Abstract
Drought is a major abiotic stress affecting crop yields. Mapping quantitative trait loci (QTLs) and mining genes for drought tolerance in rice are important for identifying gene functions and targets for molecular breeding. Here, we performed linkage analysis of drought tolerance using a recombinant inbred line population derived from Jileng 1 (drought sensitive) and Milyang 23 (drought tolerant). An ultra-high-density genetic map, previously constructed by our research team using genotype data from whole-genome sequencing, was used in combination with phenotypic data for rice grown under drought stress conditions in the field in 2017–2019. Thirty-nine QTLs related to leaf rolling index and leaf withering degree were identified, and QTLs were found on all chromosomes except chromosomes 6, 10, and 11. qLWD4-1 was detected after 32 days and 46 days of drought stress in 2017 and explained 7.07–8.19% of the phenotypic variation. Two loci, qLRI2-2 and qLWD4-2, were identified after 29, 42, and 57 days of drought stress in 2018. These loci explained 10.59–17.04% and 5.14–5.71% of the phenotypic variation, respectively. There were 281 genes within the QTL interval. Through gene functional annotation and expression analysis, two candidate genes, Os04g0574600 and OsCHR731, were found. Quantitative reverse transcription PCR analysis showed that the expression levels of these genes were significantly higher under drought stress than under normal conditions, indicating positive regulation. Notably, Os04g0574600 was a newly discovered drought tolerance gene. Haplotype analysis showed that the RIL population carried two haplotypes (Hap1 and Hap2) of both genes. Lines carrying Hap2 exhibited significantly or extremely stronger drought tolerance than those carrying Hap1, indicating that Hap2 is an excellent haplotype. Among rice germplasm resources, there were two and three haplotypes of Os04g0574600 and OsCHR731, respectively. A high proportion of local rice resources in Sichuan, Yunnan, Anhui, Guangdong and Fujian provinces had Hap of both genes. In wild rice, 50% of accessions contained Hap1 of Os04g0574600 and 50% carried Hap4; 13.51%, 59.46% and 27.03% of wild rice accessions contained Hap1, Hap2, and Hap3, respectively. Hap2 of Os04g0574600 was found in more indica rice resources than in japonica rice. Therefore, Hap2 has more potential for utilization in future drought tolerance breeding of japonica rice.
Similar content being viewed by others
Background
Drought tolerance is a complex plant abiotic stress response trait. Researchers worldwide have conducted extensive research on rice drought tolerance in terms of morphology, physiology, biochemistry, and molecular biology, with significant results. These studies have been instrumental in the development of strategies for breeding drought tolerance in rice. However, there remains a sizable disparity between current achievements and desired goals. Rapid advances in modern biotechnology have facilitated the application of tools, such as linkage and association analyses based on genomics data, in studies of fundamental trait genes. These studies have revealed the molecular mechanisms underlying plant responses to adverse abiotic stresses and opened new paths for enhancing crop tolerance to these stresses.
Over the past 30 years, researchers have used tools such as linkage analysis combined with molecular marker analysis and genome sequencing to systematically study drought tolerance and related traits of rice at different growth stages and in various populations. Vikram et al. (2011) and Ghimire et al. (2012) used linkage analysis to identify a key drought-tolerance QTL, qDTY1.1, that affects yield. This QTL was mapped to the same chromosome location in diverse study populations. Yadav et al. (2019) used high-density linkage maps for two BC1F3 genetic populations, Swarna*2/Dular and IR11N121*2/Aus 196, which they constructed utilizing genotyping-by-sequencing data, to identify six and eight QTLs, respectively. Notably, two of these QTLs (qDTY2.4 and qDTY3.3) were mapped to the same chromosomal region as the known drought tolerance QTL qDTY1.1. Pan et al. (2021) identified five QTLs related to leaf water potential at the tiller stage, each located on a different chromosome, using a recombinant inbred line (RIL) population (Nekken2/HZ). Chen et al. (2023) mapped 28 QTLs related to leaf rolling index (LRI), leaf withering degree (LWD), and leaf chlorophyll content (LCC) utilizing a RIL population and simple sequence repeat (SSR) markers. The QTLs identified from these studies have been crucial for understanding the hereditary foundation of drought tolerance in rice. These studies have also provided essential genetic information for the design of molecular markers for marker-assisted selection (MAS) breeding, transgenic analysis, and gene editing. For example, researchers have used QTLs related to traits such as root system architecture and yield for MAS breeding of rice varieties with superior yield and drought tolerance (Mishra et al. 2013; Catolos et al. 2017; Yadav et al. 2019). Furthermore, researchers have made considerable progress in the map-based cloning and functional studies of drought-tolerance genes (Zhu and Xiong 2013; Chen et al. 2014; Geng et al. 2023), including DRO1, OsbZIP46, DROT1, and SANC1 (Uga et al. 2013; Tang et al. 2016; Sun et al. 2022; Gao et al. 2023). Using the fun Rice Genes database (https://funricegenes.github.io), researchers have successfully cloned 378 genes related to rice drought tolerance. However, the number of cloned genes is relatively small compared with the number of QTLs that have been mapped. This could be attributed to the low logarithm of the odds (LOD) scores and phenotypic contribution rates of several QTLs, or the variability in performance across diverse environments and the wide confidence intervals (Geng et al. 2023). Therefore, it is critical to identify reliable and dominant QTLs using high-density genetic maps and accurate phenotypic data.
