Abstract
Background
Migraine is a highly prevalent and complex neurovascular disease. However, the currently available therapeutic drugs often fall to adequately meet clinical needs due to limited effectiveness and numerous undesirable side effects. This study aims to identify putative novel targets for migraine treatment through proteome-wide Mendelian randomization (MR).
Methods
We utilized MR to estimate the causal effects of plasma proteins on migraine and its two subtypes, migraine with aura (MA) and without aura (MO). This analysis integrated plasma protein quantitative trait loci (pQTL) data with genome-wide association studies (GWAS) findings for these migraine phenotypes. Moreover, we conducted a phenome-wide MR assessment, enrichment analysis, protein–protein interaction networks construction, and mediation MR analysis to further validate the pharmaceutical potential of the identified protein targets.
Results
We identified 35 protein targets for migraine and its subtypes (p < 8.04 × 10–6), with prioritized targets showing minimal side effects. Phenome-wide MR identified novel protein targets—FCAR, UBE2L6, LATS1, PDCD1LG2, and MMP3—that have no major disease side effects and interacted with current acute migraine medication targets. Additionally, MMP3, PDCD1LG2, and HBQ1 interacted with current preventive migraine medication targets. The causal effects of plasma protein on migraine were partly mediated by plasma metabolites (proportion of mediation from 3.8% to 21.0%).
Conclusions
A set of potential protein targets for migraine and its subtypes were identified. These proteins showed rare side effects and were responsible for biological mechanisms involved in migraine pathogenesis, indicating priority for the development of migraine treatments.
Similar content being viewed by others
Introduction
Migraine is a prevalent neurovascular disorder with a significant genetic component. It affects 15–20% of individuals over their lifetime and is the second most disabling disease globally [1,2,3]. However, current migraine treatments face challenges. First, despite the new medications targeting the CGRP system, there remains a high non-response rate of around 30% [4, 5]. Secondly, most acute therapeutic drugs for migraine still have many side effects, including nausea, paresthesia, fatigue, and mild to moderate gastrointestinal and nervous system symptoms [6, 7]. Furthermore, there is a lack of specific drug treatment targets for different subtypes of migraines, such as migraine without/with aura (MO and MA). Discovering new drug targets for migraine and developing effective, low side-effect treatments remain significant challenges with immense potential for improving patient outcomes.
Proteins are crucial biological processes and serve as important drug targets [8]. In recent years, there has been increasing interest in exploring plasma proteins as potential drug targets for human migraine [9,10,11]. However, observational studies cannot determine whether the identified proteins are causal factors or merely consequences of the disease, as they are susceptible to confounding and reverse causality. Mendelian randomization (MR) analysis has emerged as a powerful approach to address these limitations in drug target development and drug repurposing [12]. This epidemiological approach estimates the causal effect of an exposure on an outcome by leveraging genetic variations as instrumental variables, thereby reducing the impact of confounding factors and reverse causality. MR can mimic the rigor of randomized controlled trials and improve the success rate of drug development [13]. Advancements in high-throughput proteomic techniques applied to plasma have facilitated the utilization of MR-based strategies in identifying potential therapeutic targets for various diseases [14, 15].
Recent two studies have managed to identify proteins associated with migraine using MR analysis [16, 17]. They integrated plasma quantitative trait loci (pQTL) and genome-wide association studies (GWAS) data, revealing potential pharmacological targets for migraine. These findings prompt further research with larger datasets to increase statistical power and discover additional drug target proteins. Additionally, the priority protein targets for drugs with various clinical aims require more thorough assessment. For example, distinguishing between the targets of acute and preventive medications is essential for ensuring effective and precise management of migraines. Moreover, the ways in which identified drug targets discriminately affect migraine and its subtypes need further exploration to deepen our understanding of their actions and to mitigate potential side effects.
In the present study, we performed MR to identify causal effects of proteins on migraine, MA and MO, using the largest sample size of pQTL data and GWAS data for migraine. Phenome-wide MR and protein–protein interaction (PPI) analyses were used to prioritize drug targets, while functional enrichment and two-step MR analyses were combined to explore potential biological pathways of causal associations. We hypothesize that: 1) different migraine subtypes have their specific drug target proteins; 2) the potential mechanisms by which drug target proteins in different migraine subtypes operate vary; 3) identified drug target proteins exhibit distinct applicability in acute and preventive medications.
Methods
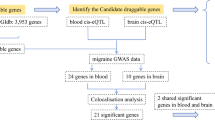
An overview of the study design is shown in Fig. 1. First, we utilized MR analysis to investigate causal relationships between exposures (plasma protein cis-pQTLs) and outcomes (migraine and its two subtypes), and we conducted enrichment analysis on identified drug target proteins to examine potential mechanisms. Second, we performed a phenome-wide MR analysis to assess potential side effects associated with these significant proteins. Third, we explored the relationships between proteins lacking significant side effects and established acute or preventive drug targets using Protein–Protein Interaction (PPI) analysis. In the final step, we applied MR mediation analysis to identify plasma metabolites that may mediate the effects between the recognized drug target proteins and the outcomes.
Informed consent was obtained from all subjects in the original genome-wide association studies. For genome-wide association study (GWAS) datasets, ethical review and approval can be accessed in the original studies.
