Abstract
Tumour necrosis factor-α (TNF-α) is critical in the regulation of inflammation and tumour progression. TNF-α-308G > A is associated with constitutively elevated TNF-α expression. The purpose of this study was to assess the association between TNF-α-308G > A and breast cancer (BC) risk by subtype and the connection between genotypes and clinical features of BC. A total of 768 patients and 565 controls were enrolled in this study and genotypes were detected using the TaqMan assay. No effect on susceptibility for any BC subtype was found for the TNF-α-308 polymorphism in our study or in the pooled meta-analysis. This polymorphism was shown to be associated with age at menarche in all BC and in progesterone receptor-negative BC. Interestingly, triple negative breast cancer (TNBC) patients with TNF-α-308A had an increased risk of distant tumour metastasis (OR = 3.80, 95% CI: 1.31–11.02, P = 0.009). Multi-regression analysis showed that TNF-α-308A was also a risk factor for distant tumour metastasis after adjustment for tumour size and lymph node metastasis status (OR= 6.26, 95% CI: 1.88–20.87, P = 0.003). These findings indicate that TNF-α might play a distinct role in the progression of TNBC, especially in distant tumour metastasis of TNBC.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the most frequent type of malignancy in women in both the developed and the developing world1. It is a heterogeneous disease in regards to its clinical, histological and molecular profile. In recent decades, there has been great progress in the diagnosis of BC, but only oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor2 (Her2) are typically used for BC diagnosis in routine clinical practice. BC intrinsic subtypes, including triple-negative breast cancer (TNBC) and Her2+, luminal A and luminal B BCs, are characterized by immunohistochemistry (IHC) and have important differences in phenotype and prognosis2,3. Inflammation within the breast tumour microenvironment is known to be correlated with increased invasiveness and poor prognosis4. Pro-inflammatory biomarkers and the immune response are related to BC risk and/or prognosis5,6. However, many cytokines are involved in the pathogenesis of BC and inflammatory processes and genes involved in cytokine-related functional pathways have gained increasing interest by researchers.
Tumour necrosis factor-α (TNF-α), a multi-functional cytokine, is involved in the promotion of inflammatory responses and plays a critical role in the pathogenesis of inflammatory, autoimmune and malignant diseases7. TNF-α is also a key molecule in the promotion of angiogenesis through the stimulation of endothelial cell proliferation and the enhancement of the expression of other pro-angiogenic factors8. Furthermore, TNF-α induces the expression of adhesion molecules involved in the invasion of metastatic tumour cells9,10. Elevated plasma levels of TNF-α have been detected in many malignancies and are often associated with poor prognoses11,12,13. Knockdown of the TNF-α gene is associated with cell proliferation inhibition and apoptosis in TNBC14.
Considerable evidence has shown that genetic variations, such as single nucleotide polymorphisms (SNP), in both tumour and host genomes have roles in the diagnosis, treatment outcome and survival of patients with cancer15,16. Increasing evidence has shown that a SNP in the promoter region of the TNF-α gene (−308G > A, rs1800629) causes genetic susceptibility to many types of tumours and autoimmune diseases, such as hepatocellular carcinoma, myeloma, lymphoma, ulcerative colitis and Crohn’s disease17,18,19. The TNF-α-308A allele appears to have higher constitutive and inducible TNF-α expression, as the −308G > A mutation affects a consensus binding site of AP-220,21. The association between the TNF-α-308 polymorphism and BC has been widely evaluated in different ethnicities; however, the results of these studies have been inconsistent, possibly due to BC heterogeneity and other factors22. It is unclear whether TNF-α-308G > A is associated with different BC subtypes and/or clinical features.
Because TNF-α is tumourigenic in vitro and in vivo, we hypothesized that the TNF-α-308G > A polymorphism may have an important function in different BC subtypes and be related to the characteristics of different BC subtypes, especially in highly aggressive BC. The current case-control study investigated the role of TNF-α-308G > A in BC susceptibility by IHC subtype and ER, PR and Her2 status and the relationship between genotypes and clinicopathological characteristics of BC.
