Abstract
The emergence and spread of bacteria resistant to commonly used antibiotics poses a critical threat to modern medical practice. Multiple classes of bacterial efflux pump systems play various roles in antibiotic resistance, and members of the resistance-nodulation-division (RND) transporter superfamily are among the most important determinants of efflux-mediated resistance in gram-negative bacteria. RND pumps demonstrate broad substrate specificities, facilitating extrusion of multiple chemical classes of antibiotics from the bacterial cell. Several newer beta-lactams and beta-lactam/beta-lactamase inhibitor combinations (BL/BLI) have been developed to treat infections caused by multidrug resistant bacteria. Here we review recent studies that suggest RND efflux pumps in clinically relevant gram-negative bacteria may play critical but underappreciated roles in the development of resistance to beta-lactams and novel BL/BLI combinations. Improved understanding of the genetic and structural basis of RND efflux pump-mediated resistance may identify new antibiotic targets as well as strategies to minimize the emergence of resistance.
Similar content being viewed by others
Introduction
The development of antibiotics is a key achievement of modern medicine. In addition to their direct therapeutic use in the management of serious bacterial infections, antibiotics have made advances in chemotherapy, transplantation, and surgery possible, which depend on the ability to control secondary bacterial infections that can occur when immune defenses are compromised. The clinical use of antibiotics, however, can generate selection pressures on the underlying treated bacterial populations that drive the evolution of resistance through a variety of mechanisms1. The emergence of antimicrobial resistance (AMR) within bacterial populations has resulted in the broad loss of efficacy of many commonly used antibiotics, and the spread of highly drug resistant bacteria poses a critical threat to public health.
Gram-negative bacteria such as Pseudomonas aeruginosa, Acinetobacter baumannii-calcoaceticus, Klebsiella pneumoniae, and other members of the order Enterobacterales (see Box 1 for a glossary explaining the technical terms used) can cause serious infections that result in significant morbidity and mortality. One of the most successful classes of antibiotics used to treat infections caused by gram-negative bacteria are the beta lactams, which act by inhibiting the DD-transpeptidase enzymes that cross-link the cell wall that is essential for viability. Thus, resistance to beta lactam antibiotics in gram-negative bacteria is of critical importance. Beta-lactam resistance in gram-negative bacteria, in turn, is generally driven by a combination of four major mechanisms: drug hydrolysis by beta-lactamases; alteration of target sites in the penicillin-binding proteins (DD-transpeptidases); alteration of drug permeability, primarily through the modification of outer membrane porin channels; and extrusion from the cell through the activity of efflux pumps2,3 (Box 2).
Over the past decade, several new beta-lactams and beta-lactam/beta-lactamase inhibitor combinations (hereafter described as novel BL/BLI) have been developed to meet the increasing threat of carbapenemase-producing organisms (CPO)4,5,6. Carbapenemases are beta lactamases that exhibit activity against carbapenems and most other beta lactams, rendering the bacteria that produce these enzymes resistant to these agents. The addition of broad spectrum carbapenemase inhibitors in novel BL/BLI combinations can restore susceptibility to the included beta-lactam, and thus these combination antibiotics are often used as a treatment for CPO infections7. These agents have also shown efficacy against other difficult to treat pathogens, such as P. aeruginosa, Stenotrophomonas maltophilia and carbapenem-resistant A. baumannii-calcoaceticus complex (CRAB) that become resistant to carbapenems through mechanisms other than acquired carbapenemases8,9. Unsurprisingly, in the years following the introduction of these new antibiotics into clinical practice, resistant bacteria have been increasingly reported. Multiple studies have described genomic changes associated with this resistance10,11,12,13,14,15,16,17,18,19,20,21, and a smaller subset have provided experimental validation of the effects of specific mutations22,23,24,25,26,27 (Box 3). Many of the observed genetic changes involve RND efflux pumps, some of which are currently under-characterized, but likely play important roles in Bl/BLI resistance. Unfortunately, there are currently no standard approaches for specific molecular or functional testing for efflux-mediated resistance in clinical microbiology laboratories, and while efflux pump inhibitors are under development, none are currently available for clinical use.
In this Perspective, we briefly introduce the structure and role of RND efflux pumps in bacteria. We then review recent work that highlights the importance of RND efflux pumps in resistance to novel BL/BLI in clinically relevant gram-negative bacteria. The novel BL/BLI combinations currently used, or in clinical development that will be covered, as well as the year of FDA approval, are ceftazidime/avibactam (CZA, 2015), ceftolozane/tazobactam (C/T, 2014), meropenem/vaborbactam (MEM/VAB, 2017), imipenem/relebactam (IMI/REL, 2019), cefepime/zidebactam (FEP/ZID, not approved, but available under compassionate use since 2021) and sulbactam/durlobactam (SUL/DUR, 2023). We will additionally cover cefiderocol (FDR, 2019), a new siderophore cephalosporin and last-line agent in clinical use.
RND efflux pumps and their regulators are commonly mutated in Gram-negative bacteria with AMR
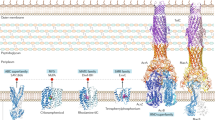
RND efflux pumps play essential roles in bacterial physiology, facilitating the extrusion of a wide range of metabolic and organic products, including molecules involved in quorum sensing28,29, the production of biofilms30, and pathogenesis and virulence31,32. These pumps are organized as large tripartite complexes that span the gram-negative envelope from inner to outer membrane, usually consisting of homomeric assemblies of three different components: an inner membrane pump (IMP), membrane fusion protein (MFP) and an outer membrane porin (OMP)33 (Fig. 1a). While most characterized RND pumps can transport a wide variety of chemically diverse substrates, individual pumps have very different substrate preferences, which have clinical consequences for antibiotic resistance34,35,36,37,38. For example, in P. aeruginosa, the MexAB-OprM efflux pump is able to transport beta-lactams, fluoroquinolones, and sulfonamides, in addition to a highly diverse set of metabolites, while the MexXY-OprM pump preferentially transports aminoglycosides and tetracyclines39. Mutations in the efflux pump regulatory systems leading to pump overexpression are among the most common efflux-related mechanisms for drug resistance described40. Meanwhile, amino acid substitutions in known structural binding sites in the IMP subunits of RND efflux pumps appear to have the greatest impact on substrate specificity36,37,41.
a Structure of the complete MexAB-OprM complex from P. aeruginosa (rendered from PDB Accession 6IOL using UCSF Chimera). OprM is the outer membrane porin in the complex and is a homotrimer. MexA is the membrane fusion protein and is a homohexamer. MexB is the inner membrane pump and is a homotrimer. b MexB trimer viewed from the intracellular face with residues associated with antibiotic resistance from Table 1 shown in spacefill representation. Colors are defined at end. c MexB trimer viewed parallel to inner membrane. d MexB monomer with individual residues corresponding to Table 1 labeled. Colors indicate residues associated mutations conferring resistance to the following agents: Purple = CZA; Dark blue = both CZA and FDR; Pink = IMI/REL; Orange = both IMI/REL and FEP/ZID; Light blue/gray = FEP/ZID.
