Abstract
Background
Recombination signal-binding protein for immunoglobulin kappa J region (RBPJ) is a transcriptional regulator that plays an important role in maintaining immune homeostasis. This study aimed to estimate the expression of RBPJ in rheumatoid arthritis (RA) patients and investigate its relationship with RA.
Methods
A total of 83 newly diagnosed RA patients and 70 healthy controls were included. mRNA was extracted from peripheral blood mononuclear cells (PBMCs), and the expression of RBPJ was detected using quantitative real-time PCR (qRT‒PCR). An RA dataset (GSE89408) was obtained from the Gene Expression Omnibus (GEO) database, and RA synovial tissues were divided into two groups. The differentially expressed genes (DEGs) were selected with the “DESeq2” R package.
Results
RBPJ expression was lower in RA patients than in health controls and was negatively correlated with the DAS28 score, C-reactive protein (CRP) level and erythrocyte sedimentation rate (ESR). RA synovial tissues from GSE89408 were classified into RBPJ-low (≤ 25%) and RBPJ-high (≥ 75%) groups according to RBPJ expression, and 562 DEGs were identified. Gene Ontology (GO) enrichment analyses revealed that the DEGs significantly affected the regulation of T cell activation and lymphocyte/mononuclear cell differentiation. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis revealed that the most enriched pathways of DEGs were the T cell receptor signaling pathway, Th1/2 and Th17 cell differentiation, the PI3K − Akt signaling pathway and cytokine‒cytokine receptor interaction. CytoHubba Plugin revealed that most of the top 10 genes were involved in osteoclast differentiation, the T cell receptor signaling pathway and cytokine‒cytokine receptor interaction.
Conclusions
RBPJ expression was significantly lower in RA patients and negatively correlated with disease activity. GEO dataset analysis demonstrated that RBPJ may be involved in osteoclast differentiation, T cell activation and differentiation, and the T cell receptor signaling pathway. Our research may contribute to understanding the potential mechanisms by which RBPJ regulates T cell differentiation and cytokine‒cytokine receptor interaction in RA patients.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disease that primarily affects the joints and has the highest incidence rate among autoimmune diseases [1]. During the progression of RA, a cellular immune imbalance develops in the synovial tissue. This imbalance in the immune system causing persistent synovial hyperplasia and inflammation, cell infiltration, production of proinflammatory cytokine, and persistent articular cartilage destruction, which ultimately leads to joint pain and loss of function. The levels of proinflammatory cytokines and chemokines are reportedly increased in the plasma of RA patients [2].
The Notch signaling pathway coordinates numerous cellular processes, including immune homeostasis, cell differentiation, proliferation, and apoptosis [3, 4]. As a transcriptional regulator of Notch signaling, recombination signal-binding protein for immunoglobulin kappa J region (RBPJ) plays a key role in maintaining cellular differentiation and homeostasis [5]. Several studies in RBPJ−/− mice have shown that RBPJ is involved in the function and development of Th17 cells, CD4+ T cells, macrophages, and other myeloid cells [6,7,8]. Research has revealed the transcriptional repression of RBPJ in the bone marrow microenvironment, and RBPJ deletion disrupts hematopoietic homeostasis and leads to the upregulation of multiple inflammatory cytokines [9]. Increased osteoclastogenesis and abnormal generation of osteoclasts lead to joint destruction in RA [10, 11]. Research has shown that RBPJ plays a crucial role in suppressing inflammatory bone resorption, osteoclast differentiation, and osteoclastogenesis [12]. Stahl’s group identified RBPJ allelic polymorphisms associated with RA susceptibility through a genome-wide association study (GWAS) [13, 14], but the mechanisms of RBPJ in the progression and pathogenesis of RA are still unclear.
In this study, we collected fresh peripheral blood from newly diagnosed RA patients and healthy controls, and then extracted peripheral blood mononuclear cells (PBMCs). The expression of RBPJ mRNA in PBMCs was detected through quantitative real-time PCR (qRT‒PCR) analysis. Moreover, we screened the differentially expressed genes (DEGs) between high and low levels of RBPJ in RA synovial tissue via analysis of the RA dataset (GSE89408) from the Gene Expression Omnibus (GEO) database and investigated potential pathways related to RBPJ. This study improves our understanding of the target genes and potential mechanisms underlying the functions of RBPJ in RA patients.
Materials and methods
Study participants
In this study, we recruited 157 newly diagnosed RA patients in our hospital. All patients underwent clinical assessment by specialists and were diagnosed according to the criteria for RA [15]. Fifty patients with no specimens or incomplete clinical information or who were under 18 years old or over 75 years old were excluded. We also excluded patients with concomitant diabetes, malignancy, infectious diseases, or other autoimmune diseases. Finally, 83 RA patients were included for testing and analysis (Fig. 1). Demographic data, including age, sex, disease duration, morning stiffness, swollen joint count, tender joint count, and laboratory indices, were collected. The disease activity of RA patients was evaluated by the Disease Activity Score in 28 Joints (DAS28 score) and subsequently divided into three groups: patients with a DAS28 ≤ 3.2 were classified into the low disease activity group, those with 3.2 < DAS28 ≤ 5.1 were classified into the moderate disease activity group, and those with a DAS28 > 5.1 were classified into the high disease activity group. In addition, seventy age- and sex-matched healthy controls were included. The present study was approved by the ethics committee of Taizhou Hospital of Zhejiang Province.
Quantitative real-time PCR analysis of PBMCs
We collected fresh peripheral blood from RA patients and healthy controls, and PBMCs were isolated from peripheral blood via density gradient centrifugation using Ficoll (Sigma − Aldrich Co. LLC). TRIzol reagent (Thermo) was used to extract total RNA from PBMCs, and the RNA quantity was measured with a NanoDrop ND-1000 (NanoDrop, Wilmington, DE, USA). A BeyoRT™ II cDNA first strand synthesis kit was used to generate complementary DNA (cDNA) from total RNA. The mRNA expression of RBPJ and six hub genes were measured by qRT‒PCR using BeyoFast™ SYBR Green qPCR mix (high Rox) with a Step-One Plus Real-Time PCR system (ABI). The procedure conditions were as follows: pre-denaturation at 95 °C for 2 min; 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 30 s; and melting curve analysis at 95 °C for 15 s, 60 °C for 15 s, and 95 °C for 15 s. GAPDH was used as the internal reference gene for mRNA. Relative mRNA expression levels were evaluated using the 2−ΔΔCT method and compared with those of the GAPDH control. The sequences of the primers were selected from PrimerBank and synthesized by Sangon Biotech (Shanghai, China), and listed in Supplementary Table S1.
