodium pentobarbital (3 wt.%, 40 mg·kg-1) by intraperitoneal injection. Then, the largest lobe of the liver was exposed and cut the cross incision (1.5 cm diameter and 0.5 cm depth), the wound was immediately covered with an AR sponge (60 mg). The hemostasis and blood loss were noted. The hemostatic process was recorded by camera. Except for the untreated control group, the cross incision was performed as above with Gauze (500 mg), Gelatin sponge (150 mg), Celox (200 mg), Surgicel (100 mg) treatment. After hemostasis, the cross-liver injury site was preserved into 4% paraformaldehyde and performed pathological observation.
Hemostasis of rat artery scission model
Male SD rats were fully anesthetized as above and exposed the deep femoral artery. Then, the femoral artery was cut and was immediately covered with an AR sponge (60 mg). And other as above with Gauze (500 mg), Gelatin sponge (150 mg), Celox (300 mg), Surgicel (100 mg) treatment. The hemostasis time and blood loss were noted.
Hemostasis in the murine tail amputation model
Male mice were fully anesthetized and approximately two-thirds of the tail length was surgically amputated. The immediate post-amputation intervention included the application of an AR sponge (20 mg) directly to the amputation site. For comparative analysis, additional groups were treated with different hemostatic agents: gauze (30 mg), Celox (30 mg), and gelatin sponge (25 mg). The hemostasis time and blood loss were recorded.
Bacteriostatic activity analysis
The bacteriostatic efficacy of the AR sponge against E. coli (ATCC25404), S. aureus (ATCC27217), and P. aeruginosa (ATCC27853) was evaluated using bacteriostasis experiments. Briefly, 100 μL of bacterial suspensions (1 × 107 colony-forming units [CFU]·mL−1) were evenly spread on Luria–Bertani (LB) agar medium. Subsequently, the cylindrical AR sponge (bottom diameter = 1 cm, ~20 mg) was centrally placed on the plate and incubated at 37 °C for 16 h. The inhibitory zone formed was measured using a Vernier caliper, specifically from the edge of the inhibition source to the nearest bacterial colony. All samples were irradiated with UV for 1 h before testing.
Bacterial growth kinetics
Bacterial suspensions adjusted to 1 × 106 CFU·mL−1 were cultured with the hemostat samples at 37 °C for 12 h (sample/bacterial suspension = 5 mg·mL−1). Absorbance data of the bacterial suspension were recorded at 600 nm at 2-hour intervals, tracking proliferation curves over 12 h. After 12 h, the treated bacterial suspensions were diluted 1000 times and 100 µL of the suspension was spread on the plate. Following a 24-hour culture at 37 °C, the concentration of the 12-hour sample-treated bacterial suspension was recorded.
Live/dead bacteria analysis
The bactericidal capacity of the AR50 sponge against E. coli, S. aureus, and P. aeruginosa was evaluated using LIVE/DEAD™ BacLight™ Bacterial Viability Kit71. Specifically, NS bacterial dilutions (4 × 107 CFU mL−1) were treated with the AR50 sponge (sponge/bacterial suspension = 5 mg·mL−1) for 2 h. Meanwhile, a mixture of SYTO 9 and PI, in equal volumes, was prepared in a microcentrifuge tube. After removing the sponge, each milliliter of the treated bacterial suspension was uniformly mixed with 1 µL of the dye mixture, followed by a 10 min incubation in the dark at room temperature. Subsequently, a slide specimen was prepared from 5 µL of the stained bacterial suspension and photographed with a semi-automated fluorescence microscope (Olympus BX53). Image J 6.4 was used to analyze and combine the photographs; the imaging settings were maintained constant within the same experimental batch.