BL/BLI resistance in P. aeruginosa is mediated by multiple chromosomal RND Efflux Pumps
P. aeruginosa is one of the most important human pathogens causing infections in hospital settings and in immunocompromised hosts42. Unique characteristics of this organism include its highly impermeable envelope relative to Enterobacterales43,44, and the presence of a large number of chromosomal resistance genes, including at least 12 distinct RND-type efflux systems45. This allows it to acquire resistance to all classes of currently used antibacterial agents through chromosomal mutations. The RND efflux pumps that are mutated display broad substrate specificities demonstrating significant overlap, as has been reviewed in detail elsewhere36,46,47,48. The major efflux pump, MexAB-OprM, plays a critical role in the development of clinical resistance against penicillins, cephalosporins, monobactams, meropenem, fluoroquinolones, macrolides, and sulfonamides, and the consequences of amino acid substitutions in MexB have been the subject of extensive study37,48,49,50. Additional notable pumps known to contribute to clinical resistance include MexCD-OprJ, involved in resistance to cefepime, macrolides, and fluoroquinolones46,51; and MexXY-OprM, which contributes to aminoglycoside, tetracycline, macrolide, and fluoroquinolone resistance46. Other less well-described pumps in the context of antibiotic resistance include MexEF-OprN, MexGHI-OpmD, MexMN-OprM, MexPQ-OpmE, MexVW-OprM, MuxABC-OpmB and TriABC-OpmH.
The novel BL and BL/BLI alternatives currently in use or development to treat carbapenem-resistant P. aeruginosa include CZA, C/T, IMI/REL, FEP/ZID, and FDR. The most common mechanisms of CZA and C/T resistance in P. aeruginosa are mutations in the chromosomal AmpC cephalosporinase (PDC), often located in the omega loop region of the enzyme52,53, or increased expression of MexAB-OprM12,54,55. Other efflux pumps are likely to be contributing as well. For example, in an in vitro experimental evolution system, it was found that in increased expression of the MexVW efflux pump due to an intergenic mutation upstream of mexV, in addition to a MexW E36K substitution, can contribute to resistance both to CZA and C/T, and to cefepime (FEP)54. These mutations spontaneously emerged in a DNA mismatch repair-deficient hypermutator background with a non-functional MexAB-OprM, and their role in conferring a 4- to 6-fold increase in minimum inhibitory concentration (MIC) to CZA, C/T and FEP was confirmed through genetic engineering in a wild type background. Of note, MexVW is more phylogenetically distant than the other major P. aeruginosa RND efflux pumps37,41, and while its ability to confer resistance to fluoroquinolones, tetracycline, chloramphenicol, erythromycin, and cefpirome in vitro has been previously demonstrated56, little attention has been paid to this pump in the context of clinical antibiotic resistance, particularly to novel BL and BL/BLI during the past decade57.
A variety of genomic variants were observed to emerge in P. aeruginosa laboratory strains in a study involving serial passaging with ceftazidime (CAZ) or CZA23. In addition to mutations occurring in relation to MexAB-OprM that were common to both antibiotic exposures, variants observed only in the CZA group included mutations associated with the MexMN-OprM efflux pump and its upstream regulators that emerged in independent lineages (Table 1). MexMN-OprM may play a role in susceptibility to diverse classes of antibiotics58, and increased expression of MexMN can contribute to resistance to imipenem and to the D13-9001 efflux pump inhibitor59. However, establishing the precise role of MexMN-OprM in resistance to new antibiotics requires further study including testing the effects of individual mutations in appropriate genetic backgrounds.
The potent antipseudomonal activity of C/T is thought to result largely from its ability to accumulate in the periplasmic space because ceftolozane is a poor substrate for MexAB-OprM. Thus, while multidrug-resistant P. aeruginosa that have been studied commonly overexpress this major RND efflux pump12,60, C/T is minimally affected by this mechanism. Mutational C/T resistance in P. aeruginosa has largely been described to emerge from substitutions in the PDC beta-lactamase61. Nonetheless, evidence is emerging demonstrating that mutations and/or overexpression of other efflux pumps can contribute to C/T resistance. For instance, a clinical isolate was described with disruptions in NfxB, a repressor of MexCD-OprJ; MexD S133G, which forms part of the serine loop; and MexD Q178R, predicted to be immediately adjacent to a short loop lining the pump’s distal binding pocket25. The role of MexCD-OprJ in C/T and CZA resistance was confirmed experimentally25. As noted above, substitutions resulting in increased expression and modifications of MexVW have also been demonstrated experimentally to result in increases in C/T MIC54.
IMI/REL is another potent agent for the treatment of carbapenem-resistant P. aeruginosa. A major mechanism of resistance to carbapenems in P. aeruginosa is disruption of the major outer membrane porin OprD44. Increased efflux through the MexAB-OprM efflux pump, particularly when overexpressed, plays an additional role in MEM and MEM/VAB resistance, while IMI is less affected by MexAB-OprM efflux, as it is a worse substrate of this major efflux pump. Nonetheless, IMI can still be hydrolyzed to some extent by the P. aeruginosa PDC62. This has been suggested to explain why the addition of REL to IMI restores susceptibility in about 80% of carbapenem-resistant P. aeruginosa62, while the combination of VAB and MEM does not appear to perform significantly better than MEM alone in a collection of similar clinical isolates63.
