Abstract
Background
The DELLA proteins, a class of GA signaling repressors, belong to the GRAS family of plant-specific nuclear proteins. Members of DELLA gene family encode transcriptional regulators with diverse functions in plant development and abiotic stress responses. To date, DELLAs have been identified in various plant species, such as Arabidopsis thaliana, Malus domestica, Populus trichocarpa, and other land plants. Most information of DELLA family genes was obtained from A. thaliana, whereas little is known about the DELLA gene family in blueberry.
Results
In this study, we identified three DELLA genes in blueberry (Vaccinium darrowii, VdDELLA) and provided a complete overview of VdDELLA gene family, describing chromosome localization, protein properties, conserved domain, motif organization, and phylogenetic analysis. Three VdDELLA members, containing two highly conserved DELLA domain and GRAS domain, were distributed across three chromosomes. Additionally, cis-acting elements analysis indicated that VdDELLA genes might play a critical role in blueberry developmental processes, hormone, and stress responses. Expression analysis using quantitative real-time PCR (qRT-PCR) revealed that all of three VdDELLA genes were differentially expressed across various tissues. VdDELLA2 was the most highly expressed VdDELLA in all denoted tissues, with a highest expression in mature fruits. In addition, all of the three VdDELLA genes actively responded to diverse abiotic stresses. Based on qRT-PCR analysis, VdDELLA2 might act as a key regulator in V. darrowii in response to salt stress, whereas VdDELLA1 and VdDELLA2 might play an essential role in cold stress response. Under drought stress, all of three VdDELLA genes were involved in mediating drought response. Furthermore, their transiently co-localization with nuclear markers in A. thaliana protoplasts demonstrated their transcriptional regulator roles.
Conclusions
In this study, three VdDELLA genes were identified in V. darrowii genome. Three VdDELLA genes were closely related to the C. moschata DELLA genes, S. lycopersicum DELLA genes, and M. domestica DELLA genes, respectively, indicating their similar biological functions. Expression analysis indicated that VdDELLA genes were highly efficient in blueberry fruit development. Expression patterns under different stress conditions revealed the differentially expressed VdDELLA genes responding to salt, drought, and cold stress. Overall, these results enrich our understanding of evolutionary relationship and potential functions of VdDELLA genes, which provide valuable information for further studies on genetic improvement of the plant yield and plant resistance.
Similar content being viewed by others
Background
DELLA proteins are plant-specific transcriptional regulators belonging to a subfamily of the GRAS family [1]. The name was coined based on the basis of a short stretch of amino acids, named D-E-L-L-A, which is highly conserved in its N-terminal region [2]. DELLA and VHYNP domains, and polymeric Ser/Thr/Val (poly S/T/V) motifs constitute the unique N-terminal “DELLA” domain [3], which is tightly conserved among all plant species and is required for gibberellic acid (GA) responses [1]. GA, an extremely important endogenous phytohormone, promotes diverse growth and developmental processes by overcoming growth restraint mediated by DELLAs [1]. The C-terminal region is conserved among all GRAS family members, containing two leucine heptad repeats (LHR1 and LHR2), a putative nuclear localization signals (NLS), and three conserved motifs: VHIID, Src-homology 2 like (SH2-like), and SAW [1]. The LHRs are essential for protein homodimerization, DELLA activity, and GA-dependent DELLA degradation [1]. Specifically, the HLR1 motif mediates the interaction between DELLAs and TFs [1], VHIID and SAW are necessary for maintaining the functions of DELLA [4] To date, DELLAs have been identified in various land plant species, such as five genes in Arabidopsis thaliana [5], seven genes in Glycine max [6], four genes in Populus trichocarpa [7], one gene in Solanum lycopersicum [8], one gene in Vitis vinifera [9], six genes in Malus domestica [10], two genes in Lactuca sativa [11], one gene in Capsicum annuum [12], and seven genes in Cucurbita moschata [13]. DELLA proteins are well-known key negative regulators of the GA signaling pathway [1]. All the information encoded by GAs is canalized through DELLAs, which modulate the activity of many transcription factors and transcriptional regulators through protein-protein interactions [14]. Therefore, DELLAs participate not only in plant growth and development, but also in abiotic stress responses. Several studies have demonstrated that DELLAs regulate plant height, stem elongation, flowering, root meristem, axillary bud formation, fruit development, seed germination, hypocotyl elongation, apical hook development, and abiotic stress responses [1].