In this study, we used an RIL population that was established by crossing the drought-sensitive cultivar Jileng 1 (JL1) and the drought-tolerant cultivar Milyang 23 (MY23). QTLs were mapped using an ultra-high-density genetic map and drought resistance phenotypes that were accurately measured over multiple years.The candidate genes for drought tolerance QTLs were verified by quantitative reverse transcription PCR (qRT-PCR) and haplotype analysis. Excellent haplotypes related to rice drought tolerance were then selected. This study provides valuable genetic resources for exploring the functions of rice drought tolerance-related genes and promoting the molecular breeding of rice drought tolerance.
Materials and Methods
Plant Materials
The research material used was a RIL population made by crossing JL1 (female parent) and MY23 (male parent). The population consisted of 253 RILs obtained using the single seed descent (SSD) method. JL1, a japonica rice variety, was drought-sensitive throughout the growth period, while MY23, an indica rice variety, exhibited strong drought tolerance throughout the growth period.
Phenotypic Analysis of Drought Tolerance
From 2017 to 2019, tests were conducted at the Wanghong Experimental Base at the Crop Research Institute of Ningxia Academy of Agriculture and Forestry Sciences to phenotype the drought-tolerant parents and 253 RILs. The area is dry and there is little rainfall. Yinchuan receives an average annual precipitation of about 200 mm. In 2017, seedlings were sown on April 28 and transplanted in the experimental field on May 30. Irrigation was stopped on June 10, and LRI and LWD were observed on days 32 (July 11) and 46 (July 25) of the drought treatment. In 2018, the seedlings were sown on April 25 and transplanted in the field on May 21. The water supply was cut off on June 1. LRI and LWD were measured on days 29 (June 29), 42 (July 12), and 57 (July 27) of the drought stress. In 2019, the seedlings were sown on April 23 and transplanted in the field on May 25. Irrigation was discontinued on June 5. LRI and LWD were measured on 33 (July 7), 49 (July 23), and 63 (August 6) of the drought treatment. The criteria used for scoring LRI and LWD are listed in Table 1. A smaller LRI and LWD indicates higher drought tolerance. The field experiment followed a random block design, with each material planted in two rows of 10 plants each, and a single plant was transplanted in each hole. The plant spacing was 9.9 cm, and the row spacing was 26.7 cm. Pest control and weeding were performed in accordance with local field practices.
Linkage Analysis
We previously created an ultra-high-density genomic map for rice using whole-genome sequencing data (Geng et al. 2023). The genotypic data were combined with phenotypic data from three consecutive years of measuring drought tolerance in the field, and the Inclusive Composite Interval Mapping (ICIM) model in IciMapping v4.2 software was used to fine-map QTLs for LRI and LWD and to assess the extent to which each QTL explained the phenotypic variation. The mapping was performed with a scanning step of 1 cM and a LOD threshold of ≥ 2.5 for determining significance. QTLs were named following the principles proposed by McCouch et al. (1997). In cases where QTLs controlled different traits within the same interval, it was envisaged a co-localization phenomenon.
RNA Extraction and qRT-PCR Analysis
Seven drought-tolerant lines were selected from the RIL family groups and analyzed for expression of genes related to drought tolerance. After germination, 30 seeds per line were sown in a seedling tray (length × width × height = 54 cm × 28 cm × 5 cm) and placed in an incubator at 28 ℃ for 4 days. The seedlings were then transplanted into two plastic boxes (length × width × height = 60 cm × 45 cm × 20 cm) with a soil depth of 18 cm, with separate treatment and control groups. After 21 days of seedling growth, the treatment group was subjected to drought stress by stopping irrigation, while the control group was provided ample water. Fresh leaves were cut from both groups when the soil water content in the treatment group reached 14%. The leaves were quickly frozen in liquid nitrogen and kept at -80 ℃. RNA was extracted using the TRIzol reagent, and 1 µg of RNA was used for reverse transcription. qPCR was performed on the StepOne Plus real-time quantitative PCR instrument (ABI Company) with a reaction system of 20 µL. The sequence-specific primers used are shown in Supplementary Table 1. The qPCR procedure consisted of two steps: denaturation for 30 s at 95 ℃, followed by 40 cycles of 95 ℃ for 10 s and 60 ℃ for 30 s. The relative gene expression was calculated using the 2-ΔΔCT method with UBI as the reference gene (Livak et al. 2001). Three biological replicates were performed.
Haplotype Analysis
To investigate the markers associated with QTLs and the superior haplotypes within the identified regions, the current paper utilized PLINK software for genotype data analysis. We obtained 1,860,935 high-quality SNP markers by excluding those with allele frequencies below 0.05. After filtering, we obtained a representative set of SNPs, which were used for additional haplotype analysis. The filtered SNP data were converted to Variant Call Format (VCF) utilizing PLINK software. The genotype data from the VCF file were then imported into Haploview 4.2 software for haplotype analysis. To investigate the relationship between haplotype and drought tolerance traits, analysis of variance (ANOVA) was performed using SPSS 27.0 software. The phenotypic data for each line were used along with haplotype data to identify significant haplotypes associated with superior drought tolerance traits.
