Abstract
Background
Penicillium oxalicum is an important fungal agent in the composting of cattle manure, but the changes that occur in the microbial community, physicochemical factors, and potential functions of microorganisms at different time points are still unclear. To this end, the dynamic changes occurring in the microbial community and physicochemical factors and their correlations during the composting of cattle manure with Penicillium oxalicum were analysed.
Results
The results showed that the main phyla observed throughout the study period were Firmicutes, Actinobacteria, Proteobacteria, Bacteroidetes, Halanaerobiaeota, Apicomplexa and Ascomycota. Linear discriminant analysis effect size (LEfSe) illustrated that Chitinophagales and Eurotiomycetes were biomarker species of bacteria and eukaryote in samples from Days 40 and 35, respectively. Bacterial community composition was significantly correlated with temperature and pH, and eukaryotic microorganism community composition was significantly correlated with moisture content and NH4+-N according to redundancy analysis (RDA). The diversity of the microbial communities changed significantly, especially that of the main pathogenic microorganisms, which showed a decreasing trend or even disappeared after composting.
Conclusions
In conclusion, a combination of high-throughput sequencing and physicochemical analysis was used to identify the drivers of microbial community succession and the composition of functional microbiota during cattle manure composting with Penicillium oxalicum. The results offer a theoretical framework for explaining microecological assembly during cattle manure composting with Penicillium oxalicum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
The beef cattle industry is one of six unique industries in the Ningxia Hui Autonomous Region. In recent years, with the rapid development of large-scale and intensive breeding operations, the amount of animal manure produced has increased sharply [1]. China's annual rate of animal waste production has reached approximately 3.8 billion tons, among which the amount of cattle manure produced is as high as 1.38 billion tons, but the comprehensive utilization rate is less than 60% [2]. Due to the large quantity of the discharged cattle manure and the lack of safe treatment technologies, the manure cannot be centralized in a timely manner and results in increasingly severe environmental pollution [3]. Therefore, finding methods to reasonably manage and use animal manure has become an urgent problem to be solved.
At present, composting is the main method to treat and utilize livestock and poultry manure [4]. Composting involves the transformation of refractory organic matter into stable and pathogen-free humus under aerobic conditions and under the joint action of various microorganisms, accompanied by a series of physical and chemical reactions [5]. Livestock manure plays an important role in improving soil fertility and crop production [6]. Cattle manure contains macronutrients (i.e., nitrogen, phosphorus, potassium) and organic matter components. Thus, it has a high potential for use as fertilizer. It is often used in the production of biofertilizers; additionally, cattle manure contains significant amounts of lignocellulosic materials such as cellulose and hemicellulose and can be used to produce solid fuels [7, 8]. However, conventional composting techniques have drawbacks, such as lengthy composting periods, sluggish cellulose decomposition rates, and low levels of humus formation [9]. Some microorganisms can secrete cellulase to hydrolyse cellulose and degrade organic agricultural waste, so they are used as compost inoculants [10]. In a previous study, to improve the efficiency of composting, the physicochemical properties of organic matter and the microbial structure of piles were altered with the addition of beneficial exogenous bacteria, and the microbial growth environment was optimized to improve the degradation efficiency and degree of humification of the organic matter [11]. Therefore, the addition of effective microbial agents in the composting process produces better results than traditional compost in terms of compost performance, the cellulose degradation rate, the nutrient retention rate, and the degree of maturation. It can also effectively reduce pathogenic microorganisms in manure and change various composting process characteristics, such as temperature, pH, and the C/N ratio [12].
Penicillium oxalicum promotes cellulose degradation. It has a short growth cycle and high temperature resistance [13]. Penicillium oxalicum has strong cellulase secretion abilities. It produces a complete cellulase enzyme system with high enzyme activity and high yield [13]. In the composting process, Penicillium oxalicum can be used as a ripening agent, and the cellulose degradation rate of inoculated material is 50% faster than that in traditional compost [14]. In addition, the maximum adsorption rates of Cu2+ and Ni2+ by Penicillium oxalicum were 94% and 80%, respectively. It has a strong tolerance to heavy metals and has strong application potential in soil heavy metal pollution remediation [15]. In fact, during the composting of cow manure and straw as raw materials, an experimental group inoculated with thermophilic fungi had a longer high-temperature period, a faster rate of decline in total organic carbon (TOC) and C/N, a significant increase in total nitrogen content, a higher degradation rate of cellulose and lignin, a higher germination index (GI) value, and greater maturation than did a control group [10]. Moreover, compared with the control group, the microbial community structure of the inoculated group significantly changed [13]. Therefore, Penicillium oxalicum has good application potential for compost production, cellulose degradation, biological control, phosphorus solubilization, heavy metal adsorption, etc., and is an effective functional microorganism.
To date, many studies have confirmed that cattle manure composting with Penicillium oxalicum results in better composting efficiency than composting without Penicillium oxalicum, and the structure of the microbial community significantly changes with inoculation [13, 14]. However, the changes that occur in the microbial community and physicochemical factors during the whole composting period for cattle manure inoculated with Penicillium oxalicum and related correlations are still unclear. Therefore, in this study, high-throughput sequencing, physicochemical property analysis, and other technologies were combined to accurately analyse dynamic changes in microbial communities and compost physicochemical properties and explore the potential functions of microorganisms at different time points in the composting of cattle manure with Penicillium oxalicum.
Results
Changes in organic matter physicochemical parameters during composting
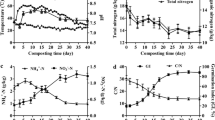
The temperature profiles at the top, middle, and bottom of the compost heap are shown in Fig. 1A. A linear increase in the temperature of the compost pile was observed for 1–4 days, and the temperature of the middle layer reached 45°C on the 4th day. The compost temperature remained above 45°C from 5–25 days. The temperature of the compost pile slowly decreased linearly from 25–34 days, and the temperature of the compost pile was basically consistent with the outside temperature from Days 35 to 40. During the composting process, the temperature on the 13th day was the highest, at approximately 60°C, and the temperature was stable (> 50°C) from 15–25 days. According to the temperature change, the composting process involved four stages: the mesophilic stage (0 d ~ 5 d), thermophilic stage (6 d ~ 25 d), cooling stage (26 d ~ 34 d) and maturation stage (35 d ~ 40 d).