GEO database analyses of RBPJ expression in RA synovial tissues
We obtained an RA dataset (GSE89408) from the GEO database to study the potential regulatory pathway of RBPJ in RA synovial tissues. We subsequently divided the RA synovial tissues into two groups according to the interquartile range of RBPJ expression: those with RBPJ expression levels ≤ 25% were classified into the RBPJ-low group (n = 38), whereas those with RBPJ expression ≥ 75% were classified into the RBPJ-high group (n = 38). The DEGs between the two groups were selected using the “DESeq2” R package (version 4.3.1) with the following thresholds: adjusted p value < 0.01 and |log2 fold change| > 1.2.
DEG enrichment and pathway analysis
The DEG enrichment analyses included Gene Ontology (GO) functional annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis and were conducted with the R package ‘clusterProfiler’. All DEGs were mapped to GO terms for functional enrichment analysis, including biological processes (BP), molecular function (MF), and cellular component (CC). KEGG pathway analysis of clusters and hub genes was also performed through KOBAS (http://kobas.cbi.pku.edu.cn/) online. Moreover, gene set enrichment analysis (GSEA) was further used for enrichment analysis, and the KEGG database was used as the reference gene set.
Construction and analysis of the protein‒protein interaction (PPI) network
The PPI network for the interactions among proteins was established using the STRING database online (https://string-db.org/) and visualized with Cytoscape (version 3.9.1). The hub genes were screened and identified by the CytoHubba plugin in Cytoscape, and the main clusters were determined by the Molecular Complex Detection (MCODE) plugin.
Statistical analysis
The relative RBPJ mRNA expression data were analyzed by SPSS 23.0 statistical software (IBM Corp., Armonk, N.Y., USA) and compared using Student’s t test. The correlations between RBPJ and laboratory indicators were determined using Spearman’s correlation analysis. The graphs were constructed with GraphPad Prism 8.0 software (GraphPad Software, La Jolla, CA, USA) or R software (version 4.3.1). An adjusted p value or p value < 0.05 was considered statistically significant.
Results
Clinical characteristics and RBPJ expression in RA patients
There were no significant differences of age and sex between the RA and healthy controls. Table 1 shows the laboratory and clinical characteristics of the RA patients. The disease duration of the RA patients was 3.0 (IQR: 2.0–6.0) months, and 23 (27.7%) patients experienced morning stiffness. Our results revealed significantly lower RBPJ expression levels in PBMCs extracted from RA patients than in those extracted from healthy controls (P < 0.001) (Fig. 2A). Among 83 newly diagnosed RA patients, 20 patients were classified in the low disease activity group, 34 patients in the moderate disease activity group, and 29 patients in the high disease activity group. RBPJ expression was significantly lower in the high disease activity group than in the moderate and low disease activity groups (P < 0.001) (Fig. 2B, C).
Correlations between RBPJ expression and the clinical parameters of RA patients
The correlations between RBPJ expression and the disease activity and clinical parameters of RA patients were investigated (Fig. 3). The results revealed a significant negative correlation between RBPJ mRNA expression and the DAS28 score (r = -0.388, P < 0.001), erythrocyte sedimentation rate (ESR) (r = -0.334, P = 0.002), C-reactive protein (CRP) (r = -0.241, P = 0.028), monocyte count (r = -0.221, P = 0.044) and complement 3 level (r = -0.290, P = 0.008). However, no relationships between RBPJ mRNA expression and anti-cyclic citrullinated peptide (anti-CCP) level, rheumatoid factor (RF) level or disease duration were detected (P > 0.05).
Identification of differentially expressed genes (DEGs)
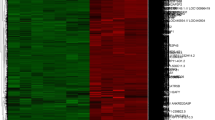
The DEGs between the RBPJ-high group and RBPJ-low group were identified according to the criteria of |log2FC| ≥ 1.2 and adjusted P value < 0.01 and determined to be statistically significant. In total, 562 DEGs meeting the criteria were identified, including 486 downregulated genes and 76 upregulated genes. The 50 most significant DEGs in accordance to the adjusted p value are shown in Fig. 4A.
Differentially expressed genes (DEGs) between RBPJ-high (n = 38) and RBPJ-low (n = 38) groups and DEG enrichment analysis. A. Heatmap for cluster analysis of top 50 DEGs between RBPJ-high and RBPJ-low, Z score normalized. DEGs were screened according to (|log2FC| ≥ 1.2 and adjusted p value < 0.01). B. GO enrichment analysis of DEGs according to the adjusted p value. C. KEGG pathway enrichment analysis of DEGs. D. Gene set enrichment analysis (GSEA) of the expression genes between the two groups
Functional enrichment analysis of DEGs
Both GO functional annotation and KEGG pathway analyses were performed on the datasets to clarify the functions of the DEGs. The results revealed that 562 DEGs were significantly enriched in 554 GO terms. The 10 most significantly enriched BP, CC, and MF GO terms according to the adjusted p value were selected (Fig. 4B). The most enriched biological processes were the regulation of T cell activation, the regulation of cell‒cell adhesion, mononuclear cell differentiation, lymphocyte differentiation, and leukocyte migration. The most enriched cellular components were the external side of plasma membrane, the collagen-containing extracellular matrix and the secretory granule membrane. The most enriched molecular functions were extracellular matrix structural constituent, kinase regulator activity, immune receptor activity and chemokine activity. The KEGG enrichment pathway analysis revealed that the most significantly enriched pathways were cytokine‒cytokine receptor interaction, the PI3K‒Akt signaling pathway, ECM‒receptor interaction, the chemokine signaling pathway, the T cell receptor signaling pathway and Th17 cell differentiation (Fig. 4C). Thirty DEGs were enriched in cytokine‒cytokine receptor interactions (hsa04060), twenty-four DEGs were enriched in the PI3K‒Akt signaling pathway (hsa04151), seventeen DEGs were enriched in the ECM‒receptor interaction (hsa04512) and chemokine signaling pathways (hsa04062), fifteen DEGs were enriched in the T cell receptor signaling pathway (hsa04660), and fourteen DEGs were enriched in Th17 cell differentiation (hsa04659). Moreover, we performed GSEA to further assess the potential signaling pathways associated with RBPJ, and the results revealed that RBPJ was also involved in ABC transporters, fatty acid metabolism, and glycolysis/gluconeogenesis (Fig. 4D).