Results and Analysis
QTL Mapping of LRI and LWD under Drought Stress
In 2017, six QTLs related to LRI were identified on chromosomes 1, 4, 5, and 7. The most significant QTL (LOD value = 20.73) detected on day 32 of drought stress was qLRI5-2 on chromosome 5 (Table 2). This QTL explained 10.34% of the phenotypic variation and had the largest effect size. Three QTLs showed positive additive effects, indicating that the beneficial alleles came from the male parent MY23. Three QTLs showed negative additive effects, indicating that the beneficial alleles originated from the female parent JL1. In 2018, nine QTLs related to LRI were mapped to chromosomes 2, 3, 4, 7, 8, and 12. qLRI2-2, the major QTL, was repeatedly detected on days 29, 42, and 57 of drought treatment, with contribution rates of 10.59–17.04%. The beneficial alleles of all nine QTLs originated from the male parent. In 2019, eight QTLs related to LRI were discovered on chromosomes 1, 2, 5, and 12. Among them, qLRI5-4 explained the most variance (11.13%), and it was the major effect QTL. Three adjacent QTLs were mapped on chromosome 12, namely qLRI12-1, qLRI12-2, and qLRI12-3, with contribution rates of 4.75–7.32%. For all of these QTLs except qLRI2-3, the allele increasing LRI was inherited from the male parent.
In 2017, five QTLs related to LWD were detected on chromosomes 1, 3, 4, and 5, explaining 3.62–9.37% of the phenotypic variation (Table 2). The additive effects of the three QTLs were positive, indicating that the beneficial alleles originating from the female parent. The additive effects of two QTLs were negative, indicating that the beneficial alleles originated from the male parent. qLWD4-1, located on chromosome 4, was detected on both day 32 and day 46 of drought stress, with contribution rates of 7.07% and 8.19%, respectively. In 2018, three QTLs related to LWD were identified on chromosomes 3, 4, and 12. Among these, qLWD4-2 on chromosome 4 was detected on days 29, 42, and 57 of drought, with contribution rates ranging from 5.14 to 5.71%. The beneficial allele for this QTL came from the male parent. qLWD12-1 was mapped to the same chromosome interval as qLRI12-3, which was detected in 2019. In 2019, eight QTLs associated with LWD were mapped to chromosomes 3, 5, 8, 9, and 12. These QTLs explained 3.95–8.40% of the observed phenotypic variation. The beneficial alleles of five of the QTLs were from the male parent.
During the tillering stage, 39 QTLs related to LRI and LWD were identified (Table 2). About three-fourths of these QTLs had confidence intervals less than 1 cM, with the smallest being 0.11 cM and the largest being 1.92 cM. The LOD scores ranged from 2.51 to 20.73, and the percent phenotypic variation ranged from 1.2 to 17.04%. Four QTLs, all of which were linked to LRI, explained more than 10% of the phenotypic variation. The positive alleles of the 25 QTLs were from the drought-tolerant parent MY23, while the positive alleles of 9 QTLs were derived from the drought-sensitive parent JL1. Overall, 23 QTLs related to LRI and 16 QTLs related to LWD were identified. Among these, qLWD5-3 and qLRI5-3, qLWD5-4 and qLRI5-4, qLWD12-2 and qLRI12-2, and qLWD12-1 and qLRI12-3 were located in the same region of the same chromosome.
Discovery of Drought-Resistance Genes and Excellent Haplotypes in Rice
There were 281 genes located in the QTL region, with 201 genes located in the region with a LOD > 3. Among these genes, 147 were up-regulated and 54 were down-regulated. The most significantly up-regulated and down-regulated genes were located on chromosome 5. The gene with the highest up-regulated expression was located in a 0.00 cM–1.28 cM region with a LOD value of 12.82. Conversely, the gene with the most down-regulated expression was found in a 2.59 cM–3.52 cM region with a LOD value of 20.73. A total of 90 genes were found in the co-localized QTLs. Of these, 31 genes were repeatedly detected during the two-year drought tolerance identification study. Three drought resistance genes, OsANN1, OsSLAC1, and OsTPS1, are located on chromosomes 2 (31665723–31765723 bp), 4 (29020325–28882817 bp), and 5 (25576289–26094452 bp), respectively (Zang et al. 2011; Qiao et al. 2015; Sun et al. 2016).
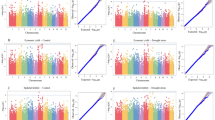
During the haplotype analysis of the RIL population, two candidate genes with significant differences were noted in the intervals of 34,757,965–34,738,491 bp on chromosome 4 and 19,640,244–19,535,141 bp on chromosome 7. The sequences of non-homogeneous SNPs in the genes Os04g0574600 (LOC_Os04g48520) and OsCHR731 (LOC_Os07g32730) were extracted to identify these genes. These two genes were designated as candidate genes because of the presence of repeated allelic variants. The coding sequence of Os04g0574600, which is 2001 bp, contains four SNPs: 28,922,253 (T→C), 28,926,734 (C→T), 28,927,682 (G→T), and 28,928,513 (T→A). In the 1107 bp coding sequence of OsCHR731, there is one SNP (A→G). For both genes, there were two haplotypes, haplotype 1 (Hap1) and haplotype 2 (Hap2), present in the RIL population, there were significant differences in the phenotypic values of LRI and LWD between RILs carrying Hap1 and those carrying Hap2 (P < 0.05, P < 0.01) (Figs. 1 and 2). Specifically, the phenotypic values of LRI and LWD were much lower in Hap2-carrying RILs than in Hap1-carrying RILs. These results suggest that RILs carrying Hap2 of either Os04g0574600 or OsCHR731 exhibit stronger drought tolerance than those carrying Hap1.
