Abstract
Background
The Lin−Sca1+c-Kit+ (LSK) fraction of the bone marrow (BM) comprises multipotent hematopoietic stem cells (HSCs), which are vital to tissue homeostasis and vascular repair. While diabetes affects HSC homeostasis overall, the molecular signature of mRNA and miRNA transcriptomic under the conditions of long-standing type 2 diabetes (T2D;>6 months) remains unexplored.
Methods
In this study, we assessed the transcriptomic signature of HSCs in db/db mice, a well-known and widely used model for T2D. LSK cells of db/db mice enriched using a cell sorter were subjected to paired-end mRNA and single-end miRNA seq library and sequenced on Illumina NovaSeq 6000. The mRNA sequence reads were mapped using STAR (Spliced Transcripts Alignment to a Reference), and the miRNA sequence reads were mapped to the designated reference genome using the Qiagen GeneGlobe RNA-seq Analysis Portal with default parameters for miRNA.
Results
We uncovered 2076 out of 13,708 mRNAs and 35 out of 191 miRNAs that were expressed significantly in db/db animals; strikingly, previously unreported miRNAs (miR-3968 and miR-1971) were found to be downregulated in db/db mice. Furthermore, we observed a molecular shift in the transcriptome of HSCs of diabetes with an increase in pro-inflammatory cytokines (Il4, Tlr4, and Tnf11α) and a decrease in anti-inflammatory cytokine IL10. Pathway mapping demonstrated inflammation mediated by chemokine, cytokine, and angiogenesis as one of the top pathways with a significantly higher number of transcripts in db/db mice. These molecular changes were reflected in an overt defect in LSK mobility in the bone marrow. miRNA downstream target analysis unveils several mRNAs targeting leukocyte migration, microglia activation, phagosome formation, and macrophage activation signaling as their primary pathways, suggesting a shift to an inflammatory phenotype.
Conclusion
Our findings highlight that chronic diabetes adversely alters HSCs’ homeostasis at the transcriptional level, thus potentially contributing to the inflammatory phenotype of HSCs under long-term diabetes. We also believe that identifying HSCs-based biomarkers in miRNAs or mRNAs could serve as diagnostic markers and potential therapeutic targets for diabetes and associated vascular complications.
Similar content being viewed by others
Background
Diabetes is a chronic debilitating metabolic disease affecting more than 537 million people worldwide, and this number is predicted to rise to 783.2 million by 2045 [1, 2]. Hematopoietic stem cells (HSCs) are bone-marrow-derived stem cells that can differentiate into myeloid, lymphoid cells, platelets, and red blood cells [3]. These bone marrow-derived HSCs are adversely affected by chronic diabetes. With the primary role of tissue maintenance, homeostasis, and vascular repair, these HSCs in diabetes lose their ability to be reparative, instead, it has been reported in multiple studies that mobilopathy, stem cell rarefaction, neuropathy, micro-angiopathy, and inflammation become more prevalent in HSCs with diabetes [4, 5]. Diabetes also manifests many micro- and macro-vascular complications, such as retinopathy, neuropathy, cardiomyopathy, and nephropathy, posing a high risk for multi-organ damage [6]. Consistently, it has been shown that low levels of HSCs in chronic diabetes accelerate the progression of diabetes-induced microvascular complications [7, 8].
The transcriptomics studies have strengthened our knowledge of genetic factors associated with the development and associated outcomes of diabetes. Importantly, different groups have utilized the insulin-targeted organs, such as the pancreas, liver, skeletal muscles, adipose tissues, etc., for gene expression studies reflecting the dominance of inflammatory immune responses in the pathogenesis of diabetes [9,10,11]; however, Lin−Sca1+c-Kit+ cells’(LSK) transcriptome under long-standing diabetes has not been studied. In addition to mRNA targets, non-coding RNAs like micro RNAs (miRNA) have been widely explored for their role in chronic diabetes progression [12]. Recent evidence has shown the potential of tissue-specific and circulating miRNAs for their capability to be used as a biomarker in diabetes and associated vascular complications [13, 14]. Therefore, studying miRNA and mRNA transcriptomic could provide critical information about LSK cell phenotype under conditions of long-standing diabetes.
It is noteworthy that HSC dysfunction is a known outcome of diabetes [4, 5]; however, the transcriptome signature of these HSCs in diabetes has not been investigated. In the present study, we isolated a highly purified fraction of HSCs; Lin−Sca1+c-Kit+ (LSK) cells from the bone marrow of db/db mice (an animal model of type 2 diabetes) with six months of diabetes followed by miRNA and mRNA sequencing. Our studies unravel previously unknown miRNAs and mRNA transcripts changed with long-term diabetes, which could have contributed to HSC mobilopathy and inflammatory phenotype.
Methods
Animals
The B6.BKS(D)-Leprdb/J (an animal model for T2D; db/db; n = 9) and Leprdb/+ db/m (heterozygotes; db/m; n = 9) mice [stock number 000697] were procured from The Jackson Laboratory (Bar Harbor, ME, USA) and housed in the animal care facility at Glick Eye Institute, Indiana university. All the animals were kept under normal physiological conditions (12-hour light/dark conditions), with access to food and water ad libitum. The mice were maintained for eight months (6 months of diabetes) before proceeding with the bone marrow experiments. All the experiments were performed per the Guiding Principles in the Care and Use of Animals (National Institutes of Health). The mice were euthanized using inhalation overdose of CO2 delivered using a gas cylinder, /regulator, and induction chamber.