Results
Because the cases and controls were frequency-matched for age, there were no significant differences in the distributions of age between the cases and controls (P = 0.275). Of the 768 BC cases, 163 were TNBC and 82 were Her2+, 183 were luminal A, 340 were luminal B, 523 were ER+, 245 were ER−, 585 were PR− and 686 were Her2− BCs. The genotype frequency of TNF-α-308G > A in the controls was in concordance with Hardy-Weinberg equilibrium (HWE) (P = 0.940).
TNF-α-308 polymorphism and BC
The allelic frequency of TNF-α-308A was 0.074 in the controls and 0.065 in all BC cases. Table 1 presents the distribution of genotypes for TNF-α-308G > A in the controls and BC cases. For all BCs, there was no association with the TNF-α-308 polymorphism (OR= 0.89, 95% CI: 0.63–1.25, P = 0.482) in the dominant genetic model. According to IHC classification, no association was found between the TNF-α-308 polymorphism and the TNBC (OR= 0.67, 95% CI: 0.38–1.19, P = 0.133), Her2+ (OR= 0.90, 95% CI: 0.41–1.72, P = 0.812), luminal A (OR= 0.67, 95% CI: 0.33–1.14, P = 0.120) or luminal B (OR= 1.20, 95% CI: 0.84–1.77, P = 0.523) subtypes. Consistent with the results for all BCs, no association was observed between the TNF-α-308 polymorphism and ER+ (OR= 0.98, 95% CI: 0.68–1.36, P = 0.852), ER− (OR= 0.72, 95% CI: 0.43–1.18, P = 0.204), PR− (OR= 0.92, 95% CI: 0.65–1.34, P = 0.867) or Her2− (OR= 0.86, 95% CI: 0.63–1.35, P = 0.531) BCs.
Due to a lack of information, we conducted an updated meta-analysis to test whether the TNF-α-308 polymorphism is associated with overall BC risk rather than specifically testing its association with the various subtypes. Eighteen studies (including the present study) were included in this analysis, with a total of 13567 BC cases and 15087 controls. The detailed characteristics of each study in the pooled analysis are summarized in Table 2. Thirteen studies were conducted in Caucasians23,24,25,26,27,28,29,30,31,32,33,34,35, while the remaining 5 were conducted in Asians36,37,38,39. No association between the TNF-α-308 polymorphism and BC risk was found in Caucasians (OR= 1.06, 95% CI: 0.88–1.27, P = 0.555; I2= 80.7%, Phet < 0.001), Asians (OR = 1.07, 95% CI: 0.58–1.96, P = 0.826; I2 = 88.7%, Phet < 0.001) or all subjects (OR= 1.04, 95% CI: 0.87–1.24, P = 0.653; I2= 84.6%, Phet < 0.001) (Table 3). Begg’s funnel plot and Egger’s test were performed to evaluate the publication bias of all included studies. In the overall analysis, no evidence of obvious asymmetry for the TNF-α-308G > A polymorphism was found (PBegg’s = 0.596). Additionally, the Egger’s test found no significant publication bias (P = 0.375).
TNF-α-308 polymorphism and clinical features of BC
We analysed the association between TNF-α-308G > A and clinical characteristics (age at diagnosis, BMI, tumour stage, tumour size, lymph node metastasis, distant metastasis, age at menarche, menopause and family history of cancer) by BC subtypes classified using IHC (Table 4). In all BCs, the GA and AA genotypes were less frequent in patients who were 15 years old or more at menarche than inpatients who were under 15 years old at menarche (OR= 0.57, 95% CI: 0.35–0.95, P = 0.029). In the TNBC group, the GA and AA genotypes were associated with distant tumour metastasis (OR= 3.80, 95% CI: 1.31–11.02, P = 0.009). After adjusting for tumour size and lymph node metastasis status, this association remained (OR= 6.26, 95% CI: 1.88–20.87, P = 0.003) and in our study, this association was also statistically significant after adjusting for all clinical characteristics (OR = 5.83, 95% CI: 1.64–20.76, P = 0.004).
We analysed the association between TNF-α-308G > A and clinical characteristics in patients with different ER, PR and Her2 statuses (Supplementary Table 1). A similar association between the GA and AA genotypes and age at menarche to that observed in all BCs was seen in PR− patients (OR= 0.56, 95% CI: 0.32–0.98, P = 0.041).
