Abstract
Meat quality traits have an increasing importance in the pig industry because of their strong impact on consumer acceptance. Herewith, we have combined phenotypic and microarray expression data to map loci with potential effects on five meat quality traits recorded in the longissimus dorsi (LD) and gluteus medius (GM) muscles of 350 Duroc pigs, i.e. pH at 24 hours post-mortem (pH24), electric conductivity (CE) and muscle redness (a*), lightness (L*) and yellowness (b*). We have found significant genome-wide associations for CE of LD on SSC4 (~104 Mb), SSC5 (~15 Mb) and SSC13 (~137 Mb), while several additional regions were significantly associated with meat quality traits at the chromosome-wide level. There was a low positional concordance between the associations found for LD and GM traits, a feature that reflects the existence of differences in the genetic determinism of meat quality phenotypes in these two muscles. The performance of an eQTL search for SNPs mapping to the regions associated with meat quality traits demonstrated that the GM a* SSC3 and pH24 SSC17 QTL display positional concordance with cis-eQTL regulating the expression of several genes with a potential role on muscle metabolism.
Similar content being viewed by others
Introduction
The physicochemical properties of the porcine muscle and its post-mortem maturation determine the organoleptic properties of fresh meat and cured products and, consequently, their acceptance by consumers1. The genetic determinism of electrical conductivity, acidity and color, which have been often used as predictors of meat quality, has been explored by performing genome-wide association studies (GWAS) in F2 populations2,3,4 as well as in purebred pigs5,6. An important limitation of using F2 intercrosses in GWAS studies is that they are not representative of the purebred populations that constitute the selection nuclei of breeding companies. On the other hand, certain breeds, such as Large White, have been strongly introgressed with Asian alleles that do not segregate in other European porcine populations7.
In a previous study, we measured electrical conductivity at 24 hours (CE), pH at 24 hours (pH24) and color (lightness or L*, redness or a*, and yellowness or b*) in gluteus medius (GM) and longissimus dorsi (LD) samples from 350 Duroc pigs (Lipgen population)8. Performance of a genome scan with 105 microsatellites revealed that the QTL maps for these two muscles were quite different8. Indeed, the only QTL that remained significant at the genome-wide level were those associated with GM a*, on Sus scrofa chromosome 13 (SSC13, 84 cM), and GM b* (SSC15, 108 cM). Unfortunately, the confidence intervals of these QTL were quite large due to the poor resolution of the microsatellite-based analysis. Moreover, we may have missed many QTL due to the relatively large spacing between markers. In the current work, we aimed to circumvent these limitations by employing a GWAS approach to identify meat quality QTL in the Lipgen population mentioned above. Taking advantage that microarray measurements of gene expression in the GM muscle were available for 104 Lipgen pigs, we have performed an additional analysis where we have investigated the co-localization between GM QTL and expression QTL in cis (cis-eQTL).
Materials and Methods
Ethics approval
The manipulation of Duroc pigs followed Spanish national guidelines and it was approved by the Ethical Committee of Institut de Recerca i Tecnologia Agroalimentàries (IRTA).
Measurement of phenotypic and expression data
Phenotypic records were collected in a commercial Duroc line of 350 barrows distributed in five half-sib families (Lipgen population). A detailed description of the management conditions of this commercial line has been previously reported9. Meat quality analyses were performed 24 h after slaughter at the IRTA-Centre of Food Technology by using 200 g samples of the LD and GM muscles. Electrical conductivity was estimated with a Pork Quality Meter (Intek GmbH) while pH24 was measured with a pH-meter equipment with a Xerolyte electrode (Crison). Meat L*, a* and b* color parameters were determined with a Minolta Chroma-Meter CR-200 (Konica Minolta) equipment (light source C and aperture 2). Microarray expression data of GM samples from 104 Duroc pigs were obtained in a previous study (data can be found in the Gene Expression Omnibus public repository, accession number: GSE19275) based on the use of GeneChip Porcine Genomic arrays (Affymetrix, Inc., Santa Clara, CA)10. A detailed description of the techniques and methods used to perform the RNA purification and microarray hybridization steps can be found in Canovas et al.10. Briefly, GM samples from 104 pigs were grinded in liquid nitrogen and homogenized with a mechanical rotor. Total RNA was purified with an acid phenol protocol11 and it was subsequently used as a template to synthesize double stranded cDNA with the One Cycle cDNA Synthesis Kit (Affymetrix, Inc.). cRNAs were purified with the GeneChip Sample Cleanup Module (Affymetrix, Inc.), fragmented and added to a hybridisation cocktail10. The GeneChip Porcine Genome Array was equilibrated to room temperature and prehybridised with 1× hybridisation buffer at 45 °C for 10 min10. The hybridisation cocktail was heated to 99 °C for 5 min in a heat block and cooled to 45 °C for 5 min. Subsequently, a hybridization step was carried out at 45 °C for 16 hours. GeneChips were washed and labeled with streptavidin phycoerythrin in a Fluidics Station 450 (Affymetrix, Inc) and they were scanned in an Agilent G3000 GeneArray Scanner (Agilent Technologies, Inc.). The “Affy” and “Sympleaffy” packages from the Bioconductor project12 were employed to establish a set of quality control metrics to assess the quality of RNA samples and the efficiencies of the labelling and hybridisation steps. Data pre-processing and normalization were carried out with the BRB-ArrayTools software version 3.7.113. Genes displaying more than 20% of expression values over ±1.5 times the median expression of all arrays were retained for further analysis.