Data sources for plasma pQTL
Plasma pQTLs were collected from the extensive GWAS conducted by the UK Biobank Pharma Proteomics Project (UKB-PPP) [18]. The UKB-PPP has conducted proteomic profiling on blood plasma samples from 54,219 participants using the Olink platform, covering 2,923 proteins [18].
Data sources for migraine, MA, and MO
For the MR analysis, summary statistics were retrieved from the largest GWAS dataset on migraine [19], which included a total of 1,339,303 individuals (cases = 79,495, controls = 1,259,808) of European ancestry. This study combined extensive GWAS data from six European populations to investigate the two main migraine subtypes, migraine with aura (MA, cases = 16,603, controls = 1,336,517) and migraine without aura (MO, cases = 11,718, controls = 1,330,747).
Mendelian randomization analysis
We performed two-sample MR analysis to evaluate the causal effect of plasma proteins on migraine and its subtypes using the ‘TwoSampleMR’ package (https://github.com/MRCIEU/TwoSampleMR). For each protein, we extracted SNPs within 1 Mb of the gene encoding the protein that were associated with plasma protein levels at genome-wide significance (p < 5 × 10⁻⁸). Then, we performed clumping process (R2 < 0.001, window size = 10,000 kb) using European samples from the 1000 Genomes Project to estimate LD between SNPs and excluded SNPs with minor allele frequency (MAF) < 0.01. To minimize weak instrument bias, only SNPs with an F value greater than 20 in the GWAS of exposure were considered as potential IVs [20, 21]. To enhance the accuracy and robustness of the genetic instruments, we proposed heterogeneity tests to detect and adjust outliers, employing the ‘ivw_radial (alpha = 0.05, weights = 1, tol = 0.0001)’ and ‘egger_radial (alpha = 0.05, weights = 1)’ functions from the RadialMR v0.4R package (https://github.com/WSpiller/RadialMR) to calculate the modified Q and Q’ tests, respectively, and discarded outliers with a nominal significance level of 0.05. We also corrected for the effect of ambiguous SNPs with non-concordant alleles (e.g., A/G vs. A/C) and palindromic SNPs with ambiguous strands (i.e., A/T or G/C), or excluded these SNPs during the harmonization process to ensure that the effect of a SNP on the exposure and the effect of the same SNP on the outcome corresponded to the same allele. We employed the IVW method with a random effects model to assess the causal impact of exposure on outcome when the number of IVs was more than 3 [22, 23], and fixed-effect inverse variance-weighted method for proteins with two or three IVs [24]. For proteins with a single IV, we relied on the Wald ratio. Odds ratios (OR) for increased risk of migraine were expressed per standard deviation (SD) increase in plasma protein levels. Bonferroni correction was used to adjust for multiple testing (p < 8.04 × 10−6, 0.05/2073/3; 2073 is the number of proteins with at least one IV and 3 represents the number of migraine types compared).
The findings of our study were rigorously validated through a comprehensive suite of sensitivity analyses. To explicitly examine the presence of horizontal pleiotropy, we employed MR-Egger regression and the MR-PRESSO Global test [25, 26]. The Cochran’s Q statistic was utilized to evaluate heterogeneity across the SNPs, ensuring a rigorous examination of effect variability [27]. Additionally, we conducted reverse MR analysis to investigate the potential associations between migraine liability and the levels of identified proteins, thereby exploring the possibility of reverse causation [28].
Phenome-wide MR analysis
To assess the potential side effects of protein targets, we conducted phenome-wide MR analyses for the identified plasma proteins using disease outcomes from the FinnGen cohort (R8, Total sample size = 342,499). The FinnGen study is a large-scale genomics initiative that has analyzed over 500,000 Finnish biobank samples and correlated genetic variation with health data to understand disease mechanisms and predispositions. Given the statistical power limitations, we selected 1421 non-headache disease traits with over 500 cases for the phenome-MR analysis. The results were corrected for multiple comparisons using Bonferroni correction. Considering the significant impact of major disease-related side effects on drug target selection, we classified proteins based on the presence of side reactions related to major diseases (cancer, benign tumors, neurological disorders, and cardiovascular diseases). Primary drug targets are proteins completely devoid of any side effect. Secondary drug targets are proteins with potential side effects but without the aforementioned significant major side effects. The proteins associated with the aforementioned major side effects were excluded from subsequent PPI analysis. This decision was made to enhance the applicability of our findings and to concentrate on proteins that may serve as potential drug targets.
Gene enrichment
We utilized GeneMANIA (http://www.genemania.org) to predict the functions and networks associated with cis-genes for proteins linked to migraine, as well as those associated with MA and MO. For a detailed description of the datasets included in GeneMANIA, kindly refer to the previous literature [29]. Pathways exhibiting enriched functions were deemed statistically significant if they had a false discovery rate less than 0.05, ensuring a robust identification of biologically relevant interactions.
We used GAMBA (http://dutchconnectomelab.nl/GAMBA.) to swiftly examine the transcriptome-neuroimaging associations for genes related to migraine and its subtypes. Gene expression and metabolic levels in the brain have been found to be closely related to migraines and contribute to migraine susceptibility [30,31,32]. Therefore, we examined the expression of the identified drug targets in the brain and investigated the metabolic mechanisms through which they might influence migraines. The brain map displays the averaged gene expression values by default, with region labels of the top 12 regions that show the highest expression levels. The cortical metabolism analysis examines the cortical metabolic differences associated with migraine-related genes, focusing on five main indicators: glycolytic index (GI), oxygen-glucose index (OGI), cerebral metabolic rate for oxygen (CMRO2), cerebral metabolic rate for glucose (CMRGlu), and cerebral blood flow (CBF). The results were corrected using Bonferroni correction.
