Abstract
Resin cement integrated with zein-incorporated magnesium oxide nanoparticles has previously been found to inhibit oral microbes and decrease bacterial biofilm. However, the bond strength and surface features of this biomaterial have yet to be investigated. The objective of this study was to evaluate the shear bond strength, mode of fracture, and surface roughness of resin cement modified with zein-incorporated magnesium oxide nanoparticles. Characterization of the cement was performed by X-ray diffraction, field emission scanning electron microscopy, and Fourier transform infrared spectroscopy. 126 human teeth were divided into 3 groups and cemented to lithium disilicate ceramic using resin cement with zein-incorporated magnesium oxide nanoparticles at concentrations of 0%, 1%, and 2% (n = 42). 21 samples of each group were subjected to the shear bond strength test, while the other 21 underwent thermocycling for 10,000 cycles before the test, after which all samples were evaluated for the mode of fracture. To assess surface roughness, resin cement disks were analyzed by a profilometer before and after undergoing thermocycling for 10,000 cycles. The shear bond strength of the cement with 1% and 2% nanoparticles was significantly higher than the control before thermocycling. The mode of fracture was found to be mainly adhesive with all groups, with the unmodified cement presenting the highest cohesive failure. There was no significant difference in surface roughness between the groups before or after thermocycling. The addition of zein-incorporated magnesium oxide nanoparticles to resin cement improved or maintained the shear bond strength and surface roughness of the resin cement.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The integration of nanotechnology for dental utilization has become increasingly prevalent, following the promising results that nanoparticles are capable of providing [1,2,3]. Nanoparticles are defined as particles of matter that are smaller than 100nm in dimension, and consequently have unique properties by virtue of their small size. Nanoparticles have a large surface-area-to-volume ratio, enabling them to interact with materials more effectively than their larger-sized counterparts. This in turns leads to improved physical, biological, mechanical, and physicochemical properties of the dental material incorporated with the nanoparticles [2, 4,5,6]. Additionally, nanoparticles can be engineered to embody distinct properties, such as an enhanced biocompatibility, biodegradability, or antibacterial activity [1, 3, 5, 7]. Nanomaterials consisting of an organic polymer and inorganic nanoparticles have especially drawn attention [8].
These promising results have spurred a flurry of interest to further expand on the currently limited information available. Dental treatment has benefited from this interest in the form of improving materials such as composites, ceramics, and adhesives, among others [3, 9]. Inorganic nanoparticles are commonly employed, with notable ones being silver, copper, and zinc nanoparticles [1, 10,11,12]. Among these inorganic nanoparticles, magnesium oxide nanoparticles (MgO NPs) have attracted interest as a result of their exceptional antimicrobial capacity as well as their ability to permeate into the spaces between the inter-polymeric chains, eventuating an increase in mechanical and physical strength of the material [6, 13,14,15,16]. Nanoparticles must be evenly distributed throughout the matrix of the material in order to achieve the desired properties, and previously, MgO NPs had been overlooked due to the nanoparticles’ tendency to agglomerate, which would impede on the dispersion of the nanoparticles, transforming them to a macro-scale filler. This would ultimately lead to a loss of the nano-properties and consequently hinder the stability and attributes of the material [1, 4, 14, 17].
However, a discovery by Naguib et al. involving the coating of the MgO NPs with the natural corn polymer zein has been shown to prevent the agglomeration without interfering with the MgO NPs’ nano-characteristics [18, 19]. The antimicrobial impact of 1% or 2% zein-coated MgO NPs was found to be highly effective against the four bacteria Streptococcus mutans, Staphylococcus aureus, Enterococcus faecalis, and Candida albicans. This antimicrobial activity has been reported to occur as a result of the nanoparticles binding and damaging the peptidoglycan cell wall and membrane of the bacteria, disrupting the synthesis of bacterial proteins, and preventing DNA duplication [20, 21]. The zein-incorporated magnesium oxide nanoparticles (zMgO NPs) have been integrated into dental biomaterials with promising results, but the data is still limited [14, 22,23,24,25,26]. Additional investigations are necessary to fully evaluate the scope of effects the zMgO NPs can exhibit on the properties of dental materials.
Dental cements are an integral part of the restorative process. They possess many dental indications and are used in various procedures such as bonding restorations, cementing fixed dental prosthesis, and sealing root canals [14, 27]. The disintegration or failure of the dental cement in its respective treatment can lead to bacterial microleakage and the consequent sequelae of recurrent caries, oral disease progression, and failure of the treatment [28, 29]. To bypass these issues, various active ingredients such as hexametaphosphate microparticles, calcium phosphate, and silver ions have been added to dental cements, as an antibacterial cement could be advantageous in defending the sensitive tooth-restoration interface against bacterial exposure from the oral environment [30,31,32]. However, the addition of nanoparticles demonstrated varying degrees of effects on the physical and mechanical properties of the dental material. Aluminum oxide was found to weaken glass ionomer cement, and titanium oxide nanoparticles similarly decreased the hardness of glass ionomer cement [33,34,35]. These limitations were often encountered with higher concentrations of nanoparticles or with nanoparticles lacking a coating or coupling agent, which would result in less dispersion of the nanoparticles and therefore negatively affect the properties of the dental material [14, 30, 32]. Compromising the properties of the cement will jeopardize the longevity of the restoration, and so a solution is required to improve the antibacterial capacity of the cement while maintaining or improving its inherent desirable qualities.
Resin cements, with their esthetic and conservative characteristics along with strong compressive and tensile strengths and low solubility, are becoming the preferred cement of choice in clinical dentistry [27]. Resin polymers with nanoparticles have been utilized to reinforce and strengthen the mechanical qualities of the cement as well as improve the bond between the adhesive and the tooth by penetrating the tubules of the dentin, reducing polymerization shrinkage, and raising the elastic modulus of the adhesive layer [8, 36]. Researchers reported that the supplementation of MgO NPs to glass ionomer cements enhanced the antimicrobial activity of the cement without hindering the mechanical properties, while others discovered that a nanostructured mixture of MgO NPs augmented the mechanical properties of the reinforced polymer [13, 37].
The use of an antimicrobial resin cement has the potential to improve dental treatment; however, a wider scope of the effects of implementing zMgO NPs into resin cement needs to be assessed. This study is part of a series of investigations regarding the physical, mechanical, and biological effects of zMgO NPs on various dental materials [14, 18, 19, 22,23,24,25,26]. To the authors’ knowledge, there is currently no data on the effects of zMgO NPs on the shear bond strength and surface roughness of resin dental cement. The objective of this study was to incorporate zMgO NPs with resin cement and evaluate the effects on the shear bond strength, mode of failure, and surface roughness of the resin cement, as well as to assess the degree of these changes before and after thermocycling. The null hypothesis stated that there will be no difference between the modified and unmodified cement in the shear bond strength and surface roughness before and after thermocycling.
