Abstract
Background
Migraine is a cyclic, neurosensory disorder characterized by recurrent headaches and altered sensory processing. The latter is manifested in hypersensitivity to visual stimuli, measured with questionnaires and sensory thresholds, as well as in abnormal cortical excitability and a lack of habituation, assessed with visual evoked potentials elicited by pattern-reversal stimulation. Here, the goal was to determine whether factors such as age and/or disease severity may exert a modulatory influence on sensory sensitivity, cortical excitability, and habituation.
Methods
Two similar experiments were carried out, the first comparing 24 young, episodic migraine patients and 28 healthy age- and gender-matched controls and the second 36 middle-aged, episodic migraine patients and 30 healthy age- and gender-matched controls. A neurologist confirmed the diagnoses. Migraine phases were obtained using eDiaries. Sensory sensitivity was assessed with the Sensory Perception Quotient and group comparisons were carried out. We obtained pattern-reversal visual evoked potentials and calculated the N1-P1 Peak-to-Peak amplitude. Two linear mixed-effects models were fitted to these data. The first model had Block (first block, last block) and Group (patients, controls) as fixed factors, whereas the second model had Trial (all trials) and Group as fixed factors. Participant was included as a random factor in both. N1-P1 first block amplitude was used to assess cortical excitability and habituation was defined as a decrease of N1-P1 amplitude across Blocks/Trials. Both experiments were performed interictally.
Results
The final samples consisted of 18 patients with episodic migraine and 27 headache-free controls (first experiment) and 19 patients and 29 controls (second experiment). In both experiments, patients reported increased visual hypersensitivity on the Sensory Perception Quotient as compared to controls. Regarding N1-P1 peak-to-peak data, there was no main effect of Group, indicating no differences in cortical excitability between groups. Finally, significant main effects of both Block and Trial were found indicating habituation in both groups, regardless of age and headache frequency.
Conclusions
The results of this study yielded evidence for significant hypersensitivity in patients but no significant differences in either habituation or cortical excitability, as compared to headache-free controls. Although the alterations in patients may be less pronounced than originally anticipated they demonstrate the need for the definition and standardization of optimal methodological parameters.
Similar content being viewed by others
Background
Migraine is often characterized as a cyclic, neurosensory disorder due to reports of altered sensory processing in both ictal (migraine attack) and interictal (attack-free) phases [1, 2]. Although sensory alterations have been observed in different modalities, the visual one remains the most highly researched [3, 4]. Furthermore, aside from the presence of ictal photophobia as a criterion for the diagnosis of migraine [5] visual alterations have also been reported interictally.
Previous studies exploring whether sensory processing is altered in migraine have focused on different processes. The first of these, sensory sensitivity, or hypersensitivity (i.e., a heightened perception or discomfort) to a variety of stimuli and stimulus characteristics, has traditionally been measured using self-report questionnaires [6,7,8] and sensory thresholds [9,10,11,12]. In the interictal phase, patients with migraine have been found to report an increased number of visual stressors in their environment, including a heightened sensitivity to glare, flicker, and contrasting patterns [6, 13] as well as decreased visual thresholds [10, 14, 15]. One of the questionnaires used to assess sensory sensitivity is the Sensory Perception Quotient (SPQ), which evaluates a variety of visual parameters and has been used in both healthy and clinical populations [16].
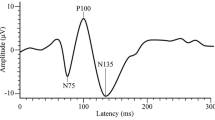
Another process, which has been assessed to understand sensory alterations in migraine is cortical excitability, with reports of abnormality (both hyper- and hypoexcitability), observed through either increased or decreased amplitudes of pattern-reversal visual evoked potentials (PR-VEPs) [17] (for a review see [18]). In fact, the combination of electroencephalography (EEG) and the Pattern-Reversal (PR) task (e.g., [18,19,20,21]), is frequently used to study visual processing, in both clinical and research applications [22]. This task consists of a black and white checkerboard with a given spatial frequency, which reverses its pattern at a predefined temporal frequency. The abrupt onset/offset stimulation constitutes a powerful tool to elicit visual evoked potentials including N1 (also referred to as N70, N75, N80) and P1 (P100). Both components have their maximum amplitude at posterior sites. N1 is a negative component peaking around 75 ms after stimulus onset/offset, sensitive to stimulus characteristics such as contrast [23], spatial frequency [24], stimulus salience, and the degree of attention [25]. P1, is a positive component, peaking around 100 ms, related to luminance [26] and contrast [23], and also modulated by stimulus unpleasantness [27]. Many studies using the PR task in migraine use a peak-to-peak difference amplitude measure (N1-P1) as an index of visual processing, given its correlation to psychophysical measures and to avoid the distortion of the amplitude of later components, such as P1, due to the earlier components, such as N1 [28]. In particular, the N1-P1 amplitude at the beginning of the experiment has frequently been used to assess cortical excitability [29]. Some studies found a decreased amplitude [30] whereas others reported an increased one [31, 32], in patients with migraine as compared to headache-free controls (despite [17, 33,34,35], for a review of the literature, see Table 1).
Finally, habituation has been studied in patients with migraine and headache-free controls, and a deficit of habituation or even potentiation of the N1-P1 peak-to-peak amplitude over time (or experimental blocks; final blocks compared to first blocks) has been found in patients with migraine interictally [19, 70]. Despite these findings being proposed as robust, some controversy remains, particularly given the presence of negative or inconsistent results [21, 31, 32, 35] (see Table 2 for a review of the literature).
The study of sensory sensitivity, cortical excitability, and habituation across the lifespan is relevant for better disease management. At a clinical level it would appear that migraine (not necessarily episodic migraine) incidence tends to peak in the late 30s [96] and then level off (although some studies report increased frequency during perimenopause and menopause) [97]. Furthermore, accompanying symptoms, in particular photophobia and phonophobia would appear to increase with age [98] up until a point after which, in older patients, over the age of 60, there is a reported decrease [99], and diminish with increasing migraine frequency [100]. In terms of sensory sensitivity, sensory thresholds have been shown to increase with older age, indicating decreased sensory sensitivity, usually in the 60s and onwards [101, 102] (for a review see [103]).
However, this remains inconclusive with regard to migraine symptomatology, given that it appears linked to the degeneration of sensory receptors ([104]; for a review see [105]). Pertaining to cortical excitability and habituation, to the best of our knowledge, no study has directly evaluated, using PR-VEPs their variation as a function of age and migraine frequency. With current data, it is not possible to establish a relationship between sensory sensitivity, cortical excitability, and habituation nor understand exactly how they are associated with measures related to age and migraine frequency.
The present study aimed to assess whether a relationship exists between visual sensitivity, often reported in episodic migraine interictally, and cortical excitability and/or habituation, and whether it might be modulated by age and disease severity. To accomplish this objective, we carried out a research study involving two experiments. Experiment 1 consisted of a sample of young adults with episodic migraine and their headache-free controls whereas Experiment 2 included middle-aged adults with episodic migraine and their headache-free controls. Given the state of the literature, we hypothesized the presence of hypersensitivity in patients with migraine in both experiments [9, 10, 12, 106] as well as, abnormal cortical excitability (hypo- or hyper-) and a deficit of habituation in patients with migraine as compared with headache-free controls [19, 20, 52] (for a review see [18]), although in the case of the latter, we accept that opposing results are entirely plausible. The novelty of our study can be found in its use of two experiments, which allowed us to collect data from patients with episodic migraine and their age- and gender-matched headache-free controls differing in age and headache frequency and a novel trial-by-trial analysis, using linear mixed-effects models (LMMs), which should help capture both individual and temporal variability.
Prior to their participation, all subjects provided written informed consent. See the Ethics approval and consent to participate section within Declarations for more information.
Experiment 1
Method
Participants
In this experiment, 63 young university students (all females, between 18–30 years old, right-handed, and with normal or corrected-to-normal vision) were included. 35 were diagnosed with episodic migraine (EM) with or without aura by a neurologist, according to the International Classification of Headache Disorders 3rd edition (ICHD-3) [5]. The remaining 28 participants constituted the headache-free control group (HC), which was age- and gender-matched to the EM group. One objective of this experiment was to have a very homogenous and clinically similar sample of participants to reduce biases and effectively compare brain responses. Diagnoses were not formally disclosed to the participants until the end of the study. Exclusion criteria included: known morphological brain abnormalities, neurological or severe psychiatric illness, chronic pain, cardiovascular disease, pregnancy, as well as the use of any pharmaceutical or non-pharmaceutical drugs that could alter the EEG waveform. Patients could not have been previously diagnosed with any other headache disorder and could not have been taking prophylactic medication. Controls could not have had any previous headache diagnosis or first-degree relatives with migraine. Considering previous literature indicating the importance of phase, particularly with regard to the habituation deficit [18], we excluded patients that were outside of the interictal phase (see Results). Specifically, patients who did not report a moderate to severe attack 24 h prior, the day of, and 24 h post-session (72-h headache-free window) were considered interictal (confirmed by a headache virtual daily calendar or eDiary).
Procedure and paradigm
Prior to the experimental session, potential participants completed a (A) sociodemographic and anthropometric questionnaire as well as a (B) migraine screening questionnaire based on ICHD-3 [5] criteria. Participants that fit the inclusion/exclusion criteria were subsequently assessed by a neurologist, assigned a diagnosis (EM or HC), and provided with a baseline, virtual, daily headache calendar (eDiary), which was used to obtain an objective measure of headache frequency and confirm interictal phase during the recording. The eDiary also contained questions relative to the presence of headache, its duration, intensity, accompanying symptoms, and acute medication as well as other medication, menses, and participant sleep–wake cycle. All of the participants filled it out for an average of 34 ± 8 days prior to the experimental session, as well as on the day of the recording and at least 24 h after to confirm interictal phase.
The session itself consisted of (i) psychiatric, clinical, and experimental session questionnaires and (ii) an EEG recording. The psychiatric questionnaires included the State-Trait Anxiety Inventory (STAI) [107, 108], ADHD Self-Report Scale (ASRS) [109], and Beck Depression Inventory-II (BDI-II) [110]. To assess sensory sensitivity, we used the SPQ, with lower scores denoting increased sensitivity [16], which evaluates sensory sensitivities across all five modalities and has been validated for use in both healthy adults and clinical populations. On the other hand, clinical questionnaires included the: Headache Impact Test-6 (HIT-6) [111], Migraine Disability Assessment Test (MIDAS) [112], and Migraine-Specific Quality of Life Questionnaire (MSQ) [113]. Participants also completed an experimental session questionnaire which inquired about headache presence and its characteristics, acute medication use, other medication use, sleep quality, fatigue, and menstruation, at the time of the experiment. The questionnaires and eDiary were hosted by Research Electronic Data Capture (REDCap) tools [114, 115], at the Vall d’Hebron Institute of Research.
The EEG recording consisted of a 5 min resting state recording followed by the PR task and was performed in a chamber with dimmed lights as well as acoustic and electromagnetic attenuation. Participants sat at a distance of 0.75 m from the computer monitor. The stimuli used for the PR task consisted of a checkerboard pattern of black and white squares (93% contrast; see Fig. 1A). The reversal frequency was set at 1.55 Hz and was based on Coppola et al. whereas the check size or spatial frequency was 6 min of arc (6’), adapted from the recommended 8’ at 1 m [61]. The stimulated visual field was 30.7 cm × 22.5 cm, under binocular presentation. A red fixation point at the center of the screen was present throughout the task to reduce ocular artifacts. Experimental stimuli were programmed and presented, using custom-made scripts, with MATLAB R2017a (The Mathworks Inc., 2017) and Psychophysics Toolbox Version 3.0.13 [116, 117], running on Windows XP. All stimuli were displayed on a Sony Multiscan G520 Trinitron Color Monitor (CRT screen, resolution: 1024 × 768, 120 Hz refresh rate, background luminance: 21 cd/m2). Accurate timing of stimuli was confirmed using the Black Box Toolkit (Accuracy of < 0.005 s (seconds); Black Box Toolkit, Ltd., Sheffield, UK). Participants were instructed to remain still and maintain their eyes on the fixation point. The task consisted of 600 trials (3.23 min total duration), segmented into six blocks of 100 trials post-recording.