LRI and LWD of lines carrying one of two haplotypes of OsCHR731. Drought stress treatment for 46 days in 2017 (A); drought stress treatment for 29 days in 2018 (B); drought stress treatment for 57 days in 2018 (C); drought stress treatment for 63 days in 2019 (D, F); drought stress treatment for 49 days in 2019 (E)
The Expression of Candidate Genes Related to Drought Tolerance Differs Between the Treatment Group and the Control Group
qRT-PCR analysis was performed to assess the difference in expression of candidate genes between the treatment and control groups. The expression levels of the candidate genes were generally higher in the treatment group than in the control group. Specifically, the expression of Os04g0574600 was significantly higher in the treatment group than in the control group in five out of seven drought-tolerant samples tested. In the treatment group, the expression level of R8 was the highest (7.68 times that of the control group). R16 had an expression level 3.86 times higher than that the control group. R20 and R26 had expression levels 1.11 and 1.20 times higher than that of the control group, respectively, but these differences were not significant. In six out of the seven drought-tolerant samples tested, the expression of OsCHR731 was considerably or extremely considerably higher than that of the control group. Like Os04g0574600, OsCHR731 was most highly expressed in the R8 treatment group (19.47 times that of the control group). The expression level of OsCHR731 in the R16 treatment group was 6.26 times that of the control group. These results suggest that Os04g0574600 and OsCHR731 play an important part in regulating rice drought tolerance (Fig. 3).
Relative expression of candidate genes in drought-tolerant groups analyzed by qRT-PCR in both control and treatment conditions. Data are means ± SD of three biological replicates. Significant differences were determined using two-tailed Student’s t-test (*P < 0.05, **P < 0.01). R is the family number
Geographical Distribution of Haplotypes for Candidate Drought-Tolerance Genes
To investigate the geographical distributions of the Os04g0574600 and OsCHR731 haplotypes, we analyzed the haplotypes of 372 rice germplasm resources from 12 provinces in the Yangtze River and southern regions. The genomic data for these rice materials were obtained from the NCBI database (Liu et al. 2022). Of the rice germplasm resources, 209 were indica rice and 163 were japonica rice. There were two haplotypes (Hap1 and Hap2) of Os04g0574600 in indica rice, accounting for 8.97% and 91.03% of accessions, respectively. In japonica rice, only Hap1 was detected. There were three haplotypes of OsCHR731 in indica rice: Hap1, Hap2, and Hap3, accounting for 1.22%, 96.95%, and 1.83% of accessions, respectively. In japonica rice, 89.37%, 10.15%, and 0.48% of accessions had Hap1, Hap2, and Hap3, respectively (Fig. 4).
Haplotype analysis of rice germplasm resources showed that the proportion of accessions with the superior Os04g0574600 haplotype Hap2 among the 12 provinces ranked as follows: Sichuan (93.33%) > Anhui (75.00%) > Yunnan (68.75%) > Fujian (66.67%) > Guangdong (64.71%) > Hunan (44.44%) > Jiangxi (37.50%) > Hubei (35.71%) > Guizhou (27.07%) > Jiangsu (25.00%) > Guangxi (23.53%) > Zhejiang (15.38%) (Fig. 5). In five of the provinces over 50% of accessions had Hap 2. The proportion of accessions with the superior haplotype of OsCHR731, Hap2, in 12 provinces ranked as follows: Sichuan (100%) > Yunnan (85.71%) > Anhui (84.62%) > Fujian (80.00%) > Guangdong (77.78%) > Hubei (50.00%) > Guangxi (47.06%) > Hunan (44.44%) > Jiangxi (43.75%) > Guizhou (37.93%) > Jiangsu (26.67%) > Zhejiang (Fig. 6). In addition, Hap2 was found in more than 50% of accessions in five other provinces. Hap2 of both genes was dominant among the local rice resources of Sichuan, Yunnan, Anhui, Fujian, and Guangdong provinces, whereas a low proportion of the local rice resources of Zhejiang carried these haplotypes (Figs. 5A and 6A).
Haplotype analysis of wild rice resources showed that 50% carried Hap1 of Os04g0574600 and 50% carried Hap4 (Fig. 5B), and 13.51%, 59.46%, and 27.03% carried Hap1, Hap2, and Hap3 of OsCHR731, respectively (Fig. 6B).
Discussion
Pleiotropic QTLs and Comparison with Previous Drought Tolerance QTLs
This study revealed that some QTLs may affect multiple traits owing to pleiotropy or tight linkage. For instance, qLRI5-3 and qLWD5-3, qLRI5-4 and qLWD5-4, qLRI12-2 and qLWD12-2, and qLRI12-3 and qLWD12-1 were mapped to the same chromosome intervals and may control LRI and LWD simultaneously because of pleiotropy. All alleles were derived from the drought-tolerant parent MY23, and qLRI5-4, which controls LRI, explained the highest percentage of phenotypic variation (11.13%). Three QTLs, qLRI12-1, qLRI12-2, and qLRI12-3, on chromosome 12 are closely linked. This suggests that these genes may interact and have a cumulative effect, resulting in a synergistic enhancement of drought tolerance. These QTLs overlap with those reported in previous studies and may be hotspots for drought tolerance in rice.