Protein–protein interaction network
We subsequently conducted a PPI network analysis on the primary and secondary proteins, alongside commonly used migraine drug targets, to elucidate potential mechanisms of these prospective drug targets. The therapeutic drugs for migraine are categorized into two types: acute therapeutic drugs and preventive therapeutic drugs. To explore the interactions between the identified proteins and existing drug targets on the market, we constructed PPI networks for migraine, MA, and MO. Based on a recent review, a total of 16 acute migraine drugs and 10 preventive drugs were included [33], and the corresponding protein targets were located in the Drugbank (https://www.drugbank.ca) [34]. The selected treatments were from medications that are Food and Drug Administration approved or cleared, as well as treatments endorsed in guidelines and quality measures. All PPI analyses were conducted using the Search Tool for the Retrieval of Interacting Genes (STRING) database, version 12.0 (https://string-db.org/), setting the minimum required interaction score at 0.4 to ensure the relevance and specificity of our findings [35].
Mediation analysis
Considering the pivotal role of the plasma metabolites in drug metabolism [36, 37] and their crucial role in the pathogenesis of migraines [38], we utilized plasma metabolites to investigate their mediating effects between potential protein targets and migraines [39]. The GWAS summary statistics (N = 8,299) of 1,091 blood metabolites and 309 metabolite ratios were considered in this study [39]. The metabolites were categorized into eight pathways according to the Kyoto Encyclopedia of Genes and Genomes (KEGG) database definitions: lipid, amino acid, xenobiotics, nucleotide, cofactor and vitamins, carbohydrate, peptide, and energy. Additionally, 241 metabolites did not belong to any of these pathways and were thus classified as unknown or "partially" characterized molecules. More details are available in the previous study [39]. We first investigated the causal effects of metabolites on migraine. Consistent with previous studies [40, 41], we relaxed the significance threshold to p < 1 × 10−5. Then, we examined the causal effects of the identified protein targets on metabolites exerting causal effects on migraine, to observe whether they mediate the effect of proteins on migraine. Finally, we quantified the indirect effect of protein targets on migraine via metabolites. The "product of coefficients" method was used to assess the indirect effects, while the "delta" method was employed to estimate their standard errors. FDR correction was conducted for each step. The mediation effect was defined as the ratio of the indirect effect to the total effect of plasma proteins on migraine. We retained only mediation effects that were consistent with the direction of the total effect.
Results
The putative causal effects of plasma proteins on migraine
MR analysis identified significant causal effects of 17 plasma proteins on migraine (p < 8.04 × 10–6) (Fig. 2A). The odds ratios for migraine ranged from 0.67 (95% confidence interval [CI], 0.56–0.79) for Neogenin (NEO1) to 1.63 (95% CI, 1.35–1.97) for Serine/threonine-protein kinase (LATS1) (Fig. 2A and Table S1). Nine proteins were inversely associated (protective factors) and eight proteins were positively associated (risk factors). The identified migraine-associated proteins demonstrated networks, particularly in terms of Co-expression and Predicted (Figure S1). The genes of migraine-related proteins are predominantly expressed in entorhinal, caudal anterior cingulate, thalamus, insula, and pallidum, and they are linked to an increase in the cortical OGI (Fig. 3A).
MR results for plasma proteins and the risk of migraine, MO and MA. The forest plot of the MR results for 2073 plasma proteins on the risk of migraine (A), MO (B) and MA (C). Each box represents the effect (i.e., OR change) per 1 SD change in the respective plasma proteins on migraine (A), MO (B) and MA (C), respectively and the error bars represent 95% CI. Arrows indicate that 95% CI exceeds the x axis. Bonfferoni corrected P = 8.04E-06 (0.05/2073/3). Abbreviations: OR, odds ratio; CI, confidence interval
Major expression regions and cortical metabolism patterns of target genes. A Brain plots of 12 major expression regions of migraine-related genes. Overview of linear regression results between migraine-related genes expression profile and five cortical metabolism properties included in GAMBA. Dark blue indicates significant (Bonferroni p < 0.05). B Brain plots of 12 major expression regions of MO-related genes. Overview of linear regression results between migraine-related genes expression profile and five cortical metabolism properties included in GAMBA. Dark blue indicates significant (Bonferroni p < 0.05). C Brain plots of 12 major expression regions of MA-related genes. Overview of linear regression results between migraine-related genes expression profile and five cortical metabolism properties included in GAMBA. Dark blue indicates significant (Bonferroni p < 0.05). Abbreviations: GI, glycolytic index; OGI, oxygen-glucose index, CMRO2, cerebral metabolic rate for oxygen; CMRGlu, cerebral metabolic rate for glucose; CBF, cerebral blood flow
The putative causal effects of plasma proteins on MA and MO
For the two migraine subtypes, MO and MA, causal associations were identified for 10 and 9 proteins, respectively. The odds ratios for MO and MA ranged from 0.82 (95% CI, 0.76–0.87) for B-cell scaffold protein with ankyrin repeats (BANK1) to 1.17 (95% CI, 1.12–1.23) for Delta and Notch-like epidermal growth factor-related receptor (DNER) and 0.85 (95% CI, 0.81–0.90) for Beta-glucuronidase (GUSB) to 1.09 (95% CI, 1.05–1.13) for Stromelysin-1 (MMP3), respectively (Fig. 2B, C and Table S1).