As one of the five major healthy foods, blueberry is a highly attractive emerging crop species due to their enriched antioxidant phytochemical content, and is recognized as the “king of the world fruit” [15]. To date, global blueberry cultivation zones, cultivated areas, and yields have increased significantly. The northern and southern highbush constitute the majority of blueberry production worldwide [16]. However, blueberry production also presents many challenges, as it varies by variety or genotype, regional adaptability, and environmental conditions. For example, the oxygen radical absorbance capacity (ORAC) and phenolics content in highbush blueberry leaf tissues are significantly higher than those in fruit tissue [17]. In addition, adverse abiotic environmental conditions such as salt, drought, cold, heat, and nutrient deficiencies limit the worldwide utilization of arable lands and negatively affect productivity [18]. In recent years, salt stress, drought stress, extreme temperatures, and flooding have limited the blueberry growth and survival. Therefore, it is importance to elucidate the stress response mechanism of blueberry for enhancing the stress resistance of Vaccinium horticultural crops. Most information of DELLA family genes was obtained from the studies in A. thaliana, S. lycopersicum, and Oryza sativa. In A. thaliana, DELLA proteins interact with SPL9 and attenuate the repressing activity of SPL9, promoting the initiation of axillary buds [19]. Fruit development can be regulated by DELLA proteins. In S. lycopersicum, SlDELLA interacted with SlARF7/SlIAA9 to regulate fruit initiation [20], and silencing of DELLA resulted in parthenocarpic fruits, which were smaller with a distinctive elongated shape when mature [21]. In O. sativa, plants overexpressing SLR1 exhibited increased tiller number, whereas SLR1 knock-down plants showed decreased tiller number [22]. Previously, studies have shown that DELLA proteins participate in abiotic stress response and improve the plant survival by regulating reactive oxygen species (ROS) levels during adverse environments [23, 24]. However, little is known about the DELLA gene family in blueberry fruit development and stress response. To investigate the role of DELLA proteins in these processes, we have identified DELLA-encoding genes from blueberry. There is potential to improve berry size, yield, and stress resistance in commercial blueberry production by introducing genetic variants of VdDELLAs into new cultivars.
In this study, three members of VdDELLA genes were identified and analyzed by phylogenetic relationship, subcellular localization, protein structure, and cis-elements in the V. darrowii genome. Additionally, we characterized expression patterns of VdDELLA genes in six different tissues, leaves, stems, flowers, small fruits, big fruits, and mature fruits by qRT-PCR. Expression patterns under different stress conditions such as salt, drought, and cold were also analyzed using qRT-PCR. These results will provide valuable information for further studies on the multiple functions of the DELLA proteins in V. darrowii and genetic modification toward developing V. darrowii variants with increased yield and stress tolerance.
Results
Genome-wide identification and chromosomal localization of DELLA family members in V. darrowii
Based on the recently sequenced V. darrowii genome [16], three VdDELLA members harboring DELLA and GRAS domains were identified from the V. darrowii genome and named VdDELLA1 to VdDELLA3 (Fig. 1). The coding sequence (CDS) lengths of VdDELLA genes ranged from 1626 bp (VdDELLA3) to 1746 bp (VdDELLA2) (Table 1). Their corresponding proteins ranged from 541 to 581 aa, with a molecular weight varying from 59.24 kDa (VdDELLA2) to 63.77 kDa (VdDELLA3) (Table 1). Moreover, the isoelectric point (pI) values of VdDELLA proteins ranged from 4.99 (VdDELLA2) to 5.7 (VdDELLA3), which showed that these proteins were highly acidic. Subcellular localization analysis of the VdDELLA proteins showed that they were localized to the nucleus, which revealed their transcriptional regulator role. (Table 1). We further analyzed the chromosomal location of VdDELLA genes. Three VdDELLA genes were distributed on 2, 7, and 8 chromosomes of V. darrowii, respectively (Fig. 2), suggesting that they underwent duplication events.
Conserved domains, motifs, and phylogenetic analysis of VdDELLA family
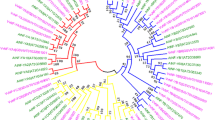
All VdDELLA proteins contained an N-terminal DELLA domain and a C-terminal GRAS domain (Fig. 3A and B). The DELLA domain consisted of two motifs (motif 1 and motif 2), whereas eight motifs (motif 3 to motif 10) constituted the GRAS domain (Fig. 3C). To investigate the evolutionary relationship among DELLAs from V. darrowii, A. thaliana, G. max, P. trichocarpa, S. lycopersicum, V. vinifera, M. domestica, L. sativa, C. annuum, and C. moschata, a phylogenetic tree was constructed. All of DELLA genes were divided into four groups (Fig. 4). Group I consisted of fifteen members, Group II consisted of ten members, Group III consisted of five members, and Group IV contained eight members. VdDELLA1, VdDELLA2, and VdDELLA3 were belonged to Groups I, II, and IV, respectively. As shown in phylogenetic tree, DELLA proteins from V. darrowii exhibited the conserved evolutionary relationship with those from S. lycopersicum, L. sativa, and M. domestica. In Group I, VdDELLA1 clustered with LsDELLA1 and LsDELLA2. In Group II, VdDELLA2 clustered with SlDELLA. In Group IV, VdDELLA3 clustered with MdRGL3a and MdRGL3b. The phylogenetic tree can provide some indications for the putative roles of VdDELLA genes in blueberry. For instance, the evolutionary relationship of VdDELLA2 with SlDELLA revealed the functional prediction of VdDELLA2 in fruit development [20].