Visual illustrations of the checkerboard pattern and resulting visual evoked potentials (VEPs) and habituation to pattern-reversal (PR) stimulation in Experiment 1. A Checkerboard pattern used in the PR task. B VEPs at the Oz electrode, with time (in ms) on the x-axis and N1-P1 peak-to-peak amplitude difference voltage on the y-axis, observed for each block (1 to 6) of the Pattern-Reversal task, and both groups (EM right, HC left). C Bar graph with Block number on the x-axis and mean N1-P1 peak-to-peak amplitude difference voltage on the y-axis, depicting habituation of the N1-P1 between Blocks 1 and 6 (green EM, blue HC). Please note the decrement in amplitude between the 1st and 6th block
EEG recordings
Continuous EEG recordings (digitized, 500 Hz sampling rate, no online filters) were acquired using a BrainAmp Standard (001 10/2008) amplifier connected to an actiCHamp Control Box (BrainVision Analyzer, Version 2.2.2, Brain Products GmbH, Gilching, Germany). 64 active electrodes (10–10 system) at standard positions were used and an online reference electrode was placed on the tip of the nose whereas a ground electrode was positioned at AFz in the cap. External electrodes consisted of left and right mastoids as well as vertical and horizontal electrooculograms. Impedances were maintained below 15 kΩ.
EEG pre-processing
EEG data analyses were performed using EEGLAB 13.5.4b [118] and ERPLAB 7.0.0 [119], as well as MATLAB R2017a (The Mathworks Inc., 2017) custom-made scripts. EEG pre-processing was carried out offline according to standard procedure, which included the use of a 50 Hz notch filter (stop-band Parks-McClellan notch, 180 order). Next, interpolation of noisy channels was done. After, data was band-pass filtered, in two steps, using a Hamming windowed sinc finite impulse response (FIR) filter (zero-phase). First, a high-pass filter of 0.1 Hz (16,501 order, -6 dB cutoff) was applied followed by a low-pass filter of 60 Hz (111 order, -6 dB cutoff). Subsequently, data was segmented to epochs of 0–300 ms time-locked to the reversals and normalized by the mean segment activity, which was re-referenced to the mean-activity of both mastoids. Finally, visual inspection and manual rejection were performed to remove epochs with noise.
Analyses
Statistical analyses were carried out using R (R Core Team, 2021, version 4.1.1) and RStudio software (RStudio Team, 2021, version 1.4.1717). The following packages were used: base, car, dgof, dplyr, emmeans, ggpubr, ggResidpanel, graphics, lattice, lme4, nlme, multiplyr, nortest, pgirmess, psych, rstatix, and stats.
Psychiatric, clinical, and SPQ questionnaires
Data from the psychiatric, clinical, and SPQ questionnaires were reported using percentages (categorical), means and standard deviations (continuous, normally distributed), or medians and interquartile ranges (continuous, not normally distributed). Group effects were evaluated with Fisher’s exact test, two-sided, unpaired t-tests of equal variance, or two-sided, nonparametric Mann–Whitney U test, respectively.
Electrophysiological data
PR-VEP. The amplitudes and latencies of N1 and P1 as well as the Peak-to-Peak amplitude difference (N1-P1) were obtained for each trial (1–600), block (1–6), and participant at the Oz electrode (active electrode in [18,19,20], among others). Only clean trials, free of artifacts, were used. Each block contained a maximum of 100 clean trials, with a mean of 89.46 ± 9.093 (range: 30–100) trials per participant per block. Participants had a grand mean of 536.78 ± 36.962 trials, post artifact rejection, out of a total of 600 (range: 435–595). Components were identified based on visual inspection and peak latencies (reversal-locked) with N1 being the most negative peak between 65–95 ms (peak: 80 ms; window: ± 15 ms) and P1 being the most positive peak between 86–126 ms (peak:106 ms; window: ± 20 ms). The amplitudes of P1 and N1 used to calculate the N1-P1 peak-to-peak were extracted using an automatic system and subsequent visual inspection [61].
Classic block analyses. The first series of analyses were comprised of classic block analyses on the N1-P1 peak-to-peak amplitude [120]. A type III two-way mixed analysis of variance (ANOVA) was used with Block (1 and 6) as the between-subject factor and Group (EM and HC) as the within-subject factor. In the event that post hoc tests were necessary, pairwise comparisons were executed, and Bonferroni-adjusted p values were obtained (padj).
Block linear mixed-effects model. LMMs were fitted to N1-P1 data, using the nlme package in R [121], to evaluate cortical excitability and habituation. The fixed effects variables were Block (1 and 6) and Group (EM and HC), and the random effects variable was Participant. We also tested an autocorrelation structure of order 1, in the form of Participant nested within Trial. To ensure that our model was the best alternative, we ran model comparisons using the Akaike Information Criterion (AIC) and a Chi-square test on the model log-likelihoods (Chisq) [122]. Using the final model, we obtained a type III ANOVA table calculating Kenward-Roger "F" tests with Satterthwaite degrees of freedom, where the within-subject factor was Block and the between-subject factor was Group (F and p-values were reported). The confidence level was set to 0.95. Visual inspection of residual plots did not reveal deviations from homoscedasticity or normality in any measure. In the presence of a significant Block x Group interaction, estimated marginal means were calculated to do post hoc, pairwise comparisons, and z ratios and p values were reported. The False Discovery Rate (FDR) was applied to adjust for multiple comparisons.
Cortical excitability was assessed by examining peak-to-peak first block amplitude, with differences in this measure suggesting either hypo- (significantly lower amplitude) or hyper-excitability (significantly greater amplitude) in patients with migraine as compared to headache-free controls [29]. Habituation on the other hand referred to a peak-to-peak amplitude decrement between the first and last block [19, 70] and was evaluated both within- and between-groups. Only data from Blocks 1 and 6 were used given that habituation was defined as the difference in the N1-P1 peak-to-peak amplitude between the first and last block (for a review see Table 5 in [71]). Please note that to perform these comparisons the presence of a Block x Group interaction was necessary.
Trial linear mixed-effects model. Using the N1-P1 peak-to-peak data, we also went one step further and fitted LMMs using the nlme package in R [121] taking into account trial-by-trial fluctuation thus increasing the precision of our measures. The first model (the Block LMM) was selected to be more similar to past literature (comparing first block and last block measures; see Table 5 in [71]) and permitted us to account for individual variability whereas the second one (trial LMM) provided a complementary trial-by-trial analysis and allowed us to consider both temporal and individual variability. Fixed effects variables were Group and Trial (numeric) with Participant as the random effects variable. We also added an autocorrelation structure of order 1, in the form of Trial nested within Participant. Model comparisons were done using the AIC and Chisq. Once again, a type III ANOVA table was obtained with Kenward-Roger “F” test statistics and Satterthwaite degrees of freedom, with Trial as the within-subject factor and Group as the between-subject factor (F and p values were reported, confidence level set to 0.95). Once again residual plots were visually inspected for deviations from homoscedasticity or normality in any measure. In this analysis, cortical excitability was approximated through a main effect of Group. In turn, habituation was confirmed in the presence of a main effect of Block (decrease confirmed through visual inspection).
Correlation analyses
The relationships between the continuous variables of age, migraine frequency (headache days/month; EM only), sensory sensitivity (SPQ Vision scores), cortical excitability (first block N1-P1 amplitude difference), and habituation (last block N1-P1 – first block N1-P1) were assessed using Spearman correlations. Correlation values (r) and p values were reported, and p values were adjusted for multiple comparisons using the FDR method.
Results
Participant demographics and migraine characteristics
Post-EEG recording, two participants were excluded due to technical problems (one HC, one EM), four due to severe depression (four EM), seven due to screening failure (seven EM), and five EM for being outside of the interictal phase. The final sample consisted of 18 EM (six patients reported aura as an accompanying symptom of migraine) and 27 HC. Groups were age- and gender-matched. No significant differences between patients with and without accompanying symptoms of aura were found, in terms of sensory sensitivity (SPQ Vision scale; t(15) = -1.037, p = 0.316), cortical excitability (first block N1-P1 amplitude difference; t(16) = 0.150, p = 0.883), and/or habituation (last block N1-P1 amplitude difference – first block N1-P1 amplitude difference; t(16) = -0.757, p = 0.460). For this reason, patients with and without accompanying symptoms of aura were collapsed for further analyses. Scores on the psychiatric measures related to anxiety, attention deficit disorder, and depression did not yield any significant differences between groups (see Table 3). Despite their relatively low headache frequency, patients reported mild to moderate disability and some impact of headache, according to the results of the clinical questionnaires (see Table 3).
Sensory perception questionnaire
Results on the SPQ indicated that EM patients had increased hypersensitivity on the Vision subscale and in particular on Vision-Brightness and Vision-Color, as compared to HC (see Table 3). No other subscales related to Vision, as well as the Total SPQ score, yielded significant differences between groups. Please note that one participant did not complete the SPQ and therefore only data from 17 patients were included in this analysis.
Electrophysiological analyses
Classic block analyses
The results of the ANOVA indicated a main effect of Block (F(1,43) = 8.895, p = 0.005) but no main effect of Group (F(1,43) = 2.279, p = 0.138) or significant Block x Group interaction (F(1,43) = 0.497, p = 0.485). The resulting main effect of Block would provide support to the presence of habituation in both groups (see Fig. 1B and C for a visual representation). Furthermore, given a lack of significant Group and interaction effects no significant differences in either cortical excitability or habituation were found between participant groups.
Block linear mixed-effects model
Here, we fitted a LMM to our data to account for individual variability. Extreme outlier trials were removed prior to fitting the model and were identified as any trial that was three times the interquartile range above the third and below the first quartile (11 trials total). The model, which best fit our data, following AIC and Chisq comparisons, was:
Results yielded a significant main effect of Block (F(1,7963) = 11.499, p = 0.0007) but no main effect of Group (F(1, 43) = 2.710, p = 0.100), or Block x Group interaction (F(1,7963) = 2.287, p = 0.130). The significant main effect of Block, in line with the classic analyses, continued to confirm the presence of habituation in both groups, through a significant decrease in N1-P1 amplitude over time (see Fig. 2A). Meanwhile, the lack of a main effect of Group or a significant Block x Group interaction indicated that EM and HC did not significantly differ regarding N1-P1 amplitude and by extension habituation and cortical excitability (confirmed through a visual inspection of Fig. 2A).
