Abstract
Background
Despite strong recommendations for colorectal cancer (CRC) screening, participation rates are low. Understanding factors that affect screening choices is essential to developing future screening strategies. Therefore, this study assessed patient willingness to use non-invasive stool or blood based screening tests after refusing colonoscopy.
Methods
Participants were recruited during regular consultations. Demographic, health, psychological and socioeconomic factors were recorded. All subjects were advised to undergo screening by colonoscopy. Subjects who refused colonoscopy were offered a choice of non-invasive tests. Subjects who selected stool testing received a collection kit and instructions; subjects who selected plasma testing had a blood draw during the office visit. Stool samples were tested with the Hb/Hp Complex Elisa test, and blood samples were tested with the Epi proColon® 2.0 test. Patients who were positive for either were advised to have a diagnostic colonoscopy.
Results
63 of 172 subjects were compliant to screening colonoscopy (37%). 106 of the 109 subjects who refused colonoscopy accepted an alternative non-invasive method (97%). 90 selected the Septin9 blood test (83%), 16 selected a stool test (15%) and 3 refused any test (3%). Reasons for blood test preference included convenience of an office draw, overall convenience and less time consuming procedure.
Conclusions
97% of subjects refusing colonoscopy accepted a non-invasive screening test of which 83% chose the Septin9 blood test. The observation that participation can be increased by offering non-invasive tests, and that a blood test is the preferred option should be validated in a prospective trial in the screening setting.
Similar content being viewed by others
Background
Colorectal cancer (CRC) has been estimated to afflict 1.36 million people worldwide, accounting for nearly 10% of cancers [1] and is the second most common cause of death due to cancer in Europe [2]. It is well established that the five-year survival rate for CRC, which is greater than 90% for early localized cancer, drops to less than 5% for late stage metastatic disease. A number of CRC screening methods aimed at early detection have been developed, and there is a substantial body of evidence supporting the benefits of CRC screening [3–5]. Paradoxically, despite the clear and long standing evidence that CRC screening reduces mortality and may reduce cancer incidence, participation rates in screening programs remains too low, at an estimated 65% in the US [6] and ranging from 1.9% to 54% across Europe [7].
In Germany, screening by annual guaiac fecal occult blood tests (gFOBT) has been recommended since the mid 1970’s, and screening by colonoscopy was introduced as a covered option in 2002 [8, 9]. While screening is encouraged, organized nationwide screening activities are limited. Both methods are available without additional cost as part of health care coverage. Estimates of test usage in Germany indicate that for use of FOBT within the past year, only 14% of men and 22% of women were compliant, and that colonoscopy use within 10 years was 23% for men and 26% for women [8] though in most instances the colonoscopy was diagnostic rather than for screening. In Germany, nationwide data on screening colonoscopy, including adenoma detection, cecal intubation and complication rates amongst others are tracked through a central registry [10]. In the city of Berlin, the quality and performance of screening colonoscopy has been tracked through the Berlin colonoscopy projects - BECOP 1&3 [9, 11].
Given the low participation in CRC screening programs despite the clear medical benefit, it is important to understand the barriers to screening to develop successful alternative approaches. Numerous studies report behavioral as well as structural barriers that limit screening participation. These include factors specific to the tests themselves, such as embarrassment, fear of the procedure, or inconvenience, as well as broader factors such as lack of access to care, limited knowledge of screening and a lack of physician recommendation [reviewed in 12]. While these findings are clearly influenced by the country or health system of the participants, many factors (e.g. fear) are consistently reported in different settings [12]. To overcome these barriers, considerable effort has gone in to developing educational and outreach programs to improve screening rates. One aspect of this has been the demonstration that offering a choice in tests has a positive impact on participation in screening programs [13].
As indicated above, surveys, focus groups and patient interviews focused on understanding the resistance to screening, demonstrate that the screening modalities themselves (fecal sampling, bowel prep, colonoscopy etc.) present significant hurdles to patients [12]. The blood based test for CRC screening provides an alternative screening method based on the detection of methylated Septin9 DNA in patient plasma, and may overcome these barriers [14]. The test uses a standard EDTA plasma sample collected at the physician’s office or diagnostic laboratory.