A recent study has provided insight into how IMI/REL resistance develops in P. aeruginosa. Serial whole genome sequencing of clinical P. aeruginosa isolates that developed increased IMI/REL MIC from five patients with ventilator-associated pneumonia treated with IMI/REL for 10-28 days found most isolates harbored disrupted OprD porins at baseline19. A pattern of emerging mutations in MexB, MexR (a major MexAB-OprM repressor), and frameshifting mutations in MexEF-OprN components or its regulators (Table 1 and Fig. 1b-d) was then observed. While individual validation of these variants was not performed, the shared targets across these independent lineages, and the genomic data associating the genetic changes to MIC increases provides supportive evidence of the involvement of the MexAB-OprM efflux pump in IMI/REL resistance. Among the observed variants, three MexB mutations – K134N, located in the serine loop; and G621S and R620C, located in the switch loop – were situated in the lining of the pump’s ligand binding pockets, further supporting a prediction of functional effect19. Most of the mutations in MexEF-OprN or its regulator MexS/T in this report (Table 1) are consistent with frameshifting indels, which would be predicted to result in truncated translation products19. The MexAB-OprM and MexEF-OprN pumps are co-regulated47,64, and it has previously been shown that disruption of MexEF-OprN can result in increased expression of MexAB-OprM47, suggesting that this increased expression may contribute to the mechanism conferring resistance. As the authors point out, given that IMI is not efficiently transported by MexAB-OprM, it is possible that increased efflux of REL by MexAB-OprM plays a role19. The role of MexAB-OprM overexpression in IMI/REL resistance in clinical isolates has been suggested in another recent case report demonstrating divergent evolution of resistance to CZA, C/T, and IMI/REL in a set of clinical isolates from a patient treated with CZA65.
However, at least one previous study found that overexpression of wild-type MexAB-OprM had little effect in IMI/REL MIC66, suggesting the possibility that MexB substitutions that modify specificity may be required for MexAB-OprM to confer resistance. In a different study, several P. aeruginosa laboratory strains, along with isogenic MutS-deficient hypermutator counterparts, underwent serial passage through increasing concentrations of IMI/REL for 7 days24. In addition to OprD inactivation, overexpression of MexB and mutations in MexR and MexB were observed in several lineages (Table 1)24. These mutations included MexB R620C, corroborating clinical variants found by Shields and colleagues and discussed above19. Out of the five MutS-deficient hypermutator lineages evolved, two were also found to develop MexY mutations, and one each had MexF, MexK, MexH and MexW variants24. As the functional consequences of these variants were not verified experimentally, establishing their significance requires further study.
FDR is a newer promising agent which demonstrates potent activity against carbapenem-resistant gram-negatives, including P. aeruginosa and organisms that harbor metallo-β-lactamases. FDR is a siderophore antimicrobial which exploits the bacterial iron transport mechanisms to gain entry into the cell and is stable against hydrolysis by most beta-lactamases67. The first resistance mechanisms described were mutations in genes encoding iron transport system components, including piuA, piuB, pvdS, pirS, pirR, tonB3, exbB, and exbD, consistent with its mode of transport17,20,21,68,69. Early in vitro studies suggested a minor role for MexAB-OprM, with a maximum of 2-fold increase in MIC in laboratory strains with MexAB overexpression70. Separately, multiple mutations outside iron transport systems that may be associated with FDR resistance were identified in clinical isolates from individuals with cystic fibrosis20. Multiple efflux genes were mutated in these isolates including mexZ (a MexXY-OprM regulator), mexY, mexA, mexC, and mexS (a MexEF-OprN regulator). Four mexA mutations in 4/6 resistant isolates were predicted to be disruptive or inactivating. The set of isolates above contained additional mutations in other known resistance mechanisms including the PmrAB two-component system, the UTP-glucose-1-phosphate uridylyltransferase, PBP2, PBP3 (DD-transpeptidases), the major porin OprD, and PDC. The relative potential roles of these individual mutations were not validated experimentally. Other studies examined the genomic changes in serial clinical isolates that developed FDR resistance, and found mutations in mexR, mexE and mexB (MexB V767G) (Table 1)17,21. As above, the specific roles of these efflux system mutations in FDR resistance were not confirmed experimentally, and thus would require further study17,20,21.
FEP/ZID is a novel BL/BLI with additional antimicrobial activity attributable to intrinsic PBP2 affinity of the BLI; FEP/ZID has thus been described as a beta-lactam/beta-lactam enhancer agent71,72. The combination is currently in late stages of development and has demonstrated in vitro activity against metallo-beta-lactamase producing gram-negative bacteria73. Genomic changes associated with resistance to FEP/ZID have been described in P. aeruginosa laboratory strains and isogenic MutS-deficient hypermutator counterparts26. Among others, mutations in the MexAB-OprM pump and its regulators emerged independently in different lineages, including several mutations in key residues lining the ligand binding pockets (F136L, T329A, V612M, M630T). In addition, increased expression of mexB was confirmed in all lineages with increased MIC, further supporting a role of this pump in FEP/ZID resistance. Less uniformly, some lineages developed overexpression of mexY and mutations in mexF (Table 1).
Overexpression and alterations of AcrAB-TolC may play roles in BL/BLI resistance in Enterobacterales
The prototype RND efflux pump in Enterobacterales, AcrAB-TolC, plays a major role in resistance to almost every antibiotic class including the beta-lactams, with the notable exception of aminoglycosides47,74. AcrD, as part of AcrAD-TolC, plays an important role in aminoglycoside efflux75. In many Enterobacterales, AcrAB-TolC is under the control of several regulators including the local repressor AcrR and global transcriptional factors MarR, MarA, RamR, RamA, SoxS, and Rob76,77,78. Some of these factors counter-regulate both AcrAB-TolC and outer membrane porins important for beta-lactam transport, including OmpC and OmpF in E. coli, and OmpK36 and OmpK35 in K. pneumoniae79,80. A combination of efflux pump expression and porin loss often confers carbapenem resistance in clinical Enterobacterales isolates that lack carbapenemases81. Several additional RND efflux pumps are present in Enterobacterales, including AcrEF and MdtABC in E. coli, and OqxAB and KpgABC in K. pneumoniae, but the roles of these additional pumps in antibiotic resistance, as well as those of others outside the RND family, have not been studied in great depth82,83.
CZA resistance in carbapenemase-producing Enterobacterales has been associated with substitutions in class A carbapenemases, particularly in the KPC enzyme84. Additionally, some reports on KPC-containing K. pneumoniae suggest a possible role of AcrAB-TolC overexpression and/or porin deficiencies in CZA resistance11,85, but other studies suggest the contribution of efflux may be minor86. AcrAB-TolC overexpression and porin defects were frequent in a collection of novel BL/BLI-resistant Enterobacterales that lacked carbapenemases15, but the contributions of these changes to resistance were not directly examined experimentally in this study. Though a number of studies have investigated expression levels of the AcrAB-TolC efflux pump, less is known about the roles of specific coding variants in resistance to BL and BL/BLI16. In one study, genomic sequencing of two serial clinical KPC-containing Enterobacter cloacae clinical isolates that developed CZA resistance during treatment found an AcrB F396L substitution as one of 6 unique variants differentiating the susceptible and resistant isolates18. These changes in aggregate were associated with a 32-fold increase in the CZA MIC (from 1 to 32 μg/mL). The F396 residue is located in the TM4 domain of AcrB, and is 70-80% conserved across AcrB homologs37. In a separate in vitro study, Enterobacterales isolates containing extended spectrum beta lactamases including CTX-M were exposed to increasing CZA to evolve resistance, followed by genomic sequencing22. Among 19 isolates with increased CZA MIC, the only change observed in two CTX-M-15-expressing E. coli isolates was an AcrB F615S substitution, conferring an 8- to 16-fold increase in CZA MIC. The F615 residue is notable for being part of the well-characterized phenylalanine-rich site lining the distal binding pocket involved in direct substrate interactions37,87 suggesting that this substitution might alter substrate affinity, binding kinetics, or specificity. However, the effect of these isolated substitutions was not experimentally validated.