2 Methodology
2.1 Ethical approval
An ethical approval was obtained from the Research Ethics Committee at King Abdulaziz University, Faculty of Dentistry (#18-03-20).
2.2 Study design
2.2.1 Shear bond strength and mode of failure tests
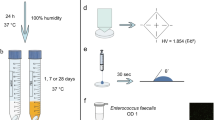
An overview of the study design for the shear bond strength (SBS) tests and the mode of fracture can be seen in Fig. 1A. 126 sound extracted human teeth (molars) were obtained (n = 126) from Dr. Rami Hasanain of ‘RadiClinic Dental and Implant Clinic’ (Malibari Center, Jeddah, Saudi Arabia) after assessment of the following criteria: caries-free, fully-formed with a closed apex, and with no previous treatment or instrumentation introduced to the pulp chamber or canals. The teeth were stored in saline mixed with 0.1% thymol solution at 4 °C prior to the experiment and were divided randomly into 3 groups (n = 42). These 3 groups were the control (Rely X unmodified resin cement paste), the 1% zMgO NPs group (Rely X resin cement paste with zMgO NP in 1% weight concentration), and the 2% zMgO NPs group (Rely X resin cement paste with zMgO NP in 2% weight concentration). Each of these groups was further divided into 2 groups of ‘before thermocycling’ and ‘after thermocycling’ (n = 21). The thermocycled samples underwent 10,000 cycles between 5 and 55 °C. Each of these samples were subjected to the ‘Shear Bond Strength’ test, after which they were evaluated for the ‘Mode of Fracture’.
2.2.2 Surface roughness test
An overview of the study design for the surface roughness test can be seen in Fig. 1B. 30 samples of resin cement were used for the surface roughness test. The samples were divided randomly into 3 groups (n = 10): the control (unmodified Rely X resin cement paste), the 1% zMgO NPs group (Rely X resin cement paste with zMgO NP in 1% weight concentration), and the 2% zMgO NPs group (Rely X resin cement paste with zMgO NP in 2% weight concentration). Each of the samples underwent assessment using the profilometer, were thermocycled for 10,000 cycles between 5 and 55 °C, and then underwent another assessment using the profilometer.
2.3 Materials preparation
IPS e.max lithium disilicate ceramic was the material used in fabricating ceramic cylinders measuring 3mm in diameter and 10mm height. For the luting cement, 3 M™ Rely X™ Ultimate Adhesive Resin Cement (3 M, Minnesota, USA) was used. Following the previously established protocol by Naguib et al. the inorganic nanoparticles were manufactured first by utilizing microwaves. The MgO NPs, 40 nm in diameter and 100 nm in length, were coated by zein particles, which were also 40nm diameter and 100 nm. pH-controlled nano-precipitation was employed to coat the MgO nanowires with zein [19]. The zein (0.02g) was added into a solution containing ethanol and 0.1 NaOH (93.7% v/v), after which the zein droplets were infused with a 15ml blend of 0.02g of MgO and polyvinyl alcohol at 0.9% (w/v) under the following parameters: temperature of 10 °C, 750W ultrasonic shear, and 20kHz frequency. The blend was magnetically stirred at a rate of 500rpm, and then centrifuged twice at a rate of 3,000rpm for 45 min to obtain pure nanoparticles and expel the polyvinyl alcohol. The supernatant was then removed.
To liquify the formed pellet, a 5ml buffer was used after which 2% (w/v) of trehalose was introduced into the blend. A lyophilizer was utilized to lyophilize the mixture. (VirTis Bench Top Lyophilizer, SP Industries, Stone Ridge, NY, USA). Subsequently, zein with polyvinyl alcohol (2:1 by weight) was prepared, and the MgO NPs were readied (4:1 by weight). The two mixtures of MgO with zein and polyvinyl alcohol were magnetically stirred for 30 min. The polyvinyl alcohol was volatilized, and the mix was again centrifuged, and finally freeze-dried. The zein-coated MgO NPs were weighed using a balance accurate to 0.0001g (BEL Engineering, Monza, Italy) and were added to the Rely X cement in the ratio of 1% and 2% by weight. These concentrations were used as they were previously found to exhibit an antimicrobial effect against the microbes S. mutans, S. aureus, E. faecalis and C. albicans [18].
2.4 Characterization of the dental cement modified with zMgO NPs
2.4.1 X-ray diffraction analysis
An X-Ray diffractometer (XRD; Rigaku, Ultima IV, Japan) was utilized to investigate crystalline structure of the Rely X resin cement alone as well as the Rely X resin cement after incorporation of the zMgO NPs. A Cu-Kα X-ray radiation (λ = 1.542 Å) was used, and the XRD spectra were scanned in the 2θ range of 10–80°.
2.4.2 Field emission scanning electron microscopy
The surface morphology and the distribution of zein-coated nanoparticles in the resin cement samples were studied through Field Emission Scanning Electron Microscopy (FESEM) (JEOL JSM-7600F, JEOL Ltd. Tokyo, Japan) under an ultra-high vacuum of ~ 10−6 mbar.
2.4.3 Fourier transform infrared spectroscopy (FTIR)
FTIR was used to scan the test samples and observe their chemical properties. The FTIR spectra of the Rely X resin cement before and after incorporation of zMgO nanoparticles were assessed. This was achieved by using an FTIR type 8000 series Fourier Transformation (Shimadzu Co., Japan) to record Fourier transformation of infrared spectra. The specifications involved KBr plates in the absorbance mode at a wavenumber range of 500–4000 cm−1 under identical conditions.
2.5 Preparation of the teeth (for the SBS and mode of fracture tests)
The 126 teeth were prepared by cutting the crown portion to expose the dentin surface using an IsoMet machine (Buehler International Inc, Illinois, USA). Teeth were examined to ensure that the pulp was not exposed. Small ceramic cylinders (IPS e.max lithium disilicate ceramic: 3mm diameter, 10mm height) were fabricated by the CAD/CAM technology using the Cerec inLab system (Sirona, Bensheim, Germany) and made ready to be luted onto the teeth surfaces using a resin luting cement (Rely X Resin Adhesive, 3M, Minnesota, USA) mixed according to the manufacturer’s instructions.
2.6 Preparation of specimens for the SBS and mode of failure tests
The 126 teeth surfaces were etched using 37% phosphoric acid, while the ceramic ingot was etched using 5% hydrofluoric acid. The etching time was done according to their respective manufacturer’s instructions. The Rely X resin cement was applied to the ceramic ingot, after which the ingot was pressed to the tooth surface using a 20N force, and appropriately light-cured using an LED light cure (3M ESPE Elipar) for 30 s on all surfaces. The type of Rely X resin cement used was according to the respective groups, with 63 of the samples (belonging to their respective groups) undergoing thermocycling using a SD Mechatronik Thermocycler THE-1200 for 10,000 cycles. All samples were stored for 24 h in distilled water at 37 °C before undergoing thermocycling and the shear bond strength test.