The performance characteristics of the Epi proColon 2.0 CE blood test have been reported to be in the range of 70% sensitivity and 90% specificity in a number of studies [14–17]. The test has no dietary or time restrictions. The objective of the current study was to determine the impact of offering a new blood based test on the participation rate for CRC screening in Berlin, Germany rather than on characterizing performance. In addition, the study aimed to determine the relationships between demographic variables and test choice, and to survey the reasons for choosing non-invasive tests.
Methods
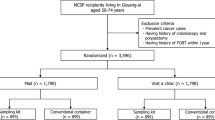
The study protocol was reviewed and approved by the Institutional Review Board of the Charite hospital. Subjects were recruited at 15 sites in Berlin (Germany) and surrounding areas, all of which were managed under the Charite hospital review board. All subjects provided written informed consent. Inclusion criteria were: age 50 to 75; Patient showing no symptoms that may indicate a tumor of the colon; Patient has no known inflammatory bowel disease; Patient has no known familial predisposition for colorectal cancer; Patient has no known strong family history or genetic predisposition to colorectal cancer; Informed Consent provided. The study design is summarized in Figure 1.
Study subjects
Subjects were recruited during regular consultations with primary care physicians or company doctors as outlined in the Additional file 1. Physicians followed a standardized recommendation script, first offering screening by colonoscopy, and for patients who refused, offered the option of non-invasive testing with the stool based immunochemical fecal occult blood test (FIT) or the Septin9 blood test. Although the protocol stated that all patients fitting the inclusion criteria be asked to participate, the total number of potential patients who could be invited to be screened was not recorded. 174 CRC screening eligible subjects were enrolled and provided demographic, health, psychological and socioeconomic data by a questionnaire (Tables 1, 2 and 3). Two subjects who were younger than 50 were removed from the analysis. Although the recommended age for screening by colonoscopy is 55 in Germany, screening by FIT/FOBT is promoted for patients aged 50+ and therefore all subjects age 50+ were included in the analysis. All subjects were advised to undergo screening by colonoscopy and the screening decision was recorded. Subjects who accepted colonoscopy were not included in the remainder of the study.
Subjects who refused screening colonoscopy were offered the option of either a FIT test (Hb / Hp–Complex ELISA, MDI Laboratorien GmbH, Berlin Germany) or an Epi proColon blood test (Epigenomics AG, Berlin, Germany). Physicians provided approved descriptive materials for each test. These subjects also filled out an additional questionnaire focused on determining the basis for their screening decisions (Additional file 1: Table S1–S11).
Laboratory testing
Subjects who selected a stool test were provided the Hb/Hp–Complex ELISA stool collection kit, instructions for sample collection, and were requested to provide the fecal sample to the testing lab. Subjects who selected the plasma test provided a blood sample as part of the physician visit. For both methods, tests were performed following the manufacturers instruction at a qualified laboratory and the test results were recorded. Individuals who were positive for either test were recommended to have a diagnostic colonoscopy.
Statistical analysis
Answers from the questionnaires were reported as simple numbers and percentages. The significance of demographic, health, psychological and medical variables between colonoscopy acceptors and refusers were analysed by the Chi squared test, (p < 0.05). To correct for age, logisitic regression models were used with acceptor/refuser as response and age as an additional variable.
Results
Of the 174 subjects enrolled, 2 were under the age of 50 and were excluded. Sixty three (36.6%) opted for screening colonoscopy and 109 (63.4%) refused (Figure 1). Demographic data for the 172 included subjects is summarized in Table 1 and the number refusing colonoscopy is indicated.