A second agent clinically used in carbapenem resistant Enterobacterales (CRE) infections is MEM/VAB. Increased AcrAB-TolC expression has been found among KPC-producing Enterobacterales with elevated MEM/VAB MIC and no metallo-β-lactamases10 as well as among carbapenemase-negative CRE15; however, the role of efflux in MEM/VAB resistance is not clear. In a different study, the change in MEM/VAB susceptibility in E. coli and K. pneumoniae strains was tested with various engineered resistance mechanisms, including carbapenemases of the four Ambler classes, porin disruptions and RamR inactivation with consequent AcrAB-TolC overexpression and OmpK35 downregulation88. In this study, the effect of RamR inactivation was comparable to OmpK35 inactivation alone and was minimal in the background of OmpK35 and/or OmpK36 inactivation. This implies that, while porins are likely to be involved in VAB entry to the cell, the effect of AcrAB-TolC upregulation did not seem to contribute meaningfully to MEM/VAB resistance.
FDR is another agent that demonstrates broad in vitro activity against most CRE70,89,90,91. Currently it is not usually the first choice of therapy as there is less clinical experience with it, and other options are available for most serine carbapenemase-producing Enterobacterales, such as CZA and MEM/VAB67,92. FDR might be most useful in the context of metallo-β-lactamase producing Enterobacterales for which other good options are scarce93. The FDR resistance mechanisms are still being characterized in Enterobacterales. Several in vitro and in vivo studies point to TonB-dependent iron transport mechanisms, including CirA and Fiu94,95, while others have reported substitutions/deletions in the AmpC beta-lactamase R2 loop13,96 or the contribution of KPC alleles that confer CZA resistance97. An examination of the genetic makeup of fourteen clinical isolates that developed increases in FDR MIC during the APEKS-NP and CREDIBLE-CR clinical trials98,99,100 identified changes in AmpC as a candidate resistance mechanism in one Enterobacter cloacae isolate, while no other changes in iron transport genes, outer membrane porins or beta-lactamases were identified in four other Enterobacterales isolates. Of note, in this study the increase in MIC remained unexplained in 10/14 isolates, and efflux pump expression or coding sequence variants were not explicitly analyzed. Overall, data on the impact of efflux pump variants or overexpression in FDR resistance in Enterobacterales remain scarce.
The role of efflux-mediated resistance to BL/BLI in CRAB and S. maltophilia remain to be elucidated
CRAB and S. maltophilia are two nosocomial pathogens of increasing importance, particularly in the context of wide-spread use of broad-spectrum antimicrobials and increase in the size of the immunocompromised population at risk6,101,102. The treatment of infections caused by these organisms poses a significant challenge, as they harbor an extensive repertoire of chromosomal resistance determinants including multiple RND efflux pumps and beta-lactamases103,104,105,106,107. A handful of studies have analyzed the mechanisms of resistance to novel BL/BLI in these organisms, with particular attention to SUL/DUR108 and FDR100,109 in the case of CRAB, and FDR27 in S. maltophilia. Efflux pump-based mechanisms of resistance were not identified in these studies; however, their involvement has not been excluded, and more work remains to done.
Conclusions and future directions
The last decade has seen a much-needed increase in new antimicrobials for treating infections caused by resistant gram-negative organisms7,110, in particular novel BL and BL/BLI agents. The wide-spread availability of genomic sequencing, in combination with in vitro evolution experiments, is also enabling a proactive approach in seeking potentially relevant resistance mechanisms that might play a role when these new agents enter clinical practice. Emerging data reviewed here suggest that both major and lesser-known RND efflux pumps represent important resistance mechanisms in critical gram-negative bacterial pathogens. We believe that the roles of RND efflux pumps in resistance to novel BL/BLI agents merit more attention, and that broader genomic sequencing of clinical isolates will provide valuable insights into currently understudied, but important mechanisms.
In addition to genomic sequencing, work involving direct genetic manipulation is needed to establish the functional roles (or lack thereof) of the now large catalog of identified efflux pump mutations in clinical BL/BLI-resistant isolates. As reviewed extensively above, a primary weakness of many current studies is the observational nature of associations between mutations in efflux pump systems, often occurring in complex genetic backgrounds, and BL/BLI resistance. Construction of isogenic mutant series in which the direct consequences of individual changes can be observed is required to prove causality. In-frame chromosomal deletions of efflux pumps with direct MIC readout are an additional valuable approach to establish the contributions of individual pumps to resistance occurring in clinical isolates. These studies remain limited, as many labs that are specialized in genomic sequencing lack the capacity to perform genetic engineering, and additionally, genetic manipulation of wild type clinical isolates is often extremely challenging. However, establishment of a verified catalog of resistance mutations may facilitate direct molecular testing in the clinical diagnostic lab. Such testing may direct future targeted therapy with pump inhibitors.
Work is also required to determine the absolute transport specificities of RND efflux pumps contributing to resistance to BL/BLI combination agents. It has been assumed that efflux-mediated BL/BLI resistance is primarily due to transport of the beta lactam antibiotic component. But for efflux pumps that efficiently transport beta lactam antibiotics, it is very reasonable to assume that they may also transport structurally related beta lactam class beta lactamase inhibitors, such as tazobactam, but this is currently uncharacterized. It also unknown whether these same pumps are capable of transporting the structurally dissimilar diazabicyclooctane class beta lactamase inhibitors, such as avibactam, vaborbactam, and relebactam, though this seems less likely. Direct assays that test transport specificities or ligand binding assays are required but are technically challenging to perform. Another related challenge is the relative lack of direct structural data for many of the RND class transporters reviewed above. This is due in part to the general difficulty of structural studies on large, multiprotein complexes that span two membranes of different lipid composition.