Conserved domains and motifs in VdDELLA proteins. (A) The conserved domains of VdDELLA proteins. DELLA domain and GRAS domain are represented by yellow and green box, respectively. (B) Sequence logos of conserved domains in VdDELLA protein sequences. (C) The conserved motifs of VdDELLA proteins. Colored boxes indicate different motifs
Cis-acting regulatory elements in the promoter region of VdDELLA genes
Transcriptional regulation plays an important role in the activation and suppression of gene expression [25]. Cis-acting elements in gene promoters are specific binding sites for proteins involved in the initiation and regulation of transcription [26]. To explore VdDELLA genes transcriptional regulation networks in blueberry, we analyzed the promoter sequences of VdDELLA genes. The analysis showed that cis-acting elements were mainly divided into hormone response, stress response, and developmental elements (Fig. 5). Among them, stress response elements were the most abundant, whereas developmental elements had the lowest abundance. Stress response elements containing ARE (for anoxic specific inducibility), LTR (for low temperature responsiveness), MBS and MYC (for drought-inducibility), STRE (for heat inducibility), TC-rich repeats (for defense and stress responsiveness) and WUN-motif (for wound responsiveness). Notably, VdDELLA1 promoter contained all the response elements. There are several known hormone response elements in the VdDELLA gene promoter, including ABRE, which is involved in the response to ABA; CGTCA-motif and TGACG-motif, which are known to respond to methyl jasmonic acid (MeJA); ERE, which is well known as the ethylene (ETH) response element; P-box, which is required for involvement in the response to GA; TCA-element, which is involved in the response to salicylic acid (SA); and TGA-element, which is required for auxin-responsiveness. Only four development elements (A-box, AAGAA-motif, CAT-box, and circadian) were distributed in the VdDELLA gene promoter. Interestingly, there was no developmental element in the promoter region of VdDELLA1, whereas the promoter region of VdDELLA2 contained only one developmental element.
Cis-acting elements in the promoters of VdDELLA genes. (A) The types of promoter cis-acting elements of VdDELLA genes. The different colored boxes represent the different types and positions of cis-acting elements in each VdDELLA genes. (B) The numbers of promoter cis-acting elements of VdDELLA genes
Expression patterns of VdDELLA genes in different tissues
The DELLA family is involved in multiple aspects of plant growth and development [1]. To explore the role of VdDELLA genes in blueberry growth and development, we evaluate the contribution of VdDELLA genes in leaves, stems, flowers, small fruits, big fruits, and mature fruits using qRT-PCR. According to these findings, the expression levels of VdDELLA genes were predominantly high in blueberry fruits (Fig. 6). VdDELLA2 and VdDELLA3 expression in mature fruits was significantly higher than in the other five tissues, and VdDELLA1 was highly expressed in small fruits. In contrast, the stems and flowers exhibited relatively low expression levels. Noticeably, VdDELLA2 expressions in all denoted tissues were the most pronounced among the VdDELLAs. These results showed the tissue-specific expression patterns of the DELLA genes in multiple blueberry tissues, indicating the functional divergence of DELLA genes in the growth and development of blueberry.
Expression patterns of the VdDELLA genes in the different tissues. (A) Samples of leaves, stems, flowers, small fruits, big fruits and mature fruits were collected from three-year-old ‘O’Neal’ plants. (B) VdDELLA gene expression patterns in leaves, stems, flowers, small fruits, big fruits, and mature fruits. Data are means of three replicates ± SD. Relative expression of VdDELLA1, VdDELLA2, and VdDELLA3 in flowers was set to 1.00. Asterisks indicate significant differences compared with flowers (*P < 0.05, **P < 0.01, *** P < 0.001, two-tailed Student’s t-test)
Expression profiles of VdDELLA genes under abiotic stress
Arabidopsis DELLA proteins integrate responses to independent hormonal and environmental signals under adverse conditions [27]. To explore the role of VdDELLA genes in the blueberry response to abiotic stress, we first evaluated the changes in their expression patterns under salt (200 mM NaCl), drought, and cold (4 °C) stress, respectively. VdDELLA2 gene showed the higher expression under all tested stresses, whereas VdDELLA3 gene exhibited the lowest expression. The expressions of all three VdDELLA genes showed an initial downregulation trend with salt, drought, and cold treatments, respectively (Fig. 7). However, the expression level of VdDELLA2 gene showed a trend of first decreased, then increased and then decreased expression after treatment with 200 mM NaCl (Fig. 7A). At 5 d post-treatment, VdDELLA2 gene had the highest expression levels, compared with normal condition, suggesting VdDELLA2 gene play a critical role in response to salinity stress. Under drought stress, all three VdDELLA genes exhibited an initial downregulation followed by almost stable expression (Fig. 7B), suggesting that VdDELLA genes play an important role in the negative regulation in response to drought stress. Additionally, VdDELLA1 and VdDELLA2 expressions were increased by 4.2- and 2.7-fold, respectively, in response to cold stress after treatment for 48 h (Fig. 7C). Specifically, VdDELLA3 exhibited a continuous downward trend over time under salt, drought, and cold stress, implying that VdDELLA3 may function as a negative regulator in response to salt, drought, and cold stress, respectively. These results suggest the involvement of DELLA genes in the response of blueberry to salt, drought, and cold stress.
Expression profiles of VdDELLA genes under salt, drought and cold stress. (A) VdDELLA gene expression patterns under salt stress. (B) VdDELLA gene expression patterns under drought stress. (C) VdDELLA gene expression patterns under cold stress. Heat map was created by TBtools using the relative expression values. Data are means of three replicates ± SD. The red and blue cells represent the highest and lowest expression levels, respectively
Subcellular localization of VdDELLA proteins
According to subcellular prediction analysis, VdDELLA proteins were found to be localized in the nucleus. To validate subcellular localization, 35S: VdDELLA1-GFP, 35S: VdDELLA2-GFP, and 35S: VdDELLA3-GFP vectors were constructed. Additionally, NLS-RFP was used as a nuclear marker. The co-localization of VdDELLA1-GFP, VdDELLA2-GFP, and VdDELLA3-GFP with NLS-RFP in Arabidopsis protoplasts was analyzed, respectively. We found that VdDELLA1-GFP, VdDELLA2-GFP, and VdDELLA3-GFP exhibited obvious co-localization with NLS-RFP in the nucleus (Fig. 8).