Discussion
Inflammatory cytokines play critical roles at different stages of tumour development and progression, including invasion and metastasis. Although several studies have reported an association between the TNF-α-308G > A polymorphism and BC risk, the results of those study were inconsistent. Moreover, little is known about this association in the Chinese. In the present study, we found that the TNF-α-308G > A polymorphism was not associated with the risk of BC in the different IHC subtypes or BCs classified based on ER, PR or Her2 status in Chinese, Asians or Caucasians. We provided evidence that the TNF-α-308 polymorphism is associated with age at menarche in all BCs and in PR− BCs. Interestingly, patients with TNBC and the TNF-α-308A allele had an increased risk of distant tumour metastasis. Our study highlights the effect of the TNF-α gene polymorphism on the progression of BC by subtype. These findings suggest a potential connection between constitutively higher TNF-α expression and the pathogenesis of TNBC.
TNF-α is mainly produced by macrophages and is also expressed in a wide variety of cells, including mast cells, lymphoid cells, endothelial cells, cardiac myocytes and fibroblasts40. Two TNF-α bioactive isoforms, a 26-kD transmembrane isoform and a 17-kD soluble isoform that under goes proteolytic cleavage by a metalloprotease, function by binding to TNF-α receptors (TNFRs)41. Two receptors (TNFR1 and TNFR2) bind TNF-α. TNFR1 is constitutively expressed in most tissues and can be activated by both transmembrane TNF-α (tmTNF-α) and soluble TNF-α (sTNF-α). When the death domain of TNFR1 interacts with the TNF-α receptor-associated death domain (TRADD), the resulting complex recruits proteins to activate apoptosis through caspase-3. TRADD can also bind TNF receptor-associated factor 2 (TRAF2) to recruit proteins that activate inhibitor of nuclear factor kappa-B kinase (IKK), germinal centre kinase (GCK) and receptor-interacting protein (RIP). These molecules then activate the nuclear factor kappa B (NF-κB), c-Jun N-terminal kinase (JNK) and mitogen-activated protein kinase (MAPK) pathways, which promote anti-apoptosis and cell survival. TNFR2 is generally expressed in cells of the immune system and can only bind sTNF-α. TNFR2 lacks a death domain and can also bind TRAF2 to activate an anti-apoptosis pathway42. Abnormal activation of JNK, MAPK and NF-κB can lead to the aberrant expression of many genes that cause chronic inflammation, which stimulates tumour growth. Thus, tmTNF-α and sTNF-α act as immunoregulatory cytokines that connect inflammation with cancer progression.
Two recent meta-analyses reported that the TNF-α-308GA and AA genotypes were significantly associated with decreased BC risk in Caucasians22,43. However, the allele frequencies in the controls of some studies44,45 included in those two meta-analyses were not in accordance with HWE. Additionally, a meta-analysis by Yang et al. included one study that compared the frequencies of the different TNF-α-308 polymorphism genotypes in patients with benign breast disease and controls46 and another study that did not provide the frequencies of each genotype47. With strict inclusion criteria, we added new individual studies and performed an updated meta-analysis; for all BCs, we found no association with this polymorphism in Asians and Caucasians. It must be noted that BC is a complex disease with multiple environmental and genetic factors contributing to its progression. The lack of an association between TNF-α-308G > A and all BCs does not indicate that TNF-α-308G > A has no effect of susceptibility in certain subtypes. Future research is needed to clarify the connection between the higher constitutive TNF-α expression observed with the TNF-α-308G > A polymorphism and the risk of BC in each BC subtype.
TNBC is frequently observed in young patients and in patients with larger and higher-grade tumours48,49. TNBC is also associated with higher recurrence rates of metastasis and death, especially within 3 years of diagnosis50. TNBCs must have some specific and common pathways involved in metastasis. Our study provided some clarification of the distinct molecular pathway of distant metastasis in TNBC. It is known that TNF-α is involved in tumour metastasis through the stimulation of chemokines, which increases cell migration and invasion and promotes proliferation and is involved in angiogenesis by increasing VEGF expression51,52. Our study suggests that higher constitutive TNF-α expression in patients with TNBC rather than other BC subtypes is associated with distant tumour metastasis. Previous studies also support our findings: knockdown of TNF-α gene expression through blockage of the NF-κB pathway inhibited cell proliferation and induced apoptosis in a TNBC cell line14; and in a murine model of TNBC, targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor 2 suppressed TNBC tumour growth and metastasis53. Although sTNF-α originates from tmTNF-α, the function of these two isoforms are not exactly the same. Accumulating evidence shows that tmTNF-α might play an opposite role to that of sTNF-α. Tumour cells that express tmTNF-α are protected from apoptosis by the activation of NF-κB by sTNF-α through reverse signalling54. In tumour cells, the suppression of NF-κB reverse signalling by tmTNF-α resulted in higher cytotoxicity of sTNF-α55. We propose that higher constitutive TNF-α expression alters the ratio of tmTNF-α to sTNF-α and promotes TNBC cell growth. Future research should focus on how these two isoforms influence BC progression in various subtypes.