Clear establishment of roles for specific alterations in specific transporters in mediating resistance will likely drive renewed structural and functional interest in these proteins. Structures and structural-functional studies will, in turn, stimulate the development of new antibiotics that are poor substrates for wild type and altered efflux pumps. They will also facilitate the development of targeted efflux pump inhibitors that may be co-administered in a manner analogous to beta lactamase inhibitors. We believe the many avenues of this work will yield both new antibiotic targets that are needed to maintain clinical efficacy of BL/BLI combination antibiotics, as well as new strategies to minimize the emergence of resistance.
References
Dekker, J. P. Within-host evolution of bacterial pathogens in acute and chronic infection. Annu Rev. Pathol. 19, 203–226 (2024).
Blair, J. M., Webber, M. A., Baylay, A. J., Ogbolu, D. O. & Piddock, L. J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol 13, 42–51 (2015).
Darby, E. M. et al. Molecular mechanisms of antibiotic resistance revisited. Nat. Rev. Microbiol 21, 280–295 (2022).
Carlet, J. et al. Ready for a world without antibiotics? The pensieres antibiotic resistance call to action. Antimicrob. Resist Infect. Control 1, 11 (2012).
Tangden, T. & Giske, C. G. Global dissemination of extensively drug-resistant carbapenemase-producing Enterobacteriaceae: clinical perspectives on detection, treatment and infection control. J. Intern Med. 277, 501–512 (2015).
Hamidian, M. & Nigro, S. J. Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii. Microb Genom 5, https://doi.org/10.1099/mgen.0.000306 (2019).
Yahav, D. et al. New beta-lactam-beta-lactamase inhibitor combinations. Clin. Microbiol Rev. 34, https://doi.org/10.1128/CMR.00115-20 (2020).
Mushtaq, S., Warner, M. & Livermore, D. M. In vitro activity of ceftazidime+NXL104 against Pseudomonas aeruginosa and other non-fermenters. J. Antimicrob. Chemother. 65, 2376–2381 (2010).
Kadri, S. S. et al. Difficult-to-treat resistance in gram-negative bacteremia at 173 US hospitals: Retrospective cohort analysis of prevalence, predictors, and outcome of resistance to all first-line agents. Clin. Infect. Dis. 67, 1803–1814 (2018).
Castanheira, M., Rhomberg, P. R., Flamm, R. K. & Jones, R. N. Effect of the beta-lactamase inhibitor vaborbactam combined with meropenem against serine carbapenemase-producing enterobacteriaceae. Antimicrob. Agents Chemother. 60, 5454–5458 (2016).
Nelson, K. et al. Resistance to ceftazidime-avibactam is due to transposition of KPC in a porin-deficient strain of klebsiella pneumoniae with increased efflux activity. Antimicrob Agents Chemother 61, https://doi.org/10.1128/AAC.00989-17 (2017).
Castanheira, M., Doyle, T. B., Smith, C. J., Mendes, R. E. & Sader, H. S. Combination of MexAB-OprM overexpression and mutations in efflux regulators, PBPs and chaperone proteins is responsible for ceftazidime/avibactam resistance in Pseudomonas aeruginosa clinical isolates from US hospitals. J. Antimicrob. Chemother. 74, 2588–2595 (2019).
Shields, R. K. et al. Clinical evolution of ampc-mediated ceftazidime-avibactam and cefiderocol resistance in enterobacter cloacae complex following exposure to cefepime. Clin. Infect. Dis. 71, 2713–2716 (2020).
Fraile-Ribot, P. A. et al. Activity of Imipenem-Relebactam against a Large Collection of Pseudomonas aeruginosa Clinical Isolates and Isogenic beta-Lactam-Resistant Mutants. Antimicrob Agents Chemother 64, https://doi.org/10.1128/AAC.02165-19 (2020).
Castanheira, M., Doyle, T. B., Deshpande, L. M., Mendes, R. E. & Sader, H. S. Activity of ceftazidime/avibactam, meropenem/vaborbactam and imipenem/relebactam against carbapenemase-negative carbapenem-resistant Enterobacterales isolates from US hospitals. Int. J. Antimicrob. Agents 58, 106439 (2021).
Sader, H. S., Mendes, R. E., Doyle, T. B., Davis, A. P. & Castanheira, M. Characterization of Enterobacter cloacae and Citrobacter freundii species complex isolates with decreased susceptibility to cephalosporins from United States hospitals and activity of ceftazidime/avibactam and comparator agents. JAC Antimicrob. Resist 3, dlab136 (2021).
Simner, P. J. et al. Cefiderocol activity against clinical pseudomonas aeruginosa isolates exhibiting ceftolozane-tazobactam resistance. Open Forum Infect. Dis. 8, ofab311 (2021).
Senchyna, F. et al. Comparative genomics of Enterobacter cloacae complex before and after acquired clinical resistance to Ceftazidime-Avibactam. Diagn. Microbiol Infect. Dis. 101, 115511 (2021).
Shields, R. K., Stellfox, M. E., Kline, E. G., Samanta, P. & Van Tyne, D. Evolution of imipenem-relebactam resistance following treatment of multidrug-resistant pseudomonas aeruginosa pneumonia. Clin. Infect. Dis. 75, 710–714 (2022).
Lopez-Causape, C. et al. Cefiderocol resistance genomics in sequential chronic Pseudomonas aeruginosa isolates from cystic fibrosis patients. Clin. Microbiol. Infect. 29, 538.e7–538.e13 (2023).
Sadek, M. et al. Progressive in vivo development of resistance to cefiderocol in Pseudomonas aeruginosa. Eur. J. Clin. Microbiol Infect. Dis. 42, 61–66 (2023).
Livermore, D. M. et al. Selection of mutants with resistance or diminished susceptibility to ceftazidime/avibactam from ESBL- and AmpC-producing Enterobacteriaceae. J. Antimicrob. Chemother. 73, 3336–3345 (2018).
Sanz-Garcia, F., Hernando-Amado, S. & Martinez, J. L. Mutation-driven evolution of pseudomonas aeruginosa in the presence of either ceftazidime or ceftazidime-avibactam. Antimicrob Agents Chemother. 62, https://doi.org/10.1128/AAC.01379-18 (2018).
Gomis-Font, M. A. et al. In vitro dynamics and mechanisms of resistance development to imipenem and imipenem/relebactam in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 75, 2508–2515 (2020).
Gomis-Font, M. A. et al. Emergence of resistance to novel cephalosporin-beta-lactamase inhibitor combinations through the modification of the pseudomonas aeruginosa MexCD-OprJ efflux pump. Antimicrob. Agents Chemother. 65, e0008921 (2021).
Barcelo, I. et al. In vitro evolution of cefepime/zidebactam (WCK 5222) resistance in Pseudomonas aeruginosa: dynamics, mechanisms, fitness trade-off and impact on in vivo efficacy. J. Antimicrob. Chemother. 76, 2546–2557 (2021).