During the composting process, the moisture content of the raw materials was approximately 53%, and the moisture content decreased linearly with increasing composting time. The moisture content of the compost product on Day 40 was approximately 32% (Fig. 1B and Table S1). The pH of the manure before composting was approximately 9.6 and decreased linearly from Days 0 to 10, and the minimum pH was approximately 8.9. From Days 10 to 40, the pH of the compost products was basically stable at approximately 9 (Fig. 1C and Table S1). The NH4+-N and NO3−-N content in the compost decreased from 1477.75 mg/kg and 874.41 mg/kg in the initial stage to 207.04 mg/kg and 282.22 mg/kg on Day 40, respectively, and they showed a linear decreasing trend (Fig. 1D, 1E and Table S1). The total potassium and total phosphorus levels decreased from 20.37 g/kg and 3.37 g/kg to 20.17 g/kg and 2.45 g/kg, respectively, during the first five days of composting. With manure composting, the levels of total potassium and total phosphorus increased. Their contents increased to 22.68 g/kg and 3.12 g/kg, respectively, on Day 40 of composting (Fig. 1F, 1G and Table S1). Crude fibre accounted for 24.55% of the raw compost materials. From Day 0 to Day 30, the crude fibre degraded rapidly, and its content decreased to 12.18%. Its content remained stable from Day 30 to Day 40. Its content at the end of composting was 12.93%. Throughout the composting process, the content of crude fibre tended to decrease (Fig. 1H and Table S1). The organic carbon content showed a downwards trend. The organic carbon content was 200 g/kg before composting and decreased to 140 g/kg after composting (Fig. 1I and Table S1). There was no difference in the composting process before and after the transformation of metal constituents such as arsenic, mercury, cadmium, copper, and zinc. The chromium content decreased significantly after composting (p < 0.01) (Fig. 1J and Table S2). After manure composting, the chloride ion concentration tended to increase, and there was a significant difference compared with that on Day 0 (p < 0.01) (Fig. 1K and Table S2).
Dynamic variations in the diversity and richness of microbial communities
Alpha diversity of microbial communities
The species accumulation curve was plotted according to the ASVs (Fig. 2). The species accumulation curve gradually flattened, and the coverage rate of each sample sequence was greater than 96%. The results showed that the sequencing efforts were sufficient, the sequencing data were representative, and the results were accurate and objective. The Shannon index of bacteria increased significantly from Day 30 to Day 40 of composting, while the Chao1 index decreased significantly with the composting process. Compared with those at other time points, the Chao1 index of bacteria on Day 5 of composting was greater (p < 0.05) (Table 1). The results showed that the species richness of bacteria increased on the 5th day of composting, and the species richness of bacteria decreased on the 30th day. Furthermore, the Chao1 index of eukaryotic microorganisms on Day 10 was significantly different (p < 0.05) (Table 2), indicating that the species richness increased compared with that at other time points. There was no significant difference between the Shannon and Simpson indices of eukaryotic microorganisms (p > 0.05).
Beta diversity of the microbial communities (NMDS analysis)
The results of the nonmetric multidimensional scaling (NMDS) analysis showed that the community structure of eukaryotic microorganisms significantly differed at the different composting time points. However, the matrix distances of the bacterial community were shorter than those of the eukaryotic community (Fig. 3). For bacteria, samples Po0 and Po5 were clustered together, far from samples Po35 and Po40, indicating that the bacterial community composition at the mesophilic stage (Day 0–5) was different from that at the maturation stage (Day 35–40) (Fig. 3A). For eukaryotic microorganisms, sample Po0 was far from Po40, and Po25 was clustered together, indicating a significant difference in the composition of the eukaryotic microorganism community between the 0th day and 40th day (Fig. 3B).
Bacterial community succession
At the phylum level, a total of 28 bacterial phyla were identified from samples collected at nine time points during the composting process. The Fig. 4 lists the top 20 phyla in terms of relative abundance. As shown in Fig. 4A, Firmicutes and Actinobacteria had the highest relative abundances. This was followed by Proteobacteria, Bacteroidetes, Halanaerobiaeota, Gemmatimonadetes, and Chloroflexi. The bacteria in nine compost samples were classified at the phylum level, and the relative abundance of bacteria tended to increase during the composting process. Firmicutes, Proteobacteria, Actinobacteria, and Bacteroidetes were the dominant phyla in Po0, and their relative abundances were 43.04%, 26.57%, 17.49%, and 11.74%, respectively. In Po5, the abundances of Firmicutes and Proteobacteria decreased by 5.95% and 10.57%, respectively, while the relative abundances of Bacteroidetes and Actinobacteria increased by 14.1% and 1.2%, respectively. With increasing temperature from Days 10 to 25, the relative abundances of Firmicutes and Bacteroidetes decreased by 26.54% and 7.71%, respectively, while the relative abundances of Actinobacteria, Proteobacteria, Gemmatimonadetes, and Chloroflexi increased by 30.25%, 5.41%, 2.36%, and 1.26%, respectively. In addition, the relative abundance of Halanaerobiaeota increased from 0% to 5.87% and then decreased to 1.58%. Compared with those in Po25, the proportions of Bacteroidetes, Chloroflexi, Gemmatimonadetes, and Deinococus Thermus increased in Po35 and Po40, while the proportions of Proteobacteria and Patescibacteria decreased. Actinobacteria were the dominant phylum in Po30, Po35 and Po40.
The changes in the bacterial communities at the genus level are shown in Fig. 4B. In the compost materials, the dominant bacterial genera were Psychrobacter (19.06%), Aequorivita (6.7%), Romboutsia (6.62%), and Corynebacterium_1 (6.41%). Paeniclostridium, Clostridium_sensu_stricto_1, and Planococcaceae were also present. The relative abundance of the dominant genus Aequorivita increased to 14.59%, whereas that of Psychrobacter decreased to 4.43% in Po5. However, when the temperature increased, the relative abundances of Psychrobacter and Aequorivita decreased and eventually decreased from 10 to 25 days, while the relative abundances of Actinomadura and Longispora steadily increased from 0.01% and 0.03% to 20.88% and 10.00%, respectively. Actinomadura became the dominant genus. In Po30, the relative abundance of Actinomadura gradually decreased to 11.09% with decreasing temperature. In Po35, the relative abundance of Longispora gradually increased to 20.82%, and it was the dominant genus. In Po40, Actinomadura was the dominant genus, and its relative abundance increased to a maximum of 20.33%.
Succession of the eukaryotic microbial community
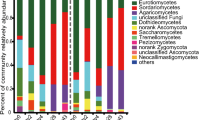
The eukaryotic microbial community structure at the phylum level is shown in Fig. 5A. The relative abundance of Apicomplexa (37.02%) in Po0 was the highest, followed by unclassified_Eukaryota, Cercozoa, Ascomycota, and Neocallimastigomycota, accounting for 26.76%, 20.12%, 7.70%, and 4.25%, respectively. As the composting process continued, the eukaryotic microbial community structure in the samples changed significantly. From Day 0 to Day 10, the relative abundance of Neocallimatigomycota declined steadily, and eventually, they were no longer detected. From Day 10 to Day 20, the relative abundance of Ascomycota increased from 0.07% to 28.33%, gradually becoming the dominant phylum. In Po30, the abundance of Ascomycota decreased to 0.99% due to the gradual decrease in temperature, but its abundance increased to 19.18% in Po35. In the Po40 treatment at the maturation stage, the relative abundance of Nematoda (71.56%) was the highest, followed by that of Arthropoda (12.48%). Moreover, compared with that of Po0, the relative abundance of Apicomplexa decreased by 34.26%.