The major strengths of this study were the comprehensive analysis of theTNF-α-308 polymorphism in relation to susceptibility for various BC subtypes and its influence on the clinical features of BC, which will greatly help improve our understanding of the role of TNF-α in BC pathogenesis. The modest sample size of each subtype, which caused suboptimal statistical power, is the main limitation of this study; however, this could not be avoided. In conclusion, the present study shows that the TNF-α-308G > A polymorphism is not associated with BC risk but is associated with distant tumour metastasis in TNBC. This association might be mediated by the constitutively higher expression of tmTNF-α and/or sTNF-α in patients with the TNF-α-308A allele, promoting tumour growth through metastasis. Our results also confirm that targeting TNF-α suppresses TNBC progression.
Patients and Methods
Study subjects
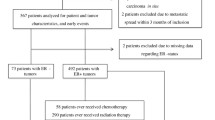
This case-control study included 768 patients with constitutive BC and 565 cancer-free controls. All subjects were unrelated ethnic Han Chinese women. Patients were recruited from January 2010 to December 2013 at the Cancer Hospital, Shandong Academy of Medical Sciences and Beijing Chao-Yang Hospital, Capital Medical University and had been diagnosed with histologically confirmed BC. In this study, we classified the BC subtypes as TNBC (ER−, PR− and Her2−), Her2+ (ER−, PR− and Her2+), luminal A(ER+, PR+ and Her2−) and luminal B (ER+, PR−/PRlow and Her2−). The controls were randomly selected based on a physical examination in the same region during the same period as patient recruitment. The selection criteria included no history of cancer and frequency matching to cases by age. At recruitment, demographic information and clinical characteristics of each participant were collected. Informed consent was obtained from all participants. This study was conducted in accordance with the approved guidelines of the Institutional Review Board of the Cancer Hospital, Shandong Academy of Medical Sciences and Beijing Chao-Yang Hospital, Capital Medical University.
TNF-α genotyping
Genomic DNA was extracted from peripheral blood lymphocytes of the study subjects. The genotypes of TNF-α at the -308 (G > A) site were analysed using a TaqMan genotyping platform (Roche LightCycler 480II, Roche Applied Science). The PCR primers were 5′-GGC CAC TGA CTG ATT TGT GTG T-3′ and 5′-CAA AAG AAA TGG AGG CAA TAG GTT-3′ and the probe sequences were 5′-FAM-CCC GTC CCC ATG CCC CTC-BHQ-3′ and 5′-TET-CTG AAC CCC GTC CTC ATG CCC-BHQ-3′. For quality control, genotyping was performed by researchers blinded to the case or control status and a 10% random sample of cases and controls was genotyped twice by different persons; the reproducibility was 99.0%.
Meta-analysis
We used three electronic databases (PubMed, Embase and Web of Knowledge) to identify relevant publications through December 2014 using key words related to the TNF-α gene polymorphism in combination with BC. The literature search was limited to English language publications on human studies. The reference lists of the relevant articles, reviews and editorials were also screened to find all additional eligible studies.
Studies were included in this meta-analysis if they met the following criteria: (1) case-control study design comparing TNF-α-308 polymorphism in BC cases and cancer-free controls; (2) cases were diagnosed histologically; (3)genomic DNA was isolated from peripheral blood leukocytes; (4) the study estimated the association between the TNF-α-308 polymorphism and BC risk; (5) sufficient data existed for calculating odds ratios (ORs) and corresponding 95% confidence intervals (CIs); and (6) the allele frequencies in the control group met HWE. If the same subject group was published in more than one study, the most complete study was selected for this pooled meta-analysis.