2.7 Preparation of the zMgO NPs disks for surface roughness assessment
30 samples of resin cement were prepared for the surface roughness test. The cement was mixed by one operator according to the manufacturer’s instructions, after which the cement was inserted into a cylindrical Teflon mold that was 6mm in diameter and 2mm thick. The cement was dispensed in single increments and pressed between polyester strips under static load on a glass slide, then light-cured per the manufacturer’s instructions. The specimens were light-cured from the other side after removal from the mold to ensure complete polymerization.
2.8 Testing phase
2.8.1 Shear bond strength test
To perform the shear bond strength test, a universal testing machine was used (Instron #5944, Instron, Massachusetts, USA). The teeth with the cemented ceramic ingot were placed into acrylic blocks, after which the acrylic blocks were mounted to the universal testing machine. The hydraulic press was then adjusted and positioned. The test was performed under shear loading at a crosshead speed of 1 mm/min and the value at which the cement failed was recorded in MPa. This was repeated for each specimen in the groups.
2.8.2 Thermocycling
Thermocycling is a typical method to simulate the hydrothermal aging of a restoration in the oral cavity. The thermal stress was applied by a thermocycling machine (JULABO GmbH, Seelbach, Germany) to represent an aged restoration. The restorations were thermocycled for approximately 10,000 cycles in baths containing distilled water at 5–55 °C, with a 32-s dwell time in each bath and a 14-s interval between baths. 10,000 cycles were selected to emulate approximately 1 year of in-vivo functioning [38].
2.8.3 Mode of fracture assessment
After furcating the 126 samples, they were examined by using a stereoscopic microscope (SZX16, OLYMPUS Co Ltd., Tokyo, Japan) to determine the failure mode at a magnification of 20x. The mode of fracture was categorized either: adhesive, cohesive, or mixed. An adhesive failure was defined as a fracture that occurred at the interface between the resin cement and the coinciding surfaces, where either the ceramic surface or the tooth surface completely contained the cement. A cohesive failure was defined as an internal fracture of the resin cement that occurred where the surface of both ceramic and tooth specimens was found to be covered by the cement. A mixed failure was defined as samples presenting with both adhesive and cohesive failures.
2.8.4 Surface roughness test
After preparing the 30 disks, a non-contact profilometer (Nanovea ps50, Nanovea Inc, South Carolina, USA) was used to measure the surface roughness of each individual sample with a scanning speed of 20 mm/s [39, 40]. A 3D image was generated for each of the samples and then compiled for assessment. After the initial assessment was completed, the 30 samples underwent thermocycling using a SD Mechatronik Thermocycler THE-1200 for 10,000 cycles. They were then subjected to another assessment using the profilometer.
2.9 Statistical analysis
All data collected through the experiments was sorted and then statistically analyzed using SPSS Software for Windows (version 23). Two-way analysis of variance (ANOVA) was used to investigate significant differences between the groups. This was followed by pairwise post-hoc test performed using the Tukey's test for multiple comparisons to determine the statistical significance of any intergroup differences. Significance was considered when p < 0.05. If p < 0.05, the null hypothesis would be rejected and the alternate hypothesis accepted.
3 Results
3.1 Characterization
3.1.1 X-ray diffraction analysis
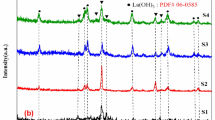
The diffraction pattern of the unmodified resin cement and the Rely X resin cement modified with the zMgO NPs was done by an X-ray diffractometer (XRD) (Rigaku, Ultima IV, Japan) (Fig. 2). The XRD spectra of the Rely X cement with 0%, 1% and 2% of zMgO NPs showed no definite diffraction peaks, as a result of the resin cement’s amorphous structure and the microscopic volume of zMgO nanoparticles present with resin specimen [41, 42]. The standard diffraction peak of the resin cement with 1% and 2% zMgO NPs was 27.3 and 26.6, respectively, while the diffraction peaks of MgO/zMgO powder were at 36.2°, 45.4° and 66.2°.
3.1.2 Field emission scanning electron microscopy
The FESEM micrographs presented the surface morphology and the distribution of the zMgO nanoparticles in the Rely X resin cement modified with 1% and 2% zMgO NPs (Fig. 3). The FESEM micrographs demonstrated that all the zMgO NPs were consistently and evenly dispersed over the entire surface of the resin cement samples. It could also be noted that the particle size was less than 100nm in all the samples.
3.1.3 Fourier transform infrared spectroscopy (FTIR)
The evaluation of the FTIR spectra of the resin cement after incorporation of the 1% and 2% zMgO nanoparticles revealed little change in the FTIR spectra when compared to that of the unmodified resin cement (control) (Fig. 4). The spectra at 1500 cm−1 corresponding to C–C band of silicate was stretched due to the presence of the zMgO NPs. Additionally, a slight stretching of the spectra appears at the 1700 cm−1 band corresponding to C=O group of ester, with a strong finger shaped stretching at 2400 cm−1 corresponding to C=O group. This signifies that the presence of the zMgO NPs did not alter the structure of the resin cement.
3.2 Shear bond strength test
The two-way ANOVA test revealed a statistically significant difference between the groups before thermocycling (p = 0.0008). A T-test followed to present where the differences indicated a significant difference between the control and 1% zMgO NPs group as well as the control and 2% zMgO NPs groups (p = 0.014, p = 0.00061, respectively). There was no statistically significant difference between the 1% and 2% zMgO groups (p = 0.15).
The mean SBS values (in MPa) for the control group without and with thermocycling were 20.3 ± 4.2 and 10.18 ± 4.5, respectively. The SBS values for the group with 1% zMgO NPs without and with thermocycling were 23.4 ± 4.5 and 13.4 ± 3.5, respectively. The SBS values (in MPa) for the last group with 2% zMgO NPs without and with thermocycling were 25.5 ± 4.3 and 15.5 ± 4.2, respectively. The SBS values (in MPa) for different groups are presented in Fig. 5.
Thermocycling resulted in a significant decrease of the SBS values for all groups. Two-way ANOVA revealed a statistically significant effect of thermocycling (p = 0.00046). There was no significant difference between the control, the 1%, and the 2% zMgO NPs groups or the 1% and the 2% zMgO NPs groups (p = 0.32, p = 0.3, p = 0.17, respectively).
3.3 Mode of failure
Adhesive failure was seen more than cohesive failure for all groups, with the highest failure being seen with the 2% zMgO NPs before and after thermocycling, being 95% and 85.72%, respectively. The 1% zMgO NPs group’s adhesive failure before and after thermocycling was 85.70% and 76.20%, respectively. Lastly, the control group’s adhesive failure before and after thermocycling was 71.50% and 61.91%, respectively (Fig. 6A).
Of the cohesive failures, the majority of them were found with the control group, being 28.50% before thermocycling and 38.09% after thermocycling. The 1% zMgO NPs group before and after thermocycling presented with 14.30% and 23.80%, respectively. Lastly, the 2% zMgO NPs group before and after thermocycling resulted with 5% and 14.28%, respectively (Fig. 6B). No samples exhibited mixed failures.
