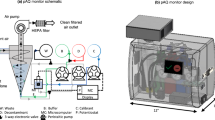
Abstract
The respiratory system is equipped with several defence mechanisms to protect the body from microorganisms and airborne pathogens. There are situations where the respiratory system can be overwhelmed or compromised and infection happens, especially in individuals with weakened immune systems. Airborne pathogens are a serious risk for human and animal health, as exemplified by the challenges faced during the COVID-19 pandemic. The list includes viruses of varying severity such as Influenza virus, SARS-CoV-2, measles virus, Varicella-Zoster Virus, or Respiratory syncytial virus among others. Smaller particles can remain suspended in the air for longer periods and may reach the lower respiratory tract, including the alveoli in the lungs. The real-time detection of these pathogens in the air presents a significant challenge. The aerosols, especially those carrying viruses, are so small that they often elude conventional air samplers, making it difficult both to detect their presence and to remove them effectively from the air. This work introduces a recent technique designed for rapid aerosol sampling, with a particular emphasis on virus sampling. The system underwent calibration using artificial ɸ29 virus aerosols and was subsequently tested with naturally emitted aerosols of SARS-CoV-2. A series of tests were conducted in diverse settings, including hospitals, farms, offices, and railway cars. The equipment is also capable of swiftly removing bioaerosols from the air, thereby facilitating effective decontamination. The relevance of this technology lies in its capability for swift detection and elimination of viruses and other kinds of aerosols from air, facilitating prompt decision-making during high-risk events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Transmission of diseases through air has been extensively studied in the recent years due to COVID-19 pandemic. Airborne transmission is traditionally defined as involving the inhalation of infectious aerosols or “droplet nuclei” smaller than 5 μm, mainly at a distance of > 1–2 m away from the infected individual, and only for “unusual” diseases. However, there is robust evidence supporting the airborne transmission of many respiratory viruses [1]. Particles up to 20 µm with favourable aerodynamic characteristics and low settling velocity or by quickly evaporating can also remain longer in the air, behaving as aerosols in suspension [2,3,4]. The list of respiratory viruses includes viruses of varying severity such as Influenza virus, SARS-CoV-2, measles virus, Varicella-Zoster Virus, or Respiratory syncytial virus and also bacteria such as tuberculosis or respiratory anthrax among others. It is essential to enhance our understanding of the airborne transmission pathway of respiratory viruses, enabling the formulation of more informed strategies to mitigate the spread of respiratory infections.
Over the years, different methodologies for sampling the air have been developed; however, not all of them are capable of collecting the finest particles such as viruses. The finest aerosols are precisely the most dangerous as they remain airborne much longer and are more difficult to filter. In this work, we elaborate on the development of a new device for the surveillance and sampling of agents present in the air. This system is called bioaerosol fast sampler (BIAFTS), and it is based in counterfog technology. Counterfog technology was developed under a FP7 programme project. It has been proposed as a rapid decontamination and disinfection technology of air and surfaces. Counterfog technology generates a dynamic jet of fog whose submicrometric droplets collide and aggregate with the smallest NBRQ aerosols [5]. It was designed for collapsing all kind of dispersed agents using a fog made of a solution that can also contain any neutralizing component. Based on this, BIAFTS has been designed as a portable device that collects airborne particles into a liquid medium in just 2 min using this principle of aggregation and collision of particles [6]. This work also showcases the results and effectiveness of BIAFTS in capturing viruses in various environments, including controlled and known conditions, hospital settings, and farm surveillance.
2 Calibration of the system
In the initial phase of developing the bioaerosol fast sampler (BIAFTS), calibration tests were conducted to determine the recovered virus quantity and, consequently, the efficiency of the system. These calibration tests played a crucial role in establishing the optimal design features of BIAFTS, including sampling distances, the methodology for liquid recovery, and minimum sampling time. The calibration tests involved a comparison with another validated methodology for air sampling—a filtration system that utilizes PTFE filters with a very small pore size. The testing took place in a simulated operational environment, specifically a railway car of 156.47 m3 with no ventilation system, mimicking real-world conditions. Prior tests in a laboratory setting were also carried out to fine-tune the system.