In addition, 16 QTLs mapped in this study overlapped with previously reported chromosomal regions (Table 3). qLRI1-1 and qLRI1-2, located on chromosome 1, are in the same chromosomal region as qLRI1-1JC and qLWD1-2B, which were mapped to an interval of 36,734,135–41,539,722 bp on chromosome 1 by Chen et al. (2023) using SSR markers. The additional nine QTLs were related to LRI and LWD under drought stress, as reported by Chen et al. (2023). Jiang et al. (2016) identified five QTLs related to drought tolerance. Three of these QTLs (qSH-1, qRL-1-3, and qRS-1) were associated with seedling height, maximum root length, and the ratio of root length to seedling height, and they are located in the same region on chromosome 1 and qLRI1-1 and qLRI1-2. Ding (2011) identified QTL5 as being related to the relative fertility rate under drought stress. In this study, qLRI5-3, qLWD5-1, and qLWD5-3 were mapped to chromosome 5. The QTL intervals from our study are significantly narrower compared with those of previous studies, which improves the localization accuracy.
We compared the QTLs mapped using an ultra-high-density genetic linkage map with those mapped in the same population using SSR markers (Chen et al. 2023). The QTLs identified by Chen et al. (2023) were considerably different from those identified in this study. Chen et al. (2023) identified QTLs on all chromosomes except 8, 9, and 11, whereas we identified QTLs on chromosomes except 6, 10, and 11. This difference may be attributed to the variation in marker density and resolution. However, the large-effect QTLs were consistent, with both found on chromosomes 2 and 5. This study dramatically narrowed the marker interval and improved detection accuracy. In crop QTL studies, the lack of molecular markers is often a major limiting factor. Traditional molecular markers, such as RFLP, RAPD, and SSR markers, are often still far apart, which limits the resolution of QTL localization. The use of high-throughput sequencing technology has led to the establishment of high-density polymorphism markers, including SNPs and insertion-deletions, which increases the accuracy of QTL localization (Geng et al. 2023).
We constructed a high-density genetic map using whole-genome resequencing data. The map had a marker density of 7.83 markers/Mb with an average interval of 0.48 cM between adjacent bin markers (Geng et al. 2023). The density of the markers used by Chen et al. was 1.28 markers/Mb with an average interval of 10.12 cM between adjacent markers (2023). The significantly higher marker density on our map, resulted in a reduction of the confidence intervals of QTLs and an improvement in the resolution of QTL localization. For instance, qLRI5-4 and qLWD5-4, which control LRI and LWD, were mapped to a 518.16 kb interval (25576289–26094452 bp) on chromosome 5. For comparison, the same QTL mapped using SSR markers was located in a 2.23 Mb interval (24121914–26356605 bp) on chromosome 5 (Chen et al. 2023). Similarly, qLWD5-1, which controls LWD, was mapped to a 70.88 kb interval (chr05: 26237907–26308755 bp) and explained 7.34% of the phenotypic variance, whereas using SSR markers, this QTL was mapped to a 747.63 kb interval (chr05: 26356605–27104234 bp) and explained 5.25% of the phenotypic variance. The high-density genetic map substantially narrowed the range of QTL intervals, allowing for more accurate location of the functional gene. QTL fine-mapping results not only increase the probability of finding functional genes, but also reflect the great advantage of performing high-density QTL mapping.
Validation of Candidate Genes for Drought Tolerance
Rice drought tolerance is quantitative trait controlled by multiple genes and a complex genetic mechanism. The rapid development of next-generation sequencing technology has accelerated QTL mapping and gene discovery in rice. These QTLs contain a large number of functional genes. Although researchers have used various techniques to mine drought-tolerance genes, only a few genes showing significant effects have been thoroughly studied or cloned. Most of these genes are were identified as being differentially expressed under drought stress conditions, and there functions were determined by reverse genetics. In this study, we cloned three drought-tolerant genes identified in the QTL regions on chromosomes 2, 4, and 5, demonstrating that resequencing technology and linkage analysis are efficient means to identify rice drought-tolerance genes. In addition, the effectiveness of this method in mining rice salt tolerance genes has also been demonstrated (Geng et al. 2023).
Of the 281 identified genes, two candidate genes, Os04g0574600 and OsCHR731, were selected based on gene function annotation, and their gene expression levels were analyzed by qRT-PCR. The expression levels of the two genes under drought stress were significantly higher than those under normal growth conditions, indicating their close association with drought tolerance. The candidate gene Os04g0574600 is located in qLWD4-2 on chromosome 4, which has frequently been detected and is located within the QTL interval identified by Zou et al. (2007). The gene OsCHR731 is located at the qLRI7-2 locus on chromosome 7 and has been recognized as a QTL by Fu et al. (2012) and Chen et al. (2023). In a previous study, qRT-PCR analysis of 14-day-old subjected to drought stress revealed that OsCHR731 was up-regulated after 12 h of treatment (Guo et al. 2022), which is consistent with our findings.