Visual illustration of both the block and linear mixed-effects models (LMMs) data from Experiments 1 and 2 with Block and Trial number on the x-axis and the N1-P1 peak-to-peak amplitude difference voltage on the y-axis. A Block LMM data, for both groups (green EM, blue HC) in Experiment 1. B Trial LMM data, for both groups (same colors) in Experiment 1. C Block LMM data, for both groups (same colors) in Experiment 2. D Trial LMM data, for both groups (same colors) in Experiment 2. Please note, that trials were grouped into bins of ten trials for both trial models (B and D) to facilitate visual inspection by reducing trial-to-trial variability
Trial linear mixed-effects model
Next, we used the Trial model to further increase our ability to account for both individual and temporal variability. To remove extreme trial outliers, we used the same criteria as for the Block analysis. The following model was determined to be optimal post-model comparisons:
In this case, the ANOVA yielded a significant main effect of Trial (F(1,24056) = 228.601, p = 2 × 10–16), no main effect of Group (F(1,43) = 1.954, p = 0.162), and no significant interaction (Trial x Group: F(1,24056) = 1.772, p = 0.183). This is consistent with the results obtained with the Block model and would support a lack of differences in habituation and cortical excitability between groups (confirmed through a visual inspection of Fig. 2B).
Correlation analyses
Given our interest in the effect of age and headache frequency on the sensory processes under examination in this research study, we wanted to see whether sensory sensitivity scores, cortical excitability measures as defined by first block N1-P1 amplitude difference, or habituation (Block 6 N1-P1 amplitude – Block 1 N1-P1 amplitude) were correlated with age or disease severity (as quantified by the number of headache days/month) or amongst themselves. In the case of HC, age was not correlated with any of the three variables (see Table 4 for a full breakdown of r and FDR-corrected p values). Additionally, sensory sensitivity scores, cortical excitability, and habituation were not correlated with each other for the HC group. On the other hand, when correlations were run taking into account EM, a significant positive correlation between age and SPQ Vision was found. Furthermore, we also checked whether sensory sensitivity (SPQ Vision score), cortical excitability (first block N1-P1 peak-to-peak amplitude difference), and habituation (Block 6 N1-P1 peak-to-peak amplitude difference – Block 1 N1-P1 peak-to-peak amplitude difference) were correlated with each other and found a significant negative correlation between cortical excitability and habituation (see Table 4 for a full breakdown of r and FDR-corrected p values). In other words, patients with a lower first block amplitude (cortical hypoexcitability) had less of a difference between Block 6 and Block 1, which may indicate less habituation. On the other hand, patients with a greater first block amplitude (cortical hyperexcitability) had a greater difference between Block 6 and Block 1, indicating more habituation. None of the other variables were significantly correlated.
Experiment 2
Method
Participants
Sixty-six participants with normal or corrected-to-normal vision, between 18 and 65 years old, were included and consisted of 36 middle-aged patients with EM (diagnosed by a neurologist using ICHD-3 criteria [5]) and 30 age- and gender-matched HC. Inclusion criteria were similar to Experiment 1 except for the recruitment location (specialized Headache Clinic) and disease severity (higher headache frequency). According to the results of Welch’s t-tests, EM in Experiment 2 were significantly older (t(19.786) = -8.867, p = 2.51 × 10–8) and had a significantly higher headache frequency (headache days/month: t(35) = -3.043, p = 0.004) than EM in Experiment 1. Given that the results from Experiment 1 did not confirm the previously described habituation deficit in patients with interictal, episodic migraine using either traditional analysis methods or newly implemented LMMs, we decided to run a second experiment with patients that were older and had a higher migraine frequency, to see whether this absence of significant effects continued to occur. Exclusion criteria were the same as in Experiment 1.
Procedure and paradigm
The procedure was very similar to Experiment 1, with participants answering the same questionnaires and completing an EEG recording comprised of a 5-min resting state and a subsequent PR task. Experimental stimuli were programmed and presented with custom-made scripts run on MATLAB R2017a (The Mathworks Inc., 2017) and Psychophysics Toolbox Version 3.0.13 [116, 117], running on Windows 10. All stimuli were presented on a BenQ XL2411P monitor with a screen size of 0.3 m height and 0.53 m width (CRT screen, resolution: 1024 × 768, 120 Hz refresh rate, background luminance: 21 cd/m2). Accurate timing was confirmed using the Black Box Toolkit (Black Box Toolkit, Ltd., Sheffield, UK).
Stimulus parameters were practically the same as in Experiment 1, with the exception of the reversal rate (3.1 Hz) and the number of blocks (12 blocks of 100 trials, divided post-recording). The reversal rate was incremented given that some authors have proposed that increasing this measure can help to detect the lack of habituation reported in migraine [71, 123]. Furthermore, several studies reporting a deficit of habituation used a reversal rate of 3.1 Hz [17, 19, 20, 52].
EEG recording
Using a BrainAmp32 Standard amplifier and a BrainVision recorder polybox BP-BM-30 actiCAP32, continuous EEG recordings (digitized, 1000 Hz sampling rate, 50 Hz online notch filter) were collected (Brain Products GmbH). The 32 active electrodes were placed in standard positions on an elastic cap. The online reference electrode was placed on the tip of the nose, and the ground electrode was inserted at the AFz point in the cap. Left and right mastoids as well as vertical and horizontal electrooculograms, were used as external electrodes. Impedances were kept below 15 kΩ.
EEG pre-processing
The same procedure was used as in Experiment 1, with the exception being that: no notch filter was applied offline as a notch filter was used during acquisition and epoch segments were from 0 to 150 ms. The metrics for the FIR band-pass filters (zero-phase) in Experiment 2 for the high-pass and low-pass filters are detailed upon continuation (high-pass: Frequency 0.1 Hz, order 33001, cutoff -6 dB; low-pass: frequency 60 Hz, order 221, cutoff -6 dB).
Analyses
Psychiatric, clinical, and SPQ questionnaires
Analyses were the same as in Experiment 1.
Electrophysiological data
PR-VEP. For all electrophysiological analyses, the same procedure was used as in Experiment 1, with a focus on the N1-P1 peak-to-peak amplitude difference at the Oz electrode. The only difference was that we considered 12 blocks and 1200 trials. Each block contained a maximum of 100 trials with a mean of 93.72 ± 6.912 (range: 56–100) clean trials per participant. Participants had a grand mean of 1124.58 ± 57.203 trials included post artifact rejection, out of a total of 1200 (range: 971–1197). Components were identified in the same way as in Experiment 1. In this case, N1 was the most negative peak between 73–101 ms (peak at 88 ms and window of ± 15 ms), and P1 was the most positive peak between 96–136 ms (peak at 116 ms and window of ± 20 ms).
Classic block analyses
Analyses were the same as in Experiment 1 with the exception that the factor Block consisted of Blocks 1 and 12. An additional analysis comparing Blocks 1 and 6 was also provided to ensure that the number of blocks did not have an effect on the results.
Block linear mixed-effects model
Analyses were the same as in Experiment 1 with the exception that the fixed-effects variable in the LMM termed Block consisted of Blocks 1 and 12 and the within-subject factor in the subsequent ANOVA also considered Blocks 1 and 12. The results assessing only Blocks 1 and 6 were also reported.
Trial linear mixed-effects model
Analyses were the same as in Experiment 1 with the exception that the fixed-effects variable Trial in the LMM consisted of Trials 1 to 1200, along with the within-subject factor Trial in the ANOVA (Trials 1 to 1200). Finally, an additional analysis examining Trials 1 to 600, to make sure that the number of trials did not have a significant influence on the results, was also reported.
Correlation analyses
Correlation analyses were carried out following the same methodology as in Experiment 1.
Results
Participant demographics and migraine characteristics
Five participants were excluded for the following reasons: three for technical problems (two EM, one HC) and two due to an insufficient number of clean trials following artifact rejection (two EM). We also had to exclude 17 EM for not being in the interictal phase, following the criteria discussed in the Method section. This resulted in a final sample of 19 EM patients (five had migraine with aura) and 29 HC. EM and HC were age- and gender-matched. Once again, patients with and without aura as an accompanying symptom did not significantly differ with regard to sensory sensitivity (t(14) = 1.412, p = 0.180), cortical excitability (t(14) = 0.232, p = 0.820), and/or habituation (t(14) = -1.965, p = 0.070). Therefore, patients were collapsed for further analyses. As expected in a sample of patients with heightened disease severity, scores related to anxiety and depression were significantly elevated in EM as compared to HC. In contrast, attention deficit disorder scores did not vary between groups. Furthermore, patients reported severe disability and severe headache impact as measured by the clinical questionnaires. See Table 3 for statistical comparisons of demographic and migraine characteristic data.
Sensory perception questionnaire
Similarly, to Experiment 1, patients with EM reported significant hypersensitivity on the Sensory Perception Questionnaire as compared to HC, on Vision, Vision-Brightness, and Vision-Acuity but not on the total score, Vision-Motion, or Vision-Color (see Table 3).
Electrophysiological analyses
Classic block analysis
The type III two-way mixed ANOVA results on N1-P1 amplitude data were examined and yielded a main effect of Block (F(1,46) = 24.082, p = 1.2 × 10–5), no main effect of Group (F(1,46) = 0.872, p = 0.355), and no significant Block x Group interaction (F(1,46) = 2.384, p = 0.129). These results would suggest habituation in both groups as supported by the main effect of Block (see Fig. 3). Furthermore, a lack of significant differences between groups with regard to cortical excitability were also found, supported by an absence of significant Group or Block x Group interaction (see Fig. 3).
Resulting visual evoked potentials (VEPs) and habituation to pattern-reversal stimulation in Experiment 2. A VEPs at the Oz electrode, with time (in ms) on the x-axis and N1-P1 peak-to-peak amplitude difference voltage on the y-axis, for each block (1 to 12) during the Pattern-Reversal task, and both groups (EM right, HC left). B Bar graph with Block number on the x-axis and mean N1-P1 peak-to-peak amplitude difference voltage on the y-axis. Habituation of the N1-P1 between Blocks 1 and 12 (green EM, blue HC)
Furthermore, to verify that the number of blocks did not influence the results, we also assessed what happened at Block 6 (similarly to Experiment 1). Similarly to the analysis using Blocks 1 and 12, we found a main effect of Block (F(1,46) = 6.603, p = 0.013) but no main effect of Group (F(1,46) = 1.012, p = 0.320) or significant Block x Group interaction (F(1,46) = 1.255, p = 0.268). Therefore, the number of Blocks did not appear to significantly affect the results.
Block linear mixed-effects model
Extreme outlier trials were removed prior to fitting the model and were identified as any trial that was ± three times the interquartile range (35 trials total). Only data from Blocks 1 and 12 were used. After performing model comparisons, the final model that best fit our data was:
First, a significant main effect of Block (F(1,8888) = 39.680, p = 2.992 × 10–10) but no significant main effect of Group (F(1,46) = 1.478, p = 0.224) was found. The interaction between Block x Group was also significant (F(1,8888) = 14.371, p = 1.501 × 10–4) and was decomposed to further explore the results. When the N1-P1 amplitude was compared between Blocks 1 and 12 as a function of Group, both HC (t = 6.299, p < 0.0001) and EM (t = 9.941, p < 0.0001) showed a significant decrease in N1-P1 amplitude between Block 1 and Block 12. This would once again appear to indicate habituation in both groups (see Fig. 3B and C and Fig. 2C for a visual representation). Furthermore, N1-P1 amplitude at Block 1 and 12 was separately compared as a function of Group, with no significant differences between Groups found at either Block 1 (t = -1.216, p = 0.345) or at Block 12 (t = -0.659, p = 0.616). Please note that the lack of differences in Block 1, would appear to indicate a lack of significant differences in cortical excitability between EM and HC.