All information extracted from the included studies, including the first author, the year of publication, the ethnicity of the subjects, the number of subjects and genotype frequencies in the case and control groups and the genotyping method, was double-checked and independently extracted from each publication by two investigators. Disagreements were resolved through discussion.
Statistical analysis
χ2 tests were used to examine the deviation in genotype frequencies from HWE in controls and the differences in demographic variables and genotype distributions for different clinical features of BC. A dominant genetic model was used to estimate the associations between the TNF-α-308 polymorphism and the risk of BC by ORs and 95% CIs, which were calculated by unconditional logistic regression adjusted for age. For the meta-analysis, subgroups of studies based on ethnicity and experimental methods were determined as described in our previous study56. All statistical analyses were performed using Statistical Analysis System software version 9.2 (SAS Institute, Cary, NC,USA) and Stata 12.0 (StataCorp, College Station, TX, USA). A P value less than 0.05 was considered statistically significant.
Additional Information
How to cite this article: Li, H.-H. et al. Tumour Necrosis Factor-α Gene Polymorphism Is Associated with Metastasis in Patients with Triple Negative Breast Cancer. Sci. Rep. 5, 10244; doi: 10.1038/srep10244 (2015).
References
Siegel, R., Ma, J., Zou, Z. & Jemal, A. Cancer statistics, 2014. CA. Cancer J. Clin. 64, 9–29 (2014).
Carey, L. A. et al. Race, breast cancer subtypes and survival in the Carolina Breast Cancer Study. JAMA 295, 2492–2502 (2006).
Blows, F. M. et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 7, e1000279 (2010).
Goldberg, J. E. & Schwertfeger, K. L. Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. Curr. Drug Targets 11, 1133–1146 (2010).
Allin, K. H., Nordestgaard, B. G., Flyger, H. & Bojesen, S. E. Elevated pre-treatment levels of plasma C-reactive protein are associated with poor prognosis after breast cancer: a cohort study. Breast Cancer Res. 13, R55 (2011).
Pierce, B. L. et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J. Clin. Oncol. 27, 3437–3444 (2009).
Bazzoni, F. & Beutler, B. The tumor necrosis factor ligand and receptor families. N. Engl. J. Med. 334, 1717–1725 (1996).
Leek, R. D. et al. Association of tumour necrosis factor alpha and its receptors with thymidine phosphorylase expression in invasive breast carcinoma. Br. J. Cancer 77, 2246–2251 (1998).
Champ, C. E. et al. Weight gain, metabolic syndrome and breast cancer recurrence: are dietary recommendations supported by the data? Int. J. Breast Cancer 2012, 506868 (2012).
Ioculano, M. et al. Tumour necrosis factor mediates E-selectin production and leukocyte accumulation in myocardial ischaemia-reperfusion injury. Pharmacol. Res. 31, 281–288 (1995).
Warzocha, K. et al. Tumor necrosis factor ligand-receptor system can predict treatment outcome in lymphoma patients. J. Clin. Oncol. 15, 499–508 (1997).
Nakashima, J. et al. Association between tumor necrosis factor in serum and cachexia in patients with prostate cancer. Clin. Cancer Res. 4, 1743–1748 (1998).
Szlosarek, P. W. & Balkwill, F. R. Tumour necrosis factor alpha: a potential target for the therapy of solid tumours. Lancet Oncol. 4, 565–573 (2003).
Pileczki, V., Braicu, C., Gherman, C. D. & Berindan-Neagoe, I. TNF-alpha gene knockout in triple negative breast cancer cell line induces apoptosis. Int. J. Mol. Sci. 14, 411–420 (2012).
Cariaso, M. & Lennon, G. SNPedia: a wiki supporting personal genome annotation, interpretation and analysis. Nucleic Acids Res. 40, D1308–1312 (2012).
Huang, Y. T. et al. Genome-wide analysis of survival in early-stage non-small-cell lung cancer. J. Clin. Oncol. 27, 2660–2667 (2009).
Wilson, A. G., di Giovine, F. S. & Duff, G. W. Genetics of tumour necrosis factor-alpha in autoimmune, infectious and neoplastic diseases. J. Inflamm. 45, 1–12 (1995).