Os04g0574600 encodes ae ZOS4-12-C2H2 zinc finger protein. C2H2 zinc finger proteins play an essential role in various developmental processes in plants, such as regulating plant growth, hormone signaling, and responses to biotic and abiotic stresses (Zhang et al. 2016). Several homologous genes of Os04g0574600 have been closely linked to drought resistance in rice, Arabidopsis, and tomato (Xu et al. 2008; Luo et al. 2012; Li et al. 2018; Feng et al. 2023). OsCHR731 encodes a Snf2 family protein. Many rice Snf2 genes exhibit tissue-specific expression patterns, and some of these genes are highly responsive to abiotic stresses, such as drought, salt, and low temperature (Guo et al. 2014, 2022). Based on these results, we speculate that Os04g0574600 and OsCHR731 may be closely related to drought tolerance in rice, and are worthy of further study.
Haplotype Analysis and Geographical Distribution of Candidate Genes
Even though whole-genome sequencing technology can provide comprehensive genetic information at the whole-genome level, it cannot distinguish allelic differences on homologous chromosomes. In contrast, haplotype analysis can more deeply analyze the combination of different genomic loci and their genetic characteristics in a single chromosome or a specific chromosome region. This is particularly important for the study of heterosis and the discovery of finer allelic variation (Ma et al. 2017). Haplotype analysis can reveal the genetic background of a trait by identifying the genetic markers associated with that trait. It can also provide more information on allelic diversity and improve genotyping accuracy (Lu et al. 2012). Haplotype analysis of the rice grain width gene GS5, rice pre-harvest sprouting gene Os04g0545200, and salt tolerance genes (SKC1/OsHKT8, qSE3/OsHAK21, and RST1/OsARF18) were analyzed by researchers (Yi et al. 2016; Min et al. 2023; Li et al. 2023) revealed haplotypes contributing to good trait values. The discovery of superior haplotypes such as these provides molecular markers for effective screening of genes related to high yield and stress resistance. It also offers significant scientific and technological support for rice breeding.
Haplotype analysis has been also been used to identify natural variants and beneficial alleles associated with drought tolerance, such as DROT1, in rice germplasm resources. Hap1 and Hap4 are haplotypes of DROT1 primarily found in japonica and indica rice, respectively. In upland rice, Hap3 is present at a much higher frequency than other haplotypes. A key SNP (C→T) in the promoter region dramatically enhances the expression of DROT1 in upland rice, thereby improving drought tolerance (Sun et al. 2022). Here, we performed haplotype analysis of 132 genes within the QTL interval. SNPs in Os04g0574600 and OsCHR731 were found in both the RIL population and rice germplasm resources. In the RIL population, the materials carrying Hap2 of Os04g0574600 or Hap2 of OsCHR731 showed strong drought tolerance. This indicates that these haplotypes have enormous potential for utilization in rice breeding. Hap2 of Os04g0574600 was found exclusively in local indica rice germplasm resources. However, Hap2 of OsCHR731 was found in significantly more indica rice accessions than japonica rice accessions, suggesting that this haplotype could be effectively utilized in japonica rice breeding.
The distribution of haplotypes (Figs. 5 and 6) indicates that the proportion of rice germplasm resources carrying the superior haplotypes (Hap2) of Os04g0574600 and OsCHR731 is higher in Sichuan, Yunnan, Anhui, Fujian, and Guangdong compared with other provinces. This distribution pattern may reflect the selection pressure exerted by specific climatic and soil conditions, as well as agricultural activities; specifically, the superior haplotypes of Os04g0574600 and OsCHR731 were selected for in local rice germplasm resources. The comparison of haplotypes between wild rice and native rice varieties revealed that the former contained Hap1 and Hap4 of Os04g0574600, whereas the latter contained Hap1 and Hap2. During rice evolution, Hap1 of the Os04g0574600 adapted to environmental stresses, such as drought stress, and anthropogenic selective pressures, and was retained, while Hap4 was eliminated. The wild rice accessions contained three haplotypes of OsCHR731: Hap1, Hap2, and Hap3. While Hap1 and Hap2 were present in local rice species, Hap3 was only found in a few provinces and was rare. It is important to emphasize that Hap3 was found in a very low proportion of rice accessions. It is possible that Hap1 and Hap2 have been conserved in rice because of their potent environmental adaptability, while Hap3, lacking such adaptability, has become a rare haplotype.
Conclusion
Thirty-nine QTLs related to drought tolerance were identified and mapped to nine chromosomes. A novel gene, Os04g0574600, which is closely associated with rice drought tolerance, was discovered. The expression levels of this gene under drought stress were much higher than those under normal conditions, indicating that it functions as a positive regulator. In the RIL population, Hap2 of this gene is closely associated with intense drought tolerance; thus, it is an excellent haplotype. In Sichuan, Yunnan, Anhui, Guangdong, and Fujian provinces, the proportion of indigenous rice varieties carrying the Hap2 haplotype was significantly higher than that in other provinces. The proportion of indica rice accessions with Hap2 is much higher than that of japonica rice accessions; thus, Hap2 has greater potential for utilization in future japonica rice drought tolerance breeding.
Data Availability
No datasets were generated or analysed during the current study.