Morphology analysis
Bacterial morphology (E. coli, S. aureus, and P. aeruginosa) was evaluated using both SEM (Hitachi Regulus 8100, Japan) and TEM (Hitachi-7800)71. A 50 mg AR sponge treated with 10 mL of bacterial suspension (4 × 108 CFU mL−1) was incubated in a shaker at 37 °C and 200 rpm for 12 h, followed by centrifugation to collect the bacteria. For SEM, the treated bacteria were fixed in glutaraldehyde (2.5 wt.%) for 2 h at 4 °C, washed with PBS, and dehydrated before further observation by SEM. For TEM, the treated bacteria were fixed with glutaraldehyde (2.5%) for 6 h at 4, washed with PBS, post-fixed in 1% osmic acid for 1 h at 4 °C, and processed according to the SEM steps described earlier. Finally, the bacteria were embedded and sliced into ultrathin sections (70–90 nm) for TEM observation.
In vitro degradation
At room temperature (22–25 °C), 50 mg cylindrical spongy riclin, AR sponges, and biodegradable gelatin sponge were soaked in 50 mL SBF (pH = 7.4) for 24 h and the degradation process was monitored. Next, 25 mg of different hemostats were soaked in 10 mL DPBS (pH = 7.4) at 37 °C for 24 h. The degradation time was recorded and photographs were captured at various periods (0 min, 15 min, 30 min, 1 h, and 24 h). After 24 h incubation, the absorbance of the treatment solution was recorded at 600 nm, using TCP as a control. Additionally, 50 mg of the AR50 cylindrical sponge was submerged in 50 mL of SBF at varying pH (pH 5–10) at 25 °C and 37 °C, and the degradation time was recorded.
In vivo absorption
Male Sprague–Dawley rats (250 g) were selected and completely anesthetized with an intraperitoneal injection of sodium pentobarbital (3 wt.%, 40 mg·kg−1). A 2 cm incision was made along the spine after disinfection. The AR50 sponge (20 mg) was implanted subcutaneously and the incision was sutured. The blank surgery group was used as a control. The rats were sacrificed after various periods (2, 5, 10, 15, 20, and 25 days) under excessive anesthesia, and the residual sponge was taken out and freeze-dried for weighing. The surrounding skin and major organs (liver, spleen, lung, heart, and kidney) were collected for H&E staining.
Hemolysis rate test
Hemostats (10 mg, 20 mg, and 30 mg) were incubated with 4 mL of the erythrocyte suspension (2 wt.%) for 1 h at 37 °C. Subsequently, a 1 mL volume of the suspension underwent a 10 min centrifugation at 1200 × g and was then photographed within an Eppendorf tube. The absorbance (ODsample) of the hemoglobin solution was recorded at 540 nm. Concurrently, PBS was introduced as the negative control (OD negative) and 2% Triton X-100 was used as the positive control (OD positive). The hemolysis ratio was determined using the following equation33:
Rabbit skin irritation test
Multiple topical applications of the AR solution (2 wt.%) were administered to assess skin irritation. The dorsal skin was shaved, exposed (5 × 5 cm), and sterilized using UV light before application. A 1 mL solution of the materials was applied to the exposed skin three times a day at 2 h intervals. The condition of the sensitized skin was continuously observed and histopathologically examined after 3 days.
Cytotoxicity and cellular ROS assay
NIH3T3 cells were incubated with PBS and AR50 (100, 200, 400, 800 µg) for 24 h, respectively. The cells were fixed and stained using Actin-Tracker Green and 4ʹ,6-diamidino-2-phenylindole (DAPI). The antioxidant effect of riclin with different degrees of oxidation was evaluated using ROS probes and ROS detection kits. NIH3T3 cells were treated as described above. The extracellular ROS scavenging of different groups was initially detected using DCFH-DA. The intracellular ROS scavenging ability of AR was then measured with an ROS assay kit. The images of samples were obtained using a Zeiss LSM880 confocal laser-scanning microscope, with constant settings maintained within the same experimental batch.