Genome-wide association analysis for meat quality and expression data
Genotyping was performed with the Porcine SNP60 BeadChip (Illumina, San Diego, CA) which contains 62,163 single nucleotide polymorphisms (SNPs). Quality genotyping analyses were carried out with the GenomeStudio software (Illumina), as previously reported14. We removed SNPs (a) mapping to the X chromosome, (b) with a rate of missing genotypes higher than 5%, (c) that did not conform Hardy-Weinberg expectations (threshold set at a P-value ≤ 0.001), (d) that had a minor allele frequency below 0.05, (e) that had a GenCall score < 0.15, (f) that had a call rate < 95% or (g) that could not be mapped to the pig genome (Sus scrofa 10.2 assembly). After filtering the raw data, a GWAS was carried out with 36,710 SNPs. Single-SNP association analyses were performed with the Genome-wide Efficient Mixed-Model Association (GEMMA) software15 under an additive genetic model that included the genomic kinship matrix to account for relatedness. The statistical model assumed in this analysis was:

where yijklm is the vector of phenotypic observations i.e. pH24, CE, L*, a* and b* measured at the GM and LD muscles of the ith individual; μ is the population mean of each trait; batchj is a systematic effect of the jth fattening batch, with 4 categories; β is the regression coefficient on the covariate weight at slaughter ( weightk); δ is the SNP allelic effect, estimated as a regression coefficient on the corresponding gl genotype (values −1, 0, 1) of the lth SNP; and eijklm is the residual effect. The statistical relevance of the systematic environmental sources of variation and the covariates included in the model were previously reported by Gallardo et al.8 and Casellas et al.16. Correction for multiple testing was implemented with a false discovery rate approach17.
Microarray data were available exclusively for GM muscle samples10. Following the strategy employed in the Genotype-Tissue Expression (GTEx) pilot analysis18, we primarily searched for cis-eQTL because they are expected to have larger effects than their trans-counterparts. We used two different strategies: Analysis 1, we retrieved 12 genes localized within GM QTL regions and we looked for cis-eQTL that might regulate their expression and Analysis 2, we made a search for cis-eQTL at a whole genome scale and we analyzed if there was a positional concordance between GWAS signals and cis-eQTL identified in this way. This second strategy made possible to identify cis-eQTL that might be located in the vicinity of GWAS signals. Genes corresponding to each probe included in the GeneChip Porcine Genomic array (Affymetrix, Inc., Santa Clara, CA) were identified in the BioMart database19. The statistical model assumed in this analysis was:

where yijklm is the vector that defines the expression of each gene in the GM muscle of the ith individual; μ is the mean expression of each gene in the population; batchjand labk are the systematic effects i.e. batchj of fattening (with 4 categories) and labk (microarray data were generated in two different laboratories); δ is the SNP allelic effect estimated as a regression coefficient on the corresponding gl genotype (values −1, 0, 1) of the lth SNP; and eijklm is the residual effect. Correction for multiple testing was implemented with a false discovery rate approach17. The threshold of significance in Analysis 1 took into consideration the number of SNPs contained within 2 Mb windows around each one of the 12 genes under consideration, while in Analysis 2 such threshold was established by taking into account the 36,710 SNPs typed in the Duroc population.