Discussion
DELLA proteins are a well-known class of GA signaling repressors. They also regulate plant growth and development as well as stress response. DELLAs are widely distributed in dicots and monocots. To date, DELLA genes have been identified and characterized in crops, horticultural plants, and woody plants. Nevertheless, the DELLAs of V. darrowii are unknown. Based on the recently sequenced V. darrowii genome [16], three non-redundant DELLA proteins distributed on three chromosomes were identified from V. darrowii genome (Figs. 1 and 2). Several species possess a single DELLA gene, such as PROCERA in S. lycopersicum [8], L1 in V. vinifera [9], LsDELLA1 in L. sativa [11], and CaGAI in C. annuum [12], whereas the DELLA genes in other plants have undergone multiplication [2]. For instance, DELLAs are encoded by four genes in P. trichocarpa [7], A. thaliana has five DELLA-encoding genes [5], ten DELLA genes are distributed in B. napus genome [28], and seven DELLA genes exist in the C. moschata genome [13]. Although the V. darrowii genome is 4.5-fold larger than the A. thaliana genome (560.5 Mb and 125 Mb, respectively), the gene number in V. darrowii is only three fifths that of A. thaliana, suggesting that there might be gene loss during genome duplication. All of VdDELLA proteins consist of N-terminal DELLA domain and C-terminal GRAS domain, which are two highly conserved domains of the DELLA family in plants and shared ten conserved motifs (Fig. 3). Interestingly, motif numbers and their composition between VdDELLAs were evenly distributed, which were inconsistent with the studies had been found in BnDELLA and CmoDELLA proteins, whereas motif numbers between BnDELLA and CmoDELLA proteins were unevenly distributed [13, 28], implying the conserved functions of VdDELLA proteins in blueberry. Moreover, the prediction of subcellular localization showed that VdDELLA proteins were mainly localized in the nucleus. Subsequently, their transiently co-localization with nuclear markers in Arabidopsis protoplasts demonstrated the subcellular location of VdDELLA proteins. All of three VdDELLA proteins apparently co-localized with the nuclear marker (Fig. 8), indicating their transcriptional regulator role. Homologous genes in different species are presumed to perform similar biological functions [29]. Phylogenetic analysis showed that VdDELLA proteins of blueberry shared higher similarity with those of S. lycopersicum, L. sativa, and M. domestica (Fig. 4), providing some indications for the putative roles of VdDELLA genes in blueberry. For instance, the evolutionary relationship of VdDELLA2 with SlDELLA revealed the functional prediction of VdDELLA2 in fruit development [20].
Various interactions between cis-acting elements and transcription factors function as molecular switches for transcription to determine transcription initiation events [30]. We found five phytohormone-related cis-acting elements, seven types of cis-acting elements related to stress response, and four types of developmental elements in VdDELLA genes promoter regions (Fig. 5). Previous reports have shown that DELLA proteins act as modulators of plant growth, by interacting with a diversity of regulatory proteins that function in various signaling pathways [31], such as GA, ABA, MeJA, SA, ETH, brassinosteroids (BRs), and auxin [1]. VdDELLA proteins contained ABRE, ERE, P-box, CGTCA-motif, TGACG-motif, TCA-element, and TGA-element, suggesting that VdDELLA genes might be responsible for plant development and stress response in blueberry by hormone signal pathway. Similar findings were also reported in M. domestica [10], G. max [6], B. napus [28], and C. moschata [13]. In B. napus, the BnaDELLA gene family contains a wide variety of stress and defense-related cis-elements compared to development, light, and hormone-responsive cis-elements [28]. Consistently, we also observed that both the types and the amount of stress-related cis-acting elements were the highest, suggesting the VdDELLA genes diverse function in response to various abiotic stresses. Accordingly, we speculate that VdDELLA proteins might be responsible for multiple environmental and hormonal signals in blueberry development.
Subsequently, we investigated VdDELLA gene expression patterns in different tissues under normal condition to understand their contribution to blueberry development. The results showed that VdDELLA genes were all expressed in leaves, stems, flowers, small fruits, big fruits, and mature fruits, and exhibited the highest expression levels in fruits. In particular, VdDELLA2 were abundantly expressed in small fruits and mature fruits, indicating that VdDELLA2 might play a significant role in fruits development and ripening. Recently, the study had reported that four of the seven C. moschata DELLA genes (CmoDELLA1, CmoDELLA4, CmoDELLA5, and CmoDELLA6) were found to be mainly expressed in fruits and associated with fruits development [13]. Furthermore, CmoDELLA1 exhibited significantly up-regulated in the pollinated fruits than that in the ovaries without pollination [32]. DELLA proteins have reported to interact with CUC2 transcription factor to promote ovule initiation [33]. Based on the expression pattern of VdDELLA genes, we speculate that VdDELLA genes are highly efficient in blueberry fruit development. Gene overexpression and gene knockout of individual VdDELLA will be essential to verify their functions.