3.4 Surface roughness
The surface roughness was calculated using the average of the surface heights and depths across the surface. The profilometer results showed no statistically significant difference in the surface roughness between the control, 1% and 2% zMgO NPs groups (p = 0.27). The mean surface roughness of the control group was 0.105 µm, the mean of surface roughness of the 1% zMgO NPs group was 0.110 µm, and lastly, the mean of surface roughness of the 2% zMgO NPs group 0.100 µm (Fig. 7). Results show that there was no significant difference between the groups before or after thermocycling (Fig. 8). However, paired T-test indicated a significant decrease in surface roughness for all groups after thermocycling (p < 0.05).
4 Discussion
The indispensable role of dental cements in the clinical setting along with the consistently increasing esthetic interest has culminated into the struggle for an ideal cement. These cements are used to facilitate various restorative, prosthetic, and orthodontic procedures such as securing fixed dental prosthesis or orthodontic bands to the tooth structure, ensuring their durability and stability [27, 43]. Due to the nature of their use, these adhesive cements should demonstrate excellent physical, mechanical, and biological properties; however, this can fail to be the case, particularly due to the ongoing issue of microleakage, which in turn can lead to bacterial invasion, soft tissue inflammation, pulpal irritation, tooth hypersensitivity, and recurrent caries [28, 44]. These drawbacks experienced with the modern-day cement are what propel the need for an improved solution, for which a nanoparticle-reinforced dental cement could be considered. Biomaterials integrated with antimicrobial nanoparticles can enhance the dental cement due to their synergistic effect of defending against bacterial contamination and extending the lifespan of the restoration [45].
Magnesium oxide has a well-documented history of antimicrobial characteristics that do not affect mammalian cells [13, 18, 46, 47]. However, MgO NPs tend to aggregate, and the formed clumps can hinder the usage of the nanoparticles’ full potential [1, 4, 48]. A previous study of Naguib et al. found that the addition of the zein, a corn-derived protein polymer commonly utilized in pharmaceutical applications, acts as a coating that prevents the agglomeration of the nanoparticles by decreasing their hydrophobic properties, thereby stabilizing them [18, 19]. This allows the nanoparticles to maintain their amorphous phase, therefore preserving the benefits of the nano-characteristics, including the prolonged antimicrobial effect [19, 49].
zMgO NPs were found to exhibit significantly less aggregation and a 20% more sustained release of active ingredients when compared to pure MgO NPs [18]. Furthermore, resin cement modified with zMgO NPs has been previously found to inhibit oral microbes as well as reduce bacterial biofilm [14]. While this property is desirable, the effect of this modification requires further research. In accordance with clinical relevance and previous studies, the shear bond strength, mode of fracture, and surface roughness were investigated [50,51,52].
The characterization of the novel material was done by XRD, FESEM, and FTIR. The XRD profile provides insight regarding the types of phases and the crystalline structures of the specimens. In our previous study, it was found that the XRD profile of the zMgO NPs alone revealed high intensity and sharp peaks, indicating the highly crystalline structure of the nanoparticles [19]. In this study, the inclusion of the nanoparticles with the resin dental cements did not generate diffraction peaks due to small weight concentration of the zMgO NPs mixed in with the cement. This is represented through Laven’s elaboration, which illustrated that the XRD analysis of nanoparticles is dependent on the elastic and inelastic scattering contributions that contain information regarding the nano-size character of the crystals in the sample, the amorphous region, and the background [53]. Furthermore, it is in accordance with Upadhyay et al. who reported that the broadening of XRD peaks occurs as a result of the miniscule size of the nanoparticles along with their quantity, particularly in certain situations involving laboratory-scale manufacturing [41]. Moreover, as the resin cement is highly amorphous in nature, it repressed the crystallinity of the zMgO, and therefore, the disappearance of the diffraction pattern subsequently demonstrated the amorphous change. This transformation could also be attributed to the hydrophilic interaction between the zein-coated inorganic nanoparticles and the resin cement [14, 54, 55].
The FESEM analysis demonstrates the surface morphology of the cement and the distribution of the zMgO NPs. Evaluation of the micrographs revealed a uniform distribution of the zMgO NPs over the entirety of the cement samples. Additionally, no aggregation of the zMgO NPs was found, and all particles were found to be less than 100nm in size, which ensures that the antimicrobial activity was present [14, 18]. The FTIR spectroscopy revealed the chemical properties of the modified resin cement when compared to the unmodified resin cement. The spectra of the resin cement with 1% and 2% zMgO NPs are almost the same as the FTIR spectra of the resin cement alone (control). This indicates that the zein-coated inorganic nanoparticles do not alter the structure of the cement, and that the modified resin cement behaves similarly to the unmodified version.
To fully assess the nature of the cement’s role in the relationship of adhesion between the tooth and the restoration, consideration needs to be given to the respective surface structures of these components. Regarding the tooth surface, it is comprised of various nanostructures such as carbonated hydroxyapatite (10–200 nm) which makes up 96% of enamel structure, intertubular dentin (60 nm, 2–5 nm thick), peritubular dentin (25 nm, 2–5 nm thick), and dentin collagen fibrils (20–75 nm). The collagen fibrils that are 80–120 nm run between the dentin into the enamel and merge with the fibrillar network of the dentin matrix, connecting the enamel and dentin [56, 57]. On the ceramic surface, the lithium disilicate’s microstructures can involve the interlaced lithium disilicate crystals (5μm long with a diameter of 0.8μm) embedded in a glass matrix at the final stage, although the variations in the size and distribution can exist according to the manufacturer and the production process [58].
The prosthesis’s clinical success is reliant on the retention, of which the cement affects the adhesion and bonding to the tooth structure [27, 51]. This can be evaluated through the shear bond strength test, which is a measure of the maximum force the adhesive cement can withstand before failing. In this study, it was found that the SBS was significantly higher with the 1% zMgO group and 2% zMgO group when compared to the control. As the concentration of the zMgO NPs increases, so did the SBS (Fig. 5). However, the SBS values decreased after thermocycling for all groups. While the SBS values of the modified cements were higher than the control after thermocycling, the difference between the groups was not statistically significant.