Statistical analysis
Data from at least three samples per experiment are expressed as means ± standard deviation; statistical significance was set at P < 0.05. Statistical analyses were performed using one-way or two-way analysis of variance and Tukey’s post-hoc test, using GraphPad Prism 6.0 and Origin Lab 8.0 software.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The data supporting the results of this study are available from the corresponding author on reasonable request.
References
Fisher, A. D., Bulger, E. M. & Gestring, M. L. Stop the bleeding: educating the public. JAMA 320, 589–590 (2018).
Rossaint, R. et al. The STOP the Bleeding Campaign. Crit. Care. 17, 136 (2013).
Hickman, D. S. A., Pawlowski, C. L., Sekhon, U. D. S., Marks, J. & Gupta, A. S. Biomaterials and advanced technologies for hemostatic management of bleeding. Adv. Mater. 30, 1700859 (2018).
Li, X. et al. Emerging materials for hemostasis. Coord. Chem. Rev. 475, 214823 (2023).
Nepal, A., Tran, H., Nguyen, N. T. & Thu Ta, H. Advances in haemostatic sponges: Characteristics and the underlying mechanisms for rapid haemostasis. Bioact. Mater. 27, 231–256 (2023).
Simpson, A., Shukla, A. & Brown, A. C. Biomaterials for hemostasis. Annu. Rev. Biomed. Eng. 24, 111–135 (2022).
Yang, X. et al. Design and development of polysaccharide hemostatic materials and their hemostatic mechanism. Biomater. Sci. 5, 2357–2368 (2017).
Chen, Y. et al. Polysaccharide based hemostatic strategy for ultrarapid hemostasis. Macromol. Biosci. 20, e1900370 (2020).
Guo, B., Dong, R., Liang, Y. & Li, M. Haemostatic materials for wound healing applications. Nat. Rev. Chem. 5, 773–791 (2021).
Du, X. et al. Microchannelled alkylated chitosan sponge to treat noncompressible hemorrhages and facilitate wound healing. Nat. Commun. 12, 4733 (2021).
Liu, F., Liu, X., Chen, F. & Fu, Q. Mussel-inspired chemistry: A promising strategy for natural polysaccharides in biomedical applications. Prog. Polym. Sci. 123, 101472 (2021).
Sun, X. et al. Diatom-inspired bionic hydrophilic polysaccharide adhesive for rapid sealing hemostasis. Acs. Nano. 17, 19121–19135 (2023).
Jiao, S. et al. Recent advances in biomimetic hemostatic materials. Mater. Today Bio. 19, 100592 (2023).
Zhao, X. et al. Antibacterial anti-oxidative electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 122, 34–47 (2017).
Teng, L. et al. Biomimetic glycopolypeptide hydrogels with tunable adhesion and microporous structure for fast hemostasis and highly efficient wound healing. Adv. Funct. Mater. 31, 2105628 (2021).
Pan, G. et al. Mussel- and barnacle cement proteins-inspired dual-bionic bioadhesive with repeatable wet-tissue adhesion, multimodal self-healing, and antibacterial capability for nonpressing hemostasis and promoted wound healing. Adv. Funct. Mater. 32, 2200908 (2022).
Yang, Y. et al. Anti-tumor activity and immunogenicity of a succinoglycan riclin. Carbohydr. Polym. 255, 117370 (2021).
Ding, Z. et al. Dietary succinoglycan riclin improves glycemia control in mice with type 2 diabetes. J. Agric. Food Chem. 70, 1819–1829 (2022).
Cheng, R. et al. In vitro and in vivo anti-inflammatory activity of a succinoglycan riclin from Agrobacterium sp. ZCC3656. J. Appl. Microbiol. 127, 1716–1726 (2019).
Kristiansen, K. A., Potthast, A. & Christensen, B. E. Periodate oxidation of polysaccharides for modification of chemical and physical properties. Carbohydr. Res. 345, 1264–1271 (2010).
Zhao, H. & Heindel, N. D. Determination of degree of substitution of formyl groups in polyaldehyde dextran by the hydroxylamine hydrochloride method. Pharm. Res. 8, 400–402 (1991).
