Abstract
Background
Ceftobiprole is a fifth-generation cephalosporin which has been reported to have broad antibacterial spectrum when tested against bacteria collected from other countries except China. This study evaluated the in vitro activity of ceftobiprole in comparison with other comparators against clinically significant isolates collected across from China.
Results
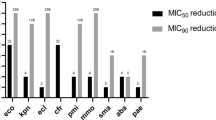
Susceptibility testing of ceftobiprole and comparators against 1163 clinically isolated Gram-positive and Gram-negative bacteria was performed with broth micro dilution method following the CLSI guidelines. All 110 S. aureus were susceptible to ceftobiprole with MIC50/90 of 1/2 mg/L for MRSA and 0.5/1 mg/L for MSSA. For Coagulase-negative staphylococci (CNS), MIC50/90 of ceftobiprole for MRCNS and MSCNS was 1/2 mg/L and 0.25/0.5 mg/L. Ceftobiprole demonstrated good potency against E. faecalis (MIC50/90 of 0.5/1 mg/L) but limited activity against E. faecium (MIC50/90 of > 32/ > 32 mg/L). Ceftobiprole demonstrated potent activity against all 39 β-hemolytic Streptococcus spp. with MIC50/90 ≤ 0.015/ ≤ 0.015–2 mg/L and 110 of PSSP with 98.2% susceptibility. Ceftobiprole inhibited all isolates of H. influenzae and M. catarrhalis at ≤ 1 mg/L. 91.8% and 98.2% of the ESBL-negative E. coli and K. pneumoniae were susceptible to ceftobiprole, but most of the ESBL-positive or carbapenem-resistant strains were also resistant to ceftobiprole. Ceftobiprole inhibited 84.2% of carbapenem-susceptible P. aeruginosa and 94.1% of carbapenem-susceptible A. baumannii at ≤ 8 mg/L, but only 52.6% of carbapenem-resistant P. aeruginosa and 5.3% of carbapenem-resistant A. baumannii.
Conclusion
Ceftobiprole demonstrated good in vitro activity against a broad range of clinically relevant contemporary Gram-positive and Gram-negative bacterial isolates.
Similar content being viewed by others
Background
Antimicrobial resistance has been a public health threat in recent years, with an increase of multi-drug resistant bacteria, such as extended-spectrum β-lactamase positive Enterobacterales, methicillin-resistant Staphylococcus aureus (MRSA), Vancomycin-resistant E. faecium and penicillin-non-susceptible S. pneumoniae (PRSP), which are listed as the important pathogens for new antibiotics by WHO [1]. Ceftobiprole is a fifth-generation parenteral cephalosporin demonstrating potent in vitro activity against Gram-positive pathogens, including MRSA and PRSP, as well as some non-carbapenemase or ESBL-producing Gram-negative pathogens commonly associated with pneumonia [2, 3]. It has obtained regulatory approval in Europe and several non-European countries for the treatment of hospital-acquired pneumonia excluding ventilator-associated pneumonia and community-acquired pneumonia in adults [4, 5]. It has been reported that ceftobiprole is generally β-lactamase stable and has a strong affinity for essential penicillin-binding proteins, including those responsible for β-lactam resistance in staphylococci and pneumococci [6]. Several studies have been reported on the spectrum and potency of ceftobiprole against Gram-positive and Gram-negative pathogens collected from Europe and surrounding countries in a variety of infection types [2,3,4, 7, 8]. In this present study, we expand upon those observations by reporting the activity of ceftobiprole and comparators against bacterial isolates obtained and tested during the 2016–2018 CHINET Antimicrobial Surveillance Network in China.