Hematopoietic stem cell (HSCs) isolation
Femur and tibia were collected for bone marrow isolation after animal sacrifice. Briefly, all the surrounding muscles were removed, and bones were cleaned and flushed with FACS buffer (PBS with 2% FBS) with the help of a needle. A cell suspension was generated by triturating the cells through the needles. Cells were then treated with ice-cold ammonium chloride for 10 min to lyse all the red blood cells, followed by washing with PBS and staining with an anti-mouse lineage cocktail, Sca1, and c-Kit/CD117 antibodies (STEMCELL Technologies) for 30 min. The samples were finally sorted for lin−Sca1-ckit+ cells using a flow cytometer. The pure population of lin−Sca1-ckit+ cells represents HSCs used for the sequencing experiments.
RNA isolation and sequencing
The RNA was extracted from the sorted HSCs using the Trizol-chloroform method followed by DNase treatment in the solution and cleaned up with an RNeasy MinElute clean-up kit from Qiagen. The purity of RNA was confirmed on the Bioanalyzer before proceeding with the sequencing. Only the samples with a RIN (RNA integrity number) of > 7 were used for the sequencing (n = 4 per group). The samples were prepared at the medical genomics core facility (Indiana University) for the paired-end mRNA and single-end miRNA seq library and sequenced on Illumina NovaSeq 6000 with a read length of 100 bp for mRNA and NextSeq 2000 with a read length of 75 bp for miRNA respectively. The mRNA sequence reads were mapped to the designated reference genome (UCSC version mm10; also used as a genome annotation file) using STAR version 2.7.10a (Spliced Transcripts Alignment to a Reference) [15]. Adaptor trimming was not required with the aligner STAR. To evaluate the quality of the RNA-seq data, the number of reads that fall into different annotated regions (exonic, intronic, splicing junction, intergenic, promoter, UTR, etc.) of the reference genes was determined with bamUtils [16]. Low-quality mapped reads (including reads mapped to multiple positions) were excluded, and featureCounts [17] were used to quantify the expression of gene level. The differential gene expression analysis for mRNA was performed with edgeR version 3.42.0 [17]. In this workflow, the statistical methodology uses negative binomial generalized linear models with likelihood ratio tests. The miRNAs raw sequencing reads were subjected to FASTQC for quality control (Table S1). No adaptor trimming was required. The miRNA sequence reads were mapped to the designated reference genome (miRBase_v22 version Mus musculus (GRCm38.101)) using the Qiagen GeneGlobe RNA-seq Analysis Portal version 4.0 with default parameters for miRNA and the differential gene expression analysis for miRNA was performed with edgeR version 3.41.9 [17]. The Benjamini-Hochberg (BH) procedure was used to correct the multiple statistical tests to get the adjusted p-values.
Pathway analysis
Ingenuity pathway analysis (IPA) software (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuity-pathway‐analysis) was used to identify biological/molecular pathways associated with the differentially expressed miRNAs and their targets. Both miRNAs and mRNA data were uploaded to the IPA and were subjected to the threshold filter of p-value − 0.05. Then, a Micro RNA target filter was applied to find the mRNA targets for the differentially expressed miRNAs. The filter selections for our analysis included p < 0.05, experimentally observed interactions, and direct and indirect relationships. mRNA targets from the IPA were overlaid with the mRNA targets from our sequencing database, and finally, pathways analysis was done for those miRNAs and mRNAs. Significant mRNAs (p < 0.05) were also subjected to Gene Ontology and Panther analysis (pantherdb.org).
qRT-PCR for miRNAs and mRNA targets
Total RNA was isolated using TRIzol reagent (Thermo Fisher Scientific) according to the manufacturer’s protocol. 10 ng of total RNA and 1 µg of RNA were reverse transcribed using TaqMan miRNA reverse transcription kit (4366596; Thermo Fisher Scientific) and cDNA Synthesis Kit (SuperScript VILO; Thermo Fisher Scientific) for miRNA and mRNA, respectively. Gene-specific primers were used along with a master mix (TaqMan Fast Universal; Thermo Fisher Scientific) and respective miRNA and mRNA levels were determined using quantitative PCR (Viia7; Thermo Fisher Scientific). All miRNAs were normalized to U6 and the mRNA genes were normalized to TBP, and data was analyzed using ΔΔCt method [18]. Primers used were Gpr89 (Mm00509312_m1), Pla2g4c (Mm01195718_m1), Cxcl12 (Mm00495553_m1), Il10 (Mm01288386_m1), Csf1 (Mm00432686_m1), Itga5 (Mm00439797_m1), Tbp (Mm00446973), mmu-miR-3968 (463749_mat), mmu-miR-1971 (121161_mat), mmu-miR-148a-3p (000470), mmu-miR-126a-5p (4427975) and miRNA U6 (001973).
Statistical analysis
All the data were expressed as Mean + SEM and analyzed using GraphPad Prism 10.0.1 for Windows (San Diego, California; www.graphpad.com). The intergroup comparison was done using a t-test and was considered significant when the p-value was less than 0.05.
Results
The bone-marrow-derived HSCs and diabetes
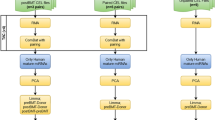
Long-term diabetes adversely affects the bone marrow’s physiological function, including the stem cell niche, [4], however, the transcriptional signature of HSCs under diabetes remains unelucidated. Therefore, we evaluated the pure population of HSCs using miRNA and mRNA sequencing (Fig. 1a). The bone marrow was first subjected to flow cytometric sorting to isolate the LSK cells. Assessment of the pure LSK cell population showed an increase in db/db mice (Fig. 1b), indicating bone marrow mobilopathy with chronic diabetes. The pure population of LSK cells was then used for the transcriptome analysis. Out of 13,708 genes with non-zero total read count; 2076 genes were significantly expressed in HSCs of db/db mice (p < 0.05) (Fig S1a). The volcano plot represents the total genes identified (Fig S1b), whereas the heatmap in Fig. 1c represents significantly changed genes between db/m and db/db mice. Of these significantly changed genes, IPA analysis revealed that 1036 were upregulated, whereas 944 were downregulated in the db/db mice (Fig S1a).