This can be explained by the key feature of nanoparticles, which is that they have an exceptionally large surface area to volume ratio [4, 5]. That large surface area gives the zMgO NPs the capacity to integrate more contact with the cement interface, as well as increase the surface area in contact with the tooth surface and lithium disilicate ceramic. Furthermore, the small particle size is associated with high surface energy, which in turn can lead to increased wettability and therefore allows the nanoparticles to distribute into the gaps within the cement [17, 59, 60]. This is further aided by the zein polymer which decreases the hydrophobic properties of the nanoparticles, and consequently aids the wettability of the tooth with the cement [14, 19, 49]. The net result is that the shear bond strength of the cement will increase due to this mechanical interlocking at the nano-level with the ceramic, enamel, and dentin, respectively. This is in accordance with the results of Felemban et al. who found a significant increase in the SBS of adhesive composite incorporated with ZrO2–TiO2 nanoparticles, and with Torres-Rosas et al. who found a significant increase in the SBS of resin adhesive with copper nanoparticles [61, 62]. Ferrando-Magraner et al. investigated the effect of incorporating antimicrobial silver compounds to glass ionomer cement’s SBS and discovered that the groups exhibited significantly higher shear strength results compared to the control material [63]. Alternatively, Thanyasiri et al. added Sr-bioactive glass nanoparticles and monocalcium phosphate monohydrate to resin cement and reported no significant effects on the SBS of the experimental resin cements, which could be due to the different nanoparticles and concentrations used [64].
To further understand the effect of the nanoparticles on the resin cement as well as further elaborate on the failure of the cement upon determining the shear bond strength, the mode of fracture was studied. The nature of bond failure was examined under a stereomicroscope and categorized into adhesive failure, which was defined as the complete detachment of the cement from the lithium disilicate or tooth structure with no remnants of the cement on the substrate, or cohesive failure, which was defined as the fracture within the cement itself, with remnants of the cement being found on both the tooth surface and the ceramic surface. Mixed failure was defined as a mix of adhesive and cohesive failure; however, no instances of mixed failure occurred in this study.
The control group before and after thermocycling demonstrated the highest cohesive failure among the groups, while the increasing concentration of the zMgO NPs with the resin cement presented with higher percentages of adhesive failure (Fig. 6A, B). This could be explained through the capacity of the nanoparticles to penetrate the voids of the cement. Their homogenous dispersion within the cement occurs due to the facilitated effort of the zein, which allows the MgO NPs to actively reinforce the amorphous phase of the cement. The zMgO nanoparticles exhibit reduced inter-particle separation, which in turn allows the uniform distribution of the zMgO in the gaps of the cement. Therefore, the material as a whole is less likely to suffer failure by the breakdown of the intermolecular forces. As such, that is why the 1% zMgO suffered less cohesive failure than the control, and why the 2% zMgO had even less cohesive failure than the others. The outcome is that the zMgO NPs incorporated cement will have a higher strength and be more likely to experience an adhesive failure.
Kheur et al. found that hydroxyapatite nanoparticle-reinforced glass ionomer and resin-modified glass ionomer cements presented with mixed type of failures while adhesive resin cement exhibited cohesive failures [65]. Torres-Rosas et al. reported that the resin cement incorporating copper nanoparticles experienced mainly cohesive failure with the unmodified adhesive experiencing a mix of adhesive and cohesive failures [61]. Thanyasiri et al. discovered that the failure mode observed from the incorporation of bioactive glass nanoparticles and monocalcium phosphate monohydrate to resin cement was mostly mixed failure [64]. The variation in the results with the previous studies could be due to the difference of the nanoparticle type, or the lack of a silane coupling agent, which aids in preventing the nanoparticles’ agglomeration and allows them to maintain their dispersion.
Surface roughness is another essential property to investigate as it directly contributes to the bonding strength of the tooth surface/cement and ceramic/cement interface, as well as the long-term success of the restoration [51]. Higher surface roughness allows for more micromechanical locking, increasing the retention of the prosthesis; however, care should be taken to ensure that no cement is exposed to the oral cavity or in an undesirable location, as that can contribute to plaque accumulation by acting as a scaffold for bacteria to latch onto, as well as the roughness of the cement acting as an irritant to the oral cavity’s soft tissue [66]. Additionally, the treatment with 37% phosphoric acid on the tooth side and 5% hydrofluoric acid on the ceramic ingot side also provides support to the cement in terms of the surface roughness of the respective substrates aiding micromechanical locking [67, 68].
In this study, upon examination of the profilometer images of the surface roughness for the different groups, no statistically significant difference was found between the control and 1% and 2% zMgO groups before thermocycling, while a significant decrease in surface roughness occurred after thermocycling in all the groups (Fig. 8). This could be due to the low level of concentration of the zMgO NPs relative to the resin cement, as well as the small particle size. Previous studies have suggested that the particle size plays a vital role in the surface roughness of a dental material, and that the use of nanosized particles could lead to a smoother surface as the nanoparticles fill the gaps in the surface [40]. While enough to significantly improve the mechanical bonding of the cement, it is not enough to significantly affect the surface properties of the cement. The uniform dispersion and integration into the cement’s gaps could be another reason as to why no irregularities were made over the surface, as it was found that there was a decrease in the surface roughness with the modified resin cement, however not statistically significant. Moreover, the forces within the nanoparticles should also be taken into consideration. All the atoms in the material will bond to their neighbors, but surface atoms have less neighbors to bind to, which results in an internally directed force towards the center of the particle [69]. This will increase the surface energy of the cement, thereby increasing its bond with zMgO NPs which consequently affects the physical properties of the cement and decreases the irregularities in the cement surface.
The reason that the surface roughness decreased after thermocycling is due to the superficial wear of the outermost layer after undergoing the effects of thermocycling. The significance is in comparison to the group before thermocycling, while the results of the control and modified resin cement after thermocycling are within range of each other and are not statistically significant for the same reasons as mentioned before. The literature on surface roughness of dental cements upon incorporation of nanoparticles is limited. In a previous study conducted by Naguib et al. it was found that the addition of 0.3%, 0.5%, 1%, 2% and 5% MgO NPs to flowable composite did not significantly change its surface roughness [17]. This is in line with the results of this study. Saadat et al. found that the surface roughness of resin-modified glass ionomer cement containing bacterial cellulose nanocrystals (0.3%, 0.5%, 1% by weight concentration) significantly reduced when compared to the control [40]. The results in our study did similarly present a reduction in surface roughness, but not in a statistically significant manner.
The inclusion of an effective antimicrobial resin cement in daily clinical practice would be a beneficial edge in combating the microbial challenge the both the dentist and patient face during restorative procedures, particularly in critical areas such as the margins of the prosthesis prone to bacterial insult. The properties investigated in this study further validate the status of the enhanced resin cement; however, further investigations into other physical and mechanical properties need to be conducted to confirm the benefits of its use over the unmodified resin cement.
The limitations of the study, as with other in vitro studies, lies in the difficulty to completely simulate the intraoral conditions. Static, chemical, and cyclic mechanical fatigue on ceramic/cement/tooth structure interfaces could not be replicated. Although lab tests can provide estimates of the clinical situation, the relationship between the bond strength and the clinical performance needs elaboration as the forces transmitted to the tooth are rarely singular. Further dynamic loading in artificial saliva before testing may more closely simulate intraoral conditions when considering the hydrolytic degradation of the bond due to pH changes of saliva. Additional evaluation of this modified resin cement should be established by long-term clinical studies, which can take the full spectrum of parameters into account. Furthermore, a previous study had investigated the cell cytotoxicity and biochemical effect of the nanoparticles in rats, but future studies are needed to comprehensively evaluate the effect in humans [22].