The community structure at each time point at the genus level is shown in Fig. 5B. Throughout the composting process, Cryptosporidium was present. In Po0, the dominant genera were Cryptosporidium (36.99%), unclassified_Eukaryota (26.76%), unclassified_Cercozoa (17.36%), and Geotrichum (4.45%). The relative abundance of Chlamydomyxa quickly increased from Day 0 to Day 5 of composting, from 0.43% to 52.62%, and it became the dominant genus in Po5, whereas the relative abundance of Cryptosporidium declined by 14.35%. The relative abundance of Chlamydomyxa peaked at 54.08% in Po10, whereas that of Cryptosporidium declined to 2.37%. As composting progressed to the thermophilic stage, the relative abundance of Chlamydomyxa decreased to 1.57% in Po15, while the relative abundance of Cryptosporidium increased to its maximum value of 48.09%. Melanocarpus (28.11%) gradually became the dominant genus in Po20 at the thermophilic stage. With decreasing temperature, the number of species of eukaryotic microorganisms decreased sharply in Po30, among which Rhabditida was the dominant genus, with a relative abundance of 11.33%. In the Po35 and Po40 strains at the maturation stage, the dominant genera were Thermomyces (16.46%) and Rhabditida (64.88%), respectively.
LEfSe of the bacterial and eukaryotic microbial communities
To elucidate the differences in the microbial community at various time points in the composting pile, biomarkers were separated using the linear discriminant analysis effect size (LEfSe) method. The LEfSe results supported LDA scores ≥ 4. The cladogram shows these indicator groupings. Throughout the composting process, different taxa of bacteria and eukaryotic microorganisms existed. As shown in Fig. 6A and B, 88 bacterial clades presented statistically significant differences during composting. LEfSe revealed that the bacterial biomarkers in Po0 and Po5 were Pseudomonadales and Bacteroidetes and were Firmicutes, Peptostreptococcaceae, Alphaproteobacteria, and Actinobacteria in Po10, Po15, Po20 and Po25. The biomarker organisms in Po30, Po35 and Po40 were Balneolaceae, Actinobacteria and Chitinophagales, respectively. When the LDA threshold was ≥ 4, 35 eukaryotic clades also showed statistically significant differences during composting. Saccharomycetes and Saccharomycetales were biomarker species in Po0. Chlamydomyxa, Conoidasida, and Ascomycota were indicator groups in Po10, Po15 and Po20 for the thermophilic stage, while Eurotiomycetes was the biomarker in Po35 at the maturation stage (Fig. 6C, D).
Cladogram showing the phylogenetic distribution of the bacterial and eukaryotic lineages associated with compost (A and C). Indicator organisms with linear discriminant analysis scores ≥ 4 in the bacterial and eukaryotic microorganism communities (B and D). The differently coloured regions represent different stages. Circles indicate phylogenetic levels from domain to genus. The diameter of each circle is proportional to the abundance of the group
Pathogenic microorganism analysis
The changes in the relative abundance of pathogenic microorganisms associated with cattle during composting are shown in Table S3 and Table S4. During the composting process, most prokaryotic pathogens showed an increasing trend from Day 0 to 10, a decreasing trend from Day 10 to 25, and an increasing trend from Day 25 to 30. There was a negative correlation between temperature and microbial changes. The results showed that low temperatures promoted the growth of microorganisms, while high temperatures reduced the content of microorganisms. Corynebacterium and Clostridium, the main pathogens that cause bovine diarrhoea, and pathogenic bacteria that cause bovine respiratory infections, including Staphylococcus, Actinomyces, Klebsiella, Proteus, and Acinetobacter, multiplied to reach large numbers within 0–10 days. However, after 10 to 25 days of high-temperature composting, the number of these bacteria decreased significantly, or they were no longer detected. However, in the composting process, the abundances of Bacillus, Streptococcus, and Escherichia Shigella decreased with increasing temperature and gradually increased with decreasing temperature from the 30th to 40th days.
With increasing temperature during composting, the relative abundances of Entamoeba, Cryptosporidium, Eimeria, Candida, Aspergillus, and other eukaryotic microorganisms decreased, or these organisms disappeared completely. The results showed that high temperature inhibits the growth of most parasites and fungi. In Po35 and Po40, the relative abundances of Rhabditida and Arachnida tended to increase.
Relationships between the microbial community and environmental factors
A link between environmental conditions and the growth of bacteria and eukaryotes was demonstrated using redundancy analysis (RDA). The arrows in the image indicate different environmental factors, and the length of the arrows shows the size of the impact. The first axis accounted for 59.15% and 28.78% of the observed bacterial (Fig. 7A) and eukaryotic (Fig. 7B) diversity, respectively, and the second axis accounted for 10.23% and 9.9%, respectively. Temperature, NH4+-N, and NO3−-N had significant effects on the bacterial community, while moisture content, NH4+-N, and NO3−-N had significant effects on the eukaryotic community composition. Angles less than 90 degrees between the environmental variables and sample dots indicate a positive correlation in the graphs. Smaller angles show stronger relationships. Temperature had a significant effect on the change in the bacterial community from the 5th to the 25th day. NH4+-N and NO3−-N had a significant impact on the composition of eukaryotic microorganism communities over the first 15 days.
Redundancy analysis (RDA) of bacterial communities and environmental factors. RDA was used to assess relationships among bacterial communities (A), between environmental factors and eukaryotic microorganism communities (B) and among environmental factors during the composting process. The values on the axes are the variabilities explained by the corresponding axes
Discussion
In this study, dynamic changes in physicochemical factors and microbial diversity and abundance were observed in different stages of cattle manure composting with Penicillium oxalicum. Stable systems were formed as composting progressed due to interactions between microbes. Not only did the relative abundance of microbes significantly differ at the nine time points, but the composition of dominant eukaryotic genera also fluctuated greatly. After composting, we found that the relative abundance of the main pathogenic microorganisms showed an overall downwards trend, or they were not detected. This also indicated that the environmental contamination potential of the organic material was reduced [13].