Self reported health information is reported in Table 2, and knowledge of cancer, colorectal cancer and general gastrointestinal health is outlined in Table 3, and the numbers refusing colonoscopy are indicated. For a number of parameters, a more detailed breakdown is reported in the Additional file 1: Table S1. Subject variables were analyzed using the Chi squared test to determine differences between colonoscopy acceptors and refusers. Significant differences were observed for the amount of weekly exercise (p ≤ 0.001) where those who excercised more frequently were also more likely to opt for a non-invasive test than a colonoscopy (Table 4). In the analysis with additional subgroups, subjects perceived CRC risk (p ≤ 0.01) was also significant, while factors such as monthly income and education level were close to significant (Table 4). While these variables were significant, no trend was observed for these variables. When further analyzed using logistic regression models with age as an additional variable, only level of exercise remained significant (p < 0.01). The remaining variables did not differ significantly at the p ≤ 0.05 level. General awareness of screening methods and prior use of tests is presented in Table 5, which illustrates that 95% of participants had an awareness of colorectal cancer screening tests.
The subjects who refused colonoscopy were offered non-invasive screening. Of the 109 subjects who rejected colonoscopy, 90 (82.6%) opted for the blood test, 16 (14.7%) opted for the Hb/Hp–Complex ELISA FOBT test, and 3 (2.8%) refused both options (Figure 1). These subjects also answered questions related to their screening decision. As indicated in the Additional file 1: Table S2, the top three reasons for rejecting colonoscopy were being uncomfortable with the bowel preparation for colonoscopy (54%), fear of colorectal cancer itself (44%) and fear that colonoscopy would be painful (32%). These results were corroborated in a follow-up question asking what would convince subjects to be screened by colonoscopy where 38% indicated an improved bowel preparation, 29% indicated cancer prevention by polypectomy and 24% indicated that overcoming fears would change their minds (Additional file 1: Tables S3). In addition, when asked why they chose one of the screening tests, 78% and 81% of subjects who had a blood and stool test respectively, indicated ease of getting the test (Additional file 1: Tables S4, S5, S7, S8). For those choosing the blood test, primary reasons for not choosing the stool test related to being uncomfortable with specimen handling (Additional file 1: Table S6). For those choosing the stool test, the primary reason related to having used a stool test in the past (Additional file 1: Table S9). As only 3 subjects rejected any form of testing, limited survey data is available (Additional file 1: Table S10 through S12).
Finally, though not the focus of this study, the two subjects who were positive for the Septin9 test and the two who were positive for FIT went on to colonoscopy.
Discussion
In this observational study, we report the impact of providing a choice of non-invasive screening tests on participation in colorectal cancer screening in Berlin, Germany and surrounding areas. We also report the results of surveys of participants addressing their perspectives on the different screening test options. In this study, 36.6% of participants chose to have a screening colonoscopy. Among the 63.4% who refused colonoscopy, 82.6% selected the Septin9 blood test, 14.7% selected the stool test and 2.8% refused any test. Thus, when all methods were considered, screening levels reached 98% (169/172 subjects).
Study recruitment was undertaken in Occupational Health and Primary Care settings, and therefore the following comparison of some of the key demographic data (Table 1) was made with census data from the German population [18]. The study enrolled a higher proportion of women (60.8%) which may be explained in part by the elevated ratio of women in the eligible age population (~53%) [18]. The age distribution was representative, with lower numbers enrolled in the 70+ age group compared to the total population (20% in the study vs 34% in the population) which reflects enrollment in the Occupational Health setting. Comparison of the migrant status of enrolled subjects was similar to that for the Berlin region, with 20.3% not German in the study, compared with 24% with a migrant background in the population [18]. The observed un-employment rate in the population was as expected (4.2% in the study, 4.6% in the age matched population) as were the rates of employed and retired subjects. Finally, the distribution of education level in the current study was higher on average than in the general population (56% no or lower education in the population compared with 40.4% in the study) though using the same metric for the Berlin region, the population estimate was 38% for no or lower education, which compares well with the 40.4% observed in the study [18]. While differences from the overall German population are not unexpected, given the sample size and the regional location of the study, the different demographic strata are represented. They are close to the observed levels for the Berlin region, thus allowing for extrapolation of the results to the region, and with caution, are also informative for the broader German population.
Given that enrollment was in a setting where CRC screening was promoted, this may account for the high degree of screening knowledge observed in the study. While the rate of acceptance of screening colonoscopy in this study (37%) was higher than the overall reported rate for the German population (~25%) [8], it remained well below that reported for the US. It is unclear why the rate of screening colonoscopy is higher than usually observed in Germany, though it may be attributed in part to participation in a study. As the study focused on the screening population, subjects were asymptomatic and representative of the broader population. It may however, also represent an overestimate, since the number refusing enrollment was not recorded.