Results and Discussion
The SNPs arrayed in the Porcine SNP60 BeadChip explain a limited amount of the phenotypic variance of meat quality traits
By using the GEMMA software, we have estimated the proportion of phenotypic variance explained by the 36,710 SNPs (h2SNP) genotyped with the Porcine SNP60 BeadChip (Table 1). In general, estimates of h2SNP ranged from low to moderate and differed between muscles. Discrepancies in the genealogic heritability (h2) estimates of meat quality traits recorded in different skeletal muscle samples were previously reported by Larzul et al.20. In this way, these authors found h2 of 0.03 and 0.23 for L* measured in the gluteus profundus and longissimus muscles, respectively. Similarly, the h2 values of pH24 measured in 4 different muscles oscillated between 0.17 (longissimus) and 0.39 (biceps femoris)20. When Gallardo et al.8 performed a QTL scan for meat quality traits in the Lipgen population, they also found that QTL maps differed markedly amongst traits recorded in the GM and LD muscles. As a whole, these results suggest that there are muscle-specific factors that modulate the genetic determinism of meat quality traits. Indeed, Quintanilla et al.21 identified remarkable differences in the gene expression patterns of the LD and GM muscles, a feature that was especially prominent for genes involved in muscle tissue development, cell proliferation and migration and muscle contraction.
Several h2SNP values obtained by us were comparable to genealogic heritabilities estimated for porcine meat quality traits in previous studies. For instance Gjerlaug-Enger et al.22 reported heritabilities for a* of 0.43 and 0.46 in Duroc and Landrace pigs, respectively. Similarly, Van Wijk et al.23 and Gjerlaug-Enger et al.22 described heritabilities of 0.11 (crossbred pigs) and from 0.12 (Landrace) to 0.27 (Duroc) for pH24. More unexpected were the null h2SNP values obtained in the current work for traits such as b* (in GM) and L* (in both muscles). We attribute these null heritabilities to our inability to detect genetic variants that may have small effects or that segregate at very low frequencies24.
Environmental variables may also obscure the contribution of genetic factors. Indeed, meat quality traits can be affected by poor on-farm handling, mixing of unfamiliar animals and high pig density and long travel distance during transportation25. Such events may increase the stress of the swine brought to the abattoir and, consequently, they may have negative consequences on meat quality25. At the abattoir, extended lairage time can increase the incidence of dark, firm and dry (DFD) meat, while a short lairage time has been associated with an increased proportion of pale, soft and exudative (PSE) meat25. Electrical stunning induces a more rapid pH fall early post mortem and an inferior water-holding capacity than CO2 stunning, while an accelerated chilling may have negative consequences on meat tenderness and water-holding capacity25. In summary, all these factors, and others that are not mentioned, can have a strong impact on the post-mortem pH, electrical conductivity and color of pig meat and “dilute” the contribution of polygenes25.
Genome-wide and chromosome-wide associations with meat quality traits in Duroc pigs
At the genome-wide level, we found significant associations between CE of LD and three genomic regions on SSC4, SSC5 and SSC13 (Table 2). The SSC4, 104 megabase (Mb) region, lies close to a previously reported QTL for CE identified by Cepica et al.26. We also found positional concordance between the SSC13 (137.0 Mb) region associated with LD CE and a semimembranosus CE QTL reported by Evans et al.27. At the chromosome-wide level, a coincidence was detected between a a* QTL on SSC3 (50–57 Mb, Table 3) and a QTL for the same trait reported by Li et al.28 on SSC3 (55 Mb). Overall, our results confirm the existence of differences in the genetic determinism of meat quality traits recorded in the GM and LD muscles. The only exception was a region on SSC5 that significantly affected CE in both LD and GM muscles (Table 3). When we compared these data with the set of QTL previously reported by Gallardo et al.8 in the same Lipgen population we found one coincidence i.e. the GWAS signal identified on SSC4 (132 Mb) for CE in LD overlapped the confidence interval of a LD CE QTL (S0097 marker, ~133 Mb) detected by these authors8.
In general the positional coincidence between GWAS signals detected by us and those reported in previous studies was weak, indicating that the majority of associations reported in the current work are new. For instance, when we compared our a*, b* and pH24 data with those described in six additional GWAS studies4,6,29,30,31,32 we only found one positional coincidence between the SSC10 (70.6 Mb) genomic region associated with LD a* in the Lipgen population (Table 3) and the SSC10 (72.8 Mb) region identified by Ma et al.4 as associated with the same trait in the semimembranosus muscle of White Duroc × Erhualian F2 pigs.