Changes in the physicochemical properties and internal microbial interactions propel the succession of microbial communities [16]. In our study, physicochemical indices played a crucial role in the evolution of microbial communities [17]. Nitrogen and organic carbon are crucial components for microbial development and energy production in the composting process [18]. In addition, the C/N ratio is thought to be a good indicator of decomposition and composting; a lower C/N ratio indicates a more mature state of decomposition and composting [19, 20]. On the 59th day after cattle manure composting under natural conditions, the C/N ratio was 17.23 [21], while on the 40th day after cattle manure composting with Penicillium oxalate, the C/N ratio reached 12.96, which satisfied the requirement for compost maturity ≤ 20 [22]. In this study, the temperature exceeded 55 °C on the 10th day, and temperatures above 55 °C were maintained for more than 10 days. The natural compost of cattle manure reached a maximum temperature of 55 °C at 41 d and remained above 55 °C for only two days [23]. This is consistent with the results of previous studies on the composting of cattle manure with Penicillium oxalicum, and the composting period was shorter than that under natural conditions [13, 21]. Moreover, pH not only affects the activity of microorganisms but also affects the presence of nitrogen in the compost system. With increasing temperature, microorganisms decompose proteins to produce NH4+, which accelerates the conversion of organic nitrogen into NH4+-N, resulting in an increase in pH. However, the acid produced by microorganisms also reduces the pH of compost piles [24]. In addition, pH is impacted by moisture content changes caused by water evaporation [25]. In our study, the pH fluctuations were largely consistent with changes in NH4+-N, indicating that microbial denitrification may be important for pH changes. In another study, mature compost prepared from cattle manure was inoculated with the lignocellulolytic fungus Penicillium expansum. Compared to uninoculated compost, inoculated compost exhibited more than 1.2 g kg−1-dw more NH4+-N, and lignocellulose degradation proceeded 57.5% faster [14]. This is consistent with the downwards trend in NH4+-N observed in this study. Furthermore, pH fluctuations may have an impact on the progress of the composting process and microorganisms [26].
Due to changes in the organic matter physicochemical properties and the degradation of compost materials, the main microorganisms in the raw compost materials changed continuously during composting. In this study, high-throughput sequencing was used to analyse changes in bacterial and eukaryotic microbial communities during the composting of cattle manure with Penicillium oxalicum. Different temperature stages typically occur during composting. The results showed that the Chao1 and Shannon indices of bacteria were significantly greater in Po5 than at the other time points and were the lowest in Po30. However, unlike bacteria, the relative abundance of eukaryotes decreased significantly in Po15 and Po25. The results showed that the bacterial and eukaryotic communities responded differently to temperature, which is consistent with the results of Tiquia et al. [27]. Therefore, this study showed that high temperatures had a significant screening effect on the eukaryotic community, while the relative abundance and diversity of the bacterial community increased under high temperatures. Due to changes in physicochemical parameters and the degradation of organic matter, the composting process significantly affects the microbial population [28]. Bacteria might be able to survive under high-temperature conditions because bacterial growth is supported by the organic matter present in the manure compost system [29]. Numerous mesophilic microorganisms die or hibernate in the thermophilic stage (Po10 ~ Po25), and thermophilic microorganisms play a crucial role in the degradation of organic waste [30]. As a result, throughout the whole composting process, the crude fibre content gradually decreased, notably during the thermophilic stage. In Po30 and Po35, the amount of crude fibre tended to remain steady. The crude fibre content gradually stabilized during the cooling and maturation stages, as there was no significant difference in crude fibre between Po30 and Po40. A similar result was reported in another study wherein thermophilic microbial consortium inoculation enhanced organic matter degradation and improved humification during composting [31]. In addition, relevant studies have shown that the degradation of organic matter is a key parameter reflecting the mineralization and conversion of organic matter in the composting process [32]. In the mesophilic stage, soluble and easily decomposed organic matter such as sugars breaks down first, resulting in a continuous heating of the pile. During the thermophilic stage, cellulose and protein decompose, and the organic matter content decreases. During the cooling stage, the residual lignin and other substances further decompose, and the organic matter content further decrease. As the composting process reaches maturity, the content of organic matter gradually stabilizes [33].
In this study, the relative abundances of Firmicutes and Proteobacteria were greater in the first 10 days of composting, which was consistent with the findings of Vidya de Gannes [34]. Firmicutes was the predominant phylum in the early stages of composting, and its relative abundance gradually increased. This bacterium can adapt to composting systems consisting of various organic wastes and has the ability to efficiently utilize carbohydrates [35]. Proteobacteria can degrade macromolecular polysaccharides such as starch and cellulose, which play important roles in the composting process [36]. In Po15, Po20 and Po25 of the thermophilic stage, Firmicutes and Actinobacteria became the dominant phyla, probably because Firmicutes can produce endospores and have the ability to resist extreme environments [37, 38]. The relative abundance of Actinobacteria gradually increased from the thermophilic stage to the maturation stage and dominated the maturation stage. This result was similar to those of previous studies [28, 39]. Actinobacteria have the ability to effectively degrade cellulose, hemicellulose, and lignin [39]. In our study, the eukaryotic community significantly changed during composting, which was consistent with the findings of Langarica Fuentes et al. [40]. There were relatively high abundances of Ascomycota throughout the entire composting process on Days 0, 20, and 35. Relevant studies have shown that Ascomycota is the dominant phylum during the composting of cow manure and corn straw [41]. This might occur because Ascomycota can effectively utilize nutrients in compost materials and release a number of enzymes that degrade cellulose and hemicellulose [42]. Our study revealed that Melanocarpus and Thermomyces were the dominant eukaryotic genera on Days 20 and 35, respectively. Melanocarpus is a thermophilic eukaryotic genus that can produce endoglucanase [43]. During the composting process, cellulose and lignin can be effectively decomposed, which may be very important for promoting the humification of compost [44]. The abundance of Thermomyces was related to the degradation of hemicellulose. Therefore, Thermomyces may have high activity, releasing heat by decomposing hemicellulose and increasing compost maturity in the late composting stage. Overall, eukaryotic communities play a crucial role in degrading different organic materials, and potential strains that effectively improve compost efficiency have been identified.
Redundancy analysis revealed that the abundance and community composition of bacteria and eukaryotes at different composting time points were significantly affected by different physicochemical parameters. The effect of NH4+-N and NO3−-N on the composition of the bacterial community was more significant than that on the composition of the eukaryotic community, indicating that bacterial community succession was more sensitive to changes in NH4+-N and NO3−-N. This might occur because NH4+-N is the preferred nitrogen source for most microorganisms [44]. In addition, the abundances of Psychrobacter, Romboutsia, Chlamydomyxa, and Cryptosporidium were significantly positively correlated with NH4+-N and NO3−-N. These results are similar to those of previous studies [26], in which NH4+-N and NO3−-N were the main environmental factors affecting the bacterial community structure in the composting process of agricultural waste. Therefore, NH4+-N and NO3−-N are important factors that affect the composting process.
There are several limitations to this study. First, we did not use a negative control to monitor the effect of environmental factors on the composting process of cattle manure without Penicillium oxalicum. However, the high efficiency of Penicillium oxalicum in cellulose degradation was confirmed in preliminary experimental studies conducted by our research group. Moreover, Penicillium oxalicum has been successfully used to decompose straw returned to fields [45]. Therefore, we used samples collected on Day 0 as controls to track changes in the physicochemical indices and microbial diversity of the piles at various time points during the composting process in the presence of Penicillium oxalicum. Second, the analysis of bacterial and eukaryotic microorganism communities was mainly based on the analysis of 16S rRNA and 18S rRNA gene sequences, which was not sufficient to reveal the functions of the strains and microorganisms [46]. Third, we did not monitor the complete transformation process of Penicillium oxalicum. Due to limitations related to 18S rRNA gene sequencing, it could not be classified at the Penicillium genus level. Therefore, Penicillium oxalicum is represented as “d_Eukaryota, p_Ascomycota, c_Eurotiomycete” [47]. Despite these shortcomings, this study helps to clarify the dynamics of bacterial and eukaryotic microbial communities during the composting of cattle manure in the presence of Penicillium oxalicum and their correlation with physicochemical indicators. Therefore, microbial additives that facilitate the decomposition of organic matter or promote the nitrogen cycle in the late stage can be developed, and physicochemical indices can be regulated to improve the efficiency of cattle manure composting and improve compost maturation rate and quality.