The associated proteins of MO demonstrated networks of Co-expression and Predicted (Figure S1). Analysis of cis-genes revealed that MO-related proteins are mainly involved in the regulation of cardiac epithelial to mesenchymal transition, ventricular cardiac muscle tissue morphogenesis, and G protein-coupled receptor binding (Table S2). The genes of MO-related proteins are predominantly expressed in the caudal anterior cingulate, entorhinal, thalamus, insula, and postcentral areas, and they are linked to an increase in OGI and a decrease in GI and CMRGlu (Fig. 3B).
The networks of MA encompassed Co-expression and Shared protein domains (Figure S1). MA-related proteins are mainly enriched in collagen metabolic process and response to UV-A (Table S2). The genes of MA-related proteins are primarily expressed in the lingual, lateral occipital, superior parietal, postcentral, and precentral areas, and they are linked to an increase in CMRO2 (Fig. 3C). Migraine and MA both share the protein MMP3, which acts as a risk factor for both conditions. In contrast, there are no overlapping proteins between MA and MO, or between MO and migraine.
Association of identified protein targets with current migraine medications
Through the phenome-wide MR analysis, we found that primary drug target proteins include migraine-related proteins LATS1, FCAR, NEO1, and UBE2L6. Secondary drug target proteins include MMP3, which is related to both migraine and MA, and HBQ1 and PDCD1LG2, which are related to MO (Bonferroni correction p < 9.77 × 10–7, 0.05/1421 diseases/36 total proteins, Figure S2 and Table S3-6). These proteins were used for subsequent PPI analysis.
Detailed information about migraine drugs and their respective targets can be found in Table S10. A total of six proteins interact with existing migraine drug protein targets. Among the migraine-related proteins, LATS1, FCAR, MMP3, and UBE2L6 are associated with acute drug targets, including non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, aspirin, diclofenac, ketorolac, and naproxen. Notably, MMP3 is found to be linked to the preventive drug target of divalproex sodium. MMP3 is also the only protein found in MA that is associated with current drugs. Among the two proteins related to MO, PDCD1LG2 and HBQ1, PDCD1LG2 is linked to both the acute drugs ibuprofen and aspirin, as well as the preventive drug divalproex sodium. In contrast, HBQ1 is only associated with the preventive drug topiramate (Fig. 4, Figure S3, S4).
Interaction between commonly used acute and preventive migraine medications targets and identified potential drug targets. The migraine-associated proteins, including LATS1, FCAR, MMP3, and UBE2L6, as well as MO-association proteins PDCD1LG2 and MA-related proteins MMP3, were linked to acute migraine drugs. The common protein MMP3 for migraine and MA, as well as the MO-related protein HBQ1, are associated with preventive migraine drugs
Mediation effect of proteins on migraine outcomes via plasma metabolites
In the mediation analysis between proteins and migraine, we found the effect of MMP3 on migraine was mediated by 21-hydroxypregnenolone disulfate and Glucuronate/Androsterone glucuronide (FDR p = 0.016 and 0.020), which was consistent with the total effect, with a mediating effect of 6.4% and 7.3%, respectively. The effect of PDCD1LG2 on MO was mediated by Gamma-glutamylglutamine, 3-hydroxysebacate, and partially characterized molecules (FDR p = 0.020, 0.039 and 0.025), which was consistent with the total effect, with a mediating effect of 11.3%, 21.0% and 14.5%, respectively. The effect of MMP3 on MA was mediated by sphingomyelin (FDR p = 0.019), which was consistent with the total effect, with a mediating effect of 3.8% (Fig. 5, Table 1 and Table S7-10).
Mediation effects of protein on migraine via plasma metabolites. Mediation analyses to quantify the effects of proteins with PPI on migraine, MO and MA outcomes via plasma metabolites. A MMP3 effect on migraine mediated by 21-hydroxypregnenolone disulfate. B MMP3 effect on migraine mediated by Glucuronate/Androsterone glucuronide; (C) PDCD1LG2 effect on MO mediated by Gamma-glutamylglutamine; (D) PDCD1LG2 effect on MO mediated by partially characterized molecules; (E) PDCD1LG2 effect on MO mediated by 3-hydroxysebacate; (F) MMP3 effect on MA mediated by sphingomyelin. βEM, effects of exposure on mediator, βMO, effects of mediator on outcome, βEO, effects of exposure on outcome
Sensitivity analysis
The results of the sensitivity analyses confirmed the robustness of the primary MR analyses. There was no evidence for heterogeneity in the association of any proteins in Table S11, as measured by Cochran Q statistics (p > 0.05). There was also no indication that the instrumental variables had horizontal pleiotropy, as assessed by the MR-Egger intercept (p > 0.05) and MR-PRESSO Global test (p > 0.05). Furthermore, there was no evidence of reverse causations (Table S12). The expected bias caused by sample overlap was quantified according to the method recommended by the previous technical study [42]. In this study, the migraine GWAS included part of the UKB population, and the plasma protein GWAS was also based on the UKB population, with an overlap rate of 4.05% at most. For each pair of causal association, the bias caused by sample overlap was less than 0.001, indicating that our causal estimates were less likely to be biased by sample overlaps (Table S13).