Neben, K. et al. Polymorphisms of the tumor necrosis factor-alpha gene promoter predict for outcome after thalidomide therapy in relapsed and refractory multiple myeloma. Blood 100, 2263–2265 (2002).
Ho, S. Y. et al. Increased risk of developing hepatocellular carcinoma associated with carriage of the TNF2 allele of the -308 tumor necrosis factor-alpha promoter gene. Cancer Causes Control 15, 657–663 (2004).
Kroeger, K. M., Carville, K. S. & Abraham, L. J. The -308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol. Immunol. 34, 391–399 (1997).
Wilson, A. G. et al. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc. Natl. Acad. Sci. USA 94, 3195–3199 (1997).
Yang, Y., Feng, R., Bi, S. & Xu, Y. TNF-alpha polymorphisms and breast cancer. Breast Cancer Res. Treat 129, 513–519 (2011).
Chouchane, L., Ahmed, S. B., Baccouche, S. & Remadi, S. Polymorphism in the tumor necrosis factor-alpha promotor region and in the heat shock protein 70 genes associated with malignant tumors. Cancer 80, 1489–1496 (1997).
Mestiri, S. et al. Genetic variation in the tumor necrosis factor-alpha promoter region and in the stress protein hsp70-2: susceptibility and prognostic implications in breast carcinoma. Cancer 91, 672–678 (2001).
Giordani, L. et al. Association of breast cancer and polymorphisms of interleukin-10 and tumor necrosis factor-alpha genes. Clin. Chem. 49, 1664–1667 (2003).
Azmy, I. A. et al. Role of tumour necrosis factor gene polymorphisms (-308 and -238) in breast cancer susceptibility and severity. Breast Cancer Res. 6, R395–400 (2004).
Kamali-Sarvestani, E., Merat, A. & Talei, A. R. Polymorphism in the genes of alpha and beta tumor necrosis factors (TNF-alpha and TNF-beta) and gamma interferon (IFN-gamma) among Iranian women with breast cancer. Cancer Lett. 223, 113–119 (2005).
Scola, L. et al. Cytokine gene polymorphisms and breast cancer susceptibility. Ann. N. Y. Acad. Sci. 1089, 104–109 (2006).
Gaudet, M. M. et al. Genetic variation in tumor necrosis factor and lymphotoxin-alpha (TNF-LTA) and breast cancer risk. Hum. Genet. 121, 483–490 (2007).
Gonullu, G. et al. Association of breast cancer and cytokine gene polymorphism in Turkish women. Saudi Med. J. 28, 1728–1733 (2007).
Sirotkovic-Skerlev, M. et al. TNF alpha promoter polymorphisms analysis in benign and malignant breast lesions. Exp. Mol. Pathol. 83, 54–58 (2007).
Risk, M.-G. C. o. G. S. f. M. H. T. R. B. C. Polymorphisms in the BRCA1 and ABCB1 genes modulate menopausal hormone therapy associated breast cancer risk in postmenopausal women. Breast Cancer Res. Treat 120, 727–736 (2010).
Karakus, N. et al. Tumor necrosis factor alpha and beta and interferon gamma gene polymorphisms in Turkish breast cancer patients. DNA Cell Biol. 30, 371–377 (2011).
Madeleine, M. M. et al. Genetic variation in proinflammatory cytokines IL6, IL6R, TNF-region and TNFRSF1A and risk of breast cancer. Breast Cancer Res. Treat 129, 887–899 (2011).
Gomez Flores-Ramos, L. et al. Association of the tumor necrosis factor-alpha −308G > A polymorphism with breast cancer in Mexican women. Genet. Mol. Res. 12, 5680–5693 (2013).
Park, K. S. et al. Polymorphisms of tumour necrosis factors A and B in breast cancer. Eur. J. Immunogenet. 29, 7–10 (2002).
Kohaar, I. et al. Association of single nucleotide polymorphisms (SNPs) in TNF-LTA locus with breast cancer risk in Indian population. Breast Cancer Res. Treat 114, 347–355 (2009).
Pooja, S. et al. Role of ethnic variations in TNF-alpha and TNF-beta polymorphisms and risk of breast cancer in India. Breast Cancer Res. Treat 126, 739–747 (2011).