PPI network analyses
We performed PPI network analyses of the DEGs in the RBPJ-high and RBPJ-low groups using the STRING database and then visualized and optimized the networks using Cytoscape software. The PPI network of the DEGs consisted of 438 nodes and 2982 edges (Fig. 5A). MCODE Plugin analysis revealed 4 main clusters (Fig. 5B-E). Cluster 1 comprised 39 genes, which are mainly involved in the plasma membrane, immune response, inflammatory response, T-cell activation and differentiation, and T cell receptor signaling pathways. The KEGG enrichment pathway analysis revealed that the genes in Cluster 1 were enriched in the T cell receptor signaling pathway, Th17 cell differentiation, cytokine‒cytokine receptor interaction and natural killer cell-mediated cytotoxicity (Fig. 6A, B). Cluster 2 contained 21 genes, which were enriched mainly in extracellular matrix organization, the PI3K-Akt signaling pathway, focal adhesion and ECM-receptor interaction (Fig. 6C, D). Cluster 3 contained 12 genes, which were enriched mainly in neutrophil chemotaxis, immune response, chemokine-mediated signaling pathway, and inflammatory response, and involved in cytokine‒cytokine receptor interactions and the TNF signaling pathway (Fig. 6E, F). Cluster 4 contained 11 genes, which were enriched mainly in the immune response, inflammatory response, transmembrane signaling receptor activity and B-cell receptor signaling pathways (Fig. 6G, H).
GO and KEGG pathway enrichment analyses of the 4 main clusters. A. GO enrichment analysis of Cluster 1. B. KEGG pathway enrichment analysis of Cluster 1 C. GO enrichment analysis of Cluster 2 D. KEGG pathway enrichment analysis of Cluster 2. E. GO enrichment analysis of Cluster 3 F. KEGG pathway enrichment analysis of Cluster 3. G. GO enrichment analysis of Cluster 4 (H). KEGG pathway enrichment analysis of Cluster 4
The hub genes of the PPI network were evaluated with the CytoHubba Plugin, and the top 10 hub genes were identified, including C-C motif chemokine receptor 7 (CCR7), Fc gamma receptor IIIa (FCGR3A), Fc gamma receptor IIIb (FCGR3B), LCK proto-oncogene (LCK), cytotoxic T-lymphocyte associated protein 4 (CTLA4), CD27 molecule (CD27), selectin L (SELL), interleukin 1 beta (IL1B), CD40 ligand (CD40LG) and granzyme B (GZMB). GO functional annotation revealed that the hub genes were enriched in the plasma membrane, immune response, transmembrane signaling receptor activity and T cell costimulation. KEGG pathway analysis of the hub genes revealed that they were mainly involved in osteoclast differentiation, the T cell receptor signaling pathway, natural killer cell-mediated cytotoxicity, and cytokine‒cytokine receptor interaction (Fig. 7).
Validation of the relationships between RBPJ and the hub genes
To verify the relationships between RBPJ and the hub genes, we detected the expression of six hub genes (CCR7, FCGR3A, LCK, GZMB, CD27 and CD40LG) in PBMCs from 40 of the 83 RA patients using qRT‒PCR and performed correlation analyses. The expression levels of RBPJ were negatively correlated with those of CCR7 (r = − 0.464, P = 0.003), LCK (r = − 0.443, P = 0.004), GZMB (r = − 0.451, P = 0.003), CD27 (r = − 0.456, P = 0.003) and CD40LG (r = − 0.670, P < 0.001) but positively correlated with FCGR3A (r = 0.569, P < 0.001) (Fig. 8).
Associations between RBPJ expression and related hub genes in RA. qRT‒PCR was performed to detect the expression levels of six hub genes and RBPJ in RA PBMCs. Spearman’s correlation analysis was used to analyze the correlations between RBPJ and CCR7, LCK1, GZMB, CD27, CD40LG and FCGR3A in RA patients
Discussion
In our study, we found that the expression level of RBPJ mRNA was lower in PBMCs from RA patients than in those from healthy controls. In RA patients, RBPJ expression was negatively correlated with DAS28 score, ESR, CRP, monocyte count and complement 3 level. In addition, we downloaded the GSE89408 dataset from the GEO database and identified DEGs related to RBPJ-high and RBPJ-low expression in RA synovial tissue. Potential pathways revealed that the DEGs were mainly involved in T cell activation and differentiation, the T cell receptor signaling pathway, Th17 cell differentiation, cytokine‒cytokine receptor interactions and the PI3K‒Akt signaling pathway. The hub genes were involved mainly in the immune response, osteoclast differentiation, the T cell receptor signaling pathway, natural killer cell-mediated cytotoxicity, and cytokine‒cytokine receptor interaction.