Discussion
This study investigated the causal effects of plasma proteins on migraine using a Two-sample MR. A total of 35 proteins were found to exert causal effects on migraine/MA/MO, and these proteins are associated with cortical metabolism. The primary drug targets associated with PPI include the migraine-related proteins FCAR, UBE2L6, and LATS1, while the secondary drug targets include proteins related to both migraine and MA, such as MMP3, as well as proteins related to MO, including PDCD1LG2 and HBQ1. Additionally, some plasma metabolites were found to partially mediate the effect of these protein targets on migraine.
Among the 35 proteins identified, only one protein, MMP3, overlaps between migraine and MA, suggesting the need to consider the pathological heterogeneity of subtypes in drug development. Animal experiments have shown that MMP3 can cause cortical spreading depression (CSD) [43] and blood–brain barrier (BBB) disruption [44], both of which are associated with migraine. Our findings provide causal evidence and enhance the reliability of translating these findings to humans. The primary expression regions in the brain for migraine-related proteins and MO-related proteins are similar, including the entorhinal cortex, caudal anterior cingulate, thalamus, and insula, all of which are involved in the pathogenesis of migraines. Interestingly, the expression regions of the MA-related proteins additionally include the lateral occipital region, which is a brain area associated with visual perception and is also the origin of CSD in the aura of MA [45]. The expression of migraine-related proteins is associated with cortical energy metabolism, including glucose metabolism and oxygen consumption. This is consistent with previous observations that chronic brain energy deficiency promote the chronicity of migraines and metabolic enhancers can improve migraine. These findings indicated that the identified protein targets for migraine and its subtypes may involve underlying neuropathology.
Three proteins—LATS1, FCAR, and UBE2L6—showed no potential side effects in the Phenome-wide MR assessment, and all of them showed protein interactions with targets of current acute migraine drugs. We found that increased levels of FCAR can reduce the risk of migraines, while the elevation of LATS1 levels increases the risk of migraines. This effect may be attributed to immune regulation. FCAR interacts with aggregated IgA (such as IgA coating invading microorganisms) and mediates several immune defense processes [46]. LATS1/2 can enhance immune responses by upregulating the expression of IFN-β and the production of these immune-induced nucleic acid exonucleases [47]. Neurogenic inflammation and neuroinflammation have been found to be associated with the mechanisms of migraine attacks [48, 49]. During migraine attacks, peripheral levels of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 were observed to further significantly increase, while levels of IL-4 and IL-5 decreased [50]. The association of pro-inflammatory cytokines and chemokines in migraines suggests that migraines have an underlying pro-inflammatory state. Furthermore, the inflammation hypothesis of migraine implies that the rapid response to acute migraine therapies can be attributed to the rapid reduction of inflammation.
The elevated levels of UBE2L6 increase the risk of migraines. UBE2L6 generally functions as a ubiquitin-conjugating enzyme, involved not only in protein ubiquitination but also in the innate immune system [51]. It has also been reported to be associated with lipid metabolism [52], which is involved in the pathogenesis of migraines. Studies have found significantly elevated levels of cholesterol, low-density lipoprotein cholesterol (LDL-C), and oxidized LDL-C in normal-weight migraine patients [53]. An elevation in total cholesterol is significantly associated with migraines, and this association increases in elderly male migraine patients [54]. Targeting UBE2L6 could potentially be an acute drug target for migraine, as it may be able to acutely reduce inflammatory responses and regulate lipid metabolism, which are both implicated in the pathogenesis of migraine.
Our Phenome-wide MR assessment found that three proteins—MMP3, HBQ1, and PDCD1LG2—showed no potential side effects of major diseases. Among them, MMP3 increased the risks of both migraine and MA. MMP3 is a member of the class of zinc-dependent proteases known to degrade the extracellular matrix. During a migraine attack without aura, MMP3 levels in the external jugular vein and cubital vein plasma are significantly lower than during the interictal phase [55]. There are three potential pathway through which MMP3 may exert effects on migraine. First, MMP3 may increase the risk of migraine through direct cellular damage. There is evidence that increased MMP activity can cause direct cellular damage in central nervous system diseases [56]. Second, MMP3 may affect migraine through inflammatory immune mechanisms. MMPs play a dual role in the regulation of inflammatory mediators, being involved in both activation (MMP3 and MMP9, and to a lesser extent MMP2) and inactivation (MMP3) [57]. Third, MMP3 may influence migraine by disrupting the BBB. It has been reported that MMP3 knockout mice have less BBB disruption compared to wild-type mice after lipopolysaccharide-induced BBB opening [44]. Additionally, CSD, which is considered the origin of aura, can increase MMP upregulation and vascular permeability changes in migraine patients [43]. MMP3 is also well-associated with existing acute and preventive drug targets, likely acting through different mechanisms. Targeting direct cellular damage and inflammatory immune responses could offer rapid effects, similar to acute nonsteroidal anti-inflammatory drugs. In contrast, targeting BBB disruption and CSD activation would be a long-term preventive treatment process. Subsequent mediation analysis found that the effect of MMP3 on migraines is partially mediated through 21-hydroxypregnenolone disulfate (a type of pregnenolone steroid) and the metabolic ratio of glucuronate/androsterone glucuronide. Pregnenolone steroid, in addition to serving as a precursor for other steroid hormones, also acts as an anti-inflammatory molecule, maintaining immune homeostasis under various inflammatory conditions [58]. In this mediation analysis, the elevated levels of MMP3 lead to a reduction in 21-hydroxypregnenolone disulfate. This causes an imbalance in immune homeostasis, implying an increase in inflammatory response, which consequently results in migraines. The effect of MMP3 on MA is partially mediated through sphingomyelin. Studies have shown that sphingolipid metabolism is altered in women with episodic migraine (EM), and the serum sphingolipid panel may have the potential to distinguish the presence or absence of EM [59]. This partially explains how MMP3 may influence MA by regulating lipid metabolism. In conclusion, drugs targeting MMP3 have great potential for the treatment of both migraine and MA.