Conclusions
In conclusion, a combination of high-throughput sequencing and physicochemical analysis was used to identify the drivers of microbial community succession and the composition of functional microbiota during the composting of cattle manure with Penicillium oxalicum. The results showed that the species and relative abundances of bacteria and eukaryotes changed significantly. The dominant bacterial phyla in the composting process included Firmicutes and Actinobacteria for bacteria and Apicomplexa and Ascomycota for eukaryotes. RDA revealed that temperature, pH, moisture content, NH4+-N, and NO3−-N had significant effects on the succession of the bacterial and eukaryotic communities. The results confirmed dynamic changes in the bacterial and eukaryotic microbial communities and relationships between the microorganisms and physicochemical indices during the whole composting period of cattle manure with Penicillium oxalicum. The results of this study provide a theoretical basis for the real-time monitoring of microbial growth and physicochemical factors in the composting of cattle manure with Penicillium oxalicum. Moreover, this study provides a scientific basis for the efficient utilization of livestock and poultry manure.
Methods
Composting experiment and sample collection
Cattle manure was collected from a cattle farm in Yinchuan, Ningxia. The microbial composting agent used was Penicillium oxalicum (KY781806.1) [45], at 1 × 108 CFUs mL−1, and the addition rate was 2%. The manure and Penicillium oxalicum were mixed evenly and piled into a trapezoid with a base width of 1.5 m, a top width of 0.8 m, and a height of 1.2 m at a composting site. The composting process was simple aerobic composting, and the composting time was 40 days.
Samples were taken from the top 1/3, 1/2, and bottom 1/3 of composted cattle manure on Days 0, 5, 10, 15, 20, 25, 30, 35, and 40. The samples were uniformly mixed and referred to as Po0, Po5, Po10, Po15, Po20, Po25, Po30, Po35, and Po40. The sample was divided into three parts, one of which was packed into a 15 mL cryopreservation tube and stored in liquid nitrogen for the detection of both prokaryotic and eukaryotic microorganisms. The other part was stored at 4 °C for the measurement of physicochemical indicators such as moisture content, pH, organic carbon, and total phosphorus. In the third part, 10% tartaric acid was added to 100 g of manure for nitrogen fixation, which was used for the determination of total nitrogen, NH4+-N, NO3−-N, and other indicators.
Determination of physicochemical parameters
The temperature of the environment and the temperature at the top 1/3, 1/2, and bottom 1/3 of the fermented cattle manure were measured once daily for 40 consecutive days. A fresh sample was placed in a beaker and allowed to dry at 105 °C to a constant weight, after which the moisture content of the sample was measured [48]. After fresh samples were shaken in water at a ratio of 1:10 (w/v) for 60 min at a speed of 120 rpm, pH was measured via a pH meter [21]. Total organic carbon (TOC) and total nitrogen (TN) content were determined by the potassium dichromate volumetric method and the Kjeldahl nitrogen determination method, respectively, and the ratio of the two was the carbon–nitrogen ratio (C/N) [49, 50]. NH4+-N and NO3−-N were extracted with 2 mol/L KCl and analysed by a dual channel flow analyser (AA3, Germany) [51]. The contents of total potassium and total phosphorus were determined by flame spectrophotometry [52]. The contents of arsenic and mercury were determined by atomic fluorescence spectrometry [53]. The contents of cadmium, lead, chromium, copper and zinc were determined by atomic absorption spectrometry [54, 55]. The chloride ion content in fertilizer was determined by mercuric nitrate titration [56].
DNA extraction and PCR amplification
Total genomic DNA was extracted from the samples using an OMEGA Soil DNA Kit (Omega Bio-Tek, Norcross, GA, USA) following the manufacturer’s instructions, and the DNA was stored at -80 °C until further analysis. The quantity and quality of the extracted DNA were measured using a Qubit 2.0 (Thermo Fisher Scientific, Waltham, MA, USA) to ensure that adequate amounts of high-quality genomic DNA had been extracted.
PCR amplification of the V3-V4 region of the bacterial 16S rRNA gene was performed using the forward primer 338F (5'-ACTCCTACGGGAGGCAGCA-3') and the reverse primer 806R (5'-GGACTACHVGGGTWTCTAAT-3', H stands for A, C, or T; V stands for A, C, or G; W stands for A or T) [57]. Sample-specific 7 bp barcodes were incorporated into the primers for multiplex sequencing. The V4 region of 18S rRNA was amplified with forward primer 547 (5’-CCAGCASCYGCGGTAATTCC-3’) and reverse primer 18SV4 (5’-ACTTTCGTTCTTGATYRA-3’, Y stands for C or T, R stands for A or G) [58]. The PCR conditions for the 16S rRNA gene V3-V4 region included pre-denaturation at 98 °C for 5 min, 25 cycles of denaturation at 98 °C for 30 s, annealing at 53 °C for 30 s, elongation at 72 °C for 45 s, and a final post-elongation cycle at 72 °C for 5 min. The PCR conditions for the 18S rRNA gene V4 region were pre-denaturation at 98 °C for 5 min, 33 cycles of denaturation at 98 °C for 30 s, annealing at 47 °C for 30 s, elongation at 72 °C for 45 s, and a final post-elongation cycle at 72 °C for 5 min. The PCR products were purified with Vazyme VAHTSTM DNA Clean Beads (Vazyme, Nanjing, China). For additional high-throughput sequencing, purified PCR products were delivered to the Illumina MiSeq platform at Shanghai Bioprofile Biotechnology Co., Ltd. (Shanghai, China). The raw sequence data with accession no. PRJNA990501 for the 16S rRNA gene and no. PRJNA990510 for the 18S rRNA gene were deposited in the NCBI Sequence Read Archive.
Sequence analysis
Illumina MiSeq™ was used to sequence samples collected at different composting time points [59,60,61]. Briefly, raw sequence data were demultiplexed using the demux plugin followed by primer cutting with the cutadapt plugin [62]. Imported paired reads were quality-filtered, denoised, dereplicated and merged using the plugin DADA2 to generate the amplicon sequence variant (ASV) feature table [63]. The feature sequence of each ASV was annotated for species by the naive Bayes classifier pretrained in QIIME2 software through the Silva database and the classification skylearn algorithm [64, 65]. The Specaccum species accumulation curves of amplicon sequence variants (ASVs) were generated to compare the abundance of ASVs between samples. Species composition, alpha diversity, and beta diversity were analysed in QIIME2 (2019.4) [66]. Additionally, linear discriminant analysis effect size (LEfSe) analysis and redundancy analysis (RDA) were conducted using R software (v3.2.0) [67, 68]. The effective sequences of the compost samples at different time points are shown in Table S5.