The level of coincidence of trait-associated regions between these six GWAS for a*, b* and pH24 traits was also quite low. Only about 20% of the regions identified as significantly associated with any of these phenotypes were shared between two studies or more, indicating that the majority of associations are population-specific. These shared regions were: (a*) SSC4 (80–85 Mb)6,30, SSC6 (17–22 Mb)4,30, SSC7 (31–32 Mb)4,31, SSC12 (58–63 Mb)30,31, SSC15 (133–136 Mb)30,31,32; (b*) SSC15 (129–133 Mb)30,32; and (pH24), SSC3 (15–19 Mb)30,31, SSC15 (133–136 Mb)29,32. This latter region on SSC15 (133–136 Mb) appeared to be associated with a*, b*, pH24, shear force and cook loss in many independent studies29,30,31,32 but not in ours. Interestingly, this SSC15 region contains the protein kinase AMP-activated non-catalytic subunit gamma 3 (PRKAG3) gene, whose polymorphism has causal effects on muscle glycogen depletion, a parameter that can have a strong influence on meat quality traits33.
Besides technical and methodological reasons, a probable cause for the lack of positional concordance between GWAS studies would be genetic heterogeneity34. Indeed, Yang et al.34 performed a GWAS for blood lipid traits in 2,400 Laiwu, Erhualian and Duroc × (Landrace × Yorkshire) pigs and they identified a total of 22 QTL. Notably, only six regions were identified in more than one population, and 16 were detected in a single population.
Positional concordance between cis-eQTL for genes expressed in the GM muscle and QTL for GM traits
In general, eQTL are highly enriched in variants with causal effects on phenotypic variation and they can provide valuable information about candidate genes to be further investigated. Integrative analyses of QTL and eQTL data have been performed in pigs, making possible to combine the power of recombination with expression studies in order to identify promising candidate genes35. For instance, multiple associations between SNPs mapping to porcine chromosomes 4 and 6 and meat quality traits have been detected30. Through an eQTL approach, it was possible to identify several genes on SSC4 (ZNF704, IMPA1 and OXSR1) and SSC6 (IH1D1, SIGLEC10, TBCB, LOC100518735, KIF1B, LOC100514845) whose variation is concomitantly associated with gene expression and phenotype data30. Similarly, Ma et al.36 used a genetical genomics approach to demonstrate that a splice mutation in the PHKG1 gene is the causal mutation for a glycolytic potential QTL mapping to SSC3.
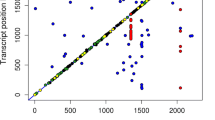
We have used this integrative strategy to identify potential candidate genes for meat quality traits in a dataset of 12 loci that mapped to GM QTL regions (Analysis 1). In doing so, we have detected 3 cis-eQTLs (Table 4) that co-localize with three chromosome-wide QTLs. One of them maps to SSC3 (16.6–17.06 Mb) and displays associations with a* (Fig. 1a); while the other two are located on SSC17 (53.1–57.2; 64.5–65.3) and show significant associations with GM pH24 (Fig. 1b and c). Interestingly, two of the three cis-regulated genes encode lysosomal enzymes, i.e. cathepsin A (CTSA) and glucuronidase β (GUSB), that might be released during the post-mortem maturation of meat37,38. Cathepsin A is a lysosomal serine protease that can also protect galactosidase β from intralysosomal proteolysis38, while glucuronidase β is mainly involved in the degradation of glycosaminoglycans39. Interestingly, there are evidences that galactosidase β and glucuronidase β might affect the degradation of the collagen mucopolysaccharide, thus having a potential impact on meat ultrastructural properties40.
Cis-eQTL (left panel) for the GUSB (1a), CTSA (1b) and FAM210B (1c) genes which map to QTL regions associated with meat quality traits recorded in the gluteus medius muscle (right panel). The x-axis represents chromosome length (Mb), and the y-axis shows the −log10 (P-value) of the associations found. The horizontal line indicates the threshold of significance (q-value ≤ 0.05). The vertical line depicts the genomic location of the GUSB, CTSA and FAM210B genes.