The overexpression of HBQ1 promotes cell proliferation by reducing basal ROS levels, highlighting its role as a protector against oxidative stress [60]. Our findings indicate that elevated expression of HBQ1 reduces the risk of MO. Oxidative stress induces neurogenic inflammation, a key event in migraines, by converting signals through the TRPA1 ion channel on meningeal pain receptors [61]. Thus, we hypothesize that increased HBQ1 expression emphasizes its antioxidant effects, reducing neurogenic inflammation and consequently lowering the risk of MO. We found that HBQ1 is associated with the preventive drug topiramate, an antiepileptic medication that reduces oxidative stress by decreasing ROS production and enhancing antioxidant defenses [62]. Therefore, HBQ1 shows potential as a preventive drug target for migraines.
PDCD1LG2 can induce various immune and non-immune cells through adaptive mechanisms, depending on the inflammatory cytokine environment [63], which increases the risk of MO. Interestingly, PDCD1LG2 exhibits potential for a dual role as both an acute therapeutic target and a preventive therapeutic target. In subsequent mediation analysis, we found that the causal relationship between PDCD1LG2 and MO is partially mediated by gamma-glutamylglutamine, 3-hydroxysebacate, and partially characterized molecules. 3-hydroxysebacate is a dicarboxylic acid dianion obtained by deprotonation of both carboxy groups of 3-hydroxysebacic acid. Increased levels of this lipid metabolite have been observed in depression subjects [64]. This explains that the effect of PDCD1LG2 on MO is partially mediated by superway peptide metabolism and lipid metabolism in plasma.
We acknowledge that our study has several limitations. First, we only focused on cis-pQTLs and did not consider trans-pQTLs, which decreases the risk of violating MR assumptions but meanwhile overlooks more complex regulatory mechanisms underlying protein expression. Second, the participants were all of European ancestry, so the findings may not be directly applicable to populations of other ethnic/racial backgrounds. Third, there may still be potential bias, though only a small percentage of samples overlap. Finally, the drug targets we identified are putative and need further validation through basic experiments and randomized controlled trials.
Conclusion
In summary, our analysis prioritized a set of circulating proteins as potential targets for migraine and its two major subtypes. Among them, FCAR, UBE2L6, and LATS1 may be attractive drug targets for treating acute migraine. HBQ1 may be attractive drug targets for preventive migraine. MMP3 and PDCD1LG2 show potential as dual-purpose drug targets, being attractive for both acute and preventive migraine treatment. The roles of these candidate proteins in migraine pathophysiology warrant further investigation to fully elucidate their therapeutic potential.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MR:
-
Mendelian randomization
- MA:
-
Migraine with aura
- MO:
-
Migraine without aura
- pQTL:
-
Protein quantitative trait loci
- GWAS:
-
Genome-wide association studies
- PPI:
-
Protein-protein interaction
- UKB-PPP:
-
UK Biobank Pharma Proteomics Project
- OR:
-
Odds ratios
- SD:
-
Standard deviation
- GI:
-
Glycolytic index
- OGI:
-
Oxygen-glucose index
- CMRO2:
-
Cerebral metabolic rate for oxygen
- CMRGlu:
-
Cerebral metabolic rate for glucose
- CBF:
-
Cerebral blood flow
- STRING:
-
Search Tool for the Retrieval of Interacting Genes
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- CSD:
-
Cortical spreading depression
- BBB:
-
Blood-brain barrier
- EM:
-
Episodic migraine
References
GBD (2019) Diseases and Injuries Collaborators (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396:1204–1222
Lipton RB, Bigal ME (2005) The epidemiology of migraine. Am J Med 118(Suppl 1):3s–10s
Steiner TJ, Stovner LJ, Jensen R et al (2020) Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain 21:137
Do TP, Guo S, Ashina M (2019) Therapeutic novelties in migraine: new drugs, new hope? J Headache Pain 20:37
Wrobel Goldberg S, Silberstein SD (2015) Targeting CGRP: A New Era for Migraine Treatment. CNS Drugs 29:443–452
Ingram EE, Bocklud BE, Corley SC et al (2023) Non-CGRP Antagonist/Non-Triptan Options for Migraine Disease Treatment: Clinical Considerations. Curr Pain Headache Rep 27:497–502
Tinsley A, Rothrock JF (2021) Safety and tolerability of preventive treatment options for chronic migraine. Expert Opin Drug Saf 20:1523–1533
Zheng J, Haberland V, Baird D et al (2020) Phenome-wide Mendelian randomization mapping the influence of the plasma proteome on complex diseases. Nat Genet 52:1122–1131