Transcriptome analysis of HSCs in diabetes. (a) Schematic presentation of the steps followed for isolating, sorting, and sequencing HSCs from the bone marrow. (b) The sorted HSCs population was found to be higher in the db/db mice. (c) Heatmap depicting the significantly changed mRNAs between db/m and db/db mice. (d) The representative procedure for the miRNA data analysis. (e) The volcano plot for the total miRNAs found in the sequencing data. The red and blue dots represent the significantly changed miRNAs. (f) The top 35 miRNAs are presented as a heatmap. The data in Figure (b) is presented as mean ± SEM, analyzed using student unpaired t-test; N = 9. The heatmaps in Figures (c) and (f) were plotted using a freely available online tool SRplot (https://www.bioinformatics.com.cn/en)
miRNA sequencing found a total of 191 miRNA, out of which 35 miRNAs were significantly changed in db/db mice (p < 0.05) (Fig. 1d). Figure 1e represents the volcano plot for a total of 191 miRNAs observed from the sequencing, whereas the top 35 miRNAs were presented in a heatmap in Fig. 1f. The differentially expressed miRNAs [35] were then subjected to Ingenuity pathway analysis (IPA) for a deeper understanding of their involvement in various molecular pathways. The core analysis from IPA showed the top 10 upregulated and downregulated miRNAs in db/db mice (Table 1). Micro RNA target filter analysis via IPA software showed that out of 35 significantly changed miRNAs, 28 miRNAs had 1974 mRNA targets. Further, these mRNA targets were traced back to the mRNAs identified in the LSK cells of the present study, which revealed that 446 mRNAs were also present in our mRNA sequencing data; on the other hand, 1528 genes or mRNA targets were unique or were absent in our mRNA sequencing data (Fig S1c). These miRNAs had several downstream targets, some unique to the individual miRNA, and some overlapping between multiple miRNAs (Fig S1d).
Transcriptome analysis of bone-marrow-derived LSK cells in chronic diabetes
Gene ontology using the online available software Panther was performed to investigate the molecular pathways associated with the differential mRNAs in the LSK cells. It was found that most of the genes were associated with inflammation mediated by chemokine and cytokine signalling pathways (Fig. 2a). Interestingly, angiogenesis was also one of the top pathways, with a significantly higher number of genes in the transcriptome of db/db mice’s LSK cells (Fig. 2a). A significant increase in the angiogenic genes, such as Vegfc, Pdgfd, Angpt1, Tgfb1i1, Pla2g4c, and Hif3a(Fig. 2b) was found in the diabetic LSK cells. We also found an increase in the expression of Cxcl12, Cxcl5, and Csf1(Fig. 2c). No significant change was observed in the expression level of Cxcr4 in LSK cells (Fig. 2c). To study inflammatory changes, we investigated our mRNA data for major inflammation-related genes, including Toll-like receptors (TLRs), interleukins (ILs), and tumour necrosis factor (TNF). The anti-inflammatory cytokine Il10 was significantly downregulated in the HSCs of db/db mice (Fig. 2d). In contrast, a significantly higher expression of Il4, Tlr4, and Tnf11α (Fig. 2e) was found in the HSCs of db/db mice.
Transcriptome analysis revealed the gene level changes in the HSCs of db/db mice. The significant changes in mRNAs were analyzed using Gene ontology/Panther to uncover the top pathways. (a) The pathways with the highest number of differentially expressed genes in the HSCs of db/db and db/m mice. Individual pathways analysis showed increased expression of (b) angiogenesis, (c) differentiation, and migration of HSCs-related genes. (d) The anti-inflammatory gene IL-10 was significantly downregulated in the db/db mice, (e) whereas there was an increase in the expression level of pro-inflammatory genes in db/db mice
Overexpression of inflammatory pathways in the HSCs of db/db mice
For further pathway analysis, we chose 446 genes that were miRNA targets and were also present in our mRNA sequencing database. Interestingly, out of 446 genes, 90 were targeted by multiple miRNAs (Fig. 3). These target mRNAs could be of more potential as their regulation is controlled by more than one miRNA. We ran a core pathway analysis on significantly changed miRNAs to investigate the potential pathways associated with differentially expressed miRNAs. The top 5 miRNAs (both upregulated and downregulated) (Table 1) were further subjected to the network analysis and overlaid with the mRNA from our sequencing database. Since our group has previously shown the role of miR-92 in diabetic retinopathy [19, 20], we also included miR-92 (fold change − 1.577 and p = 0.0065) for the network analysis. Lastly, these top miRNAs were subjected to the IPA pathway analysis.
Alluvial plot illustrating overlapped mRNA targets between multiple miRNAs. MicroRNA target filter analysis revealed a total of 1974 mRNA targets, which were then overlaid with the significantly expressed mRNA targets. 446 mRNA targets were common among two datasets, of which 206 mRNAs were targeted by multiple miRNAs, as shown in the Figure. This Figure was plotted using a freely available online tool SRplot (https://www.bioinformatics.com.cn/en)
IPA pathway and network analysis showed that each miRNA was targeting inflammation-related pathways. miRNAs are a known transcriptional regulator, which can bind to the mRNA’s 3’-UTR regions, leading to their degradation. With the differential expression of the top regulated miRNAs [miR-874-3p, miR-30c-5p, miR-3968 and miR-148a-3p (Fig. 4a-d); and miR-1-3p, miR-423-5p, miR-22-3p, miR-574-5p, and miR-92a-3p (Fig S2-S4) of the LSK cells, we found an increase in the expression of downstream targets like colony-stimulating factor 1 (CSF1), Kruppel-like factors (Klf6, Klf4, and Klf2), integrins (Itga5, Itga9, Itgav, Itgb3), and collagen (Col1a1, Col1a2, Col5a1, Col4a2) all of which held the potential to stimulate the chemotaxis, differentiation and proliferation of bone-marrow-derived monocytes/macrophages and microglia. The top canonical pathways affected by these miRNAs and their respective targets turned out to be macrophage alternative activation signalling, neuroinflammation, IL-4 signalling, TGF-β signalling, CCR5 signalling in macrophages, leukocyte extravasation signalling, VEGF family ligand-receptor interactions, neurovascular coupling signalling, neutrophil extracellular trap signalling, phagosome formation, WNT/Ca+ signalling, IL-12 signalling, natural killer cell signalling and CREB signalling in neurons (Fig. 4a and Fig S2, 3, 4). The IPA suggested a shift to the pro-inflammatory phenotype of HSCs in the db/db mice at the transcriptional level, which in turn is largely regulated by the miRNAs.