Statistical analysis
Physicochemical data analysis
The data are presented as the mean ± the standard deviation. Significant differences between the physical and chemical components of the composting process were determined using one-way ANOVA with the Bonferroni multiple comparison test. Statistical significance was defined as p < 0.05.
Bacterial and eukaryotic community structure analysis
Sequence data analyses were mainly performed using QIIME2, and R software's vegan package (v3.2.0) was used to calculate the diversity index and richness index. ASV-level alpha diversity indices, such as the Chao1 richness estimator, Shannon diversity index, Simpson index and Good’s coverage, were calculated using the ASV table in QIIME2. Beta diversity analysis was performed to investigate the structural variation in microbial communities across samples using Bray‒Curtis metrics [69] and visualized via nonmetric multidimensional scaling (NMDS) and unweighted pair-group method with arithmetic means (UPGMA) hierarchical clustering [70]. Linear discriminant analysis effect size (LEfSe) was performed to detect differentially abundant taxa across groups using default parameters [67]. Pathogenic microorganism data were assessed by one-way ANOVA with the Bonferroni multiple comparison test. For all analyses, p < 0.05 was considered significant. The redundancy analysis (RDA) method was used to analyse relationships between microbial community changes and physicochemical parameters [71].
Availability of data and materials
The data for the original contributions presented in this study are included in the article/Supplementary material, and further inquiries can be directed to the corresponding author. The raw sequence data with accession no. PRJNA990501 for the 16S rRNA gene and no. PRJNA990510 for the 18S rRNA gene were deposited in the NCBI Sequence Read Archive.
Abbreviations
- TOC:
-
Total organic carbon
- GI:
-
Germination index
- CFU:
-
Colony forming units
- PCR:
-
Polymerase chain reaction
- ASV:
-
Amplicon sequence variant
- NH4 +-N:
-
Ammonium nitrogen
- NO3 −-N:
-
Nitrate nitrogen
- NMDS:
-
Nonmetric multidimensional scaling
- LDA:
-
Linear discriminant analysis
- LEfSe:
-
Linear discriminant analysis effect size
- RDA:
-
Redundancy analysis
References
Mengqi Z, Shi A, Ajmal M, Ye L, Awais M. Comprehensive review on agricultural waste utilization and high-temperature fermentation and composting. Biomass Convers Biorefinery. 2021. https://doi.org/10.1007/s13399-021-01438-5.
Ma S, Fang C, Sun X, Han L, He X, Huang G. Bacterial community succession during pig manure and wheat straw aerobic composting covered with a semi-permeable membrane under slight positive pressure. Biores Technol. 2018. https://doi.org/10.1016/j.biortech.2018.03.054.
Shen Q, Sun H, Yao X, Wu Y, Wang X, Chen Y, et al. A comparative study of pig manure with different waste straws in an ectopic fermentation system with thermophilic bacteria during the aerobic process: performance and microbial community dynamics. Biores Technol. 2019. https://doi.org/10.1016/j.biortech.2019.01.029.
Xiuren H, Yu chen Y, Kai Z, Ganpei T, Bo L, Huan H, et al. Verification of agricultural cleaner production through rice-duck farming system and two-stage aerobic composting of typical organic waste. J Clean Product. 2022;337:130576. https://doi.org/10.1016/j.jclepro.2022.130576.
López-González JA, Suárez-Estrella F, Vargas-García MC, López MJ, Jurado MM, Moreno J. Dynamics of bacterial microbiota during lignocellulosic waste composting: studies upon its structure, functionality and biodiversity. Biores Technol. 2014. https://doi.org/10.1016/j.biortech.2014.10.123.
Ashekuzzaman SM, Poulsen TG. Optimizing feed composition for improved methane yield during anaerobic digestion of cow manure based waste mixtures. Biores Technol. 2010. https://doi.org/10.1016/j.biortech.2010.09.118.
Sfez S, De Meester S, Dewulf J. Co-digestion of rice straw and cow dung to supply cooking fuel and fertilizers in rural India: Impact on human health, resource flows and climate change. Sci Total Environ. 2017. https://doi.org/10.1016/j.scitotenv.2017.07.150.
Jurado MM, Suárez-Estrella F, López MJ, Vargas-García MC, López-González JA, Moreno J. Enhanced turnover of organic matter fractions by microbial stimulation during lignocellulosic waste composting. Biores Technol. 2015. https://doi.org/10.1016/j.biortech.2015.03.059.
Butler TA, Sikora LJ, Steinhilber PM, Douglass LW. Compost age and sample storage effects on maturity indicators of biosolids compost. J Environ Qual. 2001. https://doi.org/10.2134/jeq2001.2141.
Gou C, Wang Y, Zhang X, Lou Y, Gao Y. Inoculation with a psychrotrophic-thermophilic complex microbial agent accelerates onset and promotes maturity of dairy manure-rice straw composting under cold climate conditions. Biores Technol. 2017. https://doi.org/10.1016/j.biortech.2017.06.097.
Xie X-Y, Zhao Y, Sun Q-H, Wang X-Q, Cui H-Y, Zhang X, et al. A novel method for contributing to composting start-up at low temperature by inoculating cold-adapted microbial consortium. Biores Technol. 2017. https://doi.org/10.1016/j.biortech.2017.04.036.
Tran QNM, Mimoto H, Nakasaki K. Inoculation of lactic acid bacterium accelerates organic matter degradation during composting. Int Biodeterior Biodegradation. 2015. https://doi.org/10.1016/j.ibiod.2015.07.007.
Duan H, Fu C, Du G, Xie S, Liu M, Zhang B, et al. Dynamic microstructure assembly driven by lysinibacillus sp. LF-N1 and penicillium oxalicum DH-1 inoculants corresponds to composting performance. Microorganisms. 2022;10(4):709. https://doi.org/10.3390/microorganisms10040709.
Wang H-y, Fan B-q, Hu Q-x, Yin Z-w. Effect of inoculation with Penicillium expansum on the microbial community and maturity of compost. Biores Technol. 2011. https://doi.org/10.1016/j.biortech.2011.07.044.
Rania MAA. Optimization and statistical evaluation of copper and nickel biosorption capabilities by dry biomass of penicillium oxalicum JQ624873. Life Sci J. 2014;11(2):61–7.
Yanbo L, Haideng L, Shumei D, Zhou Z, Zhenke Z, Runna H, et al. Dynamic changes and correlations of microbial communities, physicochemical properties, and volatile metabolites during Daqu fermentation of Taorong-type Baijiu. LWT Food Sci Technol. 2022. https://doi.org/10.1016/j.lwt.2022.114290.