A known quantity of bacteriophage ɸ29, utilized as a surrogate for viruses, was intentionally dispersed throughout the entire railway car. An aliquot of 7.6 × 1011 pfu (plaque forming units) of ɸ29 was diluted in 1L of phage diluent solution (50 mM TrisClH pH 7.8, 10 mM MgCl2, 100 mM NaCl, 0.05% Tween-20). Three air pumps, each equipped with PTFE filters, were strategically placed within the railway car—two at the ends and one in the centre. Liquid samples, utilizing the BIAFTS system, were taken from one side of the railway car. Both samplers were positioned at approximately 1.5 m in height. Following nebulization, the PTFE filtration systems were activated and in-liquid samples were simultaneously collected using the BIAFTS system. Air samples were taken at various intervals over a 1-h period post-nebulization: PTFE filters (10 min, 0.3 m3/min) and BIAFTS (2 min, 1.4 m3/min). All samples were stored at 4 °C until further processing.
Furthermore, the number and size of particles were continuously monitored by a particle counter with a 10% mass concentration precision (Sensirion Particle Sensor SPS30, Sensirion, Stäfa, Switzerland). This device was positioned near the central door of the railway car at an approximate height of 0.6 m.
The results show a comparable recovery of viral particles with both samplers: BIAFTS and filtration system (Fig. 1A). Figure 1B shows the decay as a function of particle size. The figure shows that the decay is proportional to the particle size. Larger particles exhibit a steeper logarithmic decay over time. Comparing the results in Fig. 1B with the ɸ29 aerosols’ slope in Fig. 1A, it appears that the generated ɸ29 aerosols decayed according to a droplet size range of 0.5–1 µm. The decay value suggests that the BIAFTS is capable of capturing particles of at least 0.5–1 µm [6].
Results from calibration tests in railway car. A Viral titre of bacteriophage ɸ29 (PFU/m3) recovered using BIAFTS and PTFE filtration. B Natural aerosol decay measured in number of particles/cm3 for different particle sizes: ≤ 0.5 µm (dark blue), 0.5–1 µm (red), 1–2.5 µm (yellow), 2.5–4 µm (light blue), and 4–10 µm (grey). These graphics were created with Microsoft Excel
The results of these calibration tests led to improvements in the design and functionality of BIAFTS for the subsequent tests in hospital environment.
“Fast Air-to-Liquid Sampler Detects Surges in SARS-CoV-2 Aerosol Levels in Hospital Rooms” Licensed under a Creative Commons Attribution 4.0 License.
3 Hospital rooms sampling during COVID-19
After conducting initial tests with artificial aerosols of the φ29 surrogate, real-world field tests were executed. Samples of SARS-CoV-2 were collected using both technologies in rooms containing COVID-19 patients at the Hospital Universitario Fundación Alcorcón in Madrid. The sampling duration for PTFE filters ranged from 1 h and 45 min to 2 h, and the collected filters were stored in tubes containing 2 mL of phosphate-buffered saline (PBS) supplemented with 10 µg/mL bovine serum albumin (BSA), 100 µg/mL gentamicin, 200 U/mL penicillin, and 20 µg/mL streptomycin. Air samples from BIAFTS were collected in 2–4 min in 50-mL Falcon tubes using PBS medium supplemented with 0.1% BSA, 100 µg/mL gentamicin, 200 U/mL penicillin, and 20 µg/mL streptomycin. All samples were kept at 4 °C until further processing. Testing was conducted in six hospital rooms and two hospital bathrooms occupied by COVID-19 patients in the early stages of infection (less than 3 days post-infection). Both systems were positioned in the middle of the room at a considerable distance from patients' beds. After the tests, samples were taken to the laboratory and the presence of SARS-CoV-2 was determined by RT-qPCR in a CFX Opus 384 Real-Time PCR system (BIORAD).
The results of these sampling tests with both, BIAFTS and PTFE filters are presented in Table 1 [6].
Result interpretation as “indeterminate” refers to those samples with Ct > 40 for one of the N-genes (N1 or N2).