Abbreviations
- QTL:
-
Quantitative trait locus
- RIL:
-
Recombinant Inbred Line
- LRI:
-
Leaf Rolling Index
- LWD:
-
Leaf Withering Degree
- LCC:
-
Leaf Chlorophyll Content
- qRT-PCR:
-
Quantitative Reverse Transcription Polymerase Chain Reaction
- JL1:
-
Jileng 1
- MY23:
-
Milyang 23
- SSR:
-
Simple Sequence Repeat
- MAS:
-
Marker-Assisted Selection
- SSD:
-
Single Seed Descent
- LOD:
-
Logarithm of the Odds
- PVE:
-
Phenotypic Variation Explained
- VCF:
-
Variant Call Format
- ANOVA:
-
Analysis of Variance
- NCBI:
-
National Center for Biotechnology Information
- RFLP:
-
Restriction Fragment Length Polymorphism
- RAPD:
-
Random Amplified Polymorphic DNA
References
Catolos M, Sandhu N, Dixit S et al (2017) Genetic loci governing grain yield and root development under variable rice cultivation conditions. Front Plant Sci 8(9):1763. https://doi.org/10.3389/fpls.2017.01763
Chen DJ, Neumann K, Friedel S et al (2014) Dissecting the phenotypic components of Crop Plant Growth and Drought responses based on high-throughput image analysis. Plant Cell 26(12):4636–4655. https://doi.org/10.1105/tpc.114.129601
Chen L, Ma J, Ma XD et al (2023) QTL analysis of drought tolerance traits in rice during the vegetative growth period. Euphytica 219:33–49. https://doi.org/10.1007/s10681-022-03151-4
Ding XP (2011) Screening and identification of NILs for drought resistance QTL and differential responsive genes in rice. Huazhong Agricultural University, WuHan
Feng Y, Zhang SR, Li J et al (2023) Dual-function C2H2-type zinc-finger transcription factor GmZFP7 contributes to isoflavone accumulation in soybean. New Phytol 237:1794–1809. https://doi.org/10.1111/nph.18610
Fu XQ (2012) Screening indentification index of drought resistance and QTL mapping in Dongxiang wild rice (Oryza rufipogon Griff). JiangXi Agriculture University, NanChang
Gao J, Zhao Y, Zhao ZK et al (2023) RRS1 shapes robust root system to enhance drought resistance in rice. New Phytol 238(1):1146–1162. https://doi.org/10.1111/nph.18775
Geng LY, Zhang W, Zou T et al (2023) Integrating linkage mapping and comparative transcriptome analysis for discovering candidate genes associated with salt tolerance in rice. Front Plant Sci 14(1):1065334. https://doi.org/10.3389/fpls.2023.1065334
Ghimire KH, Quiatchon LA, Vikram P et al (2012) Identification and mapping of a QTL (qDTY1.1) with a consistent effect on grain yield under drought. Field Crop Res 131(2):88–96. https://doi.org/10.1016/j.fcr.2012.02.028
Guo MX, Wang RC, Wang J et al (2014) ALT1, a Snf2 family chromatin remodeling ATPase, negatively regulates alkaline tolerance through enhanced defense against oxidative stress in Rice. PLoS ONE 9(12):e112515. https://doi.org/10.1371/journal.pone.0112515
Guo ML, Zhao HM, He ZM et al (2022) Comparative expression profiling of Snf2 family genes during reproductive development and stress responses in rice. Front Plant Sci 13:910663. https://doi.org/10.3389/fpls.2022.910663
Jiang X, Ma XS, Luo LJ et al (2016) QTL mapping of phenotypic traits under Drought stress simulated by PEG-6000 in Rice Seedlings. Crops 5:31–37. https://doi.org/10.16035/j.issn.1001-7283.2016.05.006
Li Y, Chu ZN, Luo JY et al (2018) The C2H2 zinc-finger protein SlZF3 regulates AsA synthesis and salt tolerance by interacting with CSN5B. Plant Biotechnol J 16:1201–1213. https://doi.org/10.1111/pbi.12863
Li PB, Li Z, Liu X et al (2023) Haplotype analysis and marker development of five salt-tolerant–related genes in rice (Oryza sativa L.). Front. Plant Sci 14(8):1259462. https://doi.org/10.3389/fpls.2023.1259462
Liu CH, Wang TY, Chen HC et al (2022) Genomic footprints of Kam Sweet Rice domestication indicate possible migration routes of the Dong people in China and provide resources for future rice breeding. Mol Plant 16(2):415–431. https://doi.org/10.1016/j.molp.2022.12.020
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Lu YL, Xu J, Yuan ZM et al (2012) Comparative LD mapping using single SNPs and haplotypes identifies QTL for plant height and biomass as secondary traits of drought tolerance in maize. Mol Breed 30:407–418. https://doi.org/10.1007/s11032-011-9631-5
Luo X, Bai X, Zhu D et al (2012) GsZFP1, a new Cys2/His2-type zinc-finger protein, is a positive regulator of plant tolerance to cold and drought stress. Planta 235:1141–1155. https://doi.org/10.1007/s00425-011-1563-0
Ma L, Li W, Song Q et al (2017) Chromosome-range whole-genome high-throughput experimental haplotyping by single-chromosome microdissection. Methods Mol Biol 1551:161–169. https://doi.org/10.1007/978-1-4939-6750-6_9
McCouch SR, Cho YG, Yano M et al (1997) Report on QTL nomenclature. Rice Genet Newsl 14:11–13