There are only a few reports on barriers to acceptance of colonoscopy in the German screening population. In a 2009 report from the Leipzig area, the primary reasons for not being screened were a lack of awareness or recommendation for screening [19]. In a detailed survey from the Munich area, fear of the bowel preparation, lack of a physician recommendation and a lower interest in screening were associated with avoiding colonoscopy [20]. Interestingly, in that study, the demographics associated with having a colonoscopy were: lower education status, unemployment or retired, or having a primary care physician [20].
As shown in the detailed analysis in the supplement, a similar trend was observed for participants who completed Grade School compared with those completing A levels, though this was reversed for those who completed university. When these categories were aggregated, the difference was not significant. These trends differ from the US, where the lowest screening rates correlate with low socioeconomic status indicators such as income, education level and lack of work [12], and this illustrates the importance of developing an understanding of the issues at a local level. It is also interesting to note that we did not observe a correlation between refusal of colonoscopy and other health factors such as diet, alcohol consumption or smoking status.
In the current study, the primary reasons given for not having a colonoscopy were associated with fear, discomfort or concern about the bowel preparation or the colonoscopy procedure. This outcome suggests that better education about the procedure is a possible course for increasing screening by colonoscopy. In addition the primary reasons patients provided for selecting non-invasive tests related to convenience of use, and the selection of a blood test over a stool test was based on a preference not to handle stool samples. Thus, it appears that the preference for the blood test is not necessarily related to the performance of the test, but rather the convenience it offers with blood collection available at the physician’s office.
We observed that 97% of the participants who refused a colonoscopy were willing to accept a non-invasive test, despite these tests having lower performance outcomes. This aligns with the observation that educating patients with the evidence for the benefits of colonoscopy versus other screening methods had no impact on their attitude to CRC screening or their ultimate test choice [21]. Thus, understanding the patient’s motivation for screening is crucial to developing successful programs. This is underscored in a recent trial, where offering screening alternatives increased overall participation in a screening program [13]. It is further illustrated by the experience at Kaiser-Permanente in California, where, following failed efforts to implement screening by endoscopy, screening participation has consistently increased with the re-introduction of a non-invasive FIT test [22], despite the test having lower sensitivity.
It is clear from the results of the current study that offering a blood test as part of the screening menu further improves participation, as approximately 80 percent of subjects opted for a blood test. It is important to note that in the present study, the tests were provided at no cost to participants. As the blood test is not currently covered under national health care, the impact of cost to patient needs further analysis. Based on the survey data, key factors in the decision to be screened with the blood test were trust of blood tests, being comfortable with giving blood and the convenience of a blood draw compared with providing a stool sample. It is also interesting to note that providing test choice can improve screening participation, similar to what was observed by Inadomi et.al. [13] in a study in California, as well as in a discrete choice study in the Netherlands [23].
While it is clear that the ease and convenience of a blood test can improve screening participation, there are many additional factors that will determine the impact of a new screening test. In addition to performance characteristics, these include guideline recommendations, health economic considerations, and cost to patient amongst others. Despite this complex landscape, which includes significant differences in philosophy and approach by region and country, the data in this study support the idea that test convenience is an important consideration in the success of CRC screening programs.
With the limited sample size, and the observation of only a small number of patients who were positive for either non-invasive test, we did not perform statistical analysis on the test results, or whether subjects with a positive test result went on to colonoscopy. Anecdotally, the two subjects who were positive for the Septin9 test and the two who were positive for FIT went on to colonoscopy. Clearly, compliance with follow-up diagnostic colonoscopy is a critical aspect in the success of a screening program. In all four subjects, adenomas were removed completely during colonoscopy. One patient of the Septin9 group showed high grade intraepithelial neoplasia and the others had low grade intraepithelial neoplasias.