RBPJ is a key transcription factor of the Notch signaling pathway that controls numerous cellular biological functions, such as cell differentiation, proliferation, and lymphocyte development, the protein encoded by the RBPJ gene acts as both a transcriptional activator and repressor [16]. Hassed et al. identified loss of function mutations in RBPJ causing Adams-Oliver syndrome (AOS), abnormal bone development is one of the main defects of AOS [17]; The RBPJ mutations that cause AOS are located in highly conserved domains, leading to DNA binding defects [18]. Previous studies have shown that RBPJ is important for skeletal formation, osteoclastogenesis, bone metabolism and bone resorption [19,20,21]. The balance between osteoclasts and osteoblasts is disrupted in RA patients, activated osteoclasts promote bone destruction and erosion, which were central features of RA [22, 23]. Moreover, research has shown that RBPJ is involved in tumor development and growth and that RBPJ deficiency induces the activation and transformation of dermal fibroblasts into cancer-associated fibroblasts [24]. Previous studies have revealed that RBPJ is related to the pathogenesis of RA and that the allelic variants of RBPJ polymorphisms are risk factors for RA [13, 14]. The rs874040 polymorphism is located in a strong enhancer region of RBPJ gene, plays a crucial role in increasing susceptibility to RA disease [13, 25]. Previous studies have shown that CD4+ T cells in RA synovium were involved in synovial inflammation by promoting the secretion of pro-inflammatory cytokines. The pathogenic CD4+ T cells of RA patients carry the RBPJ rs874040 polymorphism, which may contribute to RA activation by enhancing T activation inflammatory responses [13, 25]. In addition, the expression level of RBPJ was significantly lower in synovial fluid macrophages from RA patients than in monocyte-derived macrophages from healthy controls and was also lower in synovial fibroblasts from RA patients than in those from arthralgia patients [12, 26]. Inoue K et al. established myeloid-specific RBPJ-deficient mice and reported increased expression of osteoclast-related genes. They also isolated CD14 positive PBMCs from osteoclast precursors of RA patients and healthy controls and reported significantly decreased expression of RBPJ in CD14+ PBMCs from RA patients [20]. These findings suggest the function of RBPJ in rheumatoid arthritis. Our study revealed decreased levels of RBPJ mRNA in RA PBMCs compared with those in healthy control PBMCs. However, the potential mechanisms of RBPJ in RA development and pathogenesis are still unclear.
Our study revealed that the DEGs were mainly involved in lymphocyte differentiation, T-cell activation and differentiation, the T cell receptor signaling pathway, Th1/2 and Th17 cell differentiation, which was consistent with previous findings. scRNA-seq analysis revealed that GZMB, IFNγ and effector-associated molecules were increased in RBPJ-deficient cells, indicating enhanced cytotoxic and effector features [27]. RBPJ conditional deletion in RBPJf/fMx-Cre mice stopped T lymphopoiesis in double-negative stage 1 and lead to the accumulation of B cells and the absence of CD4+ and CD8+ T cells in the thymus [7]. Previous studies revealed that RBPJ mediates T cell differentiation and is associated with Th1/Th2 cell differentiation [28, 29]. RBPJ is involved in coordinating colonic macrophages and Th17 cells and drives the immune response of Th17 cells to eliminate bacterial pathogens in the immune response stage of infection [6]. CD4+ T cells and their subsets, such as Th1/Th2, Th17, and Treg cells, play important roles in the development and progression of RA, and CD4+ T cells also enhance the effector functions of CD8+ T cells and NK cells [30]. Many inflammatory cells are recruited into RA synovial tissue, and CD4+ T cells account for a large proportion of inflammatory cells and contribute to synovial inflammation [31]. Moreover, several studies have indicated that Treg cells are enriched in RA synovial fluid and that Treg cells maintain their suppressive function and perform differentiated effector Treg cell functions in inflamed tissue [32].
RA is a progressive inflammatory disease that affects the synovial joints and tissues, and one of the clinical features of RA is excessive synovial hyperplasia [33]. Through the analysis of the GEO dataset, this study revealed that RBPJ may influence the pathogenesis and progression of RA. Moreover, enrichment analysis revealed that the RBPJ-related genes were significantly enriched in cytokine‒cytokine receptor interaction, ECM-receptor interaction and chemokine signaling pathway. Previous research has shown that these pathways are involved in RA chemotaxis, phagocytosis, inflammation, and synovial invasiveness [34, 35]. RA is attributed to the interaction of immune cells and synovial cells. The infiltration of activated macrophages, lymphocytes, and neutrophils in synovial tissue promotes the production and aggregation of inflammatory cytokines, thereby inducing synovial fibroblasts [36]. Chemokines are a family of inflammatory cytokines, and several lines of evidence indicate that chemokines and chemokine receptors are involved in the infiltration, migration, and accumulation of leukocytes and the inflammatory process in the synovium of RA patients [37, 38]. The extracellular matrix (ECM) plays a central role in organizing synovial cell networks in RA-affected joints by delivering abnormal proinflammatory signals to the resident cell network. The ECM can be used as a position holder for chemokines and to deliver drugs, and targeting the ECM and cell networks may be a new strategy to prevent the progression of RA [39, 40].
Furthermore, a PPI network related to RBPJ expression was constructed and the top 10 hub genes were screened with the CytoHubba plugin. Among them, most genes are involved in osteoclast differentiation, natural killer cell-mediated cytotoxicity, cytokine‒cytokine receptor interaction and the T cell receptor signaling pathway and are associated with RA pathogenicity, as reported previously. IL1B is important for maintaining the activation of inflammatory cell and immune responses in various inflammatory diseases [41]. CCR7 is widely expressed in naïve T cells, B cells, Tregs, central memory T cells and natural killer cells. Multiple studies have shown that CCR7 is involved in promoting the progression of RA and is a promising target for RA therapy, since the expression levels of CCR7 are elevated in RA patients and positively correlated with DAS28 [35, 42, 43]. Moschovakis GL et al. revealed that CCR7 is essential for the establishment of collagen-induced arthritis (CIA); CCR7 mediates cell migration, which contributes to the development of CIA [44]. LCK is expressed in all T lineage cells and is involved in TCR signaling initiation and T cell signaling, which are responsible for RA pathogenesis [45]. CD40LG, which is expressed mainly on activated T cells, B cells, NK cells and platelets, can promote the proliferation of B cells and enhance platelet activation [46]. GZMB is a serine protease. Previous studies revealed elevated expression of GZMB in an RA animal model, and GZMB silencing delayed RA inflammation, confirming the important role of GZMB in RA [47]. Moreover, Nakajima S et al. revealed that the GZMK+ GZMB+ CD8+ T-cell subtype is the dominant and strongest inflammatory phenotype in RA synovial tissue, indicating the important role of GZMB in RA [48]. In addition, increased serum soluble CD27 expression is associated with immune activity and disease activity in RA patients [49]. Neutrophil granulocytes constitute a major inflammatory cell population, and many neutrophils migrate to the joint cavity and cause joint cartilage damage in RA. The efficient influx of neutrophils into inflammatory joint tissues is based on the expression of L-selectin (SELL) [50]. In summary, molecules such as CCR7, LCK, GZMB, CD40LG, and CD27 play important roles in the occurrence and progression of RA.