Results
Ceftobiprole and comparator antibiotics activity against gram-positive bacteria
Ceftobiprole was active against 110 S. aureus (MIC range, 0.25–2 mg/L, 100% susceptibility) and 80 Coagulase-negative Staphylococci (CNS, MIC range, ≤ 0.015—4 mg/L). All S. aureus and CNS were susceptible to vancomycin and linezolid. For MRSA, susceptibility to ciprofloxacin, clindamycin, and erythromycin was 54.5%, 23.6%, and 12.7%, which was less than that of methicillin-susceptible S. aureus (MSSA), 83.6%, 72.7%, and 43.6%, respectively. Ceftobiprole was twice as active against MSSA strains with MIC50/90 of 0.5/1 mg/L than on MRSA strains with MIC50/90 of 1/2 mg/L. For methicillin-resistant Coagulase-negative Staphylococci (MRCNS), ciprofloxacin, clindamycin, and erythromycin susceptibility were 17.5%, 57.5%, and 12.5%, which were all less than that of methicillin-susceptible Coagulase-negative Staphylococci (MSCNS), 67.5%, 85%, and 32.5%, respectively. Ceftobiprole was two-fold more active on MSCNS strains with MIC50/90 of 0.25/0.5 mg/L than on MRCNS strains with MIC50/90 of 1/2 mg/L (Table 1).
Ceftobiprole was also active against 24 E. faecalis with MIC50/90 of 0.5/1 mg/L but showed no clinically relevant activity against 24 E. faecium with both MIC50 and MIC90 > 32 mg/L. All E. faecium were susceptible to vancomycin and linezolid, but 8.3% of E. faecalis was intermediate to linezolid. For E. faecalis, the resistance rate to ampicillin, ciprofloxacin, and erythromycin was much less than that for E. faecium (8.3%, 29.2%, and 62.5% VS 82.6%, 87%, and 91.3%) (Table 2).
Ceftobiprole demonstrated good activity against PSSP (susceptibility of 98.2%), which was similar to linezolid and vancomycin, whereas only half of the PISP and PRSP were susceptible to it. Erythromycin showed poor activity against all S. pneumoniae. Ceftobiprole demonstrated potent activity against all 39 Streptococcus with MIC50/90 ≤ 0.015/ ≤ 0.015–2 mg/L, which is far better than that of linezolid and vancomycin (both MIC50/90 are 0.25/0.25–0.5 mg/L). All 13 Streptococcus pyogenes were resistant to erythromycin, while 35.7% of Streptococcus agalactiae and 33.3% of Streptococcus mitis remained susceptible to it (Table 3).
Ceftobiprole and comparator antibiotics activity against gram-negative bacteria
Ceftobiprole exhibited potent activity against Haemophilus influenzae (MIC50/90, ≤ 0.015/0.5 mg/L). Ceftobiprole also showed good activity against Moraxella catarrhalis with MIC50/90 of 0.25/0.5 mg/L. All H. influenzae and M. catarrhalis were inhibited at MIC of ≤ 1 mg/L ceftobiprole, and highly susceptible to ampicillin-sulbactam, cefuroxime, ceftazidime, ceftriaxone, and ciprofloxacin with susceptibility rates ranged from 63.9% to 100% (Table 4).
Ceftobiprole had limited activity (0% and 6.9% susceptible) against most ESBL-producers, in contrast to a susceptibility rate of 91.8% and 98.2% found against non-ESBL E. coli and K. pneumoniae. For non-ESBL strains, the potency of ceftobiprole was similar to ceftazidime, ceftriaxone, cefoperazone-sulbactam, imipenem, amikacin, colistin, and tigecycline, but against ESBL-producers, ceftobiprole performed worse than these other cephalosporins. Ceftobiprole also showed no activity against carbapenem-resistant K. pneumoniae (MIC50/90, > 128/ > 128 mg/L), some of which were susceptible to amikacin (40%), colistin (91.1%), and tigecycline (100%). Ceftobiprole showed moderate activity against E. aerogenes, C. freudii, P. mirabilis, and M. morganella, with over 50% of strains inhibited at ≤ 0.06 mg/L. For E. cloacae and S. marcescens, over 50% of strains were inhibited at 0.25–0.5 mg/L. Ceftobiprole had little activity against P. vulgaris, with MIC50/90 of 32/ > 128 mg/L (Table 5a-c).