Top pathway analysis for miRNAs reflected increased inflammation-related genes in db/db mice. Top miRNAs were analyzed using IPA software for their downstream targets and associated pathways. (a) miR-874-3p and (b) miR-30c-5p were upregulated in the HSCs of db/db mice, which were traced back to leukocyte extravasation, neuroinflammation, cytokine signaling, and macrophage signaling. (c) and (d) miR-3968 and miR-148a-3p were top downregulated miRNAs, targeting VEGF signaling, formation, proliferation, and migration of bone marrow-derived macrophages and monocytes, recruitment, and migration of microglia, neuroinflammation and degeneration of photoreceptors. The data was uploaded to the IPA, and miRNAs and mRNAs with a p < 0.05 were used for the miRNA target filter and core pathway analysis
RT-PCR validation of top miRNAs and their targets
Finally, we validated two of the novel miRNAs observed in the sequencing data. Consistent with the sequencing data, we found the downregulation of miR-3968 and miR-1971 in db/db mice (Fig. 5a). Next, we also checked the qPCR expression of miR-148a-3p (top-downregulated miRNA), and miR-126a-5p (reported to be downregulated in chronic diabetes). As observed in our sequencing data, miR-126a-5p was upregulated, and miR-148a-3p was downregulated in LSK cells of db/db mice (Fig. 5a). Since these miRNAs were significantly affected, it was interesting to investigate the expression patterns of their mRNA targets. The top mRNA targets, such as Pla2g4c (miR-3968 target) and Gpr89a/b (miR-1971 target), significantly changed in gene expression analysis (Fig. 5b). Csf1 and Itga5 (miR-148a-3p target) were found to be upregulated; however, this difference was non-significant for Csf1 (Fig. 5b). Further, the gene expression of Il-10 (an anti-inflammatory gene from Fig. 2b) was significantly downregulated and Cxcl12 (participates in HSCs mobilization) was also found to be significantly upregulated in db/db mice (Fig. 5b)
Validation of novel miRNAs and mRNA using qPCR in db/db mice. The top 3 downregulated miRNAs (miR-3968, miR-1971 & and miR-148a-3p), miR-126a-5p, and their mRNA targets were analyzed using real-time quantitative PCR (qRT-PCR). (a) Gene expression analysis of miR-3968, miR-1971, miR-148a-3p, and miR-126a-5p normalized to U6 (b) Gene expression analysis of mRNA targets normalized to TBP. The figure is presented as a SuperPlot generated using GraphPad Prism, where solid dots represent biological replicates (mean ± SEM; N = 5) and small square boxes represent technical replicates. The data are analyzed on technical replicates using student unpaired t-test (N = 5*3 = 15)
Discussion
In the present study, we have investigated the transcriptional signature of bone marrow-derived HSCs under chronic diabetes, which has not been explored before. These transcriptional changes can, therefore, change the fate of circulatory HSCs. Through our transcriptional profiling, we identified two novel miRNAs (miR-3968 and miR-1971) and a distinguished set of downstream mRNAs targeting pro-inflammatory, leukocyte migration, and angiogenesis-related pathways in HSCs of diabetic animals with the potential to participate in diabetes-induced vascular complications.
Bone marrow-derived HSCs are widely studied and used as therapeutics for malignant blood disorders due to their outstanding capacity to regenerate the hemato-lymphoid system [21]. In the last two decades, multiple studies have reflected the negative outcome of diabetes on the HSCs, in terms of bone marrow function, release and migration ability of HSCs, and decreased circulation in the peripheral blood [22]. It is well-appreciated now that chronic diabetes can lead to an impaired endothelial repair system and promote stem cell mobilopathy and neuropathy [5, 23,24,25]. Consistent with the previous literature, db/db animals in the present study had a significantly higher number of entrapped HSCs in the bone marrow [25]. Stem cell mobilization is largely dependent on the CXCL12-CXCR4 signalling, where the CXCL12 upregulation is linked to impaired stem cell trafficking/mobilopathy in diabetes [26, 27]. The higher expression of Cxcl12, with the simultaneous increase in the entrapped HSCs, reflected the inability of the HSCs to be released peripherally in diabetic animals.