We also ran an additional analysis using only Blocks 1 and 6 (similarly to Experiment 1) to ascertain that the number of Blocks did not significantly affect the results. Once again, we found a main effect of Block (F(1,8888) = 5.6377, p = 0.018) but no main effect of Group (F(1,46) = 1.436, p = 0.231). This time, the Block x Group interaction proved significant (F(1,8888) = 8.140, p = 0.004). When we decomposed this interaction, we found a significant difference between Blocks 1 and Block 6 for both EM (t = 5.559, p < 0.0001) and a trend for HC (t = 2.374, p = 0.053). The comparisons between EM and HC for Block 1 (t = -1.198. p = 0.355) and Block 6 (t = -0.804, p = 0.510) amplitude were not significant. The results mirror those reported at Block 12, mainly a lack of significant differences in cortical excitability and habituation between groups.
Trial linear mixed-effects model
The final model was the same as in Experiment 1.
The results from the two-way mixed ANOVA yielded a main effect of Trial (F(1,53803) = 264.649, p = < 2 × 10–16), as well as a main effect of Group (F(1,46) = 5.529, p = 0.019), and a significant interaction between Trial x Group (F(1,53803) = 627.299, p = < 2 × 10–16). The presence of a main effect of Trial would suggest differences between some trials, however this is not unexpected and habituation is likely, as supported by comparing the first trial amplitudes to the last ones in Fig. 2D. The significant main effect of Group and Trial x Group interaction, on the other hand, might suggest that patients exhibit some general hyperexcitability as compared to healthy controls as well as potential differences in the habituation slope. Furthermore, differences in the habituation slope are not indicative of a lack of habituation in EM, on the contrary, they show that the habituation slope is different between groups most likely due to the higher amplitude on certain trials in patients as compared to controls at the beginning of the task (see Fig. 2D). These results contrast with the findings from the block LMM.
Finally, we assessed the Trial model using Trials 1 to 600 and found no main effect of Trial (F(1,27003) = 0.164, p = 0.685) or Group (F(1,46) = 1.328, p = 0.249) but a significant Trial x Group interaction (F(1,27003) = 9.236, p = 0.002). The results of this analysis in particular, might indicate the need for more trials.
Correlation analyses
With respect to the EM group, no significant correlations were found between age and/or migraine frequency and any of the measures of interest (i.e., sensory sensitivity (SPQ Vision score), cortical excitability (first block N1-P1 peak-to-peak amplitude difference), and/or habituation (Block 6 N1-P1 peak-to-peak amplitude difference – Block 1 N1-P1 peak-to-peak amplitude difference), see Table 5 for r and FDR-corrected p values). The three variables (omitting age and/or migraine frequency) were also not correlated amongst each other (see Table 5 for r and FDR-corrected p values). On the other hand, with regard to HC, age was not correlated with either sensory sensitivity, cortical excitability, and/or habituation (see Table 5 for r and FDR-corrected p values). However, cortical excitability and habituation were significantly negatively correlated, similarly to what we saw with patients in Experiment 1 (see Table 5 for r and FDR-corrected p values).
Discussion
The objective of our study was to explore visual sensitivity (using the SPQ) as well as cortical excitability and habituation (both measured with PR-VEPs), as a function of age and disease severity. Two samples of patients with episodic migraine and their headache-free controls were used. The first consisted of a group of young adults with EM and the second a middle-aged group of EM patients. The results of both experiments yielded three main findings: (i) significant hypersensitivity, as seen by lower scores on the SPQ Vision scale in EM as compared to HC, (ii) no significant differences in cortical excitability or specifically N1-P1 first block peak-to-peak amplitudes in EM and HC and, (iii) no deficit of habituation, evidenced by habituation of the N1-P1 amplitude across blocks in both EM and HC.
Visual sensitivity
Hypersensitivity to visual stimuli has been found to occur in patients with migraine, both ictally and interictally, and has been measured using different methods including self-report questionnaires and sensory thresholds [9, 12,13,14]. In this study, we selected the SPQ self-report questionnaire, a validated instrument for exploring self-reported sensory sensitivity, given its use in neurological and pain research [16, 124, 125]. In our study, we found significantly lower values on the Vision scale of the SPQ in both Experiment 1 and Experiment 2 in EM as compared to HC, denoting a general hypersensitivity to visual stimuli in patients. These results are consistent with what is found in the clinical setting, where patients frequently complain of alterations in sensory processing, including ictal and interictal sensitivities to light, as well as discomfort to certain patterns, colors, and contrasts [126, 127]. They are also in line with studies using self-report measures, indicating that migraine patients regularly report a greater number of visual sensitivities in their environment when compared to non-headache controls [6], as well as increased light sensitivity when exposed to the same stimuli of varying intensity [13]. Furthermore, and perhaps most convincingly, psychophysical studies of sensory discomfort thresholds in migraine have yielded both ictal and interictal differences in patients as compared to healthy controls, with patients demonstrating a hypersensitivity to visual stimuli, as seen through decreased visual discomfort thresholds [9, 12, 14]. The results of this study also yielded a significant positive correlation between age and sensory sensitivity (SPQ vision score), which would be in line with results from research on photophobia and phonophobia in migraine, indicating an increase of these sensory alterations with age [98]. We did not find these results in Experiment 2, with the older sample of patients, which perhaps may be indicative that age-related changes in sensory sensitivity tend to flatten out with age, which is consistent with studies indicating that in older patients (60 +) there is even a reported decrease in photophobia and phonophobia [99], most likely related to the degeneration of sensory receptors [104] (for a review see [105]). Another possible explanation may be that the difference is more headache-disability based and that in patients with more headaches other predictors matter more than age. Moreover, given that we found the same effect in both experiments and also taking into account previous literature [6, 9, 12,13,14, 126], we would propose that the presence of interictal visual hypersensitivity in migraine patients appears to be quite robust.
Cortical excitability
The PR task, coupled with EEG, has been suggested as a good tool to measure a variety of sensory cortical properties, including excitability and habituation. Regarding cortical excitability in migraine, during the interictal period, there exist two predominant theories. First, a theory of hypoexcitability, or a reduced preactivation level of sensory cortices [18, 128], has garnished growing support in recent years. In fact, reduced preactivation levels might be linked to thalamocortical dysrhythmia in patients, which may ultimately result in a lack of habituation or even potentiation [18]. Second, a theory of hyperexcitability [129, 130], postulates the opposite and is thought to be a consequence of either increased neuronal excitation or decreased inhibition (see [131] for a review). Despite numerous studies using a variety of paradigms, the results remain controversial with certain studies pointing to reduced inhibition [128] and others to heightened excitation [132]. Given that results appear to be quite divided, some authors have proposed the broader term of “cortical dysexcitability” to encompass possible alterations of cortical excitability in patients with migraine [133].
In the present research study, no significant differences in the N1-P1 peak-to-peak amplitude between EM and HC were found in either experiment at any of the first blocks. Our finding adds to a body of evidence in the literature on PR tasks in patients with migraine, which has encountered non-significant differences between this clinical population interictally and healthy controls [19, 20, 35] (for a review see [133]). Currently, and considering the results of our study, it remains difficult to establish a clear picture regarding cortical excitability in migraine patients. The most plausible explanation is that the competing theories coexist, hinting at different profiles of cortical excitability that may affect patients’ electrophysiological responses. In fact, the sum of both profiles may lead to the lack of significant differences, such as the ones found here, in certain samples when compared to healthy controls. In sum, it is not possible to completely discard the hypothesis that migraine patients could have normal cortical excitability, during the interictal period.
Habituation
Despite certain controversy [21, 31, 32, 35, 71, 77], an interictal deficit of habituation has been proposed as a hallmark of migraine electrophysiology [134, 135], supported by some past studies [18,19,20, 52, 70]. However, in both experimental EM groups, we did not find the anticipated deficit of habituation interictally in the amplitude of the N1-P1 peak-to-peak. In particular, patients continued to habituate and, in Experiment 2, even showed steeper habituation slopes as compared to controls, indicating more pronounced habituation. This result is not completely unexpected and adds to a growing body of literature reporting no habituation deficit in patients [21, 31, 32, 35], as well as a lack of replicability of the interictal habituation deficit, reported in patients with migraine [77, 136,137,138]. These results do not discard that a habituation deficit might be present in specific migraine patients [30] under specific conditions [69, 123], but given the presence of negative results in several studies, perhaps it should not be considered as a defining and general characteristic of migraine, at least in the visual domain. One potential explanation for a lack of significant results could be related to the characteristics of the sample (at least in Experiment 1), in that young patients have more metabolic resources [139, 140], which compensate for visual effort [141], making it more difficult to induce a habituation deficit. Nonetheless, even with a sample of middle-aged patients with increased disease severity, we did not find the anticipated deficit of habituation despite presumably decreased metabolic resources in this sample. Another possibility is that the habituation deficit exists but the stimulation being used, in this case, the PR, does not adequately reproduce real-world conditions.
We also found that in the EM group in Experiment 1, decreased cortical excitability (lower preactivation levels) was correlated with less habituation (and perhaps even potentiation). This is in line with several past studies [17, 18, 30, 57] that also showed that first block amplitude was negatively correlated with habituation. In fact, in a study by Coppola et al. [70], the authors proposed that these lower preactivation levels may be indicative of a hypothesized thalamocortical dysrhythmia. Interestingly, the findings could also be compatible with the ceiling theory based on Knott and Irwin [142] and applied to migraine [143], which postulates that interictally diminished pre-activation excitability levels of the sensory cortices may be related to the reported deficit of habituation in migraine. However, these explanations remain speculative given that this correlation between cortical excitability and habituation was not present in EM in Experiment 2.
Age and migraine frequency
Keeping in mind the above-mentioned concepts, we wanted to see whether a relationship between hypersensitivity and hyperexcitability and/or habituation might be modulated by factors related to age and migraine frequency. In recent years, it has been well-documented that the relationship between age and migraine frequency tends to follow an inverse U-shaped curve, in that migraine frequency usually increases with age, reaching a peak and then declining with older age, although this is not always the case. In episodic migraine, peak prevalence tends to occur between 30–39 years old [96]. Importantly, the sensory sensitivity profile follows a similar curve, in that, patients with migraine as compared to healthy controls, tend to report more hypersensitivity with increased age and migraine frequency, indicated by an increase in the mean number of visual stressors peaking around 46–60 years old [6], and then progressively declining as of 50 years old [100]. Other studies, looking at the way in which visual sensitivity changes with migraine frequency/age, found that interictal photophobia also appears to be correlated to migraine frequency, according to self-perception reports (age range: 18–55 years old; [144]) and photophobia scores (age range: 20–79 years old; [145], despite [146]). These results would support a positive association between visual sensitivity and migraine frequency, with increased disease severity being linked to heightened sensitivity.
Recently, it has been proposed that high-frequency episodic migraine patients may in fact be more clinically similar to chronic migraine patients than low-frequency episodic migraine patients and symptomatology, such as visual sensitivity, may be modulated similarly [147]. In our study, significant differences in visual hypersensitivity between EM and HC were found in both experiments, although this variable was only found to be related to age in Experiment 1 and was not correlated to migraine frequency in either experiment, according to the results of the correlation analyses. Most likely, the absence of a significant correlation between these measures was influenced by the small sample sizes (see Limitations section) but also by the homogeneity amongst the participants in each group.
Pertaining to cortical excitability and habituation in migraine patients as compared to healthy controls, to the best of our knowledge, no study has directly evaluated, using PR-VEPs their relationship to age and migraine frequency. In this light, a recent meta-analysis has highlighted an important lack of information in several papers (see [120] for list and meta-analysis criteria), which made it impossible to effectively evaluate the effect of migraine frequency on the amplitude and habituation of VEPs. Age was also discussed as a limiting factor by the same authors, to the generalization of results and they proposed that future studies should take heed to consider the effects of age on VEP attenuation [120]. Considering the lack of significant differences between patients with migraine and healthy controls and correlations between these variables in our research study, the relationship between migraine frequency, age, and cortical measures such as excitability and habituation, remains elusive.