Moreover, the MCODE plugin in Cytoscape revealed that RBPJ was associated with the immune response, inflammatory response, T-cell activation and differentiation and was enriched in the T-cell receptor signaling pathway, Th17 cell differentiation, cytokine‒cytokine receptor interaction, natural killer cell-mediated cytotoxicity and the PI3K‒Akt signaling pathway, which was consistent with previous findings [51,52,53].
Interestingly, GSEA revealed that RBPJ was associated with ABC transporters, fatty acid metabolism, and glycolysis/gluconeogenesis, which are believed to participate in the disease progression of RA. For example, several studies have revealed that fatty acid metabolism is involved in the pathogenicity of RA [54, 55]. Basal mitochondrial respiration is dependent on fatty acid oxidation, and previous studies revealed impaired mitochondrial fatty acid β-oxidation and decreased mitochondrial respiration in RA and RA-risk fibroblast-like synoviocytes (FLSs) compared with control FLSs [54]. Rodgers LC et al. revealed that fatty acid metabolism is altered in macrophages from synovial fluid and monocytes from peripheral blood of RA patients, and that fatty acid oxidation contributes to RA pathogenesis by recruiting pathogenic Th17 cells and enhancing osteoclastogenesis [56].
Several limitations of the study should be noted. First, the expression levels of RBPJ in RA patients need to be further explored in a larger sample and other specimen types including synovial fluid, synovial tissue and cartilage, with a more rigorous experimental design. Second, further experiments are needed to verify the functions of the DEGs and potential related signaling pathways. Moreover, the downstream molecules and pathways of RBPJ that may contribute to the occurrence and development of RA should be examined.
Conclusions
In summary, the present study revealed significantly lower expression levels of RBPJ in the PBMCs of RA patients, and RBPJ expression was negatively correlated with RA disease activity. GEO dataset analysis revealed that RBPJ may be involved in the regulation of T cell activation, lymphocyte/mononuclear cell differentiation, and the regulation of cell‒cell adhesion. The DEGs affected by RBPJ were enriched in pathways related to T cell receptor signaling, osteoclast differentiation, Th17 cell differentiation and cytokine‒cytokine receptor interaction. Our research provides information about the genes associated with RBPJ-mediated transcription and contributes to understanding the potential mechanisms of RBPJ in RA.
Availability of data and materials
The data and code used and/or analysed during the current study are included in the Supplementary material.
Data availability
The data were obtained from the GEO DataSets (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE89408). The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- RA:
-
Rheumatoid arthritis
- RBPJ:
-
Recombination signal-binding protein for immunoglobulin kappa J region
- PBMCs:
-
Peripheral blood mononuclear cells
- DAS28 score:
-
Disease Activity Score in 28 Joints
- CRP:
-
C-reactive protein
- ESR:
-
Erythrocyte sedimentation rate
- RF:
-
Rheumatoid factor
- Anti-CCP:
-
Anti-cyclic citrullinated peptide
- GEO:
-
Gene Expression Omnibus
- DEGs:
-
Differentially expressed genes
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- GSEA:
-
Gene set enrichment analysis
- PPI:
-
Protein-protein interaction
- IL1B:
-
Interleukin 1 beta
- CCR7:
-
C-C motif chemokine receptor 7
- FCGR3A:
-
Fc gamma receptor IIIa
- FCGR3B:
-
Fc gamma receptor IIIb
- SELL:
-
Selectin L
- LCK:
-
LCK proto-oncogene
- CTLA4:
-
Cytotoxic T-lymphocyte associated protein 4
- CD27:
-
CD27 molecule
- CD40LG:
-
CD40 ligand
- GZMB:
-
Granzyme B
References
Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–38. https://doi.org/10.1016/S0140-6736(16)30173-8.
Baker JF, England BR, George M, Cannon G, Sauer B, Ogdie A, Hamilton BC, Hunter C, Duryee MJ, Thiele G, Mikuls TR. Disease activity, cytokines, chemokines and the risk of incident diabetes in rheumatoid arthritis. Ann Rheum Dis. 2021;80(5):566–72. https://doi.org/10.1136/annrheumdis-2020-219140.
Pear WS, Radtke F. Notch signaling in lymphopoiesis. Semin Immunol. 2003;15(2):69–79. https://doi.org/10.1016/s1044-5323(03)00003-4.
Radtke F, MacDonald HR, Tacchini-Cottier F. Regulation of innate and adaptive immunity by Notch. Nat Rev Immunol. 2013;13(6):427–37. https://doi.org/10.1038/nri3445.
Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8- dendritic cells in the spleen. J Exp Med. 2007;204(7):1653–64. https://doi.org/10.1084/jem.20062648.
Kang L, Zhang X, Ji L, Kou T, Smith SM, Zhao B, Guo X, Pineda-Torra I, Wu L, Hu X. The colonic macrophage transcription factor RBP-J orchestrates intestinal immunity against bacterial pathogens. J Exp Med. 2020;217(4): e20190762. https://doi.org/10.1084/jem.20190762.
Chen ELY, Thompson PK, Zuniga-Pflucker JC. RBPJ-dependent notch signaling initiates the T cell program in a subset of thymus-seeding progenitors. Nat Immunol. 2019;20(11):1456–68. https://doi.org/10.1038/s41590-019-0518-7.
Xu H, Zhu J, Smith S, Foldi J, Zhao B, Chung AY, Outtz H, Kitajewski J, Shi C, Weber S, Saftig P, Li Y, Ozato K, Blobel CP, Ivashkiv LB, Hu X. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat Immunol. 2012;13(7):642–50. https://doi.org/10.1038/ni.2304.