Werth, B. J. et al. Evolution of cefiderocol resistance in Stenotrophomonas maltophilia using in vitro serial passage techniques. JAC Antimicrob. Resist. 4, dlac011 (2022).
Lamarche, M. G. & Deziel, E. MexEF-OprN efflux pump exports the Pseudomonas quinolone signal (PQS) precursor HHQ (4-hydroxy-2-heptylquinoline). PLoS One 6, e24310 (2011).
Minagawa, S. et al. RND type efflux pump system MexAB-OprM of Pseudomonas aeruginosa selects bacterial languages, 3-oxo-acyl-homoserine lactones, for cell-to-cell communication. BMC Microbiol 12, 70 (2012).
Sakhtah, H. et al. The Pseudomonas aeruginosa efflux pump MexGHI-OpmD transports a natural phenazine that controls gene expression and biofilm development. Proc. Natl. Acad. Sci. USA 113, E3538–E3547 (2016).
Buckley, A. M. et al. The AcrAB-TolC efflux system of Salmonella enterica serovar Typhimurium plays a role in pathogenesis. Cell Microbiol 8, 847–856 (2006).
Wang-Kan, X. et al. Lack of AcrB efflux function confers loss of virulence on salmonella enterica serovar typhimurium. mBio 8, https://doi.org/10.1128/mBio.00968-17 (2017).
Klenotic, P. A., Moseng, M. A., Morgan, C. E. & Yu, E. W. Structural and functional diversity of resistance-nodulation-cell division transporters. Chem. Rev. 121, 5378–5416 (2021).
Fernandez, L. & Hancock, R. E. Adaptive and mutational resistance: role of porins and efflux pumps in drug resistance. Clin. Microbiol Rev. 25, 661–681 (2012).
Du, D. et al. Multidrug efflux pumps: structure, function and regulation. Nat. Rev. Microbiol 16, 523–539 (2018).
Zgurskaya, H. I., Malloci, G., Chandar, B., Vargiu, A. V. & Ruggerone, P. Bacterial efflux transporters’ polyspecificity - a gift and a curse? Curr. Opin. Microbiol 61, 115–123 (2021).
Zwama, M. & Nishino, K. Ever-adapting RND efflux pumps in gram-negative multidrug-resistant pathogens: A race against time. Antibiotics (Basel) 10, https://doi.org/10.3390/antibiotics10070774 (2021).
Seyedhosseini Ghaheh, H. et al. Targeting and ultrabroad insight into molecular basis of resistance-nodulation-cell division efflux pumps. Sci. Rep. 12, 16130 (2022).
Dreier, J. & Ruggerone, P. Interaction of antibacterial compounds with RND e ffl ux pumps in Pseudomonas aeruginosa. Front Microbiol 6, 660 (2015).
Sun, J., Deng, Z. & Yan, A. Bacterial multidrug efflux pumps: mechanisms, physiology and pharmacological exploitations. Biochem Biophys. Res Commun. 453, 254–267 (2014).
Zwama, M., Yamaguchi, A. & Nishino, K. Phylogenetic and functional characterisation of the Haemophilus influenzae multidrug efflux pump AcrB. Commun. Biol. 2, 340 (2019).
Reynolds, D. & Kollef, M. The epidemiology and pathogenesis and treatment of pseudomonas aeruginosa infections: An update. Drugs 81, 2117–2131 (2021).
Angus, B. L., Carey, A. M., Caron, D. A., Kropinski, A. M. & Hancock, R. E. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild-type with an antibiotic-supersusceptible mutant. Antimicrob. Agents Chemother. 21, 299–309 (1982).
Chevalier, S. et al. Structure, function and regulation of Pseudomonas aeruginosa porins. FEMS Microbiol Rev. 41, 698–722 (2017).
Lister, P. D., Wolter, D. J. & Hanson, N. D. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin. Microbiol Rev. 22, 582–610 (2009).
Masuda, N. et al. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-oprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44, 3322–3327 (2000).
Li, X. Z., Plesiat, P. & Nikaido, H. The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria. Clin. Microbiol Rev. 28, 337–418 (2015).
Ramaswamy, V. K., Vargiu, A. V., Malloci, G., Dreier, J. & Ruggerone, P. Molecular determinants of the promiscuity of MexB and MexY multidrug transporters of pseudomonas aeruginosa. Front Microbiol. 9, 1144 (2018).
Middlemiss, J. K. & Poole, K. Differential impact of MexB mutations on substrate selectivity of the MexAB-OprM multidrug efflux pump of Pseudomonas aeruginosa. J. Bacteriol. 186, 1258–1269 (2004).
Ohene-Agyei, T., Lea, J. D. & Venter, H. Mutations in MexB that affect the efflux of antibiotics with cytoplasmic targets. FEMS Microbiol. Lett. 333, 20–27 (2012).
Gotoh, N. et al. Characterization of the MexC-MexD-OprJ multidrug efflux system in DeltamexA-mexB-oprM mutants of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 42, 1938–1943 (1998).
Lahiri, S. D. et al. Selection and molecular characterization of ceftazidime/avibactam-resistant mutants in Pseudomonas aeruginosa strains containing derepressed AmpC. J. Antimicrob. Chemother. 70, 1650–1658 (2015).
Berrazeg, M. et al. Mutations in beta-Lactamase AmpC increase resistance of pseudomonas aeruginosa isolates to antipseudomonal cephalosporins. Antimicrob. Agents Chemother. 59, 6248–6255 (2015).
Dulanto Chiang, A. et al. Hypermutator strains of Pseudomonas aeruginosa reveal novel pathways of resistance to combinations of cephalosporin antibiotics and beta-lactamase inhibitors. PLoS Biol. 20, e3001878 (2022).
Nichols, W. W., Lahiri, S. D., Bradford, P. A. & Stone, G. G. The primary pharmacology of ceftazidime/avibactam: resistance in vitro. J Antimicrob Chemother, https://doi.org/10.1093/jac/dkac449 (2023).
Li, Y. et al. A new member of the tripartite multidrug efflux pumps, MexVW-OprM, in Pseudomonas aeruginosa. J. Antimicrob. Chemother. 52, 572–575 (2003).
Poonsuk, K., Tribuddharat, C. & Chuanchuen, R. Simultaneous overexpression of multidrug efflux pumps in Pseudomonas aeruginosa non-cystic fibrosis clinical isolates. Can. J. Microbiol 60, 437–443 (2014).
Fajardo, A. et al. The neglected intrinsic resistome of bacterial pathogens. PLoS One 3, e1619 (2008).