Methodological considerations
Pertaining to PR-VEPs, several authors have highlighted the difficulty in establishing clear findings when each study uses vastly different methodological parameters, clinical samples, interictal criteria, and statistical analyses, as well as differences in blinding and task instructions. Additionally, within the literature itself, the terminology used to describe stimulation parameters is inconsistent. For example, with regard to temporal frequency, some studies use reversals/second [18, 61, 134] whereas others use Hz [17, 34, 52] and these terms do not necessarily mean the same thing across studies making it difficult to assess results and interpret, which parameters are more or less commonly used. To avoid these problems in the future, we would recommend researchers to select one metric, for example Hz, to be used accurately and consistently across studies. In our study, we selected stimulus parameters based on recommendations from previous authors [21, 32, 69, 71, 77, 123]. With regard to statistical analyses, we used a LMM approach that was methodologically superior to previously used analyses (such as repeated measures ANOVAs), to see whether this would provide more precision in uncovering subtle group differences, while also running the classic analysis methods as control analyses. Our study is, to the best of our knowledge, one of the few to analyze PR data in migraine using a statistical model approach [86, 148]. Past research studies have primarily made use of least squares slopes, linear regression slopes, or repeated measures ANOVAs of amplitude, among other methods, to study cortical excitability and habituation [30]. Compared to these methods, LMMs hold several advantages, particularly in studies of a clinical nature. First, and most importantly, all of the information and variability in the data are preserved in LMMs, especially with regard to individual and temporal factors [149, 150]. This is particularly important, given that EEG applications introduce a higher degree of complexity to the data. Furthermore, LMMs offer a superior approach to handling differences in the number of individual values (missing data), dropout in longitudinal studies, and are more robust when dealing with a smaller number of observations and/or unbalanced data [149, 150]. Given that EEG studies often carry high variability due to the nature of electrophysiological artifacts and their impact on the number of trials included in the final analysis, LMMs offer a statistically-sound approach to deal with these discrepancies [151]. Also, migraine patients tend to be quite heterogeneous [152], therefore the use of LMMs also helps to account for within-participant differences, which are often unaccounted for in traditional analyses. However, even with a statistically more powerful method, we still found negative results for both cortical excitability and habituation apart from the increased cortical excitability for the trial LMM in Experiment 2.
Relationship between sensory sensitivity, cortical excitability, and habituation
Taking into account our results, it is interesting to reflect on the apparent dissociation of the three processes of sensory sensitivity, cortical excitability, and habituation. The question remains whether hypersensitive individuals also showcase differences at a neural level, in terms of brain responses. Research on habituation and sensory sensitivity in other clinical disorders, such as obsessive–compulsive disorder and autism, would appear to suggest that deficits in habituation may reduce an individual’s ability to suppress stimuli, which may lead to the development of hypersensitivities [153, 154]. However, in the literature on migraine, only a few studies have examined the relationship between these processes and did not find an association between visual evoked potentials and sensory measures such as visual discomfort thresholds [35]. This would support the notion that a direct link between EEG and behavior is often missing and difficult to rationalize. That being said, it remains of interest to continue investigating whether the subjective experiences reported by patients with migraine as they relate to sensory perception and their subsequent impact on behavior can be connected to a more objective neural measure, especially given that these processes appear to share a link. Perhaps, cortical excitability and habituation measures cannot explain the sensory sensitivity commonly reported by patients or maybe PR-VEPs are simply unable to tap into these processes with sufficient adequacy so as to provide a tool to study a potential relationship between them.
Limitations
The main limitation was the fact that our visual stimulus used for the EEG recording was unable to measure sensory sensitivity directly, unlike our variables of cortical excitability and habituation. However, despite this limitation the PR task was chosen given its widespread use in studying visual processing in migraine patients and the ability to carefully select stimulation parameters based on recommendations from previous studies [21, 32, 69, 71, 77, 123]. In the future, it would be important to search for a paradigm that would permit us to evaluate all three concepts simultaneously. Also, stimulating with two different reversal rates may limit the ability to compare the results of both experiments amongst each other as well as to the literature. The results using the 3.1 Hz temporal frequency can be compared to some studies [17, 19, 20] whereas those using 1.55 Hz can be assessed with respect to others [61, 70, 72], although not at the same time. Nevertheless, although the results of both Experiments may not be directly comparable, they can be evaluated with respect to previous literature given that both reversal rates have been used in the past and have been found to yield both positive and negative results regarding a deficit of habituation in patients with episodic migraine interictally. In fact, the results of our study are in line with those of others [21, 32, 71, 77] that used different reversal rates and were also unable to reproduce the anticipated deficit of habituation. This would be consistent with the hypothesis proposed by Omland et al. [77] that different stimulation parameters are unable to explain the discrepant findings in previous VEP studies, which may help us clarify where the differences in the literature arise from. Likewise, we chose to use binocular stimulation, which may limit our ability to compare our results to past literature, although it is important to note that there are currently no set guidelines as far as habituation research in terms of monocular/binocular stimulation [155]. Additionally, although binocular stimulation could cause summation or subtraction phenomena in the signal affecting N1 and P1 latencies and amplitudes, Tobimatsu and Kato [156] found these effects to be more pronounced for the P50-N75 amplitude than the N1-P1 amplitude, which was the focus of the current study. Binocular summation is also not significant under transient conditions (1.5 Hz- 3.0 Hz), meaning that it should not be an issue in either Experiment 1 or 2 of this research study [157]. Finally, binocular stimulation has been previously used in with the Pattern-Reversal paradigm in patients with migraine [24, 31, 92, 158]. Furthermore, with regard to the correlation analyses, it is possible that the results were not significant due to small sample sizes and homogeneity in our participant groups. Also, another important aspect to take into account concerning age, migraine frequency, and cortical measures, is their relationship to gender. Migraine is about three times more frequent in women than in men and attacks tend to be more severe [159]. However, in the present study, we were unable to evaluate the interaction between gender, the previously mentioned factors, and our variables of interest. In Experiment 1, the entire sample consisted of women and in Experiment 2, the percentage of women and men was equivalent to that reported in the general population (approx. 3 to 1). Given that our groups were gender-matched, this avoided any potential distortions of our results. Furthermore, the effects of gender have not been accounted for in previous studies with regard to these measures, despite some studies indicating the presence of structural and functional brain differences in men and women, related to migraine [160]. For this reason, we highly encourage future studies to take into account gender and its effect on these variables. Finally, despite collecting information about aura, we did not expressly evaluate these concepts separating migraine patients into patients with and without aura due to the resulting small sample size. That being said, past literature reporting negative results with regard to cortical excitability and habituation were not limited to patients with migraine without aura, but also found normal cortical excitability and habituation in patients with migraine with aura [35, 71].
Conclusions
In conclusion, both experiments indicated a significant hypersensitivity to visual stimuli in patients with EM interictally but no differences in either cortical excitability or habituation between groups. These results would provide support for two different things. With respect to these metrics, the alterations in patients may be less pronounced than originally anticipated. At the same time, our results also clearly highlight a necessity for the standardization of methodological parameters. Further research is required to precisely define the optimal parameters for assessing sensory sensitivity, cortical excitability, and habituation in different age groups and migraine subtypes, as well as, as a function of disease severity, and other factors. Doing so would be essential in resolving the debate as to the use of these metrics as potential biomarkers of migraine.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ANOVA:
-
Analysis of Variance
- ASRS:
-
ADHD Self-Report Scale
- BDI-II:
-
Beck Depression Inventory-II
- EEG:
-
Electroencephalography
- EM:
-
Episodic Migraine (Patients)
- HC:
-
Healthy Controls
- HIT-6:
-
Headache Impact Test-6
- ICHD-3:
-
International Classification of Headache Disorders 3rd edition
- IHS:
-
International Headache Society
- LMM:
-
Linear Mixed-effects Models
- MIDAS:
-
Migraine Disability Assessment Test
- MSQ:
-
Migraine-Specific Quality of Life Questionnaire
- N1:
-
N100 electrophysiological component
- N1-P1:
-
Peak-to-peak amplitude difference of N1-P1
- P1:
-
P100 electrophysiological component
- PR:
-
Pattern-Reversal
- PR-VEP:
-
Pattern-Reversal Visual Evoked Potential
- SPQ:
-
Sensory Perception Quotient
- STAI:
-
State-Trait Anxiety Inventory
- VEP:
-
Visual Evoked Potential
References
Harriott AM, Schwedt TJ (2014) Migraine is associated with altered processing of sensory stimuli. Curr Pain Headache Rep 18:458. https://doi.org/10.1007/s11916-014-0458-8
de Tommaso M, Ambrosini A, Brighina F et al (2014) Altered processing of sensory stimuli in patients with migraine. Nat Rev Neurol 10:144–155. https://doi.org/10.1038/nrneurol.2014.14
Puledda F, Ffytche D, O’Daly O, Goadsby PJ (2019) Imaging the visual network in the migraine spectrum. Front Neurol 10:1325. https://doi.org/10.3389/fneur.2019.01325
Hadjikhani N, Vincent M (2021) Visual perception in migraine: a narrative review. Vision 5:20. https://doi.org/10.3390/vision5020020
(2018) Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38:1–211. https://doi.org/10.1177/0333102417738202
Hay KM, Mortimer MJ, Barker DC et al (1994) 1044 women with migraine: The effect of environmental stimuli. Headache 34:166–168. https://doi.org/10.1111/j.1526-4610.1994.hed3403166.x
Lévêque Y, Masson R, Fornoni L et al (2020) Self-perceived attention difficulties are associated with sensory hypersensitivity in migraine. Rev Neurol (Paris) 176:829–838. https://doi.org/10.1016/j.neurol.2020.01.360
Price A, Sumner P, Powell G (2021) Subjective sensory sensitivity and its relationship with anxiety in people with probable migraine. Headache 61:1342–1350. https://doi.org/10.1111/head.14219
Woodhouse A, Drummond PD (1993) Mechanisms of increased sensitivity to noise and light in migraine headache. Cephalalgia 13:417–421. https://doi.org/10.1046/j.1468-2982.1993.1306417.x
Main A, Dowson A, Gross M (1997) Photophobia and phonophobia in migraineurs between attacks. Headache 37:492–495. https://doi.org/10.1046/j.1526-4610.1997.3708492.x
Ashkenazi A, Mushtaq A, Yang I, Oshinsky ML (2009) Ictal and interictal phonophobia in migraine - A quantitative controlled study. Cephalalgia 29:1042–1048. https://doi.org/10.1111/j.1468-2982.2008.01834.x
Ikumi N, Cerda-Company X, Marti-Marca A et al (2022) Avoidance behaviour modulates but does not condition phonophobia in migraine. Cephalalgia 42:1305–1316. https://doi.org/10.1177/03331024221111772
Drummond PD (1986) A quantitative assessment of photophobia in migraine and tension headache. Headache 26:465–469. https://doi.org/10.1111/j.1526-4610.1986.hed2609465.x
Vanagaite J, Pareja JA, Støren O et al (1997) Light-induced discomfort and pain in migraine. Cephalalgia 17:733–741. https://doi.org/10.1046/j.1468-2982.1997.1707733.x
Peng K-P, May A (2020) Redefining migraine phases – A suggestion based on clinical, physiological, and functional imaging evidence. Cephalalgia 40:866–870. https://doi.org/10.1177/0333102419898868
Tavassoli T, Hoekstra RA, Baron-Cohen S (2014) The Sensory Perception Quotient (SPQ): Development and validation of a new sensory questionnaire for adults with and without autism. Molecular Autism 5:29. https://doi.org/10.1186/2040-2392-5-29
Afra J, Proietti Cecchini A, Sándor PS, Schoenen J (2000) Comparison of visual and auditory evoked cortical potentials in migraine patients between attacks. Clin Neurophysiol 111:1124–1129. https://doi.org/10.1016/s1388-2457(00)00271-6
Coppola G, Pierelli F, Schoenen J (2007) Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia 27:1427–1439. https://doi.org/10.1111/j.1468-2982.2007.01500.x
Schoenen J, Wang W, Albert A, Delwaide PJ (1995) Potentiation instead of habituation characterizes visual evoked potentials in migraine patients between attacks. Eur J Neurol 2:115–122. https://doi.org/10.1111/j.1468-1331.1995.tb00103.x
Afra J, Cecchini AP, De Pasqua V et al (1998) Visual evoked potentials during long periods of pattern-reversal stimulation in migraine. Brain 121:233–241. https://doi.org/10.1093/brain/121.2.233
Sand T, Zhitniy N, White LR, Stovner LJ (2008) Visual evoked potential latency, amplitude and habituation in migraine: a longitudinal study. Clin Neurophysiol 119:1020–1027. https://doi.org/10.1016/j.clinph.2008.01.009
Odom JV, Bach M, Barber C et al (2004) Visual evoked potentials standard (2004). Doc Ophthalmol 108:115–123. https://doi.org/10.1023/b:doop.0000036790.67234.22
Ellemberg D, Hammarrenger B, Lepore F et al (2001) Contrast dependency of VEPs as a function of spatial frequency: the parvocellular and magnocellular contributions to human VEPs. Spat Vis 15:99–111. https://doi.org/10.1163/15685680152692042
Shibata K, Yamane K, Iwata M, Ohkawa S (2005) Evaluating the effects of spatial frequency on migraines by using pattern-reversal visual evoked potentials. Clin Neurophysiol 116:2220–2227. https://doi.org/10.1016/j.clinph.2005.05.015
Vogel EK, Luck SJ (2000) The visual N1 component as an index of a discrimination process. Psychophysiology 37:190–203. https://doi.org/10.1111/1469-8986.3720190
Johannes S, Münte TF, Heinze HJ, Mangun GR (1995) Luminance and spatial attention effects on early visual processing. Cogn Brain Res 2:189–205. https://doi.org/10.1016/0926-6410(95)90008-X
Olofsson JK, Nordin S, Sequeira H, Polich J (2008) Affective picture processing: an integrative review of ERP findings. Biol Psychol 77:247–265. https://doi.org/10.1016/j.biopsycho.2007.11.006
Luck SJ (2005) An introduction to the event-related potential technique
Coppola G, Schoenen J (2012) Measures of Cortical Excitability', in David Borsook and others (eds), The Migraine Brain: Imaging Structure and Function (2012; online edn, Oxford Academic, 1 Sept. 2013). https://doi.org/10.1093/acprof:oso/9780199754564.003.0029. Accessed 28 June 2023.