Liu, C. et al. A highly efficient, in situ wet-adhesive dextran derivative sponge for rapid hemostasis. Biomaterials 205, 23–37 (2019).
Hu, Y. et al. Multifunctional oxidized succinoglycan/poly) N-isopropylacrylamide-co-acrylamide) hydrogels for drug delivery. Polymers. 15, 122 (2022).
Sanz-Horta, R. et al. Preparation and characterization of plasma-derived fibrin hydrogels modified by alginate di-aldehyde. Int. J. Mol. Sci. 23, 4296 (2022).
Zheng, W. et al. A novel pullulan oxidation approach to preparing a shape memory sponge with rapid reaction capability for massive hemorrhage. Chem. Eng. J. 447, 137482 (2022).
Mullay, J. Atomic and group electronegativities. J. Am. Chem. Soc. 106, 5842–5847 (1984).
Ding, W., Wang, Y. N., Zhou, J. & Shi, B. Effect of structure features of polysaccharides on properties of dialdehyde polysaccharide tanning agent. Carbohydr. Polym. 201, 549–556 (2018).
Legay, L., Budtova, T. & Buwalda, S. Hyaluronic Acid Aerogels Made Via Freeze–Thaw-Induced Gelation. Biomacromolecules 24, 4502–4509 (2023).
Corte, A. E. Particle sorting by repeated freezing and thawing. Science 142, 499–501 (1963).
Li, P. et al. Polyvinyl alcohol/sodium alginate composite sponge with 3D ordered/disordered porous structure for rapidly controlling noncompressible hemorrhage. Biomater. Adv. 134, 112698 (2022).
Wang, X. et al. Antibacterial porous sponge fabricated with capric acid-grafted chitosan and oxidized dextran as a novel hemostatic dressing. Carbohydr. Polym. 277, 118782 (2022).
Xiao, Y. et al. Peptide-immobilized starch/PEG sponge with rapid shape recovery and dual-function for both uncontrolled and noncompressible hemorrhage. Acta Biomater. 99, 220–235 (2019).
Wang, H. et al. A super tough, rapidly biodegradable, ultrafast hemostatic bioglue. Adv. Mater. 35, e2208622 (2023).
Guo, Y. et al. Snake extract-laden hemostatic bioadhesive gel cross-linked by visible light. Sci. Adv. 7, eabf9635 (2021).
Artzi, N., Shazly, T., Baker, A. B., Bon, A. & Edelman, E. R. Aldehyde-amine chemistry enables modulated biosealants with tissue-specific adhesion. Adv. Mater. 21, 3399–3403 (2009).
Mahmoodzadeh, A. et al. Biodegradable cellulose-based superabsorbent as potent hemostatic agent. Chem. Eng. J. 418, 129252 (2021).
Cui, Y. et al. Robust hemostatic bandages based on nanoclay electrospun membranes. Nat. Commun. 12, 5922 (2021).
Kalathottukaren, M. T. et al. A universal heparin antidote with negligible effect on fibrin(ogen) and plasma coagulation. Blood 124, 4231 (2014).
Mosesson, M. Fibrinogen and fibrin structure and functions. J. Thromb. Haemost. 3, 1894–1904 (2005).
Li, Z. et al. Superhydrophobic hemostatic nanofiber composites for fast clotting and minimal adhesion. Nat. commun. 10, 5562 (2019).
Cines, D. B. et al. Clot contraction: compression of erythrocytes into tightly packed polyhedral and redistribution of platelets and fibrin. Blood 123, 1596–1603 (2014).
Sharma, J. B., Gupta, N. & Mittal, S. Creation of neovagina using oxidized cellulose (surgical) as a surgical treatment of vaginal agenesis. Arch. Gynecol. Obstet. 275, 231–235 (2007).