The present study is the first of this kind, where the pure LSK cells were sorted and utilized to investigate diabetes-induced transcriptional changes in the db/db mice. Further, there is growing evidence that miRNAs play a crucial role in the transcriptional regulation of enormous molecular targets associated with metabolic disorders, including diabetes or diabetes-induced vascular complications [28,29,30,31,32,33]. However, it is noteworthy that most of the above studies have been done on circulatory or tissue-specific miRNAs, and no data is available for the HSCs miRNAs. Therefore, we investigated the LSK miRNA and mRNA targets due to their ability to change the fate of the stem cells being circulated and used for vascular repair. We found 35 significantly changed miRNAs in the LSK cells of db/db mice, with 25 being downregulated and 10 being upregulated. Interestingly, our sequencing data has uncovered 2 novel miRNAs (miR-3968, and miR-1971), which have never been reported before for any involvement with diabetes or diabetes-induced microvascular complications; previously, miR-3968 was downregulated in gestational diabetes [34] and our study presented the similar change in the LSK cells of diabetic mice. Additionally, our RT-PCR validation supported the expression pattern of these two novel miRNAs being downregulated in chronic diabetes. There were several miRNAs consistent with the reported literature, including miR-1-3p [35, 36], miR-30c-5p [37,38,39], miR-101-3p [40, 41], miR-181a-5p [42, 43], miR-340-5p [44], miR-29b-3p [43, 45, 46], miR-423-5p [47] and miR-22-3p [12, 48, 49]. Contrarily, we found three miRNAs (miR-126a-5p and miR-126a-3p [33, 50]; and miR-148a-3p [50] inconsistent with the reported miRNAs in terms of their expression pattern. Intriguingly, our quantitative real-time PCR analysis also reflected the downregulation of miR-148a-3p and upregulation of miR-126a-5p, with no change in miR-126a-3p expression. Since this study is distinguishable from the available literature due to the use of HSCs, the miRNAs observed in the data hold great potential irrespective of their expression difference with the previous studies.
The miRNAs target several downstream pathways that can manifest the fate of HSCs, to explore this phenomenon, we further analyzed the top miRNAs. We found multiple miRNAs and their downstream mRNAs targeting inflammatory and angiogenic pathways. One of the top targets of miR-3968, which is Pla2g4c, was significantly upregulated under chronic diabetes. Phospholipase A2 (PLA2) is a family of lipolytic enzymes that can produce lipid pro-inflammatory substances such as free fatty acids (FFA) that participate in inflammatory response. PLA2 family activation has been reported in diabetic retinopathy [51], diabetic nephropathy [52, 53], and cardiovascular complications. A significant upregulation of Pla2g4c in LSK cells of diabetic animals suggested the involvement of lipid-induced inflammatory changes, which could be explored further. Notably, platelet-derived growth factor (PDGF) also activates phospholipases, releasing prostaglandins [53]. Our present transcriptome analysis reflected these observations, where a simultaneous upregulation of Pdgf and Pla2g4c was found, potentially corresponding to the gonadotrophin-releasing hormone receptor pathways. The downstream target of miR-1971 was GPR89a/b, which has not been studied so far for its direct role in diabetes or diabetes-induced vascular complications. Herein, we could not validate the downregulation of Gpr89a/b with RT-PCR.
Another target is Kruppel-like factors (KLFs); a family of transcription factors that regulate macrophage differentiation and migration under the influence of inflammatory signals [54]. Interestingly, there was a significant increase in the expression levels of Klf4 and Klf6 in the LSK cells of diabetic animals, possibly contributing to the migration of inflammatory monocytes/macrophages [55,56,57]. Moreover, we also found a decrease in the expression of miR-92a-3p in the LSK cells, which was previously shown to be decreased in the angiogenic cells of diabetic retinopathy individuals [58] and retinas of diabetic mice [59]. Also, it has been shown that interactions of miR-92a-3p with KLF2 and KLF4 can modulate inflammatory macrophage signaling [60, 61], thus highlighting the possible involvement of miR-92a-3p regulated inflammatory pathways in diabetic HSCs. Likewise, studies have shown the role of colony-stimulating factor 1 (CSF1) in microglia activation and inflammatory cytokine secretion [62]. We also found a higher expression of the Csf1 gene in diabetic animals, thus promoting microglia activation and inflammation. A higher expression of pro-inflammatory genes Tlr4, Il1α, and Tnf11α and a decrease in the expression levels of anti-inflammatory cytokine Il10, in the LSK cells, also suggested a shift to inflammatory phenotype [63, 64] in diabetic HSCs. Moreover, the Ccl4 gene, which regulates macrophage activation, chemotaxis, and migration, was expressed more strongly. A simultaneous increase of these genes in the LSK cells of db/db mice marks the initiation of inflammation at transcriptional levels in the stem cells.
In addition to the changes in the inflammatory genes, we found several angiogenic genes being upregulated in the LSK cells of db/db mice. Vascular endothelial growth factor (VEGF) is a widely studied angiogenic factor with multiple isoforms [65]. VEGF-C, primarily known for lymphangiogenesis, has also been reported to play a part in pathological angiogenesis [66]. An increase in the gene expression of Vegfc in db/db mice reflects the angiogenic fate of HSCs.
Conclusion
In conclusion, we have observed HSCs mobilopathy and adverse effects on the transcriptome profile of LSK cells in chronically diabetic animals. Several miRNAs and mRNA targets reflected a shift to the inflammatory and angiogenic fate of bone-marrow-derived HSCs in diabetes. The investigation of HSCs transcriptome is clinically very relevant as these cells not only participate in the pathogenesis of disease but also play an important role in hematopoiesis and vascular repair. We believe that the present study has strengthened the current knowledge of diabetes-induced HSC dysfunction at the transcriptional levels, with the potential to contribute to vascular damage. Identifying novel miRNAs and their targets could be a potential biomarker for diabetes-induced vasculature deficits such as retinopathy. However, further studies are required to investigate the molecular mechanisms linking the HSCs miRNAs or mRNA targets to the progression of vascular disease in diabetes.
Data availability
Transcriptional data generated in this study was deposited in National Center for Biotechnology Information Gene Expression Omnibus (GEO) database under accession no. GSE261501 (mRNA data) and GSE261502 (miRNA data).