Yang X, Geng B, Zhu C, Li H, He B, Guo H. Fermentation performance optimization in an ectopic fermentation system. Biores Technol. 2018. https://doi.org/10.1016/j.biortech.2018.03.101.
Souri M, Naiji M. Nutritional value and mineral concentrations of sweet basil under organic compared to chemical fertilization. Acta scientiarum Polonorum Hortorum cultus = Ogrodnictwo. 2018.
Guo H, Zhu C, Geng B, Liu X, Ye J, Tian Y, et al. Improved fermentation performance in an expanded ectopic fermentation system inoculated with thermophilic bacteria. Biores Technol. 2015. https://doi.org/10.1016/j.biortech.2015.09.105.
Yang Y, Kumar Awasthi M, Du W, Ren X, Lei T, Lv J. Compost supplementation with nitrogen loss and greenhouse gas emissions during pig manure composting. Biores Technol. 2019. https://doi.org/10.1016/j.biortech.2019.122435.
Meng Q, Yang W, Men M, Bello A, Xu X, Xu B, et al. Microbial community succession and response to environmental variables during cow manure and corn straw composting. Front Microbiol. 2019. https://doi.org/10.3389/fmicb.2019.00529.
Keng ZX, Chong S, Ng CG, Ridzuan NI, Hanson S, Pan G-T, et al. Community-scale composting for food waste: a life-cycle assessment-supported case study. J Clean Prod. 2020. https://doi.org/10.1016/j.jclepro.2020.121220.
Zhang Z, Yang H, Wang B, Chen C, Zou X, Cheng T, et al. Aerobic co-composting of mature compost with cattle manure: organic matter conversion and microbial community characterization. Biores Technol. 2023. https://doi.org/10.1016/j.biortech.2023.129187.
Bai L, Deng Y, Li J, Ji M, Ruan W. Role of the proportion of cattle manure and biogas residue on the degradation of lignocellulose and humification during composting. Biores Technol. 2020. https://doi.org/10.1016/j.biortech.2020.122941.
Chen Q, Wang J, Zhang H, Shi H, Liu G, Che J, et al. Microbial community and function in nitrogen transformation of ectopic fermentation bed system for pig manure composting. Biores Technol. 2020. https://doi.org/10.1016/j.biortech.2020.124155.
Zhang J, Zeng G, Chen Y, Yu M, Yu Z, Li H, et al. Effects of physico-chemical parameters on the bacterial and fungal communities during agricultural waste composting. Biores Technol. 2011;102(3):2950–6.
Tiquia SM. Microbial community dynamics in manure composts based on 16S and 18S rDNA T-RFLP Profiles. Environ Technol. 2005. https://doi.org/10.1080/09593332608618482.
Insam H, Bertoldi MD. . Chapter 3 microbiology of the composting process. Waste Management. 2007;8(07):25–48.
Ren G, Xu X, Qu J, Zhu L, Wang T. Evaluation of microbial population dynamics in the co-composting of cow manure and rice straw using high throughput sequencing analysis. World J Microbiol Biotechnol. 2016. https://doi.org/10.1007/s11274-016-2059-7.
Huhe n, Jiang C, Wu Y, Cheng Y. Bacterial and fungal communities and contribution of physicochemical factors during cattle farm waste composting. MicrobiologyOpen. 2017. https://doi.org/10.1002/mbo3.518.
Xu J, Jiang Z, Li M, Li Q. A compost-derived thermophilic microbial consortium enhances the humification process and alters the microbial diversity during composting. J Environ Manage. 2019. https://doi.org/10.1016/j.jenvman.2019.05.008.
Ren S, Lu A, Guo X, Zhang Q, Wang Y, Guo X, et al. Effects of co-composting of lincomycin mycelia dregs with furfural slag on lincomycin degradation, degradation products, antibiotic resistance genes and bacterial community. Biores Technol. 2018. https://doi.org/10.1016/j.biortech.2018.10.014.
Qian Y, Shiqiu Z, Xueping L, Kun R, Jialiang L, Lihua J. Effects of microbial inoculant and additives on pile composting of cow manure. Front Microbiol. 2023. https://doi.org/10.3389/fmicb.2022.1084171.
de Gannes V, Eudoxie G, Hickey WJ. Prokaryotic successions and diversity in composts as revealed by 454-pyrosequencing. Biores Technol. 2013. https://doi.org/10.1016/j.biortech.2013.01.138.
Ariesyady HD, Ito T, Okabe S. Functional bacterial and archaeal community structures of major trophic groups in a full-scale anaerobic sludge digester. Water Res. 2007. https://doi.org/10.1016/j.watres.2006.12.036.
Tian W, Sun Q, Xu D, Zhang Z, Chen D, Li C, et al. Succession of bacterial communities during composting process as detected by 16S rRNA clone libraries analysis. Int Biodeterior Biodegradation. 2013. https://doi.org/10.1016/j.ibiod.2012.12.008.
Barnard R, Agroecologie GDR, Dijon I. Responses of soil bacterial and fungal communities to summer desiccation and rewetting in Mediterranean grasslands: summer precipitation pattern matters. Gen Inform. 2014.
de Hoon MJL, Eichenberger P, Vitkup D. Hierarchical evolution of the bacterial sporulation network. Curr Biol. 2010. https://doi.org/10.1016/j.cub.2010.06.031.
Steger K, Jarvis A, Vasara T, Romantschuk M, Sundh I. Effects of differing temperature management on development of actinobacteria populations during composting. Res Microbiol. 2007. https://doi.org/10.1016/j.resmic.2007.05.006.
Langarica-Fuentes A, Zafar U, Heyworth A, Brown T, Fox G, Robson GD. Fungal succession in an in-vessel composting system characterized using 454 pyrosequencing. FEMS Microbiol Ecol. 2014. https://doi.org/10.1111/1574-6941.12293.
Zhang L, Zhang H, Wang Z, Chen G, Wang L. Dynamic changes of the dominant functioning microbial community in the compost of a 90-m(3) aerobic solid state fermentor revealed by integrated meta-omics. Biores Technol. 2016. https://doi.org/10.1016/j.biortech.2015.12.040.
Suren S, Madlala AM, Prior BA. Thermomyces lanuginosus: properties of strains and their hemicellulases. FEMS Microbiol Rev. 2010;1:3–16.
Hirvonen M, Papageorgiou AC. Crystal structure of a family 45 endoglucanase from Melanocarpus albomyces: mechanistic implications based on the free and cellobiose-bound forms. J Mol Biol. 2003;329(3):403–10.
Yin Y, Li M, Tao X, Yang C, Zhang W, Li H, et al. Biochar enhanced organic matter transformation during pig manure composting: roles of the cellulase activity and fungal community. J Environ Manage. 2023. https://doi.org/10.1016/j.jenvman.2023.117464.
Wang G, Li C, Guo Y, Guo L, Wang M, Li T, et al. Screening of Dominant Cellulose-Degrading Microbe in Humus and optimisation of its enzyme producing conditions and application optimization of enzyme production conditions and evaluation of efficient straw degradation by penicillium oxalicum. Am J Biochem Biotechnol. 2023;19:10.