DELLA proteins are also involved in abiotic and biotic stress responses [13, 28]. The growth restraint conferred by DELLA proteins is beneficial and promotes survival [27]. Previous studies have shown that overaccumulation of DELLA proteins enhances not only salt tolerance but also cold tolerance, which causes plant fitness improvements [34]. In Camellia sinensis, the transcriptome data analysis showed that CsDELLA genes participated in response to NaCl, drought, and cold stress [35]. In B. napus, the expression patterns of BnaDELLAs varied upon salt, drought, and 4 ℃ treatments, and were associated with stress tolerance improvements [28]. In C. moschata, CmoDELLA genes mediate the stress response of pumpkin to NaCl, waterlogging, and cold [13]. Consistently, we also found VdDELLA genes were involved in response to salt, drought, and cold stress, and exhibited distinct expression patterns (Fig. 7). Induced expression was observed during salt and cold treatment. As shown in Fig. 7, VdDELLA2 showed the highly increased expression after 5 d of salt treatment (Fig. 7A), suggesting VdDELLA2 might be crucial in response to salt stress. VdDELLA1 and VdDELLA2 were significantly induced under cold stress after 48 h (Fig. 7C), indicating VdDELLA1 and VdDELLA2 might played critical roles in response to cold stress. In contrast, VdDELLA genes exhibited its reduced expression in response to drought treatment (Fig. 7B), which were consistent with studies that have been found in BnaRGL3 [28]. Taken together, VdDELLA genes might participate in the stress response of blueberry to salt, drought, and cold. However, further studies are required to explore the function of VdDELLA genes under stress condition. Moreover, it is very important to understand the molecular mechanism underling the crosstalk between VdDELLA genes and phytohormone signal transduction pathways under stress condition.
Conclusions
In this study, three VdDELLA genes were identified in V. darrowii genome. Three VdDELLA genes were closely related to the C. moschata DELLA genes, S. lycopersicum DELLA genes, and M. domestica DELLA genes, respectively, indicating their similar biological functions. Expression analysis indicated that VdDELLA genes were highly efficient in blueberry fruit development. Expression patterns under different stress conditions revealed the differentially expressed VdDELLA genes responding to salt, drought, and cold stress. Overall, these results enrich our understanding of evolutionary relationship and potential functions of VdDELLA genes, which provide valuable information for further studies on genetic improvement of the plant yield and plant resistance.
Methods
Identification of VdDELLA gene family members from blueberry genome
Five AtDELLA proteins from A. thaliana were downloaded from the TAIR database (https://www.arabidopsis.org/) [36]. To identify DELLA gene family members in V. darrowii, AtDELLA proteins were used as query sequences to perform online BLAST search against V. darrowii database (https://phytozome-next.jgi.doe.gov/blast-search) [16]. Then, the protein sequences of V. darrowii were downloaded from V. darrowii database (https://phytozome-next.jgi.doe.gov/) [16]. The Hidden Markov Model (HMM) profile of the DELLA domain (PF12041) was downloaded from the Pfam database (http://pfam.xfam.org/). HMMER3.0 program (http://hmmer.org/) [37] was used to perform hidden Markov model searches for candidate proteins containing the DELLA domain. All the candidate protein sequences gained with the above two methods were submitted to CDD (http://ncbi.nlm.nih.gov/cdd) [38] in NCBI database (https://www.ncbi.nlm.nih.gov/) [38] to reconfirm the VdDELLA proteins, and redundant proteins were manually removed. Finally, molecular weight (Mw) and theoretical isoelectric point (pI) of VdDELLAs were predicted utilizing the online software ExPASy (https://web.expasy.org/protparam/) [39]. The subcellular location information was predicted using the online software WoLF PSORT (https://wolfpsort.hgc.jp/) [40] .
Structure of VdDELLA proteins, phylogenetic and chromosome location analysis
The conserved domains were visualized with TBtools software [41]. The protein sequences of A. thaliana [5], G. max [6], P. trichocarpa [7], S. lycopersicum [8], V. vinifera [9], M. domestica [10], L.sativa [11], C. annuum [12] and C. moschata [13] were obtained from TAIR database, Soybase database (https://www.soybase.org/), Phytozome13 database (https://phytozome-next.jgi.doe.gov/), NCBI database (https://www.ncbi.nlm.nih.gov/), Ensembl database (http://plants.ensembl.org/index.html), and cucurbit genomics database (http://cucurbitgenomics.org/), respectively. Based on multiple sequence alignment, a neighbor-joining phylogenetic tree was generated using MEGA 7.0 software [42] with 1000 bootstrap replicates. The resulting phylogenetic tree was beautified with the online tool Evolview v3 [43] (https://evolgenius.info/). MEME program (http://meme-suite.org) [44] was used to detect the conserved motifs of VdDELLA proteins, and the protein structures of them were visualized with TBtools software [41]. The chromosomal distribution of VdDELLA genes was determined from the annotation file (GFF3), and visualized with using TBtools software [41].
Cis-acting element analysis
The promoter sequences of the VdDELLA genes (2000 bp DNA sequence upstream of the start codon ATG) were downloaded from Phytozome database (https://phytozome.jgi.doe.gov/pz/portal.html). Cis-acting elements in the promoter region were analyzed using online PLANTCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/), and visualized using TBtools software [41].
Plant materials and stress treatments
‘O’Neal’ plants were obtained from the Ludong University, Yantai, China by Prof. Hongxia Zhang. Seedlings from cuttings of a single ‘O’Neal’ clone were used for this study. Plants were grown in a growth chamber at 25 °C under a photoperiod of 16 h light and 8 h darkness. Leaves, stems, flowers, small fruits, big fruits, and mature fruits samples were collected from three-year-old ‘O’Neal’ plants. One-year-old ‘O’Neal’ seedlings were subjected to 200 mM NaCl, drought, and 4 °C stress, respectively. Leaves were sampled at a set time points after treatments, immediately frozen in liquid nitrogen and stored at -80 °C for further analyses.