Lisicki M, Ruiz-Romagnoli E, D’Ostilio K et al (2017) Familial history of migraine influences habituation of visual evoked potentials. Cephalalgia 37:1082–1087. https://doi.org/10.1177/0333102416673207
Oelkers R, Grosser K, Lang E et al (1999) Visual evoked potentials in migraine patients: alterations depend on pattern spatial frequency. Brain 122:1147–1155. https://doi.org/10.1093/brain/122.6.1147
Sand T, White LR, Hagen K, Stovner LJ (2009) Visual evoked potential and spatial frequency in migraine: a longitudinal study. Acta Neurol Scand Suppl 33–37. https://doi.org/10.1111/j.1600-0404.2009.01211.x
Wang W, Wang G-P, Ding X-L, Wang Y-H (1999) Personality and response to repeated visual stimulation in migraine and tension-type headaches. Cephalalgia 19:718–724. https://doi.org/10.1046/j.1468-2982.1999.019008718.x
Afra J, Ambrosini A, Genicot R et al (2000) Influence of colors on habituation of visual evoked potentials in patients with migraine with aura and in healthy volunteers. Headache 40:36–40. https://doi.org/10.1046/j.1526-4610.2000.00006.x
Sand T, Vingen JV (2000) Visual, long-latency auditory and brainstem auditory evoked potentials in migraine: relation to pattern size, stimulus intensity, sound and light discomfort thresholds and pre-attack state. Cephalalgia 20:804–820. https://doi.org/10.1046/j.1468-2982.2000.00098.x
Kennard C, Gawel M, Rudolph N de M, Rose FC (1978) Visual evoked potentials in migraine subjects. Headache Today - An Update by 21 Experts 6:73–80. https://doi.org/10.1159/000402447
Benna P, Bianco C, Costa P et al (1985) Visual evoked potentials and brainstem auditory evoked potentials in migraine and transient ischemic attacks. Cephalalgia 5:53–58. https://doi.org/10.1177/03331024850050S209
Polich J, Ehlers CL, Dalessio DJ (1986) Pattern-shift visual evoked responses and EEG in migraine. Headache 26:451–456. https://doi.org/10.1111/j.1526-4610.1986.hed2609451.x
Mariani E, Moschini V, Pastorino G et al (1988) Pattern-reversal visual evoked potentials and EEG correlations in common migraine patients. Headache 28:269–271. https://doi.org/10.1111/j.1526-4610.1988.hed2804269.x
Raudino F (1988) Visual evoked potential in patients with migraine. Headache 28:531–533. https://doi.org/10.1111/j.1526-4610.1988.hed2808531.x
Diener HC, Scholz E, Dichgans J et al (1989) Central effects of drugs used in migraine prophylaxis evaluated by visual evoked potentials. Ann Neurol 25:125–130. https://doi.org/10.1002/ana.410250204
Lai CW, Dean P, Ziegler DK, Hassanein RS (1989) Clinical and electrophysiological responses to dietary challenge in migraineurs. Headache 29:180–186. https://doi.org/10.1111/j.1526-4610.1989.hed2903180.x
Drake ME, Pakalnis A, Hietter SA, Padamadan H (1990) Visual and auditory evoked potentials in migraine. Electromyogr Clin Neurophysiol 30:77–81
Mariani E, Moschini V, Pastorino GC et al (1990) Pattern reversal visual evoked potentials (VEP-PR) in migraine subjects with visual aura. Headache 30:435–438. https://doi.org/10.1111/j.1526-4610.1990.hed3007435.x
Tsounis S, Milonas J, Gilliam F (1993) Hemi-field pattern reversal visual evoked potentials in migraine. Cephalalgia 13:267–271. https://doi.org/10.1046/j.1468-2982.1993.1304267.x
Tagliati M, Sabbadini M, Bernardi G, Silvestrini M (1995) Multichannel visual evoked potentials in migraine. Electroencephalogr Clin Neurophysiol 96:1–5. https://doi.org/10.1016/0013-4694(94)00211-3
Shibata K, Osawa M, Iwata M (1997) Pattern reversal visual evoked potentials in classic and common migraine. J Neurol Sci 145:177–181. https://doi.org/10.1016/S0022-510X(96)00258-4
Sener HO, Haktanir I, Demirci S (1997) Pattern-reversal visual evoked potentials in migraineurs with or without visual aura. Headache 37:449–451. https://doi.org/10.1046/j.1526-4610.1997.3707449.x
Shibata K, Osawa M, Iwata M (1997) Simultaneous recording of pattern reversal electroretinograms and visual evoked potentials in migraine. Cephalalgia 17:742–747. https://doi.org/10.1046/j.1468-2982.1997.1707742.x
Shibata K, Osawa M, Iwata M (1998) Pattern reversal visual evoked potentials in migraine with aura and migraine aura without headache. Cephalalgia 18:319–323. https://doi.org/10.1046/j.1468-2982.1998.1806319.x
Yücesan C, Sener Ö, Mutluer N (2000) Influence of disease duration on visual evoked potentials in migraineurs. Headache 40:384–388. https://doi.org/10.1046/j.1526-4610.2000.00058.x
Judit Á, Sándor P, Schoenen J (2000) Habituation of visual and intensity dependence of auditory evoked cortical potentials tends to normalize just before and during the migraine attack. Cephalalgia 20:714–719. https://doi.org/10.1111/j.1468-2982.2000.00122.x
Khalil NM, Legg NJ, Anderson DJ (2000) Long term decline of P100 amplitude in migraine with aura. J Neurol Neurosurg Psychiatry 69:507–511. https://doi.org/10.1136/jnnp.69.4.507
Logi F, Bonfiglio L, Orlandi G et al (2001) Asymmetric scalp distribution of pattern visual evoked potentials during interictal phases in migraine. Acta Neurol Scand 104:301–307. https://doi.org/10.1034/j.1600-0404.2001.00329.x
Yilmaz M, Bayazit YA, Erbagci I, Pençe S (2001) Visual evoked potential changes in migraine. Influence of migraine attack and aura. J Neurol Sci 184:139–141. https://doi.org/10.1016/s0022-510x(00)00503-7
Kochar K, Srivastava T, Maurya RK et al (2002) Visual evoked potential and brainstem auditory evoked potentials in acute attack and after the attack of migraine. Electromyogr Clin Neurophysiol 42:175–179
Ozkul Y, Bozlar S (2002) Effects of fluoxetine on habituation of pattern reversal visually evoked potentials in migraine prophylaxis. Headache 42:582–587. https://doi.org/10.1046/j.1526-4610.2002.02144.x
Coutin-Churchman P, de Freytez AP (2003) Vector analysis of visual evoked potentials in migraineurs with visual aura. Clin Neurophysiol 114:2132–2137. https://doi.org/10.1016/S1388-2457(03)00229-3
Ashjazadeh N, Varavipour B (2015) Abnormalitites of visual evoked potential in migraine patients. Iranian Journal of Medical Sciences 28:65–68
Spreafico C, Frigerio R, Santoro P et al (2004) Visual evoked potentials in migraine. Neurol Sci 25(Suppl 3):S288-290. https://doi.org/10.1007/s10072-004-0313-5
Coppola G, Bracaglia M, Di Lenola D et al (2015) Visual evoked potentials in subgroups of migraine with aura patients. J Headache Pain 16:92. https://doi.org/10.1186/s10194-015-0577-6
Coppola G, Ambrosini A, Di Clemente L et al (2007) Interictal abnormalities of gamma band activity in visual evoked responses in migraine: An indication of thalamocortical dysrhythmia? Cephalalgia 27:1360–1367. https://doi.org/10.1111/j.1468-2982.2007.01466.x
Di Clemente L, Coppola G, Magis D et al (2005) Nociceptive blink reflex and visual evoked potential habituations are correlated in migraine. Headache 45:1388–1393. https://doi.org/10.1111/j.1526-4610.2005.00271.x
De Marinis M, Rinalduzzi S, Accornero N (2007) Impairment in color perception in migraine with and without aura. Headache 47:895–904. https://doi.org/10.1111/j.1526-4610.2007.00774.x
Shibata K, Yamane K, Otuka K, Iwata M (2008) Abnormal visual processing in migraine with aura: a study of steady-state visual evoked potentials. J Neurol Sci 271:119–126. https://doi.org/10.1016/j.jns.2008.04.004
Boylu E, Domaç FM, Koçer A et al (2010) Visual evoked potential abnormalities in migraine patients. Electromyogr Clin Neurophysiol 50:303–308
Khalil NM, Nicotra A, Wilkins AJ (2011) Asymmetry of visual function in migraine with aura: Correlation with lateralisation of headache and aura. Cephalalgia 31:213–221. https://doi.org/10.1177/0333102410378050
Nguyen BN, McKendrick AM, Vingrys AJ (2012) Simultaneous retinal and cortical visually evoked electrophysiological responses in between migraine attacks. Cephalalgia 32:896–907. https://doi.org/10.1177/0333102412453953
Shibata K, Yamane K, Nishimura Y et al (2011) Spatial frequency differentially affects habituation in migraineurs: a steady-state visual-evoked potential study. Doc Ophthalmol 123:65–73. https://doi.org/10.1007/s10633-011-9281-2
Coppola G, Di Lorenzo C, Schoenen J, Pierelli F (2013) Habituation and sensitization in primary headaches. J Headache Pain 14:65. https://doi.org/10.1186/1129-2377-14-65
Omland PM, Nilsen KB, Uglem M et al (2013) Visual evoked potentials in interictal migraine: No confirmation of abnormal habituation. Headache 53:1071–1086. https://doi.org/10.1111/head.12006
Di Lorenzo C, Coppola G, Bracaglia M et al (2016) Cortical functional correlates of responsiveness to short-lasting preventive intervention with ketogenic diet in migraine: a multimodal evoked potentials study. J Headache Pain 17:58. https://doi.org/10.1186/s10194-016-0650-9
Viganò A, D’Elia TS, Sava SL et al (2013) Transcranial Direct Current Stimulation (tDCS) of the visual cortex: A proof-of-concept study based on interictal electrophysiological abnormalities in migraine. J Headache Pain 14:23. https://doi.org/10.1186/1129-2377-14-23
Omland PM, Uglem M, Engstrøm M et al (2014) Modulation of visual evoked potentials by high-frequency repetitive transcranial magnetic stimulation in migraineurs. Clin Neurophysiol 125:2090–2099. https://doi.org/10.1016/j.clinph.2014.01.028
Ambrosini A, Iezzi E, Perrotta A et al (2016) Correlation between habituation of visual-evoked potentials and magnetophosphene thresholds in migraine: a case-control study. Cephalalgia 36:258–264. https://doi.org/10.1177/0333102415590241