The results show the detection of SARS-CoV-2 in the air using both systems simultaneously. The concentration of SARS-CoV-2 in the air is typically low, as indicated by high Ct values in RT-qPCR analysis. This is in line with the understanding that the vast volume of air serves as a diluting factor, leading to a lower density of microorganisms compared to more concentrated sources, such as clinical samples from infected patients. PTFE filters allowed us to see the average of virus concentration in a period of 2 h, whereas BIAFTS is a fast sampler that allows punctual sampling in only a few minutes. With both systems, we obtained comparable results (Table 1). Results from each room are different because patients and the aerosol emissions were different. We can observe rooms with no or very low SARS-CoV-2-containing aerosols concentration (rooms 4 and 6) and others with higher levels of SARS-CoV-2 in aerosols (room 5). Interestingly, levels of SARS-CoV-2-containing aerosols were found to be similar in bathrooms, even when there were no patients present during the sampling, as in rooms 4 and 5. This observation highlights the transport of aerosols through the air. Surprisingly, results obtained from BIAFTS samples show that there are sudden boosts in SARS-CoV-2-containing aerosols, detecting moments of high concentration of viral genome. This is especially striking in rooms 1 and 2 (Table 1) [6].
BIAFTS has demonstrated its capability to swiftly capture naturally emitted aerosols from infected patients, showcasing promising results [6]. Detecting specific viruses in the air is challenging due to their generally low concentration. Following the tests at Hospital de Alcorcón, additional enhancements have been implemented in the BIAFTS device, and it is currently in continuous use across various environments.
“Fast Air-to-Liquid Sampler Detects Surges in SARS-CoV-2 Aerosol Levels in Hospital Rooms” Licensed under a Creative Commons Attribution 4.0 License.
4 Intensive pig farm sampling
BIAFTS has undergone enhancements and optimizations to enhance its efficiency in capturing microorganisms. An autoclavable module, consisting of a deposit and nozzle, has been developed to guarantee the sterility of the system and reliability of results. This configuration is specifically designed for the detection of airborne microorganisms, minimizing the risk of contaminations. The improved BIAFTS system has been successfully deployed in the HE FARM project from Horizon Europe programme aimed at enhancing biosecurity throughout the Farm-to-Fork chain.
HE-FARM project aims to investigate the various transmission channels through which microorganisms enter farms and how they are transported via these channels. By identifying methodologies and technologies to block these channels, we can help prevent the spread of pathogens. As part of the HE-FARM project, different types of farms have been visited, including both intensive and extensive operations as well as different species farms: pigs, cattle, poultry, snails…
BIAFTS has been employed in all these farm environments to quantify the presence of microorganisms in the air (Fig. 2).
This paper aims to highlight the evidence found in one of the farms visited, specifically an intensive mother and growing pig farm in Spain. In this farm, a positive status for PRRS (porcine reproductive and respiratory syndrome) had been identified. PRRS is one of the main pathogens targeted in HE-FARM project along with Avian Influenza Virus. This virus is one of the main threats that pig farms face nowadays along Europe, especially due to a highly virulent variant A targeted sampling campaign was organized to investigate the transmission channels of PRRS within the facility. Air samples were collected using both BIAFTS and PTFE filters, and surface samples were also obtained. PCR analysis revealed the presence of PRRS in samples obtained from various production stage areas of the farm, although not uniformly across all sections. Additionally, traces of PRRS were identified on surfaces within the facility. Figure 3 shows the viral structures observed by electron microscopy in the different samples.
Image of structural biology of PRRSV. Reference picture is from obtained from bibliography: The structural biology of PRRSV. Terje Dokland (2010) [7], compared with viral structures observed in BIAFTS and PTFE filter samples
5 Discussion
The novel device for sampling aerosols used in the present paper is distinguished by a fundamentally different operating principle compared to other air samplers documented in the literature. The latest findings of PRRS virus aerosols shown in the present paper are consistent with the prior work using the BIAFTS device that had previously sampled other viruses and other microorganisms from air.
This particularly dirty environment has been a real challenge for the processing of the samples. BIAFTS captures all types of airborne particles—including dust and smoke—and this non-specificity can pose a challenge on the isolation and growth of microorganisms, especially viruses, due to the interaction with dirt recovered from the air. Therefore, this paper demonstrates that an additional purification step for the samples is enough enabling the procedure. Other physical limitations have demonstrated to be necessarily taken into account -for example in outdoors environment where wind may reduce the sample recovery rate, although microorganism detection remains possible.
Ongoing improvements aim to enhance the preservation of samples during transport from field sites to laboratories and fast identification of the liquid sample.
The use of BIAFTS in different European projects will enable continuous development and the integration of improvements and new features, such as automated sample collection. Future directions in counterfog technology will involve testing and assessing the systems for emergency preparedness and CBRNe security. While tests have already been conducted with chemical substances, future efforts will focus on radiological and nuclear detection and counteraction.