Ranjitkar, S. et al. Target (MexB)- and efflux-based mechanisms decreasing the effectiveness of the efflux pump inhibitor D13-9001 in pseudomonas aeruginosa PAO1: Uncovering a new role for MexMN-OprM in efflux of beta-lactams and a novel regulatory circuit (MmnRS) controlling MexMN expression. Antimicrob Agents Chemother. 63, https://doi.org/10.1128/AAC.01718-18 (2019).
Kishk, R. M. et al. Efflux MexAB-Mediated Resistance in P. aeruginosa isolated from patients with healthcare associated infections. Pathogens 9, https://doi.org/10.3390/pathogens9060471 (2020).
Barnes, M. D. et al. Deciphering the evolution of cephalosporin resistance to ceftolozane-tazobactam in pseudomonas aeruginosa. mBio 9, https://doi.org/10.1128/mBio.02085-18 (2018).
Livermore, D. M., Warner, M. & Mushtaq, S. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J. Antimicrob. Chemother. 68, 2286–2290 (2013).
Zhanel, G. G. et al. Imipenem-relebactam and meropenem-vaborbactam: Two novel carbapenem-beta-lactamase inhibitor combinations. Drugs 78, 65–98 (2018).
Horna, G., Lopez, M., Guerra, H., Saenz, Y. & Ruiz, J. Interplay between MexAB-OprM and MexEF-OprN in clinical isolates of Pseudomonas aeruginosa. Sci. Rep. 8, 16463 (2018).
Alonso-Garcia, I. et al. Simultaneous and divergent evolution of resistance to cephalosporin/beta-lactamase inhibitor combinations and imipenem/relebactam following ceftazidime/avibactam treatment of MDR Pseudomonas aeruginosa infections. J. Antimicrob. Chemother. 78, 1195–1200 (2023).
Young, K. et al. In vitro studies evaluating the activity of imipenem in combination with relebactam against Pseudomonas aeruginosa. BMC Microbiol 19, 150 (2019).
McCreary, E. K., Heil, E. L. & Tamma, P. D. New perspectives on antimicrobial agents: Cefiderocol. Antimicrob. Agents Chemother. 65, e0217120 (2021).
Streling, A. P. et al. Evolution of cefiderocol non-susceptibility in pseudomonas aeruginosa in a patient without previous exposure to the antibiotic. Clin. Infect. Dis. 73, e4472–e4474 (2021).
Gupta, A., Landman, D. & Quale, J. Relationship of TonB-dependent receptors with susceptibility to cefiderocol in clinical isolates of Pseudomonas aeruginosa. J. Antimicrob. Chemother. 77, 1282–1285 (2022).
Ito, A. et al. In vitro antibacterial properties of cefiderocol, a novel siderophore cephalosporin, against gram-negative bacteria. Antimicrob. Agents Chemother. 62, e01454–17 (2018).
Moya, B. et al. WCK 5107 (Zidebactam) and WCK 5153 are novel inhibitors of PBP2 showing potent “beta-lactam enhancer” activity against pseudomonas aeruginosa, including multidrug-resistant metallo-beta-lactamase-producing high-risk clones. Antimicrob. Agents Chemother. 61, e02529–16 (2017).
Moya, B. et al. PoTent Beta-lactam Enhancer Activity Of Zidebactam and WCK 5153 aGainst Acinetobacter Baumannii, Including Carbapenemase-producing Clinical Isolates. Antimicrob. Agents Chemother. 61, e01238–17 (2017).
Sader, H. S., Rhomberg, P. R., Flamm, R. K., Jones, R. N. & Castanheira, M. WCK 5222 (cefepime/zidebactam) antimicrobial activity tested against Gram-negative organisms producing clinically relevant beta-lactamases. J. Antimicrob. Chemother. 72, 1696–1703 (2017).
Nagano, K. & Nikaido, H. Kinetic behavior of the major multidrug efflux pump AcrB of Escherichia coli. Proc. Natl. Acad. Sci. USA 106, 5854–5858 (2009).
Aires, J. R. & Nikaido, H. Aminoglycosides are captured from both periplasm and cytoplasm by the AcrD multidrug efflux transporter of Escherichia coli. J. Bacteriol. 187, 1923–1929 (2005).
Bialek-Davenet, S. et al. In vitro selection of ramR and soxR mutants overexpressing efflux systems by fluoroquinolones as well as cefoxitin in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 55, 2795–2802 (2011).
Perez, A. et al. Effect of transcriptional activators SoxS, RobA, and RamA on expression of multidrug efflux pump AcrAB-TolC in Enterobacter cloacae. Antimicrob. Agents Chemother. 56, 6256–6266 (2012).
Weston, N., Sharma, P., Ricci, V. & Piddock, L. J. V. Regulation of the AcrAB-TolC efflux pump in Enterobacteriaceae. Res. Microbiol. 169, 425–431 (2018).
Dutzler, R. et al. Crystal structure and functional characterization of OmpK36, the osmoporin of Klebsiella pneumoniae. Structure 7, 425–434 (1999).
Domenech-Sanchez, A. et al. Role of Klebsiella pneumoniae OmpK35 porin in antimicrobial resistance. Antimicrob. Agents Chemother. 47, 3332–3335 (2003).
Tenover, F. C. et al. Evaluation of the NCCLS extended-spectrum beta-lactamase confirmation methods for Escherichia coli with isolates collected during Project ICARE. J. Clin. Microbiol 41, 3142–3146 (2003).
Srinivasan, V. B. & Rajamohan, G. KpnEF, a new member of the Klebsiella pneumoniae cell envelope stress response regulon, is an SMR-type efflux pump involved in broad-spectrum antimicrobial resistance. Antimicrob. Agents Chemother. 57, 4449–4462 (2013).
Maurya, N., Jangra, M., Tambat, R. & Nandanwar, H. Alliance of efflux pumps with beta-lactamases in multidrug-resistant klebsiella pneumoniae isolates. Micro. Drug Resist 25, 1155–1163 (2019).
Hobson, C. A. et al. Klebsiella pneumoniae carbapenemase variants resistant to ceftazidime-avibactam: An evolutionary overview. Antimicrob. Agents Chemother. 66, e0044722 (2022).
Humphries, R. M. & Hemarajata, P. Resistance to ceftazidime-avibactam in klebsiella pneumoniae due to porin mutations and the increased expression of KPC-3. Antimicrob Agents Chemother 61, https://doi.org/10.1128/AAC.00537-17 (2017).
Pages, J. M., Peslier, S., Keating, T. A., Lavigne, J. P. & Nichols, W. W. Role of the outer membrane and porins in susceptibility of beta-lactamase-producing enterobacteriaceae to ceftazidime-avibactam. Antimicrob. Agents Chemother. 60, 1349–1359 (2015).