Quantitative Real-Time PCR analysis
Total RNA was extracted from all samples using the FastPure Universal Plant Total RNA Isolation Kit (Vazyme, Nanjing, China) according to the manufacturer’s protocol. First-strand cDNA was synthesized using the HiScript III 1st Strand cDNA Synthesis Kit (+ gDNA wiper) (Vazyme, Nanjing, China). RT-qPCR was performed on a CFX Connect Real-Time System (Bio-Rad) using Cham Q Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) with 10 µl PCR products. Relative gene expression values were analyzed using the 2−ΔΔCt method (each sample has three biological replicates, and each biological replicate includes three technical replicates). Tubulin beta 8 (Vda04G014670.1) [16] was used as a reference gene. Heat maps of gene expression levels were visualized with TBtools software [41]. All primer sequences were listed in Table S1.
Subcellular location analysis
Primers containing HindIII restriction sites were designed according to the CDS sequence of VdDELLA genes (removing the termination codon), and the fusion expression vectors were constructed using the Clone Express®II One Step Cloning Kit (Vazyme, Nanjing, China). Finally, the recombinant constructs (35S: VdDELLA1-GFP, 35S: VdDELLA2-GFP, and 35S: VdDELLA3-GFP) and nuclear marker plasmids (NLS-RFP) were then co-transformed into Arabidopsis protoplasts, isolating from Arabidopsis suspension culture cells. Arabidopsis suspension culture cells were obtained from the Chinese University of Hong Kong, China by Prof. Liwen Jiang. Images were taken using an LSM-710 confocal microscope (Zeiss) equipped with an argon/krypton laser.
Statistical analysis
All data were analyzed using Origin 8. A two-tailed Student’s t-test was used for statistical analysis.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. A. thaliana gene sequence data from this article can be found in the TAIR database (https://www.arabidopsis.org/) under the following accession numbers: AtGAI (At1g14920), AtRGA (At2g01570), AtRGL1 (At1g66350), AtRGL2 (At3g03450), and AtRGL3 (At5g17490); G. max gene sequence data from this article can be found in the Soybase database (https://www.soybase.org/) under the following accession numbers: GmDELLA1 (Glyma.11G216500.1), GmDELLA2 (Glyma.18G040000.1), GmDELLA3 (Glyma.08G095800.1), GmDELLA4 (Glyma.05G140400.1), GmDELLA5 (Glyma.04G150500.1), GmDELLA6 (Glyma.06G213100.1), and GmDELLA7 (Glyma.10G190200.1); C. moschata gene sequence data from this article can be found in the cucurbit genomics database (http://cucurbitgenomics.org/) under the following accession numbers: CmoDELLA1 (CmoCh01G003940.1), CmoDELLA2 (CmoCh04G022200.1), CmoDELLA3 (CmoCh04G023970.1), CmoDELLA4 (CmoCh11G005830.1), CmoDELLA5 (CmoCh14G008330.1), CmoDELLA6 (CmoCh15G007670.1), and CmoDELLA7 (CmoCh15G010000.1); P. trichocarpa and V. vinifera gene sequence data from this article can be found in the Phytozome13 database (https://phytozome-next.jgi.doe.gov/) under the following accession numbers: PtRGA (Potri.008G131700), PtRGA2 (Potri.010G110700), PtRGL1 (Potri.004G089800), PtRGL2 (Potri.017G125200), VvDELLA1 (VIT_201s0011g05260.1), VvDELLA2 (VIT_214s0006g00640.1), and VvDELLA3 (VIT_211s0016g04630.1); M. domestica and L.sativa gene sequence data from this article can be found in the NCBI database (https://www.ncbi.nlm.nih.gov/) under the following accession numbers: MdRGL1a (DQ007885), MdRGL1b (DQ007886), MdRGL2a (DQ007883), MdRGL2b (DQ007884), MdRGL3a (DQ007887), MdRGL3b (DQ007887), LsDELLA1 ( XM_023877838.3), and LsDELLA2 (XM_023886497.3); S. lycopersicum and C. annuum gene sequence data can be found in the Ensembl database (http://plants.ensembl.org/index.html) under the accession numbers: SlDELLA (Solyc11g011260.1) and CaGAI (PHT64689).
References
Xue HD, Gao X, He P, Xiao GH. Origin, evolution, and molecular function of DELLA proteins in plants. Crop J. 2022. https://doi.org/10.1016/j.cj.2021.06.005.
Locascio A, Blazquez MA, Alabadi D. Genomic analysis of DELLA protein activity. Plant Cell Physiol. 2013. https://doi.org/10.1093/pcp/pct082.
Sun TP, Gubler F. Molecular mechanism of gibberellin signaling in plants. Annu Rev Plant Biol. 2004. https://doi.org/10.1146/annurev.arplant.55.031903.141753.
Hirano K, Asano K, Tsuji H, Kawamura M, Mori H, Kitano H, Ueguchi-Tanaka M, Matsuoka M. Characterization of the molecular mechanism underlying gibberellin perception complex formation in rice. Plant Cell. 2010. https://doi.org/10.1105/tpc.110.075549.
Tyler L, Thomas SG, Hu J, Dill A, Alonso JM, Ecker JR, Sun TP. Della proteins and gibberellin-regulated seed germination and floral development in Arabidopsis. Plant Physiol. 2004. https://doi.org/10.1104/pp.104.039578.