Wang L, Zhang H, Rodriguez S, Cao L, Parish J, Mumaw C, Zollman A, Kamoka MM, Mu J, Chen DZ, Srour EF, Chitteti BR, HogenEsch H, Tu X, Bellido TM, Boswell HS, Manshouri T, Verstovsek S, Yoder MC, Kapur R, Cardoso AA, Carlesso N. Notch-dependent repression of miR-155 in the bone marrow niche regulates hematopoiesis in an NF-kappaB-dependent manner. Cell Stem Cell. 2014;15(1):51–65. https://doi.org/10.1016/j.stem.2014.04.021.
Xu H, Wang Y, Rong X, Wang D, Xie J, Huang Z, Zeng W, Fu X, Li J, Zhou Z. Ingenious synergy of a Pathology-Specific Biomimetic Multifunctional Nanoplatform for targeted therapy in rheumatoid arthritis. Small. 2023;e2305197. https://doi.org/10.1002/smll.202305197.
Ansalone C, Cole J, Chilaka S, Sunzini F, Sood S, Robertson J, Siebert S, McInnes IB, Goodyear CS. TNF is a homoeostatic regulator of distinct epigenetically primed human osteoclast precursors. Ann Rheum Dis. 2021;80(6):748–57. https://doi.org/10.1136/annrheumdis-2020-219262.
Li S, Miller CH, Giannopoulou E, Hu X, Ivashkiv LB, Zhao B. RBP-J imposes a requirement for ITAM-mediated costimulation of osteoclastogenesis. J Clin Investig. 2014;124(11):5057–73. https://doi.org/10.1172/JCI71882.
Stahl EA, Raychaudhuri S, Remmers EF, Xie G, Eyre S, Thomson BP, Li Y, Kurreeman FA, Zhernakova A, Hinks A, Guiducci C, Chen R, Alfredsson L, Amos CI, Ardlie KG, Consortium B, Barton A, Bowes J, Brouwer E, Burtt NP, Catanese JJ, Coblyn J, Coenen MJ, Costenbader KH, Criswell LA, Crusius JB, Cui J, de Bakker PI, De Jager PL, Ding B, Emery P, Flynn E, Harrison P, Hocking LJ, Huizinga TW, Kastner DL, Ke X, Lee AT, Liu X, Martin P, Morgan AW, Padyukov L, Posthumus MD, Radstake TR, Reid DM, Seielstad M, Seldin MF, Shadick NA, Steer S, Tak PP, Thomson W, van der Helm-van Mil AH, van der Horst-Bruinsma IE, van der Schoot CE, van Riel PL, Weinblatt ME, Wilson AG, Wolbink GJ, Wordsworth BP, Consortium Y, Wijmenga C, Karlson EW, Toes RE, de Vries N, Begovich AB, Worthington J, Siminovitch KA, Gregersen PK, Klareskog L, Plenge RM. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nature genetics. 2010;42(6):508–14. https://doi.org/10.1038/ng.582.
Govind N, Choudhury A, Hodkinson B, Ickinger C, Frost J, Lee A, Gregersen PK, Reynolds RJ, Bridges SL Jr, Hazelhurst S, Ramsay M, Tikly M. Immunochip identifies novel, and replicates known, genetic risk loci for rheumatoid arthritis in black South africans. Mol Med. 2014;20(1):341–9. https://doi.org/10.2119/molmed.2014.00097.
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Menard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovsky J, Wolfe F, Hawker G. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann Rheum Dis. 2010;69(9):1580–8. https://doi.org/10.1136/ard.2010.138461.
Castel D, Mourikis P, Bartels SJ, Brinkman AB, Tajbakhsh S, Stunnenberg HG. Dynamic binding of RBPJ is determined by notch signaling status. Genes Dev. 2013;27(9):1059–71. https://doi.org/10.1101/gad.211912.112.
Hassed SJ, Wiley GB, Wang S, Lee JY, Li S, Xu W, Zhao ZJ, Mulvihill JJ, Robertson J, Warner J, Gaffney PM. RBPJ mutations identified in two families affected by Adams-Oliver syndrome. Am J Hum Genet. 2012;91(2):391–5. https://doi.org/10.1016/j.ajhg.2012.07.005.
Meester JAN, Sukalo M, Schroder KC, Schanze D, Baynam G, Borck G, Bramswig NC, Duman D, Gilbert-Dussardier B, Holder-Espinasse M, Itin P, Johnson DS, Joss S, Koillinen H, McKenzie F, Morton J, Nelle H, Reardon W, Roll C, Salih MA, Savarirayan R, Scurr I, Splitt M, Thompson E, Titheradge H, Travers CP, Van Maldergem L, Whiteford M, Wieczorek D, Vandeweyer G, Trembath R, Van Laer L, Loeys BL, Zenker M, Southgate L, Wuyts W. Elucidating the genetic architecture of Adams-Oliver syndrome in a large European cohort. Hum Mutat. 2018;39(9):1246–61. https://doi.org/10.1002/humu.23567.
Gao Y, Fu Z, Guan J, Liu X, Zhang Q. The role of notch signaling pathway in metabolic bone diseases. Biochem Pharmacol. 2023;207: 115377. https://doi.org/10.1016/j.bcp.2022.115377.
Inoue K, Hu X, Zhao B. Regulatory network mediated by RBP-J/NFATc1-miR182 controls inflammatory bone resorption. FASEB J. 2020;34(2):2392–407. https://doi.org/10.1096/fj.201902227R.
Zhao B, Grimes SN, Li S, Hu X, Ivashkiv LB. TNF-induced osteoclastogenesis and inflammatory bone resorption are inhibited by transcription factor RBP-J. J Exp Med. 2012;209(2):319–34. https://doi.org/10.1084/jem.20111566.
Yang M, Zhu L. Osteoimmunology: the crosstalk between T cells, B cells, and osteoclasts in rheumatoid arthritis. Int J Mol Sci. 2024;25(5). https://doi.org/10.3390/ijms25052688.
Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol. 2012;8(11):656–64. https://doi.org/10.1038/nrrheum.2012.153.
Procopio MG, Laszlo C, Al Labban D, Kim DE, Bordignon P, Jo SH, Goruppi S, Menietti E, Ostano P, Ala U, Provero P, Hoetzenecker W, Neel V, Kilarski WW, Swartz MA, Brisken C, Lefort K, Dotto GP. Combined CSL and p53 downregulation promotes cancer-associated fibroblast activation. Nat Cell Biol. 2015;17(9):1193–204. https://doi.org/10.1038/ncb3228.
Orent W, McHenry AR, Rao DA, White C, Klein HU, Bassil R, Srivastava G, Replogle JM, Raj T, Frangieh M, Cimpean M, Cuerdon N, Chibnik L, Khoury SJ, Karlson EW, Brenner MB, De Jager P, Bradshaw EM, Elyaman W. Rheumatoid arthritis-associated RBPJ polymorphism alters memory CD4 + T cells. Hum Mol Genet. 2016;25(2):404–17. https://doi.org/10.1093/hmg/ddv474.
Ge X, Frank-Bertoncelj M, Klein K, McGovern A, Kuret T, Houtman M, Burja B, Micheroli R, Shi C, Marks M, Filer A, Buckley CD, Orozco G, Distler O, Morris AP, Martin P, Eyre S, Ospelt C. Functional genomics atlas of synovial fibroblasts defining rheumatoid arthritis heritability. Genome Biol. 2021;22(1):247. https://doi.org/10.1186/s13059-021-02460-6.
Zhou P, Shi H, Huang H, Sun X, Yuan S, Chapman NM, Connelly JP, Lim SA, Saravia J, Kc A, Pruett-Miller SM, Chi H. Single-cell CRISPR screens in vivo map T cell fate regulomes in cancer. Nature. 2023;624(7990):154–63. https://doi.org/10.1038/s41586-023-06733-x.
Amsen D, Antov A, Flavell RA. The different faces of notch in T-helper-cell differentiation. Nat Rev Immunol. 2009;9(2):116–24. https://doi.org/10.1038/nri2488.
Delacher M, Schmidl C, Herzig Y, Breloer M, Hartmann W, Brunk F, Kagebein D, Trager U, Hofer AC, Bittner S, Weichenhan D, Imbusch CD, Hotz-Wagenblatt A, Hielscher T, Breiling A, Federico G, Grone HJ, Schmid RM, Rehli M, Abramson J, Feuerer M. Rbpj expression in regulatory T cells is critical for restraining T(H)2 responses. Nat Commun. 2019;10(1):1621. https://doi.org/10.1038/s41467-019-09276-w.
Yang P, Qian FY, Zhang MF, Xu AL, Wang X, Jiang BP, Zhou LL. Th17 cell pathogenicity and plasticity in rheumatoid arthritis. J Leukoc Biol. 2019;106(6):1233–40. https://doi.org/10.1002/JLB.4RU0619-197R.
Moradi B, Schnatzer P, Hagmann S, Rosshirt N, Gotterbarm T, Kretzer JP, Thomsen M, Lorenz HM, Zeifang F, Tretter T. CD4(+)CD25(+)/highCD127low/(-) regulatory T cells are enriched in rheumatoid arthritis and osteoarthritis joints–analysis of frequency and phenotype in synovial membrane, synovial fluid and peripheral blood. Arthritis Res Therapy. 2014;16(2):R97. https://doi.org/10.1186/ar4545.
Sumida TS, Cheru NT, Hafler DA. The regulation and differentiation of regulatory T cells and their dysfunction in autoimmune diseases. Nat Rev Immunol. 2024. https://doi.org/10.1038/s41577-024-00994-x.
Rufino AT, Freitas M, Proenca C, Ferreira de Oliveira JMP, Fernandes E, Ribeiro D. Rheumatoid arthritis molecular targets and their importance to flavonoid-based therapy. Med Res Rev. 2023. https://doi.org/10.1002/med.21990.
Zhang L, Yu M, Deng J, Lv X, Liu J, Xiao Y, Yang W, Zhang Y, Li C. Chemokine signaling pathway involved in CCL2 expression in patients with rheumatoid arthritis. Yonsei Med J. 2015;56(4):1134–42. https://doi.org/10.3349/ymj.2015.56.4.1134.
McHugh J. CCL21-CCR7 axis in RA: linking inflammation and bone erosion. Nat Rev Rheumatol. 2019;15(10):576. https://doi.org/10.1038/s41584-019-0296-5.
Matsuda K, Shiba N, Hiraoka K. New insights into the role of synovial fibroblasts leading to Joint Destruction in Rheumatoid Arthritis. Int J Mol Sci. 2023;24(6): 5173. https://doi.org/10.3390/ijms24065173.
Szekanecz Z, Koch AE. Successes and failures of chemokine-pathway targeting in rheumatoid arthritis. Nat Rev Rheumatol. 2016;12(1):5–13. https://doi.org/10.1038/nrrheum.2015.157.
Chen X, Oppenheim JJ, Howard OM. Chemokines and chemokine receptors as novel therapeutic targets in rheumatoid arthritis (RA): inhibitory effects of traditional Chinese medicinal components. Cell Mol Immunol. 2004;1(5):336–42.
Buckley CD, Ospelt C, Gay S, Midwood KS. Location, location, location: how the tissue microenvironment affects inflammation in RA. Nat Rev Rheumatol. 2021;17(4):195–212. https://doi.org/10.1038/s41584-020-00570-2.
Karsdal MA, Kraus VB, Shevell D, Bay-Jensen AC, Schattenberg J, Rambabu Surabattula R, Schuppan D. Profiling and targeting connective tissue remodeling in autoimmunity - A novel paradigm for diagnosing and treating chronic diseases. Autoimmun rev. 2021;20(1): 102706. https://doi.org/10.1016/j.autrev.2020.102706.
Zhang Z, Liu W, Hu J, Qu Y, Zhao J, Pan Y, Zhang X, Quan X. Surface water extracts impair gene profiles and differentiation in human mesenchymal stem cells. Environ Int. 2019;132:104823. https://doi.org/10.1016/j.envint.2019.05.017.