In Analysis 2, we have identified three additional cis-eQTL that map near to the SSC3 QTL for a* and the SSC17 QTL for pH24 (Table 5). The ADCY3 locus, that co-localizes with the SSC3 QTL for GM a* (Fig. 2a), encodes an adenylate cyclase catalysing the conversion of ATP into cyclic adenosine-3′,5′-monophosphate (cAMP), a secondary messenger that can have broad effects on muscle metabolism41. Indeed, AMPc is an activator of the cAMP-dependent protein kinase, a molecule involved in the phosphorylation of enzymes that promote the conversion of glycogen into glucose41. Noteworthy, the amount of glycogen stored in the muscle determines the post-mortem production of lactic acid, a molecule that has strong effects on meat color. Another eQTL of interest is the one influencing the mRNA levels of the secretory leukocyte peptidase inhibitor (SLPI) gene. This cis-eQTL co-localizes with the SSC17 QTL for GM pH24 (Fig. 2b). The SLP1 gene encodes a serine-protease that inhibits protein-degrading enzymes with strong effects on meat tenderization i.e. when the skeletal muscle is being degraded and transformed into meat, SLPI attenuates muscle proteolysis by binding to proteases and rendering them inactive42. Finally, the co-localization of the IGKC cis-eQTL and the SSC3 QTL for a* (Fig. 2c) does not have an obvious biological interpretation because this gene is mainly related with humoral immunity.
Co-localization of cis-eQTL (left panel) for the ADCY3 (2a), SLP1 (2b) and IGKC (2c) genes and QTL for meat quality traits recorded in the gluteus medius muscle (right panel). The x-axis represents chromosome length (Mb), and the y-axis shows the –log10 (P-value) of the associations found. The horizontal line indicates the threshold of significance (q-value ≤ 0.05). The vertical line depicts the genomic location of the ADCY3, SLP1 and IGKC genes.
Conclusions
We have detected genome-wide and chromosome-wide significant QTL for meat quality traits recorded in a Duroc commercial line with a population size that was moderate but comparable to the ones used in other porcine GWAS43,44,45. The limited positional concordance between the set of QTL detected by us and those reported by other authors in purebred populations suggests the existence of a significant amount of genetic heterogeneity for meat quality traits in porcine breeds. We have found remarkable differences between the QTL maps for the LD and GM muscles, suggesting that meat quality is determined to a great extent by genetic factors that are muscle-specific. Finally, we have observed a number of cis-eQTL that co-localize with meat quality QTL regions. Several of these cis-eQTL regulate the expression of genes which may play important roles in muscle physiology and post-mortem meat maturation. Sequencing of the regulatory regions of these loci might be useful to uncover the identity of the causal mutations explaining the existence of these QTLs.
Additional Information
How to cite this article: González-Prendes, R. et al. Joint QTL mapping and gene expression analysis identify positional candidate genes influencing pork quality traits. Sci. Rep. 7, 39830; doi: 10.1038/srep39830 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Ciobanu, D. C., Lonergan, S. M. & Huff-Lonergan, E. J. Genetics of meat and carcass traits In The Genetics of the Pig. 2nd edn. (ed. Rothschild, M. F. & Ruvinsky, A. ) 356 (CABI, 2011).
Luo, W. et al. Genome-wide association analysis of meat quality traits in a porcine Large White × Minzhu intercross population. Int J Biol Sci. 8, 580–595 (2012).
Nonneman, D. et al. Genome-wide association of meat quality traits and tenderness in swine. J. Anim. Sci. 91, 4043–4050 (2013).
Ma, J. et al. Genome-wide association study of meat quality traits in a White Duroc × Erhualian F2 intercross and Chinese Sutai pigs. PloS One 8, e64047 (2013).
Becker, D., Wimmers, K., Luther, H., Hofer, A. & Leeb, T. A Genome-wide association study to detect QTL for commercially important traits in Swiss Large White boars. PloS One 8, e5595 (2013).
Sanchez, M. P. et al. Genome-wide association study of production traits in a commercial population of Large White pigs: evidence of haplotypes affecting meat quality. Genet Sel Evol. 46, 12 (2014).
Fang, M. & Andersson, L. Mitochondrial diversity in European and Chinese pigs is consistent with population expansions that occurred prior to domestication. Proc. Biol. Sci. 273, 1803–1810 (2006).
Gallardo, D. et al. Quantitative trait loci analysis of a Duroc commercial population highlights differences in the genetic determination of meat quality traits at two different muscles. Anim. Genet. 43, 800–804 (2012).