Ceftobiprole also had limited activity against P. aeruginosa, independent of susceptibility to carbapenems, with MIC50/90 8/64- > 128 mg/L. Interestingly, for carbapenem-susceptible A. baumanni, 94.1% of strains were inhibited at ≤ 4 mg/L, showing the potency of ceftobiprole which was comparable to that of amikacin, cefoperazone-sulbactam, imipenem, colistin and tigecycline (MIC50/90 was 1/4, 1/2, 0.125/0.25 and 0.5/1 mg/L, respectively). However, for the carbapenem-resistant A. baumanni, ceftobiprole had negligible activity with a MIC50/90 of > 128 mg/L. For all P. aeruginosa and A. baumannii, colistin retained excellent in vitro activity (MIC50/90, 0.5–1/1–2 mg/L) (Table 6).
The MIC distribution of ceftobiprole is presented in Table 7a-b.
Discussion
As one of the limited new effective antibiotics approved for treating infection caused by resistant Gram-positive and Gram-negative bacteria, ceftobiprole has been evaluated in several studies in different medical centers around the world [7, 9, 10]. However, the published literature for its efficacy against contemporary clinical isolates from China is limited. In this study, we report on the activity of ceftobiprole and comparators against recent clinical isolates collected from hospitalized patients from 2016–2018 in China through the China Antimicrobial Surveillance Program. Our study suggest that ceftobiprole has high antibacterial activity against Staphylococcus (including MRSA) similar to the results from Europe and the United States [11]. We observed that MSSA strains were more susceptible to ceftobiprole than MRSA strains with one-fold lower MIC90. When compared to the earlier studies, the data reported in our study are comparable for ceftobiprole concerning the target gram-positive pathogens, such as Staphylococcus, E. faecalis, Streptococcus, supporting that ceftobiprole has a high susceptibility [9]. Ceftobiprole’s in vitro activity demonstrates potent binding against PBPs of gram-positive bacteria, including those with decreased β-lactam sensitivity, such as PBP2x and PBP2b in PRSP and, PBPa, which confers methicillin resistance to S. aureus strains [12].
Besides gram-positive bacteria, ceftobiprole also has good antibacterial activity against non-MDR gram-negative bacteria. Ceftobiprole exhibits a high affinity for PBPs in Enterobacterales but is labile to hydrolysis by common extended spectrum β-lactamases and carbapenemases. ESBL-negative E. coli and K. pneumoniae, MICs50/90 were both 0.03/0.06 mg/L in Europe and the USA, consistent with < = 0.06/0.25 mg/L in the current study. Previous MIC results, including the SENTRY Antimicrobial Surveillance Program in the U.S. (2016) and in Europe (2015), demonstrated the potency of ceftobiprole against Pseudomonas aeruginosa (MIC50/90, 2/ > = 16 mg/L) and had limited activity against Acinetobacter spp. (MIC50/90, > = 16/ > = 16 mg/L) [11, 13]. The data reported here showed a little difference in these two non-fermentative gram-negative bacteria with MICs50/90 were 8/ > 128 mg/L for carbapenem-susceptible P. aeruginosa and 0.5/4 mg/L for carbapenem-susceptible A. baumanni.
There were some limitations to our study. Firstly, ceftobiprole is approved for the treatment of community-acquired pneumonia and hospital-acquired pneumonia except for ventilator-associated pneumonia, but there is no relevant clinical disease information for the strains in our study. Secondly, there are a few strains of some Streptococcus spp, which may not fully demonstrate the antibacterial activity of cefpirome against such Streptococcus spp..
Conclusion
Our study indicated that ceftobiprole showed potent in vitro activity against clinical significant pathogens including MRSA, MRCNS, E. faecalis, PRSP, H. influenzae, M. catarrhalis, ESBL-negative Enterobacterales, even carbapenem-susceptible A. baumanni, which could be a considerable choice for treating infections caused by those pathogens in healthcare facilities.