Abbreviations
- LSK:
-
Lin−Sca1+c-Kit+ cells
- HSCs:
-
Hematopoietic Stem Cells
- miRNA:
-
Micro RNA
- T2D:
-
Type 2 Diabetes
- IPA:
-
Ingenuity pathway analysis
- TLRs:
-
Toll-like receptors
- ILs:
-
Interleukins
- TNF:
-
Tumour necrosis factor
- KLFs:
-
Kruppel-like factors
- CSF1:
-
Colony-stimulating factor 1
- VEGF:
-
Vascular endothelial growth factor
References
Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract [Internet]. 2022 [cited 2023 Aug 28];183. https://doi.org/10.1016/j.diabres.2021.109119
IDF. IDF Diabetes Atlas 2021 _ IDF Diabetes Atlas. IDF Off website [Internet]. 2021 [cited 2023 Aug 28];1–4. https://diabetesatlas.org/atlas/tenth-edition/
Hanoun M, Frenette PS. This niche is a maze; an amazing niche. Cell Stem Cell. 2013;12(4):391–2.
Vinci MC, Gambini E, Bassetti B, Genovese S, Pompilio G. When good guys turn bad: bone marrow’s and hematopoietic stem cells’ role in the pathobiology of diabetic complications. Int J Mol Sci. 2020;21(11):1–22.
Fadini GP, Albiero M. Impaired hematopoietic Stem/Progenitor cell traffic and multi-organ damage in diabetes. Stem Cells. 2022;40(8):716–23.
Paul S, Ali A, Katare R. Molecular complexities underlying the vascular complications of diabetes mellitus – A comprehensive review. J Diabetes Complications [Internet]. 2020;34(8):107613. https://doi.org/10.1016/j.jdiacomp.2020.107613
Albiero M, Bonora BM, Fadini GP. Diabetes pharmacotherapy and circulating stem/progenitor cells. State art Evid gaps. 55, Current Opinion in Pharmacology. 2020.
Fadini GP, Ciciliot S, Albiero M. Concise Review: perspectives and clinical implications of bone marrow and circulating stem cell defects in diabetes. 35, Stem Cells. 2017.
Tonyan ZN, Nasykhova YA, Danilova MM, Barbitoff YA, Changalidi AI, Mikhailova AA et al. Overview of Transcriptomic Research on Type 2 Diabetes: Challenges and Perspectives. Vol. 13, Genes. 2022.
Lin Q, Zhou W, Wang Y, Huang J, Hui X, Zhou Z et al. Abnormal peripheral neutrophil transcriptome in newly diagnosed type 2 diabetes patients. J Diabetes Res. 2020;2020.
Corbi SCT, de Vasconcellos JF, Bastos AS, Bussaneli DG, da Silva BR, Santos RA et al. Circulating lymphocytes and monocytes transcriptomic analysis of patients with type 2 diabetes mellitus, dyslipidemia and periodontitis. Sci Rep. 2020;10(1).
He X, Kuang G, Wu Y, Ou C. Emerging roles of exosomal miRNAs in diabetes mellitus. Clin Transl Med. 2021;11(6).
Liang Z, Gao KP, Wang YX, Liu ZC, Tian L, Yang XZ, et al. RNA sequencing identified specific circulating mirna biomarkers for early detection of diabetes retinopathy. Am J Physiol - Endocrinol Metab. 2018;315(3):E374–85.
Smit-McBride Z, Morse LS. MicroRNA and diabetic retinopathy—biomarkers and novel therapeutics. Ann Transl Med [Internet]. 2021 [cited 2023 Aug 28];9(15):1280–1280. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8421969/
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics [Internet]. 2013 Jan [cited 2024 Jun 11];29(1):15–21. https://pubmed.ncbi.nlm.nih.gov/23104886/
Breese MR, Liu Y. NGSUtils: a software suite for analyzing and manipulating next-generation sequencing datasets. Bioinformatics [Internet]. 2013 Feb 15 [cited 2024 Jun 11];29(4):494–6. https://doi.org/10.1093/bioinformatics/bts731
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics [Internet]. 2010 Nov 11 [cited 2024 Jun 11];26(1):139–40. https://pubmed.ncbi.nlm.nih.gov/19910308/
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods. 2001.
Luo Q, Bhamidipalli SS, Eckert GJ, Bhatwadekar AD. Hypermethylation of miRNA-17-92 cluster in peripheral blood mononuclear cells in diabetic retinopathy. Diabetes Metab Syndr Clin Res Rev. 2022;16(2).
Luo Q, Hajrasouliha AR, Bhatwadekar AD. miR-92a and integrin expression in fibrovascular membranes in proliferative diabetic retinopathy. Front Ophthalmol. 2023;3.
Epah J, Schäfer R. Implications of hematopoietic stem cells heterogeneity for gene therapies. Vol. 28, Gene Therapy. 2021.
Fadini GP, Sartore S, Agostini C, Avogaro A. Significance of endothelial progenitor cells in subjects with diabetes. Vol. 30, Diabetes Care. 2007.
Fadini GP, Albiero M, De Kreutzenberg SV, Boscaro E, Cappellari R, Marescotti M et al. Diabetes impairs stem cell and proangiogenic cell mobilization in humans. Diabetes Care. 2013;36(4).
Bhatwadekar AD, Duan Y, Korah M, Thinschmidt JS, Hu P, Leley SP et al. Hematopoietic stem/progenitor involvement in retinal microvascular repair during diabetes: implications for bone marrow rejuvenation. Vis Res. 2017;139.
Busik JV, Tikhonenko M, Bhatwadekar A, Opreanu M, Yakubova N, Caballero S et al. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med. 2009;206(13).
Fadini GP, Fiala M, Cappellari R, Danna M, Park S, Poncina N et al. Diabetes limits stem cell mobilization following G-CSF but not plerixafor. Diabetes. 2015;64(8).