Rauschel V, Ruscheweyh R, Krafczyk S, Straube A (2016) Test-retest reliability of visual-evoked potential habituation. Cephalalgia 36:831–839. https://doi.org/10.1177/0333102415613613
Omland PM, Uglem M, Hagen K et al (2016) Visual evoked potentials in migraine: Is the “neurophysiological hallmark” concept still valid? Clin Neurophysiol 127:810–816. https://doi.org/10.1016/j.clinph.2014.12.035
Verroiopoulos GV, Nitoda E, Ladas ID et al (2016) Ophthalmological assessment of OCT and electrophysiological changes in migraine patients. J Clin Neurophysiol 33:431–442. https://doi.org/10.1097/WNP.0000000000000256
El-Shazly AAEF, Farweez YA, Hamdi MM, El-Sherbiny NE (2017) Pattern visual evoked potential, pattern electroretinogram, and retinal nerve fiber layer thickness in patients with migraine during and after aura. Curr Eye Res 42:1327–1332. https://doi.org/10.1080/02713683.2017.1319490
Kalita J, Uniyal R, Misra UK, Bhoi SK (2018) Neuronal dysexcitability may be a biomarker of migraine: a visual evoked potential study. Clin EEG Neurosci 49:342–350. https://doi.org/10.1177/1550059417734106
Susvirkar AA, Velusami D, Srinivasan N (2020) Evaluation of habituation to visual evoked potentials using pattern reversal among migraine individuals – A cross-sectional study. J Basic Clin Physiol Pharmacol;31. https://doi.org/10.1515/jbcpp-2019-0217
Coppola G, Di Lorenzo C, Di Lenola D et al (2021) Visual evoked potential responses after photostress in migraine patients and their correlations with clinical features. J Clin Med 10:982. https://doi.org/10.3390/jcm10050982
Kalita J, Misra UK, Kumar M et al (2022) Is palinopsia in migraineurs a phenomenon of impaired habituation of visual cortical neurons? Clin EEG Neurosci 53:196–203. https://doi.org/10.1177/1550059421991707
Sándor PS, Afra J, Proietti-Cecchini A et al (1999) Familial influences on cortical evoked potentials in migraine. NeuroReport 10:1235–1238. https://doi.org/10.1097/00001756-199904260-00015
Bohotin V, Fumai A, Vandenheede M et al (2003) Excitability of visual V1–V2 and motor cortices to single transcranial magnetic stimuli in migraine: a reappraisal using a figure-of-eight coil. Cephalalgia 23:264–270. https://doi.org/10.1046/j.1468-2982.2003.00475.x
Fumal A, Coppola G, Bohotin V et al (2006) Induction of long-lasting changes of visual cortex excitability by five daily sessions of repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers and migraine patients. Cephalalgia 26:143–149. https://doi.org/10.1111/j.1468-2982.2005.01013.x
Magis D, Allena M, Coppola G et al (2007) Search for correlations between genotypes and electrophysiological patterns in migraine: The MTHFR C677T polymorphism and visual evoked potentials. Cephalalgia 27:1142–1149. https://doi.org/10.1111/j.1468-2982.2007.01412.x
Coppola G, Currà A, Sava SL et al (2010) Changes in visual-evoked potential habituation induced by hyperventilation in migraine. J Headache Pain 11:497–503. https://doi.org/10.1007/s10194-010-0239-7
Coppola G, Currà A, Serrao M et al (2010) Lack of cold pressor test-induced effect on visual-evoked potentials in migraine. J Headache Pain 11:115–121. https://doi.org/10.1007/s10194-009-0177-4
Coppola G, Crémers J, Gérard P et al (2011) Effects of light deprivation on visual evoked potentials in migraine without aura. BMC Neurol 11:91. https://doi.org/10.1186/1471-2377-11-91
Hansen JM, Bolla M, Magis D et al (2011) Habituation of evoked responses is greater in patients with familial hemiplegic migraine than in controls: A contrast with the common forms of migraine. Eur J Neurol 18:478–485. https://doi.org/10.1111/j.1468-1331.2010.03190.x
Bednář M, Kubová Z, Kremláček J (2014) Lack of visual evoked potentials amplitude decrement during prolonged reversal and motion stimulation in migraineurs. Clin Neurophysiol 125:1223–1230. https://doi.org/10.1016/j.clinph.2013.10.050
Ambrosini A, Kisialiou A, Coppola G et al (2017) Visual and auditory cortical evoked potentials in interictal episodic migraine: an audit on 624 patients from three centres. Cephalalgia 37:1126–1134. https://doi.org/10.1177/0333102416665224
Ince F, Erdogan-Bakar E, Unal-Cevik I (2017) Preventive drugs restore visual evoked habituation and attention in migraineurs. Acta Neurol Belg 117:523–530. https://doi.org/10.1007/s13760-017-0749-z
Lisicki M, Ruiz-Romagnoli E, Piedrabuena R et al (2018) Migraine triggers and habituation of visual evoked potentials. Cephalalgia 38:988–992. https://doi.org/10.1177/0333102417720217
Bigal ME, Liberman JN, Lipton RB (2006) Age-dependent prevalence and clinical features of migraine. Neurology 67:246–251. https://doi.org/10.1212/01.wnl.0000225186.76323.69
Martin VT, Pavlovic J, Fanning KM et al (2016) Perimenopause and Menopause Are Associated With High Frequency Headache in Women With Migraine: Results of the American Migraine Prevalence and Prevention Study. Headache 56:292–305. https://doi.org/10.1111/head.12763
Wöber-Bingöl C, Wöber C, Karwautz A et al (2004) Clinical features of migraine: a cross-sectional study in patients aged three to sixty-nine. Cephalalgia 24:12–17. https://doi.org/10.1111/j.1468-2982.2004.00621.x
Martins KM, Bordini CA, Bigal ME, Speciali JG (2006) Migraine in the Elderly: A Comparison With Migraine in Young Adults. Headache 46:312–316. https://doi.org/10.1111/j.1526-4610.2006.00343.x
Kelman L (2006) Migraine changes with age: IMPACT on migraine classification. Headache 46:1161–1171. https://doi.org/10.1111/j.1526-4610.2006.00444.x
Owsley C, Sekuler R, Siemsen D (1983) Contrast sensitivity throughout adulthood. Vision Res 23:689–699. https://doi.org/10.1016/0042-6989(83)90210-9
Humes LE, Busey TA, Craig JC, Kewley-Port D (2009) The effects of age on sensory thresholds and temporal gap detection in hearing, vision, and touch. Atten Percept Psychophys 71:860–871. https://doi.org/10.3758/APP.71.4.860
Gibson SJ, Farrell M (2004) A review of age differences in the neurophysiology of nociception and the perceptual experience of pain. Clin J Pain 20:227–239. https://doi.org/10.1097/00002508-200407000-00004
Ochoa J, Mair WG (1969) The normal sural nerve in man. II. Changes in the axons and Schwann cells due to ageing. Acta Neuropathol 13:217–239. https://doi.org/10.1007/BF00690643
Yezierski RP (2012) The effects of age on pain sensitivity: preclinical studies. Pain Med 13(Suppl 2):S27-36. https://doi.org/10.1111/j.1526-4637.2011.01311.x
Friedman DI, De Ver Dye T (2009) Migraine and the environment. Headache 49:941–952. https://doi.org/10.1111/j.1526-4610.2009.01443.x
Spielberger C, Gorsuch R, Lushene R et al (1983) Manual for the State-Trait Anxiety Inventory (Form Y1–Y2). Consulting Psychologists Press, Palo Alto, CA
Spielberger CD, Gorsuch RL, Lushene RE, et al (2011) STAI: Cuestionario de ansiedad estado-rasgo: Manual, 8a. ed. rev. y amp., [nuevos baremos]. TEA, Madrid
Kessler RC, Adler L, Ames M et al (2005) The World Health Organization adult ADHD self-report scale (ASRS): A short screening scale for use in the general population. Psychol Med 35:245–256. https://doi.org/10.1017/S0033291704002892
Beck A, Steer R, Brown G (1996) Manual for the Beck Depression Inventory-II (BDI-II). The Psychological Corporation, San Antonio, TX
Kosinski M, Bayliss MS, Bjorner JB et al (2003) A six-item short-form survey for measuring headache impact: The HIT-6™. Qual Life Res 12:963–974. https://doi.org/10.1023/A:1026119331193
Stewart WF, Lipton RB, Kolodner KB et al (2000) Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain 88:41–52. https://doi.org/10.1016/S0304-3959(00)00305-5
Jhingran P, Osterhaus JT, Miller DW et al (1998) Development and validation of the Migraine-Specific Quality of Life questionnaire. Headache 38:295–302. https://doi.org/10.1046/j.1526-4610.1998.3804295.x
Harris PA, Taylor R, Minor BL et al (2019) The REDCap consortium: Building an international community of software platform partners. J Biomed Inform 95:103208. https://doi.org/10.1016/j.jbi.2019.103208
Harris PA, Taylor R, Thielke R et al (2009) Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42:377–381. https://doi.org/10.1016/j.jbi.2008.08.010
Brainard DH (1997) The Psychophysics toolbox. Spat Vis 10:433–436
Kleiner M, Brainard D, Pelli D et al (2007) What’s new in psychtoolbox-3. Perception 36:1–16
Delorme A, Makeig S (2004) EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134:9–21. https://doi.org/10.1016/j.jneumeth.2003.10.009
Lopez-Calderon J, Luck SJ (2014) ERPLAB: an open-source toolbox for the analysis of event-related potentials. Front Hum Neurosci 8:213. https://doi.org/10.3389/fnhum.2014.00213
Sezai T, Murphy MJ, Riddell N et al (2022) Visual processing during the interictal period between migraines: a meta-analysis. Neuropsychol Rev. https://doi.org/10.1007/s11065-022-09562-3
Pinheiro J, Bates D, R Core Team (2022) nlme: Linear and nonlinear mixed effects models
Pinheiro JC, Bates DM (2000) Linear mixed-effects models: Basic concepts and examples. Mixed-effects models in S and S-PLUS. Springer, New York, NY, pp 3–56
Omland PM, Nilsen KB, Sand T (2011) Habituation measured by pattern reversal visual evoked potentials depends more on check size than reversal rate. Clin Neurophysiol 122:1846–1853. https://doi.org/10.1016/j.clinph.2011.02.025
Isaacs D, Key AP, Cascio CJ et al (2020) Sensory hypersensitivity severity and association with obsessive-compulsive symptoms in adults with tic disorder. Neuropsychiatr Dis Treat 16:2591–2601. https://doi.org/10.2147/NDT.S274165