Gallardo, D. et al. Polymorphism of the pig acetyl-coenzyme A carboxylase α gene is associated with fatty acid composition in a Duroc commercial line. Anim. Genet. 40, 410–417 (2009).
Canovas, A., Quintanilla, R., Amills, M. & Pena, R. N. Muscle transcriptomic profiles in pigs with divergent phenotypes for fatness traits. BMC Genomics 11, 372 (2010).
Chomczynski, P. & Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156–159 (1987).
Gentleman, R. C. et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 5, R80 (2004).
Xu, X., Zhao, Y. & Simon, R. Gene set expression comparison kit for BRB-Array tools. Bioinformatics 24, 137–139 (2008).
Manunza, A. et al. A genome-wide association analysis for porcine serum lipid traits reveals the existence of age-specific genetic determinants. BMC Genomics 15, 758 (2014).
Zhou, X. & Stephens, M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 44, 821–824 (2012).
Casellas, J. et al. Bayes factor analyses of heritability for serum and muscle lipid traits in Duroc pigs. J. Anim. Sci. 88, 2246–2254 (2010).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300 (1995).
GTEx Consortium. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science 348, 648–660 (2015).
Smedley, D. et al. The BioMart community portal: an innovative alternative to large centralized data repositories. Nucleic Acids Res. 43, W589–598 (2015).
Larzul, C. et al. Selection for reduced muscle glycolytic potential in Large White pigs. II. Correlated responses in meat quality and muscle compositional traits. Genet. Sel. Evol. 31, 61–76 (1999).
Quintanilla, R., Pena, R. N., Cánovas, A. & Amills, M. Differential gene expression profile between two porcine skeletal muscles: longissimus dorsi and gluteus medius . In Book of abstracts of the 32th International Conference on Animal Genetics (2010).
Gjerlaug-Enger, E., Aass, L., Ødegard, J. & Vangen, O. Genetic parameters of meat quality traits in two pig breeds measured by rapid methods. Animal 4, 1832–1843 (2010).
Van Wijk, H. J. et al. Genetic parameters for carcass composition and pork quality estimated in a commercial production chain. J. Anim. Sci. 83, 324–333 (2005).
Zuk, O., Hechter, E., Sunyaev, S. & Lander, E. The mystery of missing heritability: Genetic interactions create phantom heritability. Proc. Natl. Acad. Sci. USA 109, 1193–1198 (2012).
Rosenvold, K. & Andersen, H. J. Factors of significance for pork quality-a review. Meat Sci. 64, 219–237 (2003).
Cepica, S. et al. Linkage and QTL mapping for sus scrofa chromosome 4. J. Anim. Breed. Genet. 120, 28–37 (2003).
Evans, G. J. et al. Identification of quantitative trait loci for production traits in commercial pig populations. Genetics 164, 621–627 (2003).
Li, H. D. et al. Quantitative trait loci analysis of swine meat quality traits. J. Anim. Sci. 88, 2904–2912 (2010).
Bernal Rubio, Y. L. et al. Implementing meta-analysis from genome-wide association studies for pork quality traits. J. Anim. Sci. 93, 5607–5617 (2015).
Ponsuksili, S., Murani, E., Trakooljul, N., Schwerin, M. & Wimmers, K. Discovery of candidate genes for muscle traits based on GWAS supported by eQTL-analysis. Int. J. Biol. Sci. 10, 327–337 (2014).
Liu, X. et al. Genome-wide association analyses for meat quality traits in Chinese Erhualian pigs and a Western Duroc × (Landrace × Yorkshire) commercial population. Genet. Sel. Evol. 47, 44 (2015).
Zhang, C. et al. Genome-wide association studies (GWAS) identify a QTL close to PRKAG3 affecting meat pH and colour in crossbred commercial pigs. BMC Genetics 16, 33 (2015).
Milan, D. et al. A mutation in PRKAG3 associated with excess glycogen content in pig skeletal muscle. Science 288, 1248–1251 (2000).
Yang, H. et al. Genome-wide association analysis for blood lipid traits measured in three pig populations reveals a substantial level of genetic heterogeneity. PloS One 10, e0131667 (2015).
Ernst, C. W. & Steibel, J. P. Molecular advances in QTL discovery and application in pig breeding. Trends Genet. 29, 215–224 (2013).