5 Conclusion
While it has been previously found that the antimicrobial properties of zMgO NPs can be incorporated into resin cement, this study further established that the shear bond strength and surface roughness of the cement will not be hindered by this modification. The shear bond strength improved, while the surface roughness was not affected. Further in vitro investigations into the modified cement’s characteristics are crucial to ensure that the dental material will behave similarly or superiorly in the patient’s mouth when compared to standard resin cement. Particularly, the effect on the mechanical properties with dynamic and cyclic forces as well as the involvement of saliva should be determined. This should be followed up by clinical studies to solidify the validity of incorporating these inorganic nanoparticles.
Data availability
Data is available from the corresponding author upon reasonable request.
Abbreviations
- MgO NPs:
-
Magnesium oxide nanoparticles
- zMgO NPs:
-
Zein-incorporated magnesium oxide nanoparticles
- SBS:
-
Shear bond strength
- XRD:
-
X-ray diffraction
- FESEM:
-
Field emission scanning electron microscopy
- FTIR:
-
Fourier transform infrared spectroscopy
References
Jandt KD, Watts DC. Nanotechnology in dentistry: present and future perspectives on dental nanomaterials. Dent Mater. 2020;36:1365–78. https://doi.org/10.1016/j.dental.2020.08.006.
Priyadarsini S, Mukherjee S, Mishra M. Nanoparticles used in dentistry: a review. J Oral Biol Craniofac Res. 2018;8:58–67. https://doi.org/10.1016/j.jobcr.2017.12.004.
Sreenivasalu PKP, Dora CP, Swami R, Jasthi VC, Shiroorkar PN, Nagaraja S, et al. Nanomaterials in dentistry: current applications and future scope. Nanomaterials. 2022. https://doi.org/10.3390/NANO12101676.
Schmalz G, Hickel R, van Landuyt KL, Reichl FX. Nanoparticles in dentistry. Dent Mater. 2017;33:1298–314. https://doi.org/10.1016/J.DENTAL.2017.08.193.
Gronwald B, Kozłowska L, Kijak K, Lietz-Kijak D, Skomro P, Gronwald K, et al. Nanoparticles in dentistry—current literature review. Coatings. 2023;13:102. https://doi.org/10.3390/COATINGS13010102.
Bdewi SF, Abdullah OG, Aziz BK, Mutar AAR. Synthesis, structural and optical characterization of mgo nanocrystalline embedded in PVA matrix. J Inorg Organomet Polym Mater. 2016;26:326–34. https://doi.org/10.1007/S10904-015-0321-3/TABLES/2.
Amin F, Rahman S, Khurshid Z, Zafar MS, Sefat F, Kumar N. Effect of nanostructures on the properties of glass ionomer dental restoratives/cements: a comprehensive narrative review. Materials. 2021. https://doi.org/10.3390/MA14216260.
Karpacheva GP. Hybrid magnetic nanocomposites containing polyconjugated polymers. Polym Sci Ser C. 2016;58:131–46. https://doi.org/10.1134/S1811238216010045.
Elkassas D, Arafa A. The innovative applications of therapeutic nanostructures in dentistry. Nanomedicine. 2017;13:1543–62. https://doi.org/10.1016/J.NANO.2017.01.018.
Gutiérrez MF, Alegría-Acevedo LF, Méndez-Bauer L, Bermudez J, Dávila-Sánchez A, Buvinic S, et al. Biological, mechanical and adhesive properties of universal adhesives containing zinc and copper nanoparticles. J Dent. 2019;82:45–55. https://doi.org/10.1016/J.JDENT.2019.01.012.
Swetha D, Vinay C, Uloopi K, Rojaramya K, Chandrasekhar R. Antibacterial and mechanical properties of pit and fissure sealants containing zinc oxide and calcium fluoride nanoparticles. Contemp Clin Dent. 2019;10:477–82. https://doi.org/10.4103/CCD.CCD_805_18.
Jowkar Z, Jowkar M, Shafiei F. Mechanical and dentin bond strength properties of the nanosilver enriched glass ionomer cement. J Clin Exp Dent. 2019;11: e275. https://doi.org/10.4317/JCED.55522.
Noori AJ, Kareem FA. The effect of magnesium oxide nanoparticles on the antibacterial and antibiofilm properties of glass-ionomer cement. Heliyon. 2019. https://doi.org/10.1016/J.HELIYON.2019.E02568.
Naguib GH, Nassar HM, Hamed MT. Antimicrobial properties of dental cements modified with zein-coated magnesium oxide nanoparticles. Bioact Mater. 2022;8:49–56. https://doi.org/10.1016/j.bioactmat.2021.06.011.
Suresh S. Investigations on synthesis, structural and electrical properties of MgO nanoparticles by sol-gel method. J Ovonic Res. 2014;10:205–10.
Sharifian S, Loghmani A, Nayyerain S, Javanbakht S, Daneii P. Application of magnesium oxide nanoparticles in dentistry: a literature review. Eur J Gen Dent. 2023;12:001–6. https://doi.org/10.1055/s-0042-1760673.
Naguib GH, Nasser M, Alturki BN, Bakhsh TA. Surface characteristics of composite resin enhanced by new antibacterial nanofillers. Int J Curr Adv Res. 2018;7:15965–9. https://doi.org/10.24327/ijcar.2018.
Naguib GH, Hosny KM, Hassan AH, Al Hazmi F, Al Dharrab A, Alkhalidi HM, et al. Zein based magnesium oxide nanoparticles: Assessment of antimicrobial activity for dental implications. Pak J Pharm Sci 2018;31.
Naguib GH, Hassan AH, Al-Hazmi F, Kurakula M, Al-Dharrab A, Alkhalidi HM, et al. Zein based magnesium oxide nanowires: effect of anionic charge on size, release and stability. Dig J Nanomater Biostruct. 2017;12:741–9.
Sharmin S, Rahaman MM, Sarkar C, Atolani O, Islam MT, Adeyemi OS. Nanoparticles as antimicrobial and antiviral agents: a literature-based perspective study. Heliyon. 2021. https://doi.org/10.1016/J.HELIYON.2021.E06456.
Haleem A, Javaid M, Singh RP, Rab S, Suman R. Applications of nanotechnology in medical field: a brief review. Glob Health J. 2023;7:70–7. https://doi.org/10.1016/J.GLOHJ.2023.02.008.
Naguib GH, Abd El-Aziz GS, Mously HA, Bukhary SM, Hamed MT. Assessment of the dose-dependent biochemical and cytotoxicity of zein-coated MgO nanowires in male and female albino rats. Ann Med. 2021;53(1):1850–62. https://doi.org/10.1080/07853890.2021.1991587.
Naguib GH, El-Aziz GSA, Mously HA, Alhazmi WA, Alnowaiser AM, Hassan AH, et al. In vitro investigation of the antimicrobial activity of mouth washes incorporating zein-coated magnesium oxide nanoparticles. Clin Cosmet Investig Dent. 2021;13:395–403. https://doi.org/10.2147/CCIDE.S327912.