Welch KM, Brandes AW, Salerno L et al (2006) C-reactive protein may be increased in migraine patients who present with complex clinical features. Headache 46:197–199
Fava A, Pirritano D, Consoli D et al (2014) Chronic migraine in women is associated with insulin resistance: a cross-sectional study. Eur J Neurol 21:267–272
Togha M, Rahimi P, Farajzadeh A et al (2022) Proteomics analysis revealed the presence of inflammatory and oxidative stress markers in the plasma of migraine patients during the pain period. Brain Res 1797:148100
Reay WR, Cairns MJ (2021) Advancing the use of genome-wide association studies for drug repurposing. Nat Rev Genet 22:658–671
Finan C, Gaulton A, Kruger FA et al (2017) The druggable genome and support for target identification and validation in drug development. Sci Transl Med 9(383):eaag1166
Lin J, Zhou J, Xu Y (2023) Potential drug targets for multiple sclerosis identified through Mendelian randomization analysis. Brain 146:3364–3372
Zhang N, Li Y, Sundquist J et al (2023) Identifying actionable druggable targets for breast cancer: Mendelian randomization and population-based analyses. EBioMedicine 98:104859
Sun X, Chen B, Qi Y et al (2024) Multi-omics Mendelian randomization integrating GWAS, eQTL and pQTL data revealed GSTM4 as a potential drug target for migraine. J Headache Pain 25:117
Niu PP, Zhang R, Zhang C et al (2024) Identifying novel proteins for migraine by integrating proteomes from blood and CSF with genome-wide association data. CNS Neurosci Ther 30:e14817
Sun BB, Chiou J, Traylor M et al (2023) Plasma proteomic associations with genetics and health in the UK Biobank. Nature 622:329–338
Bjornsdottir G, Chalmer MA, Stefansdottir L et al (2023) Rare variants with large effects provide functional insights into the pathology of migraine subtypes, with and without aura. Nat Genet 55:1843–1853
Palmer TM, Lawlor DA, Harbord RM et al (2012) Using multiple genetic variants as instrumental variables for modifiable risk factors. Stat Methods Med Res 21:223–242
Zhao L, Tang Y, Tu Y et al (2024) Genetic evidence for the causal relationships between migraine, dementia, and longitudinal brain atrophy. J Headache Pain 25:93
Burgess S, Butterworth A, Thompson SG (2013) Mendelian randomization analysis with multiple genetic variants using summarized data. Genet Epidemiol 37:658–665
Hartwig FP, Davey Smith G, Bowden J (2017) Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol 46:1985–1998
Borenstein M, Hedges LV, Higgins JP et al (2010) A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 1:97–111
Bowden J, Davey Smith G, Burgess S (2015) Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 44:512–525
Verbanck M, Chen CY, Neale B et al (2018) Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet 50:693–698
Kulinskaya E, Dollinger MB (2015) An accurate test for homogeneity of odds ratios based on Cochran’s Q-statistic. BMC Med Res Methodol 15:49
Davey Smith G, Hemani G (2014) Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 23:R89-98
Warde-Farley D, Donaldson SL, Comes O et al (2010) The GeneMANIA prediction server: biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res 38:W214-220
Estevez M, Gardner KL (2004) Update on the genetics of migraine. Hum Genet 114:225–235
Sutherland HG, Jenkins B, Griffiths LR (2024) Genetics of migraine: complexity, implications, and potential clinical applications. Lancet Neurol 23:429–446
Gomez-Pilar J, Martínez-Cagigal V, García-Azorín D et al (2022) Headache-related circuits and high frequencies evaluated by EEG, MRI, PET as potential biomarkers to differentiate chronic and episodic migraine: Evidence from a systematic review. J Headache Pain 23(1):95
Robbins MS (2021) Diagnosis and Management of Headache: A Review. JAMA 325:1874–1885
Wishart DS, Feunang YD, Guo AC et al (2018) DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res 46:D1074-d1082
Szklarczyk D, Gable AL, Lyon D et al (2019) STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47:D607-d613
Obach RS (2013) Pharmacologically active drug metabolites: impact on drug discovery and pharmacotherapy. Pharmacol Rev 65:578–640
Hendset M, Haslemo T, Rudberg I et al (2006) The complexity of active metabolites in therapeutic drug monitoring of psychotropic drugs. Pharmacopsychiatry 39:121–127
Tanha HM, Sathyanarayanan A, Nyholt DR (2021) Genetic overlap and causality between blood metabolites and migraine. Am J Hum Genet 108:2086–2098
Chen Y, Lu T, Pettersson-Kymmer U et al (2023) Genomic atlas of the plasma metabolome prioritizes metabolites implicated in human diseases. Nat Genet 55:44–53
Lv Y, Cheng X, Dong Q (2024) SGLT1 and SGLT2 inhibition, circulating metabolites, and cerebral small vessel disease: a mediation Mendelian Randomization study. Cardiovasc Diabetol 23:157