Min MH, Khaing AA, Chu SH et al (2023) Exploring the genetic basis of pre-harvest sprouting in rice through genome-wide association study based haplotype analysis. J Integr Agric 23(8):2525-2540. https://doi.org/10.1016/j.jia.2023.12.004
Mishra KK, Vikram P, Yadaw RB et al (2013) qDTY12.1: a locus with a consistent effect on grain yield under drought in rice. BMC Genet 14:12. https://doi.org/10.1186/1471-2156-14-12
Pan CY, Zhang Y, Lin H et al (2021) QTL mapping and Candidate Gene Analysis on Rice Leaf Water potential. Chin Bull Bot 56(3):275–283. https://doi.org/10.11983/CBB21039
Qiao B, Zhang Q, Liu DL et al (2015) A calcium-binding protein, rice annexin OsANN1, enhances heat stress tolerance by modulating the production of H2O2. J Exp Bot 66(19):5853–5866. https://doi.org/10.1093/jxb/erv294
Sun SJ, Qi GN, Gao QF et al (2016) Protein kinase OsSAPK8 functions as an essential activator of S-type anion channel OsSLAC1, which is nitrate-selective in rice. Planta 243:489–500. https://doi.org/10.1007/s00425-015-2418-x
Sun XM, Xiong HY, Jiang CH et al (2022) Natural variation of DROT1 confers drought adaptation in upland rice. Nat Commun 13(7):4265. https://doi.org/10.1038/s41467-022-31844-w
Tang N, Ma SQ, Zong W et al (2016) MODD mediates deactivation and degradation of OsbZIP46 to negatively regulate ABA signaling and Drought Resistance in Rice. Plant Cell 28(9):2161–2177. https://doi.org/10.1105/tpc.16.00171
Teng S (2002). Genetics analysis of physiological traits related to yield in rice (Oryza sativa L.).Hangzhou: ZheJiang University
Uga Y, Sugimoto K, Ogawa S et al (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet 45(9):1097–1102. https://doi.org/10.1038/ng.2725
Vikram P, Swamy BM, Dixit S et al (2011) qDTY 1.1, a major QTL for rice grain yield under reproductive-stage drought stress with a consistent effect in multiple elite genetic backgrounds. BMC Genet 12(1):89. https://doi.org/10.1186/1471-2156-12-89
Xu DQ, Huang J, Guo SQ et al (2008) Over ex- pression of a TFIIIA-type zinc finger protein gene ZFP252 enhances drought and salt tolerance in rice (Oryza sativa L). FEBS Lett 582:1037–1043. https://doi.org/10.1016/j.febslet.2008.02.052
Yadav S, Sandhu N, Singh VK et al (2019) Genotyping-by-sequencing based QTL mapping for rice grain yield under reproductive stage drought stress tolerance. Sci Rep 9:14326. https://doi.org/10.1038/s41598-019-50880-z
Yi CD, Wang DR, Jiang W et al (2016) Development of functional markers and identification of haplotypes for rice grain width gene GS5. Chin J Rice Sci (in Chinese) 30(5):487–492. https://doi.org/10.16819/j.1001-7216.2016.6038
Yue B, Xue WY, Xiong LZ et al (2006) Genetic analysis for drought resistance of rice at reproductive stage in field with different types of soil. Genetics 172:1213–1228. https://doi.org/10.1007/s00122-005-0040-1
Zang BS, Li HW, Li WJ et al (2011) Analysis of trehalose-6-phosphate synthase (TPS) gene family suggests the formation of TPS complexes in rice. Plant Mol Biol 76:507–522. https://doi.org/10.1007/s11103-011-9781-1
Zhang X, Zhang B, Li MJ et al (2016) OsMSR15 encoding a rice C2H2-type zinc finger protein confers enhanced drought tolerance in transgenic a rabidopsis. J Plant Biol 59:271–281. https://doi.org/10.1007/s12374-016-0539-9
Zhu XY, Xiong LZ (2013) Putative megaenzyme DWA1 plays essential roles in drought resistance by regulating stress-induced wax deposition in rice. Proc Natl Acad Sci USA 110:17790–17795. https://doi.org/10.1073/pnas.1316412110/-/DCSupplemental
Zou GH (2007) Study on the water requirement of Paddy and Upland rice and the genetic basis of drought tolerance at reproductive stage rice. HuaZhong Agriculture University, WuHan
Funding
This work was supported by the Key R&D Program of Ningxia Hui Autonomous Region (2023BCF01010), the National Key Research and Development Program of China (2021YFD1200500), the CAAS Science and Technology Innovation Program, and the National Crop Germplasm Resources Center (NCGRC-2023-02).
Author information
Authors and Affiliations
Contributions
AJ conducted the data analysis and wrote the manuscript. LC and JM carried out the field work and generated the phenotypic data. BH provided assistance with the field work. XM and DC were responsible for collecting the genotype data. JS and LH designed the experiment, guided the experiments, and revised the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
This study complied with the ethical standards of China where this research was performed.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Jiao, A., Chen, L., Ma, X. et al. Linkage Mapping and Discovery of Candidate Genes for Drought Tolerance in Rice During the Vegetative Growth Period. Rice 17, 53 (2024). https://doi.org/10.1186/s12284-024-00733-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12284-024-00733-9