6 Conclusions
In conclusion, the air-to-liquid sampler−BIAFTS is an effective and fast tool for capturing airborne matter, including bioaerosols, in a very short time, which allows fast decision-making when detecting any kind of hazard in the air. In this work, it is presented its capacity for capturing viruses present in the air. Due to the generally small size of the viruses and the low concentration of them in the air, BIAFTS is a very promising technology. Low virus concentration in the air is attributed to significant dilution within a spacious environment. The results obtained with BIAFTS are very powerful since similar results to those obtained with other air-sampling technologies, such as PTFE filtration, were obtained in much less time. PTFE filters measure the mean of the virus concentration over a period of 2 h, while BIAFTS is rapid sampler for spot sampling and it detects transient levels of aerosols. BIAFTS allows monitoring a large volume of air in a short period of time and offers the possibility of understanding aerosol dynamics by detecting aerosol levels at very specific times.
Another aspect to take into account, regarding the virus levels detected in aerosols, is the cleaning capacity of BIAFTS as it captures and collapses airborne agents, removing them from the air with an airflow of 1.44 m3/min.
Data Availability Statement
No data associated in the manuscript.
References
C.C. Wang, K.A. Prather, J. Sznitman, J.L. Jimenez, S.S. Lakdawala et al., Airborne transmission of respiratory viruses. Science 373(6558), 9149 (2021)
P.P. Moschovis, L.M. Yonker, J. Shah, D. Singh, P. Demokritou, T.B. Kinane, Aerosol transmission of SARS-CoV-2 by children and adults during the COVID-19 pandemic. Pediatr. Pulmonol. 56(6), 1389–1394 (2021)
J. Gralton, E. Tovey, M.L. McLaws, W.D. Rawlinson, The role of particle size in aerosolised pathogen transmission: a review. J. Infect. 62, 1–13 (2010)
J. Sánchez García-Casarrubios, F.J. Llerena-Aguilar, J.L. Pérez-Díaz, Fog Dynamics, in Enhancing CBRNE Safety & Security: Proceedings of the SICC 2017 Conference (2018).
J.L. Pérez Díaz, J. Sánchez García-Casarrubios, P. Méndez-Vigo Carranza, E.M. Ruiz Navas, A. Alcamí Pertejo et al., Fast surface disinfection with COUNTERFOG® SDR-F05A+. Eur. Phys. J. Plus 136, 1–8 (2021)
C. del Álamo, A. Vázquez-Calvo, A. Sanchiz, G. Rodríguez-Caravaca, R. Martín, B. Hernáez, J.L. Pérez-Díaz et al., Fast air-to-liquid sampler detects surges in SARS-CoV-2 aerosol levels in hospital rooms. Int. J. Environ. Res. Public Health 20(1), 576 (2023)
T. Dokland, The structural biology of PRRSV. Virus Res. 154(1–2), 86–97 (2010)
Acknowledgements
We would like to thank CAF the provision of infrastructure and assistance during the railway car assays. We also thank Gregorio Bonilla Zafra, and María Pilar Arribas Sancho from nursing department of Hospital Universitario Fundación Alcorcón for facilitating the logistics for taking air samples in hospital rooms. We extend our gratitude to the farms participating in the HE-FARM project, offering the use of their facilities and the valuable assistance of their staff.
Funding
This work has received funding from EU Horizon Europe under HE-FARM project with Grant number 101084097, H2020 INNO4COV19 grant 101016203, and Nextgeneration EU, and from Consejo Superior de Investigaciones Científicas (PTI Salud Global).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
José Luis Pérez-Díaz and Juan Sánchez-García-Casarrubios are shareholders of COUNTERFOG S.L, a spin-off of the FP7 Counterfog project. José Luis Pérez-Díaz and Juan Sánchez-García-Casarrubios report that they are inventors of patents US 15/799/545, ES2869723, PCT/ES2021/070232, EP 21793657.4, and US 17/972,951. All remaining authors report no other actual or potential conflicts of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pérez-Díaz, J.L., del Álamo Toraño, C., Alcamí, A. et al. Experimental assessment of counterfog bioaerosol fast sampler for virus detection and decontamination. Eur. Phys. J. Plus 139, 795 (2024). https://doi.org/10.1140/epjp/s13360-024-05441-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/s13360-024-05441-3