Van Raemdonck K, Umar S, Shahrara S. The pathogenic importance of CCL21 and CCR7 in rheumatoid arthritis. Cytokine Growth Factor Rev. 2020;55:86–93. https://doi.org/10.1016/j.cytogfr.2020.05.007.
Han L, Zhang L. CCL21/CCR7 axis as a therapeutic target for autoimmune diseases. Int Immunopharmacol. 2023;121: 110431. https://doi.org/10.1016/j.intimp.2023.110431.
Moschovakis GL, Bubke A, Friedrichsen M, Ristenpart J, Back JW, Falk CS, Kremmer E, Forster R. The chemokine receptor CCR7 is a promising target for rheumatoid arthritis therapy. Cell Mol Immunol. 2019;16(10):791–9. https://doi.org/10.1038/s41423-018-0056-5.
Kumar Singh P, Kashyap A, Silakari O. Exploration of the therapeutic aspects of lck: a kinase target in inflammatory mediated pathological conditions. Biomed Pharmacother. 2018;108:1565–71. https://doi.org/10.1016/j.biopha.2018.10.002.
Pucino V, Gardner DH, Fisher BA. Rationale for CD40 pathway blockade in autoimmune rheumatic disorders. Lancet Rheumatol. 2020;2(5):e292-301. https://doi.org/10.1016/S2665-9913(20)30038-2.
Zhang Y, Cai X, Wang B, Zhang B, Xu Y. Exploring the molecular mechanisms of the involvement of GZMB-Caspase-3-GSDME pathway in the progression of rheumatoid arthritis. Mol Immunol. 2023;161:82–90. https://doi.org/10.1016/j.molimm.2023.07.013.
Nakajima S, Tsuchiya H, Ota M, Ogawa M, Yamada S, Yoshida R, Maeda J, Shirai H, Kasai T, Hirose J, Ninagawa K, Fujieda Y, Iwasaki T, Aizaki Y, Kajiyama H, Matsushita M, Kawakami E, Tamura N, Mimura T, Ohmura K, Morinobu A, Atsumi T, Tanaka Y, Takeuchi T, Tanaka S, Okamura T, Fujio K. Synovial tissue heterogeneity in Japanese patients with rheumatoid arthritis elucidated using a cell-type Deconvolution Approach. Arthritis Rheumatol. 2023;75(12):2130–6. https://doi.org/10.1002/art.42642.
Shi L, Yang J, Xu J, Dai J, Li J. Elevated serum soluble CD27 levels are associated with both disease activity and humoral immune activity in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2024. https://doi.org/10.55563/clinexprheumatol/fq5d3i.
Sarraj B, Ludanyi K, Glant TT, Finnegan A, Mikecz K. Expression of CD44 and L-selectin in the innate immune system is required for severe joint inflammation in the proteoglycan-induced murine model of rheumatoid arthritis. J Immunol. 2006;177(3):1932–40. https://doi.org/10.4049/jimmunol.177.3.1932.
Zhang X, Smith SM, Wang X, Zhao B, Wu L, Hu X. Three paralogous clusters of the miR-17 ~ 92 family of microRNAs restrain IL-12-mediated immune defense. Cell Mol Immunol. 2021;18(7):1751–60. https://doi.org/10.1038/s41423-020-0363-5.
Zhou M, Qu R, Yin X, Qiu Y, Peng Y, Liu B, Gao Y, Bi H, Guo D. Prednisone acetate modulates Th1/Th2 and Th17/Treg cell homeostasis in experimental autoimmune uveitis via orchestrating the notch signaling pathway. Int Immunopharmacol. 2023;116: 109809. https://doi.org/10.1016/j.intimp.2023.109809.
Webb LMC, Fra-Bido S, Innocentin S, Matheson LS, Attaf N, Bignon A, Novarino J, Fazilleau N, Linterman MA. Ageing promotes early T follicular helper cell differentiation by modulating expression of RBPJ. Aging Cell. 2021;20(1): e13295. https://doi.org/10.1111/acel.13295.
de Jong TA, Semmelink JF, Denis SW, van de Sande MGH, Houtkooper RHL, van Baarsen LGM. Altered lipid metabolism in synovial fibroblasts of individuals at risk of developing rheumatoid arthritis. J Autoimmun. 2023;134: 102974. https://doi.org/10.1016/j.jaut.2022.102974.
Wei J, Huang X, Zhang X, Chen G, Zhang C, Zhou X, Qi J, Zhang Y, Li X. Elevated fatty acid beta-oxidation by leptin contributes to the proinflammatory characteristics of fibroblast-like synoviocytes from RA patients via LKB1-AMPK pathway. Cell Death Dis. 2023;14(2):97. https://doi.org/10.1038/s41419-023-05641-2.
Rodgers LC, Cole J, Rattigan KM, Barrett MP, Kurian N, McInnes IB, Goodyear CS. The rheumatoid synovial environment alters fatty acid metabolism in human monocytes and enhances CCL20 secretion. Rheumatology. 2020;59(4):869–78. https://doi.org/10.1093/rheumatology/kez378.
Funding
This research was supported by Zhejiang Provincial Natural Science Foundation of China (Grant No: LTGY24H200003, LTGY23H200007), Medical Science and Technology Project of Zhejiang Province (Grant No: 2021KY1205).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. S.Y.C., Y.L.Z. and S.S.C. determined the methods. W.B.Z. and S.Y.C collected the clinical information. J.P.D., B.S. and J.L. analyzed and interpreted the data. S.S.C. and W.B.Z. wrote the first draft of the manuscript and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The research was conducted in accordance with the Declaration of Helsinki. This study was approved by the ethics committee of Taizhou Hospital of Zhejiang Province. Written informed consents were obtained from all individuals who participated in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Chen, S., Zhao, W., Du, J. et al. The expression of RBPJ and its potential role in rheumatoid arthritis. BMC Genomics 25, 899 (2024). https://doi.org/10.1186/s12864-024-10804-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-024-10804-2