Naguib GH, Bakhsh TA, Turkistani AA, Mously HA, Fattouh M, Hamed MT. Noninvasive adaptation appraisal of antimicrobial nano-filled composite. Int Dent J. 2022. https://doi.org/10.1016/J.IDENTJ.2022.11.004.
Heboyan A, Vardanyan A, Karobari MI, Marya A, Avagyan T, Tebyaniyan H, et al. Dental luting cements: an updated comprehensive review. Molecules. 2023. https://doi.org/10.3390/MOLECULES28041619.
Dennison JB, Sarrett DC. Prediction and diagnosis of clinical outcomes affecting restoration margins. J Oral Rehabil. 2012;39:301–18. https://doi.org/10.1111/J.1365-2842.2011.02267.X.
Ebadian B, Fathi A, Savoj M. In vitro evaluation of the effect of different luting cements and tooth preparation angle on the microleakage of zirconia crowns. Int J Dent. 2021. https://doi.org/10.1155/2021/8461579.
Hosida TY, Delbem ACB, Morais LA, Moraes JCS, Duque C, Souza JAS, et al. Ion release, antimicrobial and physio-mechanical properties of glass ionomer cement containing micro or nanosized hexametaphosphate, and their effect on enamel demineralization. Clin Oral Investig. 2019;23:2345–54. https://doi.org/10.1007/S00784-018-2674-9/TABLES/3.
Bapat RA, Chaubal TV, Joshi CP, Bapat PR, Choudhury H, Pandey M, et al. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater Sci Eng C. 2018;91:881–98. https://doi.org/10.1016/J.MSEC.2018.05.069.
Shieh TM, Hsu SM, Chang KC, Chen WC, Lin DJ. Calcium phosphate cement with antimicrobial properties and radiopacity as an endodontic material. Materials. 2017. https://doi.org/10.3390/MA10111256.
Naguib G, Maghrabi AA, Mira AI, Mously HA, Hajjaj M, Hamed MT. Influence of inorganic nanoparticles on dental materials’ mechanical properties. A narrative review. BMC Oral Health. 2023. https://doi.org/10.1186/S12903-023-03652-1.
Gjorgievska E, Nicholson JW, Gabrić D, Guclu ZA, Miletić I, Coleman NJ. Assessment of the impact of the addition of nanoparticles on the properties of glass–ionomer cements. Materials. 2020;13:276. https://doi.org/10.3390/MA13020276.
Garcia-Contreras R, Scougall-Vilchis RJ, Contreras-Bulnes R, Sakagami H, Morales-Luckie RA, Nakajima H. Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J Appl Oral Sci. 2015;23:321. https://doi.org/10.1590/1678-775720140496.
Liu F, Wang R, Shi Y, Jiang X, Sun B, Zhu M. Novel Ag nanocrystals based dental resin composites with enhanced mechanical and antibacterial properties. Progress Nat Sci Mater Int. 2013;23:573–8. https://doi.org/10.1016/J.PNSC.2013.11.011.
Sayed AME, Abdelghany AM, Abou EA. Structural, optical, mechanical and antibacterial properties of mgo/poly(vinyl acetate)/poly(vinyl chloride) nanocomposites. Braz J Phys. 2022;52:1–12. https://doi.org/10.1007/S13538-022-01156-X/FIGURES/11.
Naguib GH, Bakhsh T, Mazhar J, Turkistani A, Mira A, Aljawi R, et al. Noninvasive assessment of novel nanohybrid resin cement adaptation using cross-polarization optical coherence tomography. Dent Mater. 2024. https://doi.org/10.1016/J.DENTAL.2024.02.004.
Ghavami-Lahiji M, Firouzmanesh M, Bagheri H, Kashi TSJ, Razazpour F, Behroozibakhsh M. The effect of thermocycling on the degree of conversion and mechanical properties of a microhybrid dental resin composite. Restor Dent Endod. 2018. https://doi.org/10.5395/RDE.2018.43.E26.
Miličević A, Goršeta K, Van Duinen RNV, Glavina D. Surface roughness of glass ionomer cements after application of different polishing techniques. Acta Stomatol Croat. 2018;52:314–21. https://doi.org/10.15644/asc52/4/5.
Saadat M, Moradian M, Mirshekari B. Evaluation of the surface hardness and roughness of a resin-modified glass ionomer cement containing bacterial cellulose nanocrystals. Int J Dent. 2021. https://doi.org/10.1155/2021/8231473.
Upadhyay S, Parekh K, Pandey B. Influence of crystallite size on the magnetic properties of Fe3O4 nanoparticles. J Alloy Compd. 2016;678:478–85. https://doi.org/10.1016/J.JALLCOM.2016.03.279.
Stoica M, Sarac B, Spieckermann F, Wright J, Gammer C, Han J, et al. X-ray diffraction computed nanotomography applied to solve the structure of hierarchically phase-separated metallic glass. ACS Nano. 2021;15:2386–98. https://doi.org/10.1021/ACSNANO.0C04851/SUPPL_FILE/NN0C04851_SI_001.PDF.
Sakaguchi RL, Ferracane JL, Powers JM. Craig’s restorative dental materials. 2018.
Jia S, Chen D, Wang D, Bao X, Tian X. Comparing marginal microleakage of three different dental materials in veneer restoration using a stereomicroscope: an in vitro study. BDJ Open. 2017;3:1–5. https://doi.org/10.1038/bdjopen.2016.10.
Lazouzi G, Vuksanović MM, Tomić NZ, Mitrić M, Petrović M, Radojević V, et al. Optimized preparation of alumina based fillers for tuning composite properties. Ceram Int. 2018;44:7442–9. https://doi.org/10.1016/J.CERAMINT.2018.01.083.
Bose S, Tarafder S, Bandyopadhyay A. Effect of chemistry on osteogenesis and angiogenesis towards bone tissue engineering using 3D printed scaffolds. Ann Biomed Eng. 2017;45:261. https://doi.org/10.1007/S10439-016-1646-Y.
Ke D, Tarafder S, Vahabzadeh S, Bose S. Effects of MgO, ZnO, SrO, and SiO2 in tricalcium phosphate scaffolds on in vitro genes expression and in vivo osteogenesis. Mater Sci Eng Mater Biol Appl. 2019;96:10. https://doi.org/10.1016/J.MSEC.2018.10.073.
Krishnamoorthy K, Manivannan G, Kim SJ, Jeyasubramanian K, Premanathan M. Antibacterial activity of MgO nanoparticles based on lipid peroxidation by oxygen vacancy. J Nanoparticle Res. 2012. https://doi.org/10.1007/S11051-012-1063-6.
Podaralla S, Perumal O. Influence of formulation factors on the preparation of zein nanoparticles. AAPS PharmSciTech. 2012;13:919. https://doi.org/10.1208/S12249-012-9816-1.