White, J. G., Burris, S. M. & Escolar, G. Platelet adhesion plaques: anchors for contraction of the hemostatic plug. Blood 114, 5128 (2009).
Cate, J. W. T. & De Vries, S. I. Platelet adhesiveness and haemostatic defects. Lancet 296, P1196 (1970).
Akkerman, J. W. & Holmsen, H. Interrelationships among platelet responses: studies on the burst in proton liberation, lactate production, and oxygen uptake during platelet aggregation and Ca2+ secretion. Blood 57, 956–966 (1981).
Wong, C. S. et al. Adhesion protein GMPl40 inhibits superoxiderelease by human neut rophils. PNAS 88, 2397–2401 (1991).
Cheng, H. et al. Chitin/corn stalk pith sponge stimulated hemostasis with erythrocyte absorption, platelet activation, and Ca2+-binding capabilities. Carbohydr. Polym. 284, 118953 (2022).
Menter, D. G. et al. Platelet “first responders” in wound response, cancer, and metastasis. Cancer Metastasis. Rev. 36, 199–213 (2017).
Bouchareb, R. et al. Activated platelets promote an osteogenic programme and the progression of calcific aortic valve stenosis. Eur. Heart J. 40, 1362–1373 (2019).
Danaee, A. et al. Patients with splanchnic vein thrombosis demonstrate significantly increased platelet activity. Blood 128, 1430 (2016).
Liu, J. et al. Abnormal platelet phenotypes in patients with myelodysplastic syndromes. Blood 134, 5408 (2019).
Maher, K. N., Han, X., Neeves, K. B., Nieman, M. T. & Di Paola, J. Modulation of thrombin-induced platelet activation by defibrotide. Blood. 134, 3614 (2019).
Sturm, A., Hebestreit, H. & Grossmann, R. Platelet proinflammatory and hemostatic function is differentially regulated in cystic fibrosis. Blood 108, 3948 (2006).
Panteleev, M. A., Ananyeva, N. M., Greco, N. J., Ataullakhanov, F. I. & Saenko, E. L. Two subpopulations of thrombin-activated platelets differ in their binding of the components of the intrinsic factor X-activating complex. J. Thromb. Haemost. 3, 2545–2553 (2005).
Feng, Y. et al. Assembly of clay nanotubes on cotton fibers mediated by biopolymer for robust and high-performance hemostatic dressing. Adv. Healthc. Mater. 12, e2202265 (2023).
Leonhardt, E. E., Kang, N., Hamad, M. A., Wooley, K. L. & Elsabahy, M. Absorbable hemostatic hydrogels comprising composites of sacrificial templates and honeycomb-like nanofibrous mats of chitosan. Nat. Commun. 10, 2307 (2019).
He, H. et al. Efficient, biosafe and tissue adhesive hemostatic cotton gauze with controlled balance of hydrophilicity and hydrophobicity. Nat. Commun. 13, 552 (2022).
Tan, L. et al. Dynamic hydrogel with environment-adaptive autonomous wound-compressing ability enables rapid hemostasis and inflammation amelioration for hemorrhagic wound healing. Nano. Today 52, 101962 (2023).
Wei, X. et al. Polysaccharides-modified chitosan as improved and rapid hemostasis foam sponges. Carbohydr. Polym. 264, 118028 (2021).
Zgurskaya, H. I. Introduction: transporters, porins, and efflux pumps. Chem. Rev. 121, 5095–5097 (2021).
Aziz, M. A., Cabral, J. D., Brooks, H. J. L., Moratti, S. C. & Hanton, L. R. Antimicrobial properties of a chitosan dextran-based hydrogel for surgical use. Antimicrob. Agents Chemother. 56, 280–287 (2012).
Simons, C., Walsh, S. E., Maillard, J. Y. & Russell, A. D. A note: ortho-phthalaldehyde: proposed mechanism of action of a new antimicrobial agent. Appl. Microbiol. 31, 299–302 (2000).