Limitations of the study
The study was subject to a number of limitations. 1) It was designed to enroll 100 subjects who refused colonoscopy. While this sample size was deemed sufficient to assess general preference for the two non-invasive screening modalities, it allows only limited observational analysis of subgroups. 2) By protocol, the study was designed to enroll all eligible subjects in each practice. However, the study did not include a mechanism to record the total number of subjects asked to participate or the number who refused to participate. In this respect, the results cannot be presented in the context of ‘intention to screen’. As a result, there may be bias in the study, resulting in the higher than expected estimation of participation rates. 3) The study was performed in a limited geographical setting (Berlin, Germany) and therefore, extrapolation to a broader national or broader European context should be done with caution.
Conclusions
The results of this study support the contention that providing non-invasive screening choices can augment participation in programs for colorectal cancer screening. The success of a screening program depends on having tests with acceptable performance, but also on the willingness of the target population to participate. This study demonstrates that offering non-invasive test options significantly increases compliance to colorectal cancer screening. Furthermore the addition of a convenient blood test that can be provided in the physician’s office, has the potential to improve screening participation.
Future studies will focus on characterization of complete screening programs, from the invitation to screening through to the completion of colonoscopy for patients with positive tests.
References
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F: GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: [http://globocan.iarc.fr] accessed on 13/05/2014
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F: Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013, 49 (6): 1374-1403. 10.1016/j.ejca.2012.12.027.
Kuipers EJ, Rosch T, Bretthauer M: Colorectal cancer screening – optimizing current strategies and new directions. Nat Rev Clin Oncol. 2013, 10 (3): 130-142.
Brenner H, Chang-Claude J, Jansen L, Knebel P, Stock C, Hoffmeister M: Reduced risk of colorectal cancer up to 10 y after screening, surveillance, or diagnostic colonoscopy. Gastroenterology. 2013, doi:10.1053/j.gastro.2013.09.001
U.S. Preventive Services Task Force: Screening for Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. AHRQ Publication 08-05124-EF-3, October 2008. [http://www.uspreventiveservicestaskforce.org/uspstf08/colocancer/colors.htm]
Joseph DA, King JB, Miller JW, Richardson LC: Prevalence of colorectal cancer screening among adults–behavioral risk factor surveillance system, United States, 2010. MMWR Morb Mortal Wkly Rep. 2012, 61 (Suppl): 51-56.
OECD: Screening, survival and mortality for colorectal cancer. Health at a Glance: Europe 2012. 2012, OECD Publishing, [http://dx.doi.org/10.1787/9789264183896-48-en]
Stock C, Ihle P, Brenner H: Colonoscopy and fecal occult blood test use in Germany: results from a large insurance-based cohort. Endoscopy. 2011, 43: 771-779. 10.1055/s-0030-1256504.
Adler A, Roll S, Marowski B, Drossel R, Rehs HU, Willich SN, Riese J, Wiedenmann B, Rösch T, Berlin Private-Practice Gastroenterology Working Group: Appropriateness of colonoscopy in the era of colorectal cancer screening: a prospective, multicenter study in a private-practice setting (Berlin Colonoscopy Project 1, BECOP 1). Dis Colon Rectum. 2007, 50: 1628-1638. 10.1007/s10350-007-9029-y.
Adler A, Lieberman D, Aminalai A, Aschenbeck J, Drossel R, Mayr M, Mroß M, Scheel M, Schröder A, Keining C, Stange G, Wiedenmann B, Gauger U, Altenhofen L, Rösch T: Data quality of the German screening colonoscopy registry. Endoscopy. 2013, 45 (10): 813-818.
Adler A, Wegscheider K, Lieberman D, Aminalai A, Aschenbeck J, Drossel R, Mayr M, Mroß M, Scheel M, Schröder A, Gerber K, Stange G, Roll S, Gauger U, Wiedenmann B, Altenhofen L, Rosch T: Factors determining the quality of screening colonoscopy: a prospective study on adenoma detection rates, from 12,134 examinations (Berlin colonoscopy project 3, BECOP-3). Gut. 2013, 62 (2): 236-241. 10.1136/gutjnl-2011-300167.