Zhang W, Sun J, Yu H et al (2024) Causal relationship between type 2 diabetes mellitus and aortic dissection: insights from two-sample Mendelian randomization and mediation analysis. Front Endocrinol (Lausanne) 15:1405517
Burgess S, Davies NM, Thompson SG (2016) Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol 40:597–608
Gursoy-Ozdemir Y, Qiu J, Matsuoka N et al (2004) Cortical spreading depression activates and upregulates MMP-9. J Clin Invest 113:1447–1455
Gurney KJ, Estrada EY, Rosenberg GA (2006) Blood-brain barrier disruption by stromelysin-1 facilitates neutrophil infiltration in neuroinflammation. Neurobiol Dis 23:87–96
Lauritzen M (1994) Pathophysiology of the migraine aura. The spreading depression theory Brain 117(Pt 1):199–210
Herr AB, Ballister ER, Bjorkman PJ (2003) Insights into IgA-mediated immune responses from the crystal structures of human FcalphaRI and its complex with IgA1-Fc. Nature 423:614–620
Zhang Q, Meng F, Chen S et al (2017) Hippo signalling governs cytosolic nucleic acid sensing through YAP/TAZ-mediated TBK1 blockade. Nat Cell Biol 19:362–374
Moskowitz MA (1993) Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology 43:S16-20
Cavestro C, Ferrero M, Mandrino S et al (2019) Novelty in Inflammation and Immunomodulation in Migraine. Curr Pharm Des 25:2919–2936
Munno I, Centonze V, Marinaro M et al (1998) Cytokines and migraine: increase of IL-5 and IL-4 plasma levels. Headache 38:465–467
Gao J, Li C, Li W et al (2021) Increased UBE2L6 regulated by type 1 interferon as potential marker in TB. J Cell Mol Med 25:11232–11243
Wei W, Li Y, Li Y et al (2021) Adipose-specific knockout of ubiquitin-conjugating enzyme E2L6 (Ube2l6) reduces diet-induced obesity, insulin resistance, and hepatic steatosis. J Pharmacol Sci 145:327–334
Gruber HJ, Bernecker C, Pailer S et al (2010) Lipid profile in normal weight migraineurs - evidence for cardiovascular risk. Eur J Neurol 17:419–425
Monastero R, Pipia C, Cefalù AB et al (2008) Association between plasma lipid levels and migraine in subjects aged > or =50 years: preliminary data from the Zabùt Aging Project. Neurol Sci 29(Suppl 1):S179-181
Ashina M, Tvedskov JF, Lipka K et al (2010) Matrix metalloproteinases during and outside of migraine attacks without aura. Cephalalgia 30:303–310
Trysberg E, Blennow K, Zachrisson O et al (2004) Intrathecal levels of matrix metalloproteinases in systemic lupus erythematosus with central nervous system engagement. Arthritis Res Ther 6:R551-556
Ito A, Mukaiyama A, Itoh Y et al (1996) Degradation of interleukin 1beta by matrix metalloproteinases. J Biol Chem 271:14657–14660
Murugan S, Jakka P, Namani S et al (2019) The neurosteroid pregnenolone promotes degradation of key proteins in the innate immune signaling to suppress inflammation. J Biol Chem 294:4596–4607
Peterlin BL, Mielke MM, Dickens AM et al (2015) Interictal, circulating sphingolipids in women with episodic migraine: A case-control study. Neurology 85:1214–1223
Kim K, Choi EY, Ahn HM et al (2023) Hemoglobin Subunit Theta 1 Promotes Proliferation by Reducing Reactive Oxygen Species in Lung Adenocarcinoma. Cancers (Basel) 15(23):5504
Borkum JM (2018) The Migraine Attack as a Homeostatic, Neuroprotective Response to Brain Oxidative Stress: Preliminary Evidence for a Theory. Headache 58:118–135
Nazıroğlu M, Yürekli VA (2013) Effects of antiepileptic drugs on antioxidant and oxidant molecular pathways: focus on trace elements. Cell Mol Neurobiol 33:589–599
Baumeister SH, Freeman GJ, Dranoff G et al (2016) Coinhibitory Pathways in Immunotherapy for Cancer. Annu Rev Immunol 34:539–573
Steffens DC, Wei J, Krishnan KR et al (2010) Metabolomic differences in heart failure patients with and without major depression. J Geriatr Psychiatry Neurol 23:138–146
Acknowledgements
We would like to acknowledge the participants and investigators of the UK Biobank, the FinnGen study, the Canadian Longitudinal Study on Aging (CLSA) and all the other studies.
Funding
This study was supported by the National Natural Science Foundation of China (grant numbers: 32170752, 91849104, and 31770800).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. ZHX and LZ wrote the original draft. ZHX, LZ, YLM, DQ, XSL, PZ and MTZ analyzed the data. ZHX and LZ prepared Figures and tables. JC and YGW reviewed and edited the final draft. All authors contributed to the article and approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Xiong, Z., Zhao, L., Mei, Y. et al. Proteome-wide Mendelian randomization identified potential drug targets for migraine. J Headache Pain 25, 148 (2024). https://doi.org/10.1186/s10194-024-01853-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-024-01853-9