Pang, Z., Raudonis, R., Glick, B. R., Lin, T. J. & Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 37, 177–192 (2019).
Bremer, E. & Krämer, R. Responses of microorganisms to osmotic stress. Annu. Rev. Microbiol. 73, 313–334 (2019).
Blount, P., Schroeder, M. J. & Kung, C. Mutations in a bacterial mechanosensitive channel change the cellular response to osmotic stress. J. Biol. Chem. 272, 32150–32157 (1997).
Bisset, K. A. & Vickerstaff, J. Significance of the characteristic chemical pattern of Gram positive and Gram negative bacterial cell walls. Nature 215, 1286 (1967).
Qi, G., Zhang, D., Liu, F. H., Qiao, Z. Y. & Wang, H. An “on-site transformation” strategy for treatment of bacterial infection. Adv. Mater. 29, 1703461 (2017).
Chu, X. et al. Brush polymer coatings with hydrophilic main-chains for improving surface antibacterial properties. Acs. Macro. Lett. 12, 428–432 (2023).
Sut, T. N. et al. Engineered lipid bicelle nanostructures for membrane-disruptive antibacterial applications. Appl. Mater. Today 22, 100947 (2021).
Dalheim, M. Ø. et al. Degradation kinetics of peptide-coupled alginates prepared via the periodate oxidation reductive amination route. Carbohydr. Polym. 157, 1844–1852 (2017).
Qiao, Z. et al. Biomimetic electrodynamic nanoparticles comprising ginger-derived extracellular vesicles for synergistic anti-infective therapy. Nat. Commun. 13, 7164 (2022).
Ito, T., Yeo, Y., Highley, C. B., Bellas, E. & Kohane, D. S. Dextran-based in situ cross-linked injectable hydrogels to prevent peritoneal adhesions. Biomaterials 28, 3418–3426 (2007).
Miao, Y. et al. Exopolysaccharide riclin and anthocyanin-based composite colorimetric indicator film for food freshness monitoring. Carbohydr. Polym. 314, 120882 (2023).
Deng, T. et al. A natural biological adhesive from snail mucus for wound repair. Nat. Commun. 14, 396 (2023).
Bao, G. et al. Liquid-infused microstructured bioadhesives halt non-compressible hemorrhage. Nat. Commun. 13, 5035 (2022).
Acknowledgements
We extend our gratitude to Dr. Jianfa Zhang for providing the riclin exopolysaccharides. This work received primary support from a research project of Anhui Provincial Key Research and Development Project (No. 202104j07020017), Natural Science Research Project of Anhui Educational Committee (No. 2022AH030139, No. 2022AH051498, No. 2023AH051923), Natural Science Foundation of Anhui Province (No. 2308085QH251), and National Natural Science Foundation of China (No. 32000822).
Author information
Authors and Affiliations
Contributions
J.Z. (Jinrun Zhang), Q.S., and J.L. conceived the project, designed the experiments, and wrote the paper; J.Z. (Jinrun Zhang), Z.C., and D.Z. conducted the synthesis and characterization of the samples; Z.C., X.Z., and N.L. obtained scanning electron microscopy and transmission electron microscopy images; J.Z. (Jinrun Zhang), J.L., Y.X., and Y.F. carried out all the in vitro and in vivo experiments; D.Z., Y.X., and J.Z. (Jinrun Zhang) performed the antibacterial experiments; Q.S. and J.Z. (Jianfa Zhang) revised the draft; Y.F., X.L., X.S., S.Z., and J.Z. (Jianfa Zhang) analyzed the data and contributed to interpretation of the results. All authors discuss and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Materials thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Steven Caliari and Jet-Sing Lee. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Chen, Z., Zeng, D. et al. Antibacterial and rapidly absorbable hemostatic sponge by aldehyde modification of natural polysaccharide. Commun Mater 5, 129 (2024). https://doi.org/10.1038/s43246-024-00579-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43246-024-00579-0
- Springer Nature Limited