Bala O, Arisu HD, Yikilgan I, Arslan S, Gullu A. Evaluation of surface roughness and hardness of different glass ionomer cements. Eur J Dent. 2012;6:79–86. https://doi.org/10.1055/S-0039-1698934.
Brajkovic D, Antonijevic D, Milovanovic P, Kisic D, Zelic K, Djuric M, et al. Surface characterization of the cement for retention of implant supported dental prostheses: in vitro evaluation of cement roughness and surface free energy. Appl Surf Sci. 2014;311:131–8. https://doi.org/10.1016/J.APSUSC.2014.05.027.
Rohr N, Bertschinger N, Fischer J, Filippi A, Zitzmann NU. Influence of material and surface roughness of resin composite cements on fibroblast behavior. Oper Dent. 2020;45:528–36. https://doi.org/10.2341/19-113-L.
Laven P. Separating diffraction from scattering: the million-dollar challenge. J Nanophotonics. 2010;4: 041593. https://doi.org/10.1117/1.3374327.
Igawa K, Yoshinari N, Okumura M, Ohtsu H, Kawano M, Konno T. Crystalline-amorphous-crystalline transformation in a highly brilliant luminescent system with trigonal-planar gold(I) centers. Sci Rep. 2016;6:1–8. https://doi.org/10.1038/srep26002.
Alshehri SM, Tiwari RV, Alsulays BB, Ashour EA, Alshetaili AS, Almutairy B, et al. Investigation of the combined effect of MgO and PEG on the release profile of mefenamic acid prepared via hot-melt extrusion techniques. Pharm Dev Technol. 2017;22:740. https://doi.org/10.3109/10837450.2016.1138129.
Goldberg M, Kulkarni AB, Young M, Boskey A. Dentin: structure, composition and mineralization: the role of dentin ECM in dentin formation and mineralization. Front Biosci (Elite Ed). 2011;3:711. https://doi.org/10.2741/E281.
Pokrowiecki R, Pałka K, Mielczarek A. Nanomaterials in dentistry: a cornerstone or a black box? Nanomedicine (Lond). 2018;13(6):639–67. https://doi.org/10.2217/NNM-2017-0329.
Willard A, Gabriel Chu TM. The science and application of IPS e.Max dental ceramic. Kaohsiung J Med Sci. 2018;34:238–42. https://doi.org/10.1016/J.KJMS.2018.01.012.
Vaiyshnavi W, Jei J, Kumar B. Effect of silver nanoparticles on wettability, anti-fungal effect, flexural strength, and color stability of injection-molded heat-cured polymethylmethacrylate in human saliva. J Indian Prosthodont Soc. 2022;22:368–76. https://doi.org/10.4103/JIPS.JIPS_574_21.
Kasraei S, Azarsina M. Addition of silver nanoparticles reduces the wettability of methacrylate and silorane-based composites. Braz Oral Res. 2012;26:505–10. https://doi.org/10.1590/S1806-83242012000600004.
Torres-Rosas R, Torres-Gómez N, García-Contreras R, Scougall-Vilchis RJ, Domínguez-Díaz LR, Argueta-Figueroa L. Copper nanoparticles as nanofillers in an adhesive resin system: an in vitro study. Dent Med Probl. 2020;57:239–46. https://doi.org/10.17219/DMP/121973.
Felemban NH, Ebrahim MI. The influence of adding modified zirconium oxide-titanium dioxide nano-particles on mechanical properties of orthodontic adhesive: an in vitro study. BMC Oral Health. 2017;17:1–8. https://doi.org/10.1186/S12903-017-0332-2/TABLES/4.
Ferrando-Magraner E, García-Sanz V, Bellot-Arcís C, Marín-Gozalbo A, Cabedo-Mas L, Mínguez-Vega G, et al. Improving the antibacterial properties of dental bonding materials loaded with silver compounds. Antibiotics. 2023;12:1721. https://doi.org/10.3390/ANTIBIOTICS12121721.
Thanyasiri S, Naruphontjirakul P, Padunglappisit C, Mirchandani B, Young AM, Panpisut P. Assessment of physical/mechanical properties and cytotoxicity of dual-cured resin cements containing Sr-bioactive glass nanoparticles and calcium phosphate. Dent Mater J. 2023;42:806–17. https://doi.org/10.4012/DMJ.2023-127.
Kheur M, Kantharia N, Iakha T, Kheur S, Husain NAH, Özcan M. Evaluation of mechanical and adhesion properties of glass ionomer cement incorporating nano-sized hydroxyapatite particles. Odontology. 2020;108:66–73. https://doi.org/10.1007/S10266-019-00427-5/METRICS.
Marc M. Impact of dental cements on soft tissue in dental implants: a comprehensive overview. Dentistry. 2023;13:1–2. https://doi.org/10.35248/2161-1122.23.13.636.
Sofan E, Sofan A, Palaia G, Tenore G, Romeo U, Migliau G. Classification review of dental adhesive systems: from the IV generation to the universal type. Annali di Stomatologia (Roma). 2017;8:1. https://doi.org/10.11138/ADS/2017.8.1.001.
Menees TS, Lawson NC, Beck PR, Burgess JO. Influence of particle abrasion or hydrofluoric acid etching on lithium disilicate flexural strength. J Prosthet Dent. 2014;112:1164–70. https://doi.org/10.1016/j.prosdent.2014.04.021.
Cheng L, Zhang K, Zhou CC, Weir MD, Zhou XD, Xu HHK. One-year water-ageing of calcium phosphate composite containing nano-silver and quaternary ammonium to inhibit biofilms. Int J Oral Sci. 2016;8:172. https://doi.org/10.1038/IJOS.2016.13.
Acknowledgements
Special thanks to Mohanned Majdi Toras, Rayan Hussain Alwadei, Faisal Mohammed Almasoudi, and Ali Abdullah Alghamdi for assisting in data collection.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conceptualization: G.N, A.B. and M.T.H.; Methodology and data collection: G.N., J.M., A.B., I.K., H.M., M.Z. and M.T.H.; Contributed data/analysis tools and formal analysis: G.N., A.B., H.M., I.K., M.Z., and M.T.H.; Investigation: G.N, H.M., J.M., I.K., A.M., M.Z., and M.T.H.; Data curation: G.N., J.M., A.B., H.M., M. H., and M.T.H.; Writing—original draft preparation: G.N., A.B., J.M, and M.T.H.; Writing—review and editing: G.N., J.M., and M.T.H.; Visualization: G.N., H.M., J.M., and M.T. H.; Funding acquisition: A.B., H.M. and M.T.H.
Corresponding author
Ethics declarations
Competing interests
No funds, grants, or other support was received. The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Naguib, G., Mously, H., Mazhar, J. et al. Bond strength and surface roughness assessment of novel antimicrobial polymeric coated dental cement. Discover Nano 19, 123 (2024). https://doi.org/10.1186/s11671-024-04074-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s11671-024-04074-w