Xu, F. et al. Association of TNF-alpha, TNFRSF1A and TNFRSF1B Gene Polymorphisms with the Risk of Sporadic Breast Cancer in Northeast Chinese Han Women. PLoS One 9, e101138 (2014).
Spriggs, D. R., Deutsch, S. & Kufe, D. W. Genomic structure, induction and production of TNF-alpha. Immunol. Ser. 56, 3–34 (1992).
Yu, M. et al. Targeting transmembrane TNF-alpha suppresses breast cancer growth. Cancer Res. 73, 4061–4074 (2013).
Chu, W. M. Tumor necrosis factor. Cancer Lett. 328, 222–225 (2013).
Wang, J. et al. Tumour necrosis factor alpha -308G/A polymorphism and risk of the four most frequent cancers: a meta-analysis. Int. J. Immunogenet. 38, 311–320 (2011).
Smith, K. C., Bateman, A. C., Fussell, H. M. & Howell, W. M. Cytokine gene polymorphisms and breast cancer susceptibility and prognosis. Eur. J. Immunogenet. 31, 167–173 (2004).
Skerrett, D. L., Moore, E. M., Bernstein, D. S. & Vahdat, L. Cytokine genotype polymorphisms in breast carcinoma: associations of TGF-beta1 with relapse. Cancer Invest. 23, 208–214 (2005).
Gallicchio, L. et al. Body mass, polymorphisms in obesity-related genes and the risk of developing breast cancer among women with benign breast disease. Cancer Detect. Prev. 31, 95–101 (2007).
Erdei, E. et al. Polymorphisms in cytokine genes and serum cytokine levels among New Mexican women with and without breast cancer. Cytokine 51, 18–24 (2010).
Bauer, K. R. et al. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer 109, 1721–1728 (2007).
Rakha, E. A. et al. Prognostic markers in triple-negative breast cancer. Cancer 109, 25–32 (2007).
Dent, R. et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res. 13, 4429–4434 (2007).
Kulbe, H. et al. The inflammatory cytokine tumor necrosis factor-alpha regulates chemokine receptor expression on ovarian cancer cells. Cancer Res. 65, 10355–10362 (2005).
Hamaguchi, T. et al. TNF inhibitor suppresses bone metastasis in a breast cancer cell line. Biochem. Biophys. Res. Commun. 407, 525–530 (2011).
Malin, D. et al. Enhanced metastasis suppression by targeting TRAIL receptor 2 in a murine model of triple-negative breast cancer. Clin. Cancer Res. 17, 5005–5015 (2011).
Yan, D. et al. Expression of TNF-alpha leader sequence renders MCF-7 tumor cells resistant to the cytotoxicity of soluble TNF-alpha. Breast Cancer Res. Treat 116, 91–102 (2009).
Zhang, H. et al. Transmembrane TNF-alpha mediates "forward" and "reverse" signaling, inducing cell death or survival via the NF-kappaB pathway in Raji Burkitt lymphoma cells. J. Leukoc Biol. 84, 789–797 (2008).
Zhai, K., Ding, J. & Zhou, Y. Different role of tumor necrosis factor-alpha polymorphism in non-Hodgkin lymphomas among Caucasian and Asian populations: a meta-analysis. Int. J. Mol. Sci. 15, 7684–7698 (2014).
Acknowledgements
This work was supported by the Scientific Research Program of Shandong Academy of Medical Sciences (No. 2013-26) and the Startup Foundation of Shandong Cancer Hospital.
Author information
Authors and Affiliations
Contributions
K.Z. and Z.H.W. conceived the study and were responsible for the study design, oversaw the entire study, interpreted the results and wrote parts of the manuscript. H.H.L. performed the project and obtained financial support. H.Z., L.S.L., Y.H., J.G., J.L. and C.X.C. were responsible for subject recruitment and sample preparation. X.P.S. performed the statistical analyses.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Li, HH., Zhu, H., Liu, LS. et al. Tumour Necrosis Factor-α Gene Polymorphism Is Associated with Metastasis in Patients with Triple Negative Breast Cancer. Sci Rep 5, 10244 (2015). https://doi.org/10.1038/srep10244
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep10244
- Springer Nature Limited