Ferraro F, Lymperi S, Méndez-Ferrer S, Saez B, Spencer JA, Yeap BY et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med. 2011;3(104).
Zampetaki A, Mayr M. MicroRNAs in vascular and metabolic disease. 110, Circul Res. 2012.
Kolfschoten IGM, Roggli E, Nesca V, Regazzi R. Role and therapeutic potential of microRNAs in diabetes. Diabetes, Obes Metab. 2009;11(SUPPL. 4).
Ismail A, El-Mahdy HA, Eldeib MG, Doghish AS. miRNAs as cornerstones in diabetic microvascular complications. Volume 138. Molecular Genetics and Metabolism; 2023.
Weale CJ, Matshazi DM, Davids SFG, Raghubeer S, Erasmus RT, Kengne AP et al. Circulating miR-30a-5p and miR-182-5p in Prediabetes and Screen-Detected Diabetes Mellitus. Diabetes Metab Syndr Obes [Internet]. 2020 [cited 2024 Jun 11];13:5037–47. https://pubmed.ncbi.nlm.nih.gov/33376373/
Mensà E, Giuliani A, Matacchione G, Gurău F, Bonfigli AR, Romagnoli F et al. Circulating miR-146a in healthy aging and type 2 diabetes: Age- and gender-specific trajectories. Mech Ageing Dev [Internet]. 2019 Jun 1 [cited 2024 Jun 11];180:1–10. https://pubmed.ncbi.nlm.nih.gov/30880174/
Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M et al. Plasma MicroRNA profiling reveals loss of endothelial MiR-126 and other MicroRNAs in type 2 diabetes. Circ Res. 2010;107(6).
Dong D, Zhang Y, Reece EA, Wang L, Harman CR, Yang P. microRNA expression profiling and functional annotation analysis of their targets modulated by oxidative stress during embryonic heart development in diabetic mice. Reprod Toxicol [Internet]. 2016 Oct 1 [cited 2024 Jun 11];65:365. /pmc/articles/PMC5288404/
Morales-Sánchez P, Lambert C, Ares-Blanco J, Suárez-Gutiérrez L, Villa-Fernández E, Garcia AV et al. Circulating miRNA expression in long-standing type 1 diabetes mellitus. Sci Rep. 2023;13(1).
Liu J, Chen S, Biswas S, Nagrani N, Chu Y, Chakrabarti S et al. Glucose-induced oxidative stress and accelerated aging in endothelial cells are mediated by the depletion of mitochondrial SIRTs. Physiol Rep. 2020;8(3).
Mazzeo A, Beltramo E, Lopatina T, Gai C, Trento M, Porta M. Molecular and functional characterization of circulating extracellular vesicles from diabetic patients with and without retinopathy and healthy subjects. Exp Eye Res. 2018;176.
Dong N, Wang Y. MiR-30a regulates S100A12-induced retinal microglial activation and inflammation by targeting NLRP3. Curr Eye Res. 2019;44(11).
Pordzik J, Jakubik D, Jarosz-Popek J, Wicik Z, Eyileten C, De Rosa S et al. Significance of circulating microRNAs in diabetes mellitus type 2 and platelet reactivity: Bioinformatic analysis and review. Vol. 18, Cardiovascular Diabetology. 2019.
Higuchi C, Nakatsuka A, Eguchi J, Teshigawara S, Kanzaki M, Katayama A et al. Identification of circulating miR-101, miR-375 and miR-802 as biomarkers for type 2 diabetes. Metabolism. 2015;64(4).
Santos AS, Cunha Neto E, Fukui RT, Ferreira LRP, Silva MER. Increased expression of circulating microRNA 101-3p in type 1 diabetes patients: New insights into miRNA-Regulated pathophysiological pathways for type 1 diabetes. Front Immunol. 2019;10.
Cao J, Zhao C, Gong L, Cheng X, Yang J, Zhu M et al. MiR-181 enhances proliferative and migratory potentials of retinal endothelial cells in Diabetic Retinopathy by Targeting KLF6. Curr Eye Res. 2022;47(6).
The Profiling. and Role of miRNAs in diabetes Mellitus. J Diabetes Clin Res. 2019;1(1).
Zhu Y, Yang X, Zhou J, Chen L, Zuo P, Chen L et al. MiR-340-5p Mediates Cardiomyocyte Oxidative Stress in Diabetes-Induced Cardiac Dysfunction by Targeting Mcl-1. Oxid Med Cell Longev. 2022;2022.
Zeng Y, Cui Z, Liu J, Chen J, Tang S. MicroRNA-29b-3p promotes human retinal microvascular endothelial cell apoptosis via blocking SIRT1 in Diabetic Retinopathy. Front Physiol. 2020;10.
Karolina DS, Armugam A, Tavintharan S, Wong MTK, Lim SC, Sum CF et al. Correction: MicroRNA 144 impairs insulin signaling by inhibiting the expression of insulin receptor substrate 1 in type 2 diabetes Mellitus. PLoS ONE. 2011;6(9).
Blum A, Meerson A, Rohana H, Jabaly H, Nahul N, Celesh D et al. MicroRNA-423 may regulate diabetic vasculopathy. Clin Exp Med. 2019;19(4).
Zhou L, Li FF, Wang SM. Circ-ITCH restrains the expression of MMP-2, MMP-9 and TNF-α in diabetic retinopathy by inhibiting miR-22. Exp Mol Pathol. 2021;118.
Parker DC, Wan M, Lohman K, Hou L, Nguyen AT, Ding J et al. Monocyte miRNAs are Associated with type 2 diabetes. Diabetes. 2022;71(4).
Seyhan AA, Nunez Lopez YO, Xie H, Yi F, Mathews C, Pasarica M et al. Pancreas-enriched miRNAs are altered in the circulation of subjects with diabetes: a pilot cross-sectional study. Sci Rep. 2016;6.