Liang S, Chen QS, Zhu ZK, Li DD, Qi ZM, Xin DW. Identification and analysis of soybean DELLA gene family. Chin J Oil Crop Sci. 2022. https://doi.org/10.19802/j.issn.1007-9084.2021224.
Fan D, Ran L, Hu J, Ye X, Xu D, Li J, Su H, Wang X, Ren S, Luo K. miR319a/TCP module and DELLA protein regulate trichome initiation synergistically and improve insect defenses in Populus tomentosa. New Phytol. 2020. https://doi.org/10.1111/nph.16585.
Shohat H, Illouz-Eliaz N, Kanno Y, Seo M, Weiss D. The tomato DELLA protein PROCERA promotes abscisic acid responses in guard cells by upregulating an abscisic acid transporter. Plant Physiol. 2020. https://doi.org/10.1104/pp.20.00485.
Boss PK, Thomas MR. Association of dwarfism and floral induction with a grape ‘green revolution’ mutation. Nature. 2002. https://doi.org/10.1038/416847a.
Foster T, Kirk C, Jones WT, Allan AC, Espley R, Karunairetnam S, Rakonjac J. Characterisation of the DELLA subfamily in apple (Malus domestica Borkh). Tree Genet Genomes. 2007. https://doi.org/10.1007/s11295-006-0047-z.
Sawada Y, Umetsu A, Komatsu Y, Kitamura J, Suzuki H, Asami T, Fukuda M, Honda I, Mitsuhashi W, Nakajima M, et al. An unusual spliced variant of DELLA protein, a negative regulator of gibberellin signaling, in lettuce. Biosci Biotech Bioch. 2012. https://doi.org/10.1271/bbb.110847.
Cao YC, Zhang ZH, Wang LH, Sul XL, Zhang ZX, Zhang BX. Cloning and characterization of CaGID1s and CaGAI in Capsicum annuum L. J Integr Agr. 2016. https://doi.org/10.1016/S2095-3119(15)61275-8.
Luo WR, Zhao ZX, Chen HZ, Ao WH, Lu L, Liu JJ, Li XZ, Sun YD. Genome-wide characterization and expression of DELLA genes in Cucurbita moschata reveal their potential roles under development and abiotic stress. Front Plant Sci. 2023. https://doi.org/10.3389/fpls.2023.1137126.
Blanco-Touriñán N, Serrano-Mislata A, Alabadí D. Regulation of DELLA proteins by post-translational modifications. Plant Cell Physiol. 2020. https://doi.org/10.1093/pcp/pcaa113.
Duan Y, Tarafdar A, Chaurasia D, Singh A, Bhargava PC, Yang J, Li Z, Ni X, Tian Y, Li H, et al. Blueberry fruit valorization and valuable constituents: a review. Int J Food Microbiol. 2022. https://doi.org/10.1016/j.ijfoodmicro.2022.109890.
Cui FQ, Ye XX, Li XX, Yang YF, Hu ZB, Overmyer K, Brosché M, Yu H, Salojärvi J. Chromosome-level genome assembly of the diploid blueberry provides insights into its subtropical adaptation and cuticle synthesis. Plant Commun. 2022. https://doi.org/10.1016/j.xplc.2022.100307.
Ehlenfeldt MK, Prior RL. Oxygen radical absorbance capacity (ORAC) and phenolic and anthocyanin concentrations in fruit and leaf tissues of highbush blueberry. J Agric Food Chem. 2001. https://doi.org/10.1021/jf0013656.
Zhang HM, Zhu JH, Gong ZZ, Zhu JK. Abiotic stress responses in plants. Nat Rev Genet. 2022. https://doi.org/10.1038/s41576-021-00413-0.
Zhang QQ, Wang JG, Wang LY, Wang JF, Wang Q, Yu P, Bai MY, Fan M. Gibberellin repression of axillary bud formation in Arabidopsis by modulation of DELLA-SPL9 complex activity. J Integr Plant Biol. 2020. https://doi.org/10.1111/jipb.12818.
Hu JH, Israeli A, Ori N, Sun TP. The Interaction between DELLA and ARF/IAA mediates crosstalk between gibberellin and auxin signaling to control fruit initiation in tomato. Plant Cell. 2018. https://doi.org/10.1105/tpc.18.00363.
Martí C, Orzáez D, Ellul P, Moreno V, Carbonell J, Granell A. Silencing of DELLA induces facultative parthenocarpy in tomato fruits. Plant J. 2007. https://doi.org/10.1111/j.1365-313X.2007.03282.x.
Liao Z, Yu H, Duan J, Yuan K, Yu C, Meng X, Kou L, Chen M, Jing Y, Liu G, et al. SLR1 inhibits MOC1 degradation to coordinate tiller number and plant height in rice. Nat Commun. 2019. https://doi.org/10.1038/s41467-019-10667-2.
Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P. The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell. 2008. https://doi.org/10.1105/tpc.108.058941.
Achard P, Renou JP, Berthomé R, Harberd NP, Genschik P. Plant DELLAs restrain growth and promote survival of adversity by reducing the levels of reactive oxygen species. Curr Biol. 2008. https://doi.org/10.1016/j.cub.2008.04.034.
Zou C, Sun K, Mackaluso JD, Seddon AE, Jin R, Thomashow MF, Shiu SH, S A. Cis- regulatory code of stress-responsive transcription in Arabidopsis thaliana. Proc Natl Acad Sci U. 2011. https://doi.org/10.1073/pnas.1103202108.