Gimeno García AZ: Factors influencing colorectal cancer screening participation. Gastroenterol Res Pract. 2012, 2012: 483417-doi:10.1155/2012/483417. Epub 2011 Dec 1
Inadomi J, Vijan S, Janz NK, Fagerlin A, Thomas JP, Lin YV, Munoz R, Lau C, Somsouk M, El-Nachef N, Hayward RA: Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med. 2012, 172 (7): 575-582. 10.1001/archinternmed.2012.332.
Weiss G, Rösch T: Potential of a new blood test for colorectal cancer screening – the septin 9 gene biomarker. Eur Oncol. 2010, 6 (1): 51-54.
Payne S: From discovery to the clinic: the novel DNA methylation biomarker mSEPT9 for the detection of colorectal cancer in blood. Epigenomics. 2010, 2 (4): 575-585. 10.2217/epi.10.35.
Warren JD, Xiong W, Bunker AM, Vaughn CP, Furtado LV, Roberts WL, Fang JC, Samowitz WS, Heichman KA: Septin 9 methylated DNA is a sensitive and specific blood test for colorectal cancer. BMC Med. 2011, 9: 133-10.1186/1741-7015-9-133.
Tóth K, Sipos F, Kalmár A, Patai AV, Wichmann B, Stoehr R, Golcher H, Schellerer V, Tulassay Z, Molnár B: Detection of methylated SEPT9 in plasma is a reliable screening method for both left- and right-sided colon cancers. PLoS One. 2012, 7 (9): e46000-10.1371/journal.pone.0046000. doi:10.1371/journal.pone.0046000. Epub 2012 Sep 25
German Census 2011 Data. [https://ergebnisse.zensus2011.de/?locale=en#] accessed Sept. 15th, 2014
Schoppmeyer K, Spieker H, Mossner J: Failure of screening or failure to screen?. Dtsch Arztebl Int. 2009, 106 (12): 195-201.
Ziegler M, Shubrin-Giese B, Buhner M: Attitude to secondary prevention and concerns about colonoscopy are independent predictors of acceptance of screening colonoscopy. Digestion. 2010, 81: 120-126. 10.1159/000223448.
Steckelberg A, Hulfenhaus C, Haastert B, Muhlhauser I: Effect of evidence based risk information on “informed choice” in colorectal cancer screening: randomized controlled trial. BMJ. 2011, 342: d3193-10.1136/bmj.d3193. doi:10.1136/bmj.d3193
Moiel D, Thompson J: Early detection of colon cancer-the Kaiser Permanente northwest 30-year history: how do we measure success? Is it the test, the number of tests, the stage, or the percentage of screen-detected patients?. Perm J. 2011, 4: 30-38.
Benning TM, Dellaert BGC, Dirksen CD, Severens JL: Preferences for potential innovations in non-invasive colorectal cancer screening: a labeled discrete choice experiment for a Dutch screening campaign. Acta Oncol. 2014, doi:10.3109/0284186X.2013.877159
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-230X/14/183/prepub
Acknowledgements
The authors wish to acknowledge the support of Thomas Koenig for data analysis, and Melanie Martini for data collection and review. The authors also wish to acknowledge the support of Abbott GmbH & Co. for partial sponsorship of the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
PS – At the time of the study, PS was an employee of Epigenomics, a study sponsor. TdV – At the time of the study TdV was an employee of Epigenomics, a study sponsor. JD – At the time of the study JD was an employee Abbott Molecular, a study sponsor. All other authors have no competing interests. The study was funded by grants from Epigenomics AG and Abbott Molecular.
Authors’ contributions
AA – study PI, study design, drafted manuscript. SG – study design, AK – study implementation, data collection, data review. HB – study design. PS – study design, data analysis, manuscript drafting. TdV – data analysis, manuscript drafting. JD – study design. MZ – analysis of test results. RT – analysis of test results. BW – study design and approval. All authors read and approved the final manuscript.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Adler, A., Geiger, S., Keil, A. et al. Improving compliance to colorectal cancer screening using blood and stool based tests in patients refusing screening colonoscopy in Germany. BMC Gastroenterol 14, 183 (2014). https://doi.org/10.1186/1471-230X-14-183
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-230X-14-183