Dorris ER, Maccarthy J, Simpson K, McCarthy GM (2022) Sensory Perception Quotient reveals visual, scent and touch sensory hypersensitivity in people with fibromyalgia syndrome. Front Pain Res (Lausanne) 3:926331. https://doi.org/10.3389/fpain.2022.926331
Marcus DA, Soso MJ (1989) Migraine and stripe-induced visual discomfort. Arch Neurol 46:1129–1132. https://doi.org/10.1001/archneur.1989.00520460125024
Shepherd AJ (2000) Visual contrast processing in migraine. Cephalalgia 20:865–880. https://doi.org/10.1046/j.1468-2982.2000.00119.x
Mulleners WM, Chronicle EP, Palmer JE et al (2001) Suppression of perception in migraine: Evidence for reduced inhibition in the visual cortex. Neurology 56:178–183. https://doi.org/10.1212/wnl.56.2.178
Aurora SK, Ahmad BK, Welch KM et al (1998) Transcranial magnetic stimulation confirms hyperexcitability of occipital cortex in migraine. Neurology 50:1111–1114. https://doi.org/10.1212/wnl.50.4.1111
Antal A, Temme J, Nitsche M et al (2005) Altered motion perception in migraineurs: Evidence for interictal cortical hyperexcitability. Cephalalgia 25:788–794. https://doi.org/10.1111/j.1468-2982.2005.00949.x
Vecchia D, Pietrobon D (2012) Migraine: a disorder of brain excitatory–inhibitory balance? Trends Neurosci 35:507–520. https://doi.org/10.1016/j.tins.2012.04.007
Wilkinson F, Karanovic O, Wilson HR (2008) Binocular rivalry in migraine. Cephalalgia 28:1327–1338. https://doi.org/10.1111/j.1468-2982.2008.01696.x
Valeriani M, Le Pera D (2007) Abnormal brain excitability in migraine: a debated subject. Migraine disorders research trends Nova Science, New York, pp 35–60
Coppola G, Pierelli F, Schoenen J (2009) Habituation and migraine. Neurobiol Learn Mem 92:249–259. https://doi.org/10.1016/j.nlm.2008.07.006
Brighina F, Palermo A, Fierro B (2009) Cortical inhibition and habituation to evoked potentials: relevance for pathophysiology of migraine. J Headache Pain 10:77–84. https://doi.org/10.1007/s10194-008-0095-x
Sand T (2014) We were blind, so now we can see: The EP/ERP story in migraine. Clin Neurophysiol 125:433–434. https://doi.org/10.1016/j.clinph.2013.10.006
Brighina F, Cosentino G, Fierro B (2016) Habituation or lack of habituation: what is really lacking in migraine? Clin Neurophysiol 127:19–20. https://doi.org/10.1016/j.clinph.2015.05.028
Magis D, Lisicki M, Coppola G (2016) Highlights in migraine electrophysiology: are controversies just reflecting disease heterogeneity? Curr Opin Neurol 29:320–330. https://doi.org/10.1097/WCO.0000000000000335
Martin AJ, Friston KJ, Colebatch JG, Frackowiak RS (1991) Decreases in regional cerebral blood flow with normal aging. J Cereb Blood Flow Metab 11:684–689. https://doi.org/10.1038/jcbfm.1991.121
Goyal MS, Vlassenko AG, Blazey TM et al (2017) Loss of Brain Aerobic Glycolysis in Normal Human Aging. Cell Metab 26:353-360.e3. https://doi.org/10.1016/j.cmet.2017.07.010
Wong-Riley MTT (2010) Energy metabolism of the visual system. Eye Brain 2:99–116. https://doi.org/10.2147/EB.S9078
Knott JR, Irwin DA (1973) Anxiety, stress, and the contingent negative variation. Arch Gen Psychiatry 29:538–541. https://doi.org/10.1001/archpsyc.1973.04200040080013
Schoenen J (1996) Deficient habituation of evoked cortical potentials in migraine: a link between brain biology, behavior and trigeminovascular activation? Biomed Pharmacother 50:71–78. https://doi.org/10.1016/0753-3322(96)84716-0
Pinheiro CF, Moreira JR, Carvalho GF et al (2021) Interictal photophobia and phonophobia are related to the presence of aura and high frequency of attacks in patients with migraine. Appl Sci 11:2474. https://doi.org/10.3390/app11062474
Pearl TA, Dumkrieger G, Chong CD et al (2020) Sensory hypersensitivity symptoms in migraine with vs without aura: Results from the American registry for migraine research. Headache 60:506–514. https://doi.org/10.1111/head.13745
Chong CD, Starling AJ, Schwedt TJ (2016) Interictal photosensitivity associates with altered brain structure in patients with episodic migraine. Cephalalgia 36:526–533. https://doi.org/10.1177/0333102415606080
Torres-Ferrús M, Quintana M, Fernandez-Morales J et al (2017) When does chronic migraine strike? A clinical comparison of migraine according to the headache days suffered per month. Cephalalgia 37:104–113. https://doi.org/10.1177/0333102416636055
Bohotin V, Fumal A, Vandenheede M et al (2002) Effects of repetitive transcranial magnetic stimulation on visual evoked potentials in migraine. Brain 125:912–922. https://doi.org/10.1093/brain/awf081
Molenberghs G, Verbeke G (2000) Linear mixed models for longitudinal data. Springer, New York, NY
Janmaat CJ, van Diepen M, Tsonaka R et al (2019) Pitfalls of linear regression for estimating slopes over time and how to avoid them by using linear mixed-effects models. Nephrol Dial Transplant 34:561–566. https://doi.org/10.1093/ndt/gfy128
Tibon R, Levy DA (2015) Striking a balance: analyzing unbalanced event-related potential data. Front Psychol 6:555. https://doi.org/10.3389/fpsyg.2015.00555
Nappi G, Costa A, Tassorelli C, Santorelli FM (2000) Migraine as a complex disease: heterogeneity, comorbidity and genotype-phenotype interactions. Funct Neurol 15:87–93
Sinha P, Kjelgaard MM, Gandhi TK et al (2014) Autism as a disorder of prediction. Proc Natl Acad Sci USA 111:15220–15225. https://doi.org/10.1073/pnas.1416797111
Podoly TY, Ben-Sasson A (2020) Sensory habituation as a shared mechanism for sensory over-responsivity and obsessive-compulsive symptoms. Front Integr Neurosci 14:17. https://doi.org/10.3389/fnint.2020.00017
Magis D, Ambrosini A, Bendtsen L et al (2007) Evaluation and proposal for optimalization of neurophysiological tests in migraine: part 1–electrophysiological tests. Cephalalgia 27:1323–1338. https://doi.org/10.1111/j.1468-2982.2007.01440.x
Tobimatsu S, Kato M (1996) The effect of binocular stimulation on each component of transient and steady-state VEPs. Electroencephalogr Clin Neurophysiol 100:177–183. https://doi.org/10.1016/0168-5597(95)00273-1
Katsumi O, Hirose T, Tanino T (1988) Objective evaluation of binocular function with pattern reversal VER. IV. Effect of spatial and temporal frequency. Acta Ophthalmol (Copenh) 66:194–200. https://doi.org/10.1111/j.1755-3768.1988.tb04011.x
Shibata K, Yamane K, Iwata M (2006) Change of excitability in brainstem and cortical visual processing in migraine exhibiting allodynia. Headache 46:1535–1544. https://doi.org/10.1111/j.1526-4610.2006.00612.x
Al-Hassany L, Haas J, Piccininni M et al (2020) Giving researchers a headache - Sex and gender differences in migraine. Front Neurol 11:549038
Maleki N, Becerra L, Upadhyay J et al (2012) Direct optic nerve pulvinar connections defined by diffusion MR tractography in humans: Implications for photophobia. Hum Brain Mapp 33:75–88. https://doi.org/10.1002/hbm.21194
Acknowledgements
We wish to extend our gratitude to the participants that took part in this research study.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: AMM salary has been partially financed by a predoctoral grant from the “Fundacio Institut de Recerca Hospital Universitari Vall d’Hebron” (VHIR/BEQUESPREDOC/2020/MARTI). AVB salary has been partially financed by a Juan de la Cierva-Formacion grant (FJC2018-036804-I) and a Juan de la Cierva-Incorporación grant (IJC2020-043139-I) from the Spanish Ministry of Science and Innovation. XCC salary has been co-funded by the European Regional Development Fund (001-P-001682) under the framework of the FEDER Operative Programme for Catalunya 2014–2020, with 1,527,637.88 euros. EC salary has been funded by Rıo Hortega grant Accion Estrategica en Salud 2017–2020, Instituto de Salud Carlos III (CM20/00217). SSF has been supported by grants from the Ministerio de Ciencia e Innovación (PID2019-108531 GB-I00 AEI/FEDER) and AGAUR Generalitat de Catalunya (2021 SGR 00911). The project leading to these results has received funding from “la Caixa” Foundation under the project code “LCF/PR/PR16/51110005”.
Author information
Authors and Affiliations
Contributions
The substantial contributions were as follows: Concept or design of the work: AMM, AVB, XCC, NI, MT, SSF, PPR. Participant recruitment: AMM, NI, EC, and MTF. Data acquisition: AMM and AVB. Data analyses: AMM and AVB. Data interpretation: AMM, AVB, NI, XCC, VJG, MT, and SSF. Drafted the article: AMM and AVB. Critically revised the article: all authors. Supervision, project administration: PPR and SSF. Funding acquisition: PPR.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Both experiments were approved by the Ethics Committee at the Vall d’Hebron Hospital (Experiment 1: PR(AG) 376/2017), Experiment 2: EudraCT 2019–002224-32). Participants in both experiments provided their informed written consent and received monetary compensation. In Experiment 1, all participants received 25 euros upon completion and in Experiment 2 headache-free controls received 35 euros as compensation whereas patients were administered preventive treatment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Marti-Marca, A., Vilà-Balló, A., Cerda-Company, X. et al. Exploring sensory sensitivity, cortical excitability, and habituation in episodic migraine, as a function of age and disease severity, using the pattern-reversal task. J Headache Pain 24, 104 (2023). https://doi.org/10.1186/s10194-023-01618-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-023-01618-w