Kobylka, J., Kuth, M. S., Muller, R. T., Geertsma, E. R. & Pos, K. M. AcrB: A mean, keen, drug efflux machine. Ann. N. Y Acad. Sci. 1459, 38–68 (2020).
Lomovskaya, O. et al. Vaborbactam: Spectrum of beta-lactamase inhibition and impact of resistance mechanisms on activity in enterobacteriaceae. Antimicrob Agents Chemother 61, https://doi.org/10.1128/AAC.01443-17 (2017).
Hackel, M. A. et al. In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of gram-negative bacilli collected worldwide in 2014 to 2016. Antimicrob Agents Chemother. 62, https://doi.org/10.1128/AAC.01968-17 (2018).
Kazmierczak, K. M. et al. In vitro activity of cefiderocol, a siderophore cephalosporin, against a recent collection of clinically relevant carbapenem-non-susceptible Gram-negative bacilli, including serine carbapenemase- and metallo-beta-lactamase-producing isolates (SIDERO-WT-2014 Study). Int. J. Antimicrob. Agents 53, 177–184 (2019).
Jacobs, M. R. et al. ARGONAUT-I: Activity of Cefiderocol (S-649266), a Siderophore Cephalosporin, against Gram-Negative Bacteria, Including Carbapenem-Resistant Nonfermenters and Enterobacteriaceae with Defined Extended-Spectrum beta-Lactamases and Carbapenemases. Antimicrob Agents Chemother 63, https://doi.org/10.1128/AAC.01801-18 (2019).
Tamma, P. D. et al. Infectious diseases society of america guidance on the treatment of ampc beta-lactamase-producing enterobacterales, carbapenem-resistant acinetobacter baumannii, and stenotrophomonas maltophilia infections. Clin. Infect. Dis. 74, 2089–2114 (2022).
Tamma, P. D. et al. Infectious Diseases Society of America 2022 Guidance on the Treatment of Extended-Spectrum beta-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. 75, 187–212 (2022).
Lan, P. et al. Catecholate siderophore receptor CirA impacts cefiderocol susceptibility in Klebsiella pneumoniae. Int J. Antimicrob. Agents 60, 106646 (2022).
Moon, S. H., Udaondo, Z., Jun, S. R. & Huang, E. Cefiderocol heteroresistance in Klebsiella pneumoniae is linked to mutations in the siderophore receptor cirA and beta-lactamase activities. Int. J. Antimicrob. Agents 60, 106635 (2022).
Kawai, A. et al. Structural basis of reduced susceptibility to ceftazidime-avibactam and cefiderocol in enterobacter cloacae due to AmpC R2 loop deletion. Antimicrob Agents Chemother 64, https://doi.org/10.1128/AAC.00198-20 (2020).
Hobson, C. A. et al. Cross-resistance to cefiderocol and ceftazidime-avibactam in KPC beta-lactamase mutants and the inoculum effect. Clin. Microbiol. Infect. 27, 1172 e1177–1172 e1110 (2021).
Bassetti, M. et al. Efficacy and safety of cefiderocol or best available therapy for the treatment of serious infections caused by carbapenem-resistant Gram-negative bacteria (CREDIBLE-CR): a randomised, open-label, multicentre, pathogen-focused, descriptive, phase 3 trial. Lancet Infect. Dis. 21, 226–240 (2021).
Wunderink, R. G. et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of Gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect. Dis. 21, 213–225 (2021).
Nordmann, P. et al. Mechanisms of reduced susceptibility to cefiderocol among isolates from the CREDIBLE-CR and APEKS-NP clinical trials. Micro. Drug Resist 28, 398–407 (2022).
Brooke, J. S. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clin. Microbiol. Rev. 25, 2–41 (2012).
Hu, L. F. et al. Surveillance of antimicrobial susceptibility patterns among Stenotrophomonas maltophilia isolated in China during the 10-year period of 2005-2014. J. Chemother. 30, 25–30 (2018).
Heritier, C., Poirel, L. & Nordmann, P. Cephalosporinase over-expression resulting from insertion of ISAba1 in Acinetobacter baumannii. Clin. Microbiol. Infect. 12, 123–130 (2006).
Rumbo, C. et al. Contribution of efflux pumps, porins, and beta-lactamases to multidrug resistance in clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 57, 5247–5257 (2013).
Vila, J., Marti, S. & Sanchez-Cespedes, J. Porins, efflux pumps and multidrug resistance in Acinetobacter baumannii. J. Antimicrob. Chemother. 59, 1210–1215 (2007).
Chang, Y. T., Lin, C. Y., Chen, Y. H. & Hsueh, P. R. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Front Microbiol. 6, 893 (2015).
Mojica, M. F. et al. Population structure, molecular epidemiology, and beta-lactamase diversity among stenotrophomonas maltophilia isolates in the United States. mBio 10, e00405–19 (2019).
Findlay, J., Poirel, L., Bouvier, M. & Nordmann, P. In vitro activity of sulbactam-durlobactam against carbapenem-resistant Acinetobacter baumannii and mechanisms of resistance. J. Glob. Antimicrob. Resist 30, 445–450 (2022).
Malik, S., Kaminski, M., Landman, D. & Quale, J. Cefiderocol resistance in acinetobacter baumannii: Roles of beta-lactamases, siderophore receptors, and penicillin binding protein 3. Antimicrob. Agents Chemother. 64, e01221–20 (2020).
Prasad, N. K., Seiple, I. B., Cirz, R. T. & Rosenberg, O. S. Leaks in the pipeline: A failure analysis of gram-negative antibiotic development from 2010 to 2020. Antimicrob. Agents Chemother. 66, e0005422 (2022).
Acknowledgements
This work was supported by the Intramural Research Program of the National Institute of Infectious Diseases. The content and views expressed in this work are those of the authors and do not necessarily represent the official views of the NIH or the U.S. Government.
Funding
Open access funding provided by the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
A.D.C. and J.P.D. conceived of this work. A.D.C. and J.P.D. co-wrote the manuscript. ADC created Table 1 and critically assessed the associated literature cited within it. J.P.D. created Fig. 1. Both authors critically reviewed and edited the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Communications Medicine thanks Ariel Blocker, Jacqueline Findlay and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dulanto Chiang, A., Dekker, J.P. Efflux pump-mediated resistance to new beta lactam antibiotics in multidrug-resistant gram-negative bacteria. Commun Med 4, 170 (2024). https://doi.org/10.1038/s43856-024-00591-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s43856-024-00591-y
- Springer Nature Limited