Siddiqui MK, Kennedy G, Carr F, Doney ASF, Pearson ER, Morris AD et al. Lp-PLA2 activity is associated with increased risk of diabetic retinopathy: a longitudinal disease progression study. Diabetologia [Internet]. 2018 Jun 1 [cited 2024 Jun 11];61(6):1344. /pmc/articles/PMC6447502/
Zhai Y, Cao X, Liu S, Shen Y. The diagnostic value of lipoprotein-associated phospholipase A2 in early diabetic nephropathy. Ann Med [Internet]. 2023 [cited 2024 Jun 11];55(2):2230446. /pmc/articles/PMC10424594/
Boi R, Ebefors K, Henricsson M, Borén J, Nyström J. Modified lipid metabolism and cytosolic phospholipase A2 activation in mesangial cells under pro-inflammatory conditions. Sci Reports 2022 121 [Internet]. 2022 May 5 [cited 2024 Jun 11];12(1):1–13. https://www.nature.com/articles/s41598-022-10907-4
Cao Z, Sun X, Icli B, Wara AK, Feinberg MW. Role of Krüppel-like factors in leukocyte development, function, and disease. Vol. 116, Blood. 2010.
Alder JK, Georgantas RW, Hildreth RL, Kaplan IM, Morisot S, Yu X et al. Kruppel-like factor 4 is essential for inflammatory monocyte differentiation in vivo. J Immunol. 2008;180(8).
Feinberg MW, Wara AK, Cao Z, Lebedeva MA, Rosenbauer F, Iwasaki H, et al. The Kruppel-like factor KLF4 is a critical regulator of monocyte differentiation. EMBO J. 2007;26:18.
Date D, Das R, Narla G, Simon DI, Jain MK, Mahabeleshwar GH. Kruppel-like transcription factor 6 regulates inflammatory macrophage polarization. J Biol Chem. 2014;289(15).
Bhatwadekar AD, Yan Y, Stepps V, Hazra S, Korah M, Bartelmez S, Chaqour B, Grant MB. miR-92a Corrects CD34+ Cell Dysfunction in Diabetes by Modulating Core Circadian Genes Involved in Progenitor Differentiation. Diabetes. 2015 Dec;64(12):4226-37. doi: 10.2337/db15-0521. Epub 2015 Aug 17. PMID: 26283734; PMCID: PMC4876760.
Kovacs B, Lumayag S, Cowan C, Xu S. microRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Investig Ophthalmol Vis Sci [Internet]. 2011 [cited 2023 Aug 28];52(7):4402–9. https://iovs.arvojournals.org/article.aspx?articleid=2187854
Fang Y, Davies PF. Site-specific microRNA-92a regulation of Krüppel-like factors 4 and 2 in atherosusceptible endothelium. Arterioscler Thromb Vasc Biol. 2012;32(4).
Georgantas RW, Hildreth R, Morisot S, Alder J, Liu CG, Heimfeld S et al. CD34 + hematopoietic stem-progenitor cell microRNA expression and function: a circuit diagram of differentiation control. Proc Natl Acad Sci U S A. 2007;104(8).
Kokona D, Ebneter A, Escher P, Zinkernagel MS. Colony-stimulating factor 1 receptor inhibition prevents disruption of the blood-retina barrier during chronic inflammation. J Neuroinflammation. 2018;15(1).
Yue T, Shi Y, Luo S, Weng J, Wu Y, Zheng X. The role of inflammation in immune system of diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Front Immunol. 2022;13.
D’alessandra Y, Chiesa M, Vigorelli V, Ricci V, Rurali E, Raucci A et al. Diabetes induces a transcriptional signature in bone marrow– derived CD34 + hematopoietic stem cells predictive of their progeny dysfunction. Int J Mol Sci. 2021;22(3).
Melincovici CS, Boşca AB, Şuşman S, Mărginean M, Mihu C, Istrate M et al. Vascular endothelial growth factor (VEGF) – key factor in normal and pathological angiogenesis. 59, Rom J Morphol Embryol. 2018.
Nagai N, Minami T. Emerging Role of VEGFC in Pathological Angiogenesis. Vol. 2, EBioMedicine. 2015.
Acknowledgements
We would also like to thank Flow Cytometry core and the Centre for Medical Genomics (CMG) core of Indiana University for helping us with the cell sorting and sequencing experiments.
Funding
The authors would like to acknowledge the funding support from the National Institute of Health (NIH)—National Eye Institute (NEI) grants, R01EY027779, R01EY027779-S1 and R01EY032080 to AB. Additionally, the Department of Ophthalmology is supported by a Challenge Grant from Research to Prevent Blindness (RPB).
Author information
Authors and Affiliations
Contributions
Conceptualization: AB, NM; Validation: NM, AB; Formal Analysis: NM, AB; Investigation: NM, QL; Resources: AB; Data Curation: NM, QL, SA; Writing-Original Draft: NM; Writing-Review & Editing: AB, NM; Visualization: NM, AB; Supervision: QL, AB; Project Administration: AB, QL, NM; Funding Acquisition: AB.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC), Indiana University [Approval no. 21061]. We declare that all the experiments performed were per the Guiding Principles in the Care and Use of Animals (National Institutes of Health) and in compliance with the ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
AB is an ad hoc District Support Pharmacist at CVS Health/Aetna. The contents of this study do not reflect those of CVS Health/Aetna. NM, QL, and SA do not have any conflicts to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Mahajan, N., Luo, Q., Abhyankar, S. et al. Transcriptomic Profile of Lin−Sca1+c-kit (LSK) cells in db/db mice with long-standing diabetes. BMC Genomics 25, 782 (2024). https://doi.org/10.1186/s12864-024-10679-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-024-10679-3