Hernandez-Garcia CM, Finer JJ. Identification and validation of promoters and cis-acting regulatory elements. Plant Sci. 2014. https://doi.org/10.1016/j.plantsci.2013.12.007.
Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP. Integration of plant responses to environmentally activated phytohormonal signals. Science. 2006. https://doi.org/10.1126/science.1118642.
Sarwar R, Jiang T, Ding P, Gao Y, Tan X, Zhu K. Genome-wide analysis and functional characterization of the DELLA gene family associated with stress tolerance in B. Napus. BMC Plant Biol. 2021. https://doi.org/10.1186/s12870-021-03054-x.
Cai H, Tian S, Dong H. Large scale in silico identification of MYB family genes from wheat expressed sequence tags. Mol Biotechnol. 2012. https://doi.org/10.1007/s12033-011-9486-3.
Yamaguchi-Shinozaki K, Shinozaki K. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters. Trends Plant Sci. 2005. https://doi.org/10.1016/j.tplants.2004.12.012.
Xu H, Liu Q, Yao T, Fu X. Shedding light on integrative GA signaling. Curr Opin Plant Biol. 2014. https://doi.org/10.1016/j.pbi.2014.06.010.
Luo WR, Li YY, Sun YD, Lu L, Zhao ZX, Zhou JG, Li XZ. Comparative RNA-seq analysis reveals candidate genes associated with fruit set in pumpkin. Sci Hortic-Amsterdam. 2021. https://doi.org/10.1016/j.scienta.2021.110255.
Barro-Trastoy D, Gomez MD, Blanco-Touriñán N, Tornero P, Perez-Amador MA. Gibberellins regulate ovule number through a DELLA-CUC2 complex in Arabidopsis. Plant J. 2022. https://doi.org/10.1111/tpj.15607.
Yang DLDW, Zhang YY, He ZH. Gibberellins modulate abiotic stress tolerance in plants. Sci Sin. 2013. https://doi.org/10.1360/052013-321.
Han YXDH, Zheng ST, Tong HR, Yuan LY. Identification and expression analysis of the DELLA gene family in camellia sinensis (L.) O. Ktze. Plant Sci J. 2020. https://doi.org/10.11913/PSJ.2095-0836.2020.50644.
Reiser L, Subramaniam S, Zhang P, Berardini T. Using the Arabidopsis Information Resource (TAIR) to find information about Arabidopsis genes. Curr Protoc Bioinf. 2022. https://doi.org/10.1002/cpz1.574.
Finn RD, Clements J, Eddy SR. HMMER web server: interactive sequence similarity searching. Nucleic Acids Res. 2011. https://doi.org/10.1093/nar/gkr367.
Marchler-Bauer A, Derbyshire MK, Gonzales NR, Lu S, Chitsaz F, Geer LY, Geer RC, He J, Gwadz M, Hurwitz DI, et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015. https://doi.org/10.1093/nar/gku1221.
Gasteiger E, Gattiker A, Hoogland C, Ivanyi I, Appel RD, Bairoch A, ExPASy. The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res. 2003. https://doi.org/10.1093/nar/gkg563.
Horton P, Park KJ, Obayashi T, Fujita N, Harada H, Adams-Collier CJ, Nakai K. WoLF PSORT: protein localization predictor. Nucleic Acids Res. 2007. https://doi.org/10.1093/nar/gkm259.
Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R, TBtools. An integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020. https://doi.org/10.1016/j.molp.2020.06.009.
Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016. https://doi.org/10.1093/molbev/msw054.
Subramanian B, Gao S, Lercher MJ, Hu S, Chen WH. Evolview v3: a webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019. https://doi.org/10.1093/nar/gkz357.
Bailey TL, Johnson J, Grant CE, Noble WS. The MEME suite. Nucleic Acids Res. 2015. https://doi.org/10.1093/nar/gkv416.
Acknowledgements
We are grateful for the support of the National Natural Science Foundation of China for this project.
Funding
This research was supported by the National Natural Science Foundation of China (Grant No. 32001330).
Author information
Authors and Affiliations
Contributions
Conception: Houjun Zhou and Lei Yang. Interpretation or analysis of data: Houjun Zhou, Yanwen Wang, Xinyu Wang, and Rui Cheng. Preparation of the manuscript: Houjun Zhou, Yanwen Wang, Xinyu Wang, and Rui Cheng. Revision for important intellectual content: Houjun Zhou, Hongxia Zhang, and Lei Yang. Supervision: Houjun Zhou and Lei Yang. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable. The sampling of plant material was performed in compliance with institutional guidelines. The research conducted in this study required neither approval from an ethics committee, nor involved any human or animal subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
12864_2024_10721_MOESM1_ESM.xlsx
Supplementary Material 1: Table S1 Protein sequences used for Phylogenetic analysis. Table S2 Primer sequences used for analyses.
12864_2024_10721_MOESM2_ESM.xlsx
Supplementary Material 2: Table S3 Expression levels of VdDELLAs genes in the different tissues. Table S4 Expression levels of VdDELLAs genes under salt stress. Table S5 Expression levels of VdDELLAs genes under drought stress. Table S6 Expression levels of VdDELLAs genes under cold stress
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhou, H., Wang, Y., Wang, X. et al. Genome-wide characterization of DELLA gene family in blueberry (Vaccinium darrowii) and their expression profiles in development and response to abiotic stress. BMC Genomics 25, 815 (2024). https://doi.org/10.1186/s12864-024-10721-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-024-10721-4