Ma, J. et al. A Splice mutation in the PHKG1 gene causes high glycogen content and low meat quality in pig skeletal muscle. PLoS Genet. 10, e1004710 (2014).
Dutson, T. R. & Lawrie, R. A. Release of lysosomal enzymes during post mortem conditioning and their relationship to tenderness. Int. J. Food Sci. Tech. 9, 43–50 (2007).
Hiraiwa, M. Cathepsin A/protective protein: an unusual lysosomal multifunctional protein. Cell. Mol. Life Sci. 56, 894–907 (1999).
Naz, H. et al. Human β-glucuronidase: structure, function, and application in enzyme replacement therapy. Rejuvenation Res. 16, 352–363 (2013).
Chang, H. J., Xu, X. L., Zhou, G. H., Li, C. B. & Huang, M. Effects of characteristics changes of collagen on meat physicochemical properties of beef semitendinosus muscle during ultrasonic processing. Food Bioprocess. Technol. 5, 285–297 (2009).
Hong, S. et al. Upregulation of adenylate cyclase 3 (ADCY3) increases the tumorigenic potential of cells by activating the CREB pathway. Oncotarget 4, 1791–1803 (2013).
Urso, L. et al. Alterations in mRNA expression and protein products following spinal cord injury in humans. J. Physiol. 579, 877–892 (2007).
Ramayo-Caldas, Y. et al. Genome-wide association study for intramuscular fatty acid composition in an Iberian × Landrace cross. J. Anim. Sci. 90, 2883–2893 (2012).
Fontanesi, L. et al. A genome wide association study for backfat thickness in Italian Large White pigs highlights new regions affecting fat deposition including neuronal genes. BMC Genomics 13, 583 (2012).
Becker, D., Wimmers, K., Luther, H., Hofer, A. & Leeb, T. A genome-wide association study to detect QTL for commercially important traits in Swiss Large White boars. PLoS One 8, e55951 (2013).
Acknowledgements
The authors are indebted to Selección Batallé S.A. for providing the animal material. We gratefully acknowledge to J. Reixach (Selección Batallé), I. Díaz (IRTA) and J. Soler (IRTA) for their collaboration in the experimental protocols. Thanks to Anna Mercadé and Anna Castelló for their technical support. This research was partially funded with projects AGL2013-48742-C2-1-R and AGL2013-48742-C2-2-R, awarded by the Spanish Ministry of Economy and Competitiveness (MINECO). We also acknowledge the support of the Spanish Ministry of Economy and Competitiveness for the Center of Excellence Severo Ochoa 2016–2019 (SEV-2015-0533) grant awarded to the Center for Research in Agricultural Genomics. Rayner Gonzalez-Prendes was funded by a FPU Ph.D. grant from the Spanish Ministerio de Educación (FPU12/00860). Thanks also to the CERCA Programme of the Generalitat de Catalunya.
Author information
Authors and Affiliations
Contributions
R.Q., M.A., J.L.N., A.M. and J.J. conceived the study and designed the experiment; R.Q. and J.L.N. produced the animal material and collected the phenotypic data; T.F. contributed to molecular tasks; R.G.-P. carried out the genome-wide association analyses for meat quality phenotypes and expression data; R.N.P. and A.C. contributed to the analysis of microarray data; R.G.-P. and M.A. wrote the manuscript. All authors helped to draft the manuscript and read and approved its final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
González-Prendes, R., Quintanilla, R., Cánovas, A. et al. Joint QTL mapping and gene expression analysis identify positional candidate genes influencing pork quality traits. Sci Rep 7, 39830 (2017). https://doi.org/10.1038/srep39830
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep39830
- Springer Nature Limited
This article is cited by
-
The downregulation of genes encoding muscle proteins have a potential role in the development of scrotal hernia in pigs
Molecular Biology Reports (2024)
-
Genetic regulation and variation of expression of miRNA and mRNA transcripts in fetal muscle tissue in the context of sex, dam and variable fetal weight
Biology of Sex Differences (2022)
-
Evidence for and localization of proposed causative variants in cattle and pig genomes
Genetics Selection Evolution (2021)
-
Expression quantitative trait loci in sheep liver and muscle contribute to variations in meat traits
Genetics Selection Evolution (2021)
-
Development and comparison of RNA-sequencing pipelines for more accurate SNP identification: practical example of functional SNP detection associated with feed efficiency in Nellore beef cattle
BMC Genomics (2020)