Materials and Methods
Clinical strains
A total of 1163 strains were selected randomly from 49 hospitals across China from 2016-to 2018, relying on the China Antimicrobial Surveillance Network (CHINET). Strains included methicillin-resistant S. aureus (MRSA, n = 55), methicillin-susceptible S. aureus (MSSA, n = 55), methicillin-resistant Coagulase negative Staphylococci (MRCNS, n = 40), methicillin-susceptible Coagulase negative Staphylococci (MSCNS, n = 40), E. faecalis (n = 24), E. faecium (n = 23), Streptococcus pyogenes (n = 13), Streptococcus agalactiae (n = 14), Streptococcus mitis (n = 12), Streptococcus pneumonia (MIC of Penicillin ≤ 2 mg/L, PSSP, n = 110), Streptococcus pneumonia (MIC of Penicillin = 4 mg/L, PISP, n = 25), Streptococcus pneumonia (MIC of Penicillin ≥ 8 mg/L, PRSP, n = 13), Haemophilus influenzae (n = 53), Moraxella catarrhalis (n = 49), Escherichia coli (ESBL-, n = 49), Escherichia coli (ESBL + , n = 50), Klebsiella pneumoniae (ESBL-, n = 56), Klebsiella pneumoniae (ESBL + , n = 58), Enterobacter cloacae (n = 49), Enterobacter aerogenes (n = 55), Citrobacter freudii (n = 53), Proteus mirabilis (n = 52), Proteus vulgaris (n = 35), Morganella morganella (n = 53), Serratia marcescens (n = 53), Pseudomonas aeruginosa (n = 38) and Acinetobacter baumanni (n = 36). Species identification was performed at the microbial laboratory of Huashan Hospital by the matrix-assisted laser desorption ionization-time-of-flight mass spectrometry (MALDI-TOF, Vitek MS; bioMérieux). E. coli ATCC 25,922, P. aeruginosa ATCC 27,853, S. pneumoniae ATCC 49,619, H. influenzae ATCC 49,766 and ATCC 49,247, S. aureus ATCC29213 and E. faecalis ATCC 29,212 were used as the quality control strains in antimicrobial susceptibility testing.
Antimicrobial susceptibility testing
MICs were determined by the reference broth microdilution method recommended by the Clinical and Laboratory Standards Institute (CLSI) [14]. Ceftobiprole, linezolid, vancomycin, ampicillin, penicillin, oxacillin, ciprofloxacin, clindamycin, and erythromycin were tested for all Gram-positive bacteria; Ceftobiprole, ampicillin, ampicillin-sulbactam, cefuroxime, ceftazidime, ceftriaxone, ciprofloxacin, azithromycin, cefoperazone-sulbactam, imipenem, amikacin, colistin, and tigecycline were tested for Gram-negative bacteria as needed. Quality control and interpretation of the results were based on 2019 CLSI break-points for all the antimicrobial agents except tigecycline, for which CLSI criteria are not available [14]. Tigecycline MICs were interpreted using U.S. FDA MIC breakpoints for Enterobacterales (susceptible, ≤ 2 g/ml; resistant, ≥ 8 g/ml) (https://www.fda.gov/drugs/development-resources/tigecycline-injection-products).
Availability of data and materials
All data involved in this study are available from the corresponding author by email if needed.
References
Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–27. https://doi.org/10.1016/S1473-3099(17)30753-3.
Barbour A, Schmidt S, Rand KH, Derendorf H. Ceftobiprole: a novel cephalosporin with activity against Gram-positive and Gram-negative pathogens, including methicillin-resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents. 2009;34(1):1–7. https://doi.org/10.1016/j.ijantimicag.2008.12.012.
Hodille E, Delouere L, Bouveyron C, Meugnier H, Bes M, Tristan A, et al. In vitro activity of ceftobiprole on 440 Staphylococcus aureus strains isolated from bronchopulmonary infections. Med Mal Infect. 2017;47(2):152–7. https://doi.org/10.1016/j.medmal.2016.10.004.
Falco V, Burgos J, Almirante B. Ceftobiprole medocaril for the treatment of community-acquired pneumonia. Expert Opin Pharmacother. 2018;19(13):1503–9. https://doi.org/10.1080/14656566.2018.1516749.
Giacobbe DR, De Rosa FG, Del Bono V, Grossi PA, Pea F, Petrosillo N, et al. Ceftobiprole: drug evaluation and place in therapy. Expert Rev Anti Infect Ther. 2019;17(9):689–98. https://doi.org/10.1080/14787210.2019.1667229.