Gong P, Gao D, Hu X, Tan J, Wu L, Liu W, et al. Changes of bacterial and fungal communities and relationship between keystone taxon and physicochemical factors during dairy manure ectopic fermentation. PLoS ONE. 2022. https://doi.org/10.1371/journal.pone.0276920.
Ramos SMS, Cruz R, Barbosa RdN, Machado AR, Costa AFd, Motta CMdS, et al. Penicillium and Talaromyces Communities of Sugarcane Soils (Saccharum officinarum L.): Ecological and Phylogenetic Aspects. J Agricul Sci. 2018. https://doi.org/10.5539/jas.v10n4p335.
Abid N, Sayadi S. Detrimental effects of olive mill wastewater on the composting process of agricultural wastes. Waste Manage. 2005. https://doi.org/10.1016/j.wasman.2005.06.015.
Sun Y, Sheng S, Jiang X, Bello A, Wu X, Meng Q, et al. Genetic associations as indices for assessing nitrogen transformation processes in co-composting of cattle manure and rice straw. Biores Technol. 2019. https://doi.org/10.1016/j.biortech.2019.121815.
Bremner JM. Determination of nitrogen in soil by the Kjeldahl method. J Agric Sci. 2009. https://doi.org/10.1017/s0021859600021572.
Sun Y, Liu X, Sun L, Men M, Wang B, Deng L, et al. Microecological insight to fungal structure and key fungal communities regulating nitrogen transformation based on spatial heterogeneity during cow manure composting by multi-angle and multi-aspect analyses. Waste Manage. 2022. https://doi.org/10.1016/j.wasman.2022.02.013.
Gao XS, Xiao Y, Deng LJ, Li QQ, Wang CQ, Li B, et al. Spatial variability of soil total nitrogen, phosphorus and potassium in Renshou County of Sichuan Basin, China. J Integ Agriculture. 2019; https://doi.org/10.1016/s2095-3119(18)62069-6.
Wang Q, Zhang J, Xin X, Zhao B, Ma D, Zhang H. The accumulation and transfer of arsenic and mercury in the soil under a long-term fertilization treatment. J Soils Sediments. 2015. https://doi.org/10.1007/s11368-015-1227-y.
Naylor SJ, Moccia RD, Durant GM. The chemical composition of settleable solid fish waste (Manure) from commercial rainbow trout farms in Ontario. Canada North American J Aquacul. 1999. https://doi.org/10.1577/1548-8454(1999)061%3c0021:tccoss%3e2.0.co;2.
Wolf M, Baretta D, Becegato VA, Almeida VdC, Paulino AT. Copper/Zinc Bioaccumulation and the Effect of Phytotoxicity on the Growth of Lettuce ( Lactuca sativa L.) in Non-contaminated, Metal-Contaminated and Swine Manure-Enriched Soils. Water Air Soil Pollut. 2017. https://doi.org/10.1007/s11270-017-3345-1.
Li C, Zhu T, Su G. Determination of chloride content for compound fertilizers. General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China. 2010:1–2.
Liu J-h, Zhang M-l, Zhang R-y, Zhu W-y, Mao S-y. Comparative studies of the composition of bacterial microbiota associated with the ruminal content, ruminal epithelium and in the faeces of lactating dairy cows. Microb Biotechnol. 2016. https://doi.org/10.1111/1751-7915.12345.
Yeh Y-C, McNichol J, Needham DM, Fichot EB, Berdjeb L, Fuhrman JA. Comprehensive single-PCR 16S and 18S rRNA community analysis validated with mock communities, and estimation of sequencing bias against 18S. Environ Microbiol. 2021. https://doi.org/10.1111/1462-2920.15553.
Wang K, Yin X, Mao H, Chu C, Tian Y. Changes in structure and function of fungal community in cow manure composting. Biores Technol. 2018. https://doi.org/10.1016/j.biortech.2018.01.064.
Tian X, Yang T, He J, Chu Q, Jia X, Huang J. Fungal community and cellulose-degrading genes in the composting process of Chinese medicinal herbal residues. Biores Technol. 2017. https://doi.org/10.1016/j.biortech.2017.05.116.
Sun Y, Men M, Xu B, Meng Q, Bello A, Xu X, et al. Assessing key microbial communities determining nitrogen transformation in composting of cow manure using illumina high-throughput sequencing. Waste Manage. 2019. https://doi.org/10.1016/j.wasman.2019.05.007.
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. Embnet J. 2011;17(1):10–2.
Callahan BJ, Mcmurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods.
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013. https://doi.org/10.1093/nar/gks1219.
Zheng A, Liu J, Wang M, Bu N, Liu D, Wei C. Footprint analysis of CO2 in microbial community succession of raw milk and assessment of its quality. Front Nutr. 2023. https://doi.org/10.3389/fnut.2023.1285653.
Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Caporaso JG. QIIME 2: Reproducible, interactive, scalable, and extensible microbiome data science. 2018.
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011. https://doi.org/10.1186/gb-2011-12-6-r60.
Birhanu L, Bekele T, Tesfaw B, Demissew S. Relationships between topographic factors, soil and plant communities in a dry Afromontane forest patches of Northwestern Ethiopia. PLoS ONE. 2021. https://doi.org/10.1371/journal.pone.0247966.
Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecological monographs 27. Ecolog Monographs. 1957;27(4):325–49.
Ramette A. Multivariate analyses in microbial ecology. FEMS Microbiol Ecol. 2007. https://doi.org/10.1111/j.1574-6941.2007.00375.x.
Zhang J, Zeng G, Chen Y, Yu M, Yu Z, Li H, et al. Effects of physico-chemical parameters on the bacterial and fungal communities during agricultural waste composting. Biores Technol. 2010. https://doi.org/10.1016/j.biortech.2010.11.089.
Acknowledgements
We sincerely thank all the authors for their help in this work and Shanghai Bioprofile Technology Company Ltd. for their technological assistance.
Funding
This research was funded by the Key R & D project of the Ningxia Hui Autonomous Region (2022BBF03024), the Science and Technology Innovation Demonstration Project for High Quality Agricultural Development and Ecological Protection of the Ningxia Academy of Agriculture and Forestry Sciences (NGSB-2021–12-05), and the Foundation of Major Science and Technology Projects of Henan Province of China (191110110600).
Author information
Authors and Affiliations
Contributions
M.Y. and Y.G. designed the methodology, conducted the test and wrote the manuscript; M.W. isolated Penicillium oxalicum; J.W. and F.Y. composted and collected the samples; Y.G. revised the manuscript; S.H. and X.l. provided resources and made the review. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Yang, M., Guo, Y., Yang, F. et al. Dynamic changes in and correlations between microbial communities and physicochemical properties during the composting of cattle manure with Penicillium oxalicum. BMC Microbiol 24, 301 (2024). https://doi.org/10.1186/s12866-024-03449-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-024-03449-4