Morosini MI, Díez-Aguilar M, Cantón R. Mechanisms of action and antimicrobial activity of ceftobiprole. Rev Esp Quimioter. 2019;32 Suppl 3(Suppl 3):3–10.
Flamm RK, Duncan LR, Hamed KA, Smart JI, Mendes RE, Pfaller MA. Ceftobiprole activity against bacteria from skin and skin structure infections in the United States from 2016 through 2018. Antimicrob Agents Chemother. 2020;64(6):e02566-e2619. https://doi.org/10.1128/AAC.02566-19.
Cillóniz C, Dominedò C, Garcia-Vidal C, Torres A. Ceftobiprole for the treatment of pneumonia. Rev Esp Quimioter. 2019;32 Suppl 3(Suppl 3):17–23.
Pfaller MA, Flamm RK, Mendes RE, Streit JM, Smart JI, Hamed KA, et al. Ceftobiprole activity against gram-positive and -negative pathogens collected from the United States in 2006 and 2016. Antimicrob Agents Chemother. 2019;63(1):e01566-e1618. https://doi.org/10.1128/AAC.01566-18.
Horner C, Mushtaq S, Livermore DM, BSAC Resistance Surveillance Standing Committee. Activity of ceftaroline versus ceftobiprole against staphylococci and pneumococci in the UK and Ireland: analysis of BSAC surveillance data. J Antimicrob Chemother. 2020;75(11):3239–43. https://doi.org/10.1093/jac/dkaa306.
Pfaller MA, Huband MD, Streit JM, Flamm RK, Sader HS. Surveillance of tigecycline activity tested against clinical isolates from a global (North America, Europe, Latin America and Asia-Pacific) collection (2016). Int J Antimicrob Agents. 2018;51(6):848–53. https://doi.org/10.1016/j.ijantimicag.2018.01.006.
Farrell DJ, Flamm RK, Sader HS, Jones RN. Activity of ceftobiprole against methicillin-resistant Staphylococcus aureus strains with reduced susceptibility to daptomycin, linezolid or vancomycin, and strains with defined SCCmec types. Int J Antimicrob Agents. 2014;43(4):323–7. https://doi.org/10.1016/j.ijantimicag.2013.11.005.
Pfaller MA, Flamm RK, Duncan LR, Streit JM, Castanheira M, Sader HS. Antimicrobial activity of ceftobiprole and comparator agents when tested against contemporary Gram-positive and -negative organisms collected from Europe (2015). Diagn Microbiol Infect Dis. 2018;91(1):77–84. https://doi.org/10.1016/j.diagmicrobio.2017.12.020.
CLSI. Performance Standards for Antimicrobial Susceptibility Testing. 29th ed. CLSI supplement M100. Wayne: Clinical and Laboratory Standards Institute; 2019.
Acknowledgements
We gratefully acknowledge the contributions of the members of CHINET for collection of the isolates tested in this study.
Funding
This work was funded by the National Key Research and Development Program of China (2021YFC2701803), the China Antimicrobial Surveillance Network (Independent Medical Grants from Pfizer, 2018QD100), and Shanghai Antimicrobial Surveillance Network (3030231003).
Author information
Authors and Affiliations
Contributions
WS and ZYG performed the major work of antibiotics susceptibility testing; GY and YY performed the major work of strains collection; YDD analyzed and interpreted the susceptibility data and was a major contributor in writing the manuscript; ZDM and HFP contributed to the study design and the manuscript review. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
We confirmed that all methods were carried out in accordance with relevant guidelines and regulations; all experimental protocols were approved by the Institutional Review Board of Huashan Hospital, Fudan University (No.2017–321). None of human participants were directly involved in the study, so the informed consent was not applicable here.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dandan, Y., Shi, W., Yang, Y. et al. Antimicrobial activity of ceftobiprole and comparator agents when tested against gram-positive and -negative organisms collected across China (2016–2018). BMC Microbiol 22, 282 (2022). https://doi.org/10.1186/s12866